Abstract
Background: In daily clinical practice, patients often refer temporomandibular or cervical complaints after different oral procedures, especially in lengthy procedures that can result in iatrogenic postures or trauma that can affect the temporomandibular joint (TMJ). This study aimed to evaluate the clinical and functional changes of the temporomandibular joint (TMJ) and cervical region immediately after a session of root canal therapy. Methods: Twenty-nine subjects who received a session of root canal therapy were included. Clinical assessments included mouth opening, cervical mobility, pain intensity, pressure pain thresholds (PPTs), and myofascial trigger points (MTrPs) of the jaw and neck muscles. Results: After the intervention, a significant reduction in mouth opening (41.90 mm; SD = 6.21) was observed compared to baseline (46.28 mm; SD = 6.17) (p < 0.001). A significant reduction in PPTs and cervical mobility (p < 0.05), and an increase in MTrPs (p = 0.002–0.026) were demonstrated after the intervention. Conclusions: A session of root canal therapy can produce an immediate significant reduction in mouth opening, PPTs, and cervical mobility, and an increase in MTrPs. The risk can be higher if there is a previous TMJ limitation.
1. Introduction
In daily clinical practice, patients often experience temporomandibular or cervical complaints after different oral procedures [1]. Root canal therapy, one of the most common dental procedures, is a lengthy treatment, especially for multirooted teeth. The temporomandibular joint (TMJ) and its related structures may be impaired as a consequence of prolonged dental procedures through iatrogenic posture or trauma [2,3,4]. There have been reports of complications after third molar extraction ranging from 3.4% to 4.6% [2,5]. and this type of extraction appears to increase the risk of developing posterior temporomandibular disorders (TMDs) [6]. However, there is no consensus on the prevalence of TMDs after endodontic procedures.
Temporomandibular disorders are a subclass of craniofacial pain problems affecting the TMJ, masticatory muscles, and related musculoskeletal tissues in the head and neck [7]. TMDs are regarded as one of the major contributors to non-odontogenic pain affecting the bones, ligaments, joints, and muscles of the orofacial region. Nevertheless, myofascial pain, characterized by muscle tenderness associated with pain, is the most common disorder within TMDs [8]. TMDs have a complex etiology that includes biomechanical, neuromuscular, bio-psychosocial, and biological aspects [9]. Iatrogenic injuries during any dental procedure involving prolonged mouth opening may act as a precipitating or predisposing factor for TMDs [10].
Decreased mouth opening is a well-known side effect of prolonged dental procedures, including root canal therapy [1,11]. Because of its importance, decreased mouth opening has negative effects on function and quality of life. Speaking, swallowing, and chewing are all controlled by the TMJ. Several therapies and health professionals can help patients with TMDs [12], and approximately 50% of TMDs could benefit from musculoskeletal treatment [13]. Considering the anatomical and neurophysiological relationship between the TMJ and the cervical spine, the cervical component should be treated in order to improve TMDs [7].
Although dental procedures are common, and their potential relationship with TMDs is widely recognized in clinical practice, evidence of temporomandibular and cervical complications after various oral procedures is lacking. Therefore, the purpose of this study was to investigate the effects and risk factors of 90 min lengthy root canal therapy on the TMJ and its related structures.
2. Materials and Methods
2.1. Study Design
This longitudinal, prospective, quasi-experimental study was approved by the Clinical Research Ethics Committee of Aragón under protocol number (PI18/370) and conducted in accordance with the principles of the Declaration of Helsinki of the World Medical Association. All participants signed an informed consent form before their participation in the study.
2.2. Participants
Twenty-nine consecutive subjects (13 women and 16 men with a mean age of 45.6 years (SD = 16.4)) participated in the study. The recruitment period lasted from January 2021 to May 2021. Subjects were recruited from among patients scheduled to undergo endodontic surgery at the Servicio de Prácticas Odontológicas at the University of Zaragoza (Huesca). The inclusion criteria were age > 18 years and the requirement for endodontic intervention. Participants were excluded if they had undergone TMJ or cervical treatment in the past month, or if they showed any red flags, neurological or cognitive impairment (inability to understand questionnaires or examinations). Patients underwent root canal therapy in a maximum mouth opening posture for 90 min on average.
2.3. Measurements
After patient inclusion, demographic information, including age, sex, height, weight, and body mass index (BMI), was gathered. In addition, cranio-cervico-mandibular questionnaires, including TMD Pain Screener [14], Helkimo Index [15], Jaw Functional Limitation Scale (JFLS20) [16], Neck Disability Index (NDI) [17], and Headache Impact Test (HIT-6) [18] were collected in order to analyze patients’ conditions at baseline. Maximum active mouth opening was considered the primary variable. Secondary variables included cervical range of motion, pain intensity, and pressure pain thresholds. Two evaluators with more than ten years of experience in cervical spine and TMJ assessment took measurements before the intervention (baseline) and immediately after the intervention. Both evaluators were blinded to the measurements and the study goals.
2.3.1. Maximum Active Mouth Opening
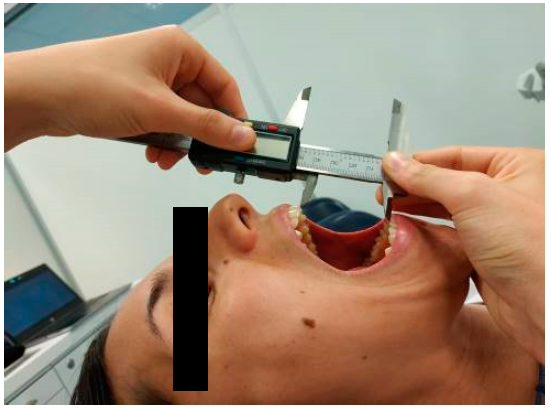
Maximum active mouth opening was measured in supine position using an electronic caliper (HIBOK DC-516). Patients were told to expand their mouths as widely as they could without experiencing pain [19,20]. The distance between the upper and lower central incisors (interincisal distance) was measured in mm [21] (Figure 1). Measurements were repeated twice and the mean was calculated for later analysis. This measurement has demonstrated a high reliability (ICC = 0.9–0.98) [22] and the smallest detectable difference is 5 mm [23].
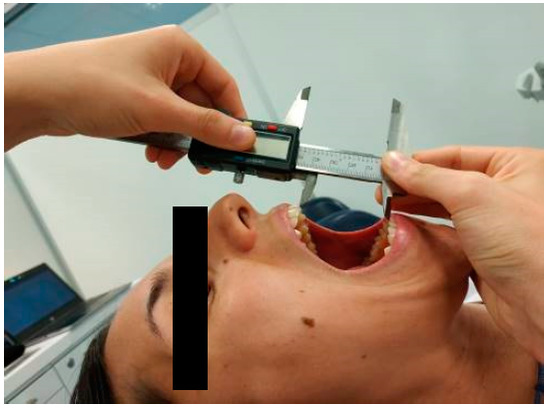
Figure 1.
Measurement of maximum active mouth opening in supine position.
2.3.2. Cervical Spine Range of Movement
The cervical spine’s range of motion was measured using a regular goniometer and the Clinometer App (version 2.4) for smartphones. Cervical flexion, extension, right/left-side bending, right/left rotation, and upper cervical spine flexion and extension were measured. In order to evaluate cervical mobility, the Clinometer has proven to be a feasible and reliable tool (ICC = 0.68–0.97) [24]. For cervical spine testing, patients were seated with their spines supported against a high back chair in a neutral spine position. Participants were told to move their head and neck as far as they could without experiencing pain in all directions, including flexion, extension, right/left lateral flexion, and rotation to the right/left. In order to assess the upper cervical spine, patients were instructed to produce the greatest amount of flexion and extension without experiencing any pain while standing, with their thoracic spine and occiput touching the wall [25] (Figure 2). Two repeated measures were made, and the mean was calculated for statistical analysis.

Figure 2.
Starting positions for sagittal plane, frontal plane and horizontal plane measurements.
2.3.3. Pain
A visual analogue scale (VAS) from 0 to 10 was used to quantify the intensity of the pain (where 0 is absence of pain and 10 is the worst pain imaginable). This assessment has proven to be valid and reliable [26], with a minimum clinically significant difference between 13 and 28 mm depending on baseline pain severity [27,28].
2.3.4. Myofascial Trigger Points (MTrPs)
Six temporomandibular and cervical muscles were evaluated bilaterally through palpation: masseter, temporalis, sternocleidomastoid, upper trapezius, splenius capitis and suboccipital muscles. The diagnosis of MTrPs was performed following the criteria described by Travell and Simons [29]: (1) presence of a taut band within the muscle; (2) presence of a hypersensitive tender spot in the taut band; (3) local twitch response elicited by the snapping palpation of the taut band; (4) and reproduction of the referred pain pattern of the muscle. When the pain produced by palpation reproduced the patient’s typical symptoms, MTrPs were considered active; however, if the pain produced by palpation was unfamiliar to the patient, MTrPs were considered latent [30].
2.3.5. Pressure Pain Threshold
The pressure pain threshold (PPT) is defined as the minimum intensity of pressure that is perceived as painful [31]. A handheld algometer (Wagner, Model FPK) with a round surface area of 1 cm2 was used to measure PPTs (Wagner Force Dial FDK 20) [32]. The PPT was assessed bilaterally in the masseter, temporalis, upper trapezius, splenius capitis and suboccipital muscles, as well as the temporomandibular joint after a washout period of one minute. In order to standardize the measurement point, the central part of each muscle belly and the center of the articular line of the TMJ were located and marked with an ink dot. In order to assess central sensitization (general pain hypersensitivity) in a non-interventional anatomical region, the thenar eminence was also assessed. The pressure on the algometer was increased at a constant rate of 1 kg/cm2/s while it was held perpendicular to the muscle being evaluated. When the pressure sensation turned into pain, the patients were instructed to raise their hands. Mechanosensitivity can be measured using the PPT examination, which has been proven to be valid and reliable [22,33].
2.4. Procedure
The study was conducted in three stages. These stages were developed during the same consultation and were as follows:
- Pre-endodontic treatment assessment: Two blinded evaluators (evaluator 2 and evaluator 3), carried out the assessment of the outcome measures. This evaluation was developed prior to root canal therapy and took approximately 10 min.
- Endodontic treatment: After the assessment (baseline) the subjects received endodontic treatment for 90 min.
- Post-endodontic treatment assessment: Once the endodontic treatment was completed, the second measurement of the dependent variables was performed by evaluators 2 and 3. This assessment lasted approximately 10 min.
Data analysis was carried out by a researcher who was blind of the nature of the intervention and the date of data collection, in order to minimize the risk of bias in the interpretation of results.
2.5. Statistical Analysis
A statistical software package, SPSS for Windows v20.0 (SPSS Inc., Chicago, IL, USA), was used to analyze the data. For quantitative variables, mean and standard deviation were determined. For qualitative variables, frequencies and percentages were obtained. The normal distribution of the variables was examined using the Kolmogorov–Smirnov test (p > 0.05). The paired samples Student’s t-test or the paired samples Wilcoxon test, for normally distributed data or non-normally distributed data, respectively, were used to evaluate intra-group comparisons of quantitative variables. In order to evaluate the differences in nominal variables, the chi-square test was utilized. To figure out the magnitude of the changes, Cohen’s d effect size was determined. The potential risk factors were selected based on the assumed risk to influence the primary outcome variable: mouth opening (group ≤ 35 mm; group > 35 mm) [34]. The level of significance was established at p < 0.05.
3. Results
The sample was composed of 29 subjects (55% men) with a mean age of 45.6 years (SD = 16.4) and a mean weight and height of 71.1 kg (SD = 14.1) and 167.6 cm (SD = 8.2), respectively. Of the total sample, 31% were smokers. Subjects showed a pre-intervention mean mouth opening of 46.28 mm (SD = 6.17), an average score in the TMD Pain Screener of 1.76 (SD = 2.10), in the JFLS 20 of 0.84 (SD = 1.15), in the Helkimo Index of 1.10 (SD = 1.82), in the HIT-6 of 42.2 (SD = 8.5), and in the NDI of 4.3 (SD = 5.5).
3.1. Maximum Active Mouth Opening
The range of motion of the TMJ was significantly reduced immediately after the endodontic intervention (41.90 mm; SD = 6.21) compared to baseline (46.28 mm; SD = 6.17) (p < 0.001) (Table 1).

Table 1.
Range of motion of the TMJ and cervical spine at baseline and after the intervention. Values are expressed as mean ± standard deviation or mean (min–max).
3.2. Cervical Spine Range of Movement
After the endodontic intervention, participants evidenced a significant reduction in general cervical mobility (flexion, extension, side bending, and rotation to the left) to baseline (p < 0.05). No significant differences were found for right cervical rotation and upper cervical mobility after endodontic intervention (p = 0.210–0.421). Table 1 shows the range of motion of the cervical spine at baseline and post-intervention.
3.3. Myofascial Trigger Points
Immediately after the endodontic intervention, there was a significant change in the distribution of MTrPs in masseter and upper trapezius muscles (bilaterally), and in splenius and suboccipital muscles (left side) (p = 0.002–0.026). This change in the distribution pattern of MTrPs reflected a significant tendency to activate latent MTrPs and to generate new MTrPs (Table 2).

Table 2.
Myofascial Trigger Points in masticatory and cervical muscles. Values are expressed as percentage (total number).
3.4. Pain and Pressure Pain Thresholds
The pain intensity measured by VAS did not change immediately after the intervention (p = 0.177). Nevertheless, PPTs of masseter, temporalis, upper trapezius, suboccipital, splenius capitis (left side) muscles, TMJ, and thenar eminence were significantly reduced immediately after the intervention (Table 3).

Table 3.
Pressure pain thresholds in masticatory muscles, neck muscles, TMJ and thenar eminence. Values are expressed as mean ± standard deviation or mean (min-max).
3.5. Potential Risk Factors for Limitation of Mouth Opening after an Endodontic Intervention
The potential risk factors that could predict a limitation of mouth opening (range of motion less than or equal to 35 mm) after an endodontic procedure were a previously reduced range of mouth opening and higher scoring in the Helkimo Index (Table 4).

Table 4.
Descriptive and comparative values of patients with/without limitation in mouth opening after endodontic intervention.
4. Discussion
To the best of our knowledge, this is the first study to examine temporomandibular and cervical dysfunctions immediately after root canal therapy. Our results demonstrate that 90 min of root canal therapy can produce a significant reduction in mouth opening, PPTs, and cervical mobility, as well as an increase in MTrPs.
4.1. Temporomandibular Dysfunctions
In the current study, mouth opening was significantly reduced by 5 mm immediately after the endodontic intervention (from 46.28 ± 6.17 mm at baseline to 41.90 ± 6.21 mm after the intervention). This range of mouth opening is above the cut-off for trismus (35 mm). However, mouth opening limitation has been demonstrated to reduce functioning and quality of life [35,36]. These findings support previous studies that have evidenced a reduction in mouth opening after other dental procedures (lower third molar extractions) [37]. In oncologic patients, the amount of mouth opening after a dental procedure directly affects quality of life and can become a serious burden that affects chewing, eating, or swallowing [34]. This finding could be explained because of the release of inflammatory mediators secondary to the dental procedure, which can cause pain, edema, and spasm of the masticatory muscles, limiting mouth opening.
Regarding MTrPs and PPTs in the temporomandibular region, the results of the present study evidenced a significant activation of MTrPs of the masseter and a reduction in PPTs in the masseter and temporalis muscles. These findings could be explained by an innate protective mechanism caused by primary hyperalgesia, a consequence of the long period of maximal mouth opening required for the intervention (90 min), leading to an increase in the tone of the masticatory muscles [38]. Moraes et al. (2015) found no alterations in muscle tone of the masseter and temporalis muscles after a dental intervention [39]. However, Buesa-Bárez et al. (2018) demonstrated electromyographic changes in masseter and temporalis muscles after dental procedures (lower third molar extractions) [37]. This contrast may reflect differences in the duration or timing of the dental procedure, emphasizing shorter and more careful extraction procedures [39].
Although some authors consider that a causal relationship between dental procedures and TMJ injury is currently poorly established, Huang et al. (2006) demonstrated in a total sample of 34,491 subjects that third molar extraction appears to be a risk factor for TMD, with a relative risk of 1.6 (CI = 1.3–2.0) [6]. Holding the maximum mouth opening for an extended period of time and the micro/macro trauma exerted during the procedure can lead to TMJ dysfunction [3,40]. This risk should be taken into account in order to minimize mechanical stress to the joint during dental procedures.
4.2. Cervical Dysfunctions
In the present study, general cervical mobility and PPTs of cervical muscles were significantly reduced immediately after the endodontic intervention. These findings were accompanied by activation of the MTrPs of the upper trapezius, suboccipital, and splenius muscles. To the best of our knowledge, this is the first study to examine the immediate cervical implications of dental treatment. Cervical dysfunction immediately after the endodontic intervention can be explained by multiple anatomical and neurophysiological connections between the cervical and temporomandibular regions through the trigeminocervical nucleus [41]. Previous studies have demonstrated that patients with temporomandibular disorders present a reduction in PPTs in cervical muscles [42,43,44]. The current study also evidenced a reduction in thenar eminence PPTs (remote from the point of intervention), which may reflect the interaction of another neurophysiological mechanism, a widespread hyperalgesia caused by central sensitization [45].
4.3. Risk Factors
Previous studies have demonstrated risk factors associated with complications after dental procedures, including age, gender, medical or dental history, smoking, surgical time, and technique aspects [5,46]. Sahebi et al. (2010) found that women experience more pain than men after an endodontic procedure, which could be due to a smaller mouth opening, biological, or cultural factors [11]. In this study, a previous mouth opening restriction and a higher score in the Helkimo index (indicating TMD) are presented as potential risk factors for predicting mouth opening restriction (≤35 mm) after endodontic procedures. These risk factors predicting a reduction in mouth opening after an endodontic intervention will allow for the development of a multivariable predictive model on larger sample sizes in future studies.
4.4. Clinical Implications and Future Perspectives
The results of the present study suggest relevant clinical implications. The aim of this study was to investigate the incidence and risk factors of temporomandibular and cervical complications after endodontic procedures.
Root canal therapy is a common treatment that has shown great benefits in relieving symptoms and restoring function to patients. However, this study showed that very prolonged procedures can cause TMJ and cervical symptoms in patients. A decrease in mouth opening, PPTs, and cervical mobility, and an increase in MTrPs were demonstrated, with higher risk in patients with previous mouth opening limitation and TMDs. These results should be considered in the preventive and post-interventional management of these patients.
Several treatment methods have been proposed to treat the musculoskeletal symptoms of TMDs, including manual therapy, exercise, education, or dry needling [12]. However, there is limited evidence concerning definitive management of mouth opening restrictions and cervical dysfunction [47,48], so prevention should be the primary focus. Earlier recognition of these patients could help to develop preventive interventions. Care should be taken in the use of mechanical stress for the TMJ and cervical spine during some dental procedures. Different devices could be used to stabilize the mandible, such as a bite block [46].
The development of a prediction model could be useful to adopt preventive measures in patients with a high risk of temporomandibular complications after endodontic procedures. The results of this study should be validated, and can be used to develop a preventive program for temporomandibular and cervical complications after endodontic procedures. Exploring the combination of multidisciplinary treatment between dentists and physiotherapists could be a potential solution to decrease post-intervention pain or even find alternatives for conducting physiotherapy treatment during the same session.
4.5. Limitations
A quasi-experimental study without a control group was conducted, which represents a limitation of the present study. The absence of a control group means that it is not possible to compare the outcome of the experimental group with a group that has not been exposed to the intervention or treatment, which can make it difficult to determine whether the observed changes are the result of the intervention or simply the result of external or random factors.
Furthermore, the main limitation of studies that aimed at identifying risk factors for developing a complication after a treatment intervention is their retrospective design. Although this study has a prospective design, an important limitation is its relatively small sample size, as in other prospective studies [34,49]. Consequently, it has been impossible to statistically predict the longitudinal course of the main variable, as several risk factors could be present. Multiple regression analyzes should be performed with larger sample sizes in order to examine associations between TMD and the variables of interest. On the other hand, another limitation is that only immediate effects were assessed. The immediate examination of this study represents an advantage over previous studies, since it is possible that neurophysiological mechanisms underlying muscle activation have an immediate pattern. However, these results cannot be generalized to the mid/long term.
5. Conclusions
The results of the present study demonstrate that a 90 min endodontic procedure can result in a significant decrease in mouth opening ability, PPTs, and cervical mobility, and an increase in MTrPs, with a higher risk in patients with a previous restriction of mouth opening and presence of TMD (higher score in the Helkimo Index). Functional examination of the TMJ and cervical spine should be considered before root canal therapy.
Author Contributions
Conceptualization, M.M.-U. and Ó.A.-E.; methodology, I.A.-C., M.M.-U. and A.C.-U.; formal analysis, S.C.-S. and I.A.-C.; investigation, S.P.-S., S.C.-S., A.C.-U. and I.A.-C.; data curation, Ó.A.-E. and S.C.-S.; writing—original draft preparation, M.M.-U., S.C.-S., A.C.-U. and I.A.-C.; writing—review and editing, all authors; supervision, A.C.-U. and M.M.-U. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Clinical Research Ethics Committee of Aragón (protocol code PI18/370).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Juhl, G.I.; Jensen, T.S.; Norholt, S.E.; Svensson, P. Incidence of symptoms and signs of TMD following third molar surgery: A controlled, prospective study. J. Oral Rehabil. 2009, 36, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Contar, C.; De Oliveira, P.; Kanegusuku, K.; Berticelli, R.S.; Azevedo-Alanis, L.-R.; Machado, M.-A.-N. Complications in third molar removal: A retrospective study of 588 patients. Med. Oral Patol. Oral Cir. Bucal 2010, 15, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.J.; LeResche, L.; Critchlow, C.W.; Martin, M.D.; Drangsholt, M.T. Risk factors for diagnostic subgroups of painful Temporomandibular Disorders (TMD). J. Dent. Res. 2002, 81, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Lago-Méndez, L.; Diniz-Freitas, M.; Senra-Rivera, C.; Gude-Sampedro, F.; Gándara Rey, J.M.; García-García, A. Relationships Between Surgical Difficulty and Postoperative Pain in Lower Third Molar Extractions. J. Oral Maxillofac. Surg. 2007, 65, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Bui, C.H.; Seldin, E.B.; Dodson, T.B. Types, Frequencies, and Risk Factors for Complications after Third Molar Extraction. J. Oral Maxillofac. Surg. 2003, 61, 1379–1389. [Google Scholar] [CrossRef]
- Huang, G.J.; Rue, T.C. Third-molar extraction as a risk factor for temporomandibular disorder. J. Am. Dent. Assoc. 2006, 137, 1547–1554. [Google Scholar] [CrossRef]
- De Leeuw, R.; Klasser, G.D. Orofacial Pain: Guidelines for Assessment, Diagnosis, and Management, 5th ed.; American Academy of Orofacial Pain, Ed.; Quintessence Publishing Co., Inc.: Chicago, IL, USA, 2018. [Google Scholar]
- Manfredini, D.; Guarda-Nardini, L.; Winocur, E.; Piccotti, F.; Ahlberg, J.; Lobbezoo, F. Research diagnostic criteria for temporomandibular disorders: A systematic review of axis i epidemiologic findings. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2011, 112, 453–462. [Google Scholar] [CrossRef]
- Chisnoiu, A.M.; Picos, A.M.; Popa, S.; Chisnoiu, P.D.; Lascu, L.; Picos, A.; Chisnoiu, R. Factors Involved in the Etiology of Temporomandibular Disorders—A Literature Review. Clujul. Med. 2015, 88, 473–478. [Google Scholar] [CrossRef]
- Sharma, S.; Pal, U.; Gupta, D.; Jurel, S. Etiological factors of temporomandibular joint disorders. Natl. J. Maxillofac. Surg. 2011, 2, 116–119. [Google Scholar] [CrossRef]
- Sahebi, S.; Moazami, F.; Afsa, M.; Nabavi Zade, M.R. Effect of lengthy root canal therapy sessions on temporomandibular joint and masticatory muscles. J. Dent. Res. Dent. Clin. Dent. Prospect. 2010, 4, 95–97. [Google Scholar]
- Wright, E.F.; North, S.L. Management and Treatment of Temporomandibular Disorders: A Clinical Perspective. J. Man. Manip. Ther. 2009, 17, 247–254. [Google Scholar] [CrossRef]
- Cooper, B.C.; Kleinberg, I. Examination of a Large Patient Population for the lar Disorders. J. Craniomandib. Pract. 2007, 25, 114–126. [Google Scholar]
- González, Y.; Schiffmann, E.; Gordon, S.; Seago, B.; Truelove, E.; Slade, G.; Ohrbach, R. Development of a brief and effective temporomandibular disorder pain screening questionnaire: Reliability and validity. J. Am. Dent. Assoc. 2011, 142, 1183–1191. [Google Scholar] [CrossRef]
- Rani, S.; Pawah, S.; Gola, S.; Bakshi, M. Analysis of Helkimo index for temporomandibular disorder diagnosis in the dental students of Faridabad city: A cross-sectional study. J. Indian Prosthodont. Soc. 2017, 17, 48–52. [Google Scholar] [CrossRef]
- Ohrbach, R.; Larsson, P. The Jaw Functional Limitation Scale: Development, Reliability, and Validity of 8-Item and 20-Item Versions. J. Orofac. Pain 2008, 22, 219–230. [Google Scholar]
- Vernon, H.; Mior, S. The Neck Disability Index: A study of reliability and validity. J. Manip. Physiol. Ther. 1991, 14, 409–415. [Google Scholar]
- Martin, M.; Blaisdell, B.; Kwong, J.W.; Bjorner, J.B. The Short-Form Headache Impact Test (HIT-6) Was Psychometrically Equivalent in Nine Languages. J. Clin. Epidemiol. 2004, 57, 1271–1278. [Google Scholar] [CrossRef]
- Bretischwerdt, C.; Rivas-Cano, L.; Palomeque-del-Cerro, L.; Fernández-de-las-Peñas, C.; Alburquerque-Sendín, F. Immediate Effects of Hamstring Muscle Stretching on Pressure Pain Sensitivity and Active Mouth Opening in Healthy Subjects. J. Manip. Physiol. Ther. 2010, 33, 42–47. [Google Scholar] [CrossRef]
- George, J.W.; Fennema, J.; Maddox, A.; Nessler, M.; Skaggs, C.D. The effect of cervical spine manual therapy on normal mouth opening in asymptomatic subjects. J. Chiropr. Med. 2007, 6, 141–145. [Google Scholar] [CrossRef]
- Mansilla-Ferragut, P.; Fernández-de-las Peñas, C.; Alburquerque-Sendín, F.; Cleland, J.A.; Boscá-Gandía, J.J. Immediate Effects of Atlanto-Occipital Joint Manipulation on Active Mouth Opening and Pressure Pain Sensitivity in Women with Mechanical Neck Pain. J. Manip. Physiol. Ther. 2009, 32, 101–106. [Google Scholar] [CrossRef]
- Goulet, J.-P.; Clark, G.T.; Flack, V.F.; Liu, C. The Reproducibility of Muscle and Joint Tenderness Detection Methods and Maximum Mandibular Movement Measurement for the Temporomandibular System. J. Orofac. Pain 1998, 12, 17–26. [Google Scholar] [PubMed]
- Kropmans, T.J.B.; Dijkstra, P.U.; Stegenga, B.; Stewart, R.; De Bont, L.G.M. Smallest Detectable Difference in Outcome Variables Related to Painful Restriction of the Temporomandibular Joint. J. Dent. Res. 1999, 78, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sanz, J.; Carrasco-Uribarren, A.; Cabanillas-Barea, S.; Hidalgo-García, C.; Fanlo-Mazas, P.; Lucha-López, M.O.; Tricás-Moreno, J.M. Validity and reliability of two Smartphone applications to measure the lower and upper cervical spine range of motion in subjects with chronic cervical pain. J. Back Musculoskelet. Rehabil. 2018, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Strimpakos, N. The assessment of the cervical spine. Part 1: Range of motion and proprioception. J. Bodyw. Mov. Ther. 2011, 15, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.C.R.; De Azevedo, L.R.; De Souza, N.V.W.; Ferreira, F.V. Pain measurement in TMD patients: Evaluation of precision and sensitivity of different scales. J. Oral Rehabil. 2001, 28, 534–539. [Google Scholar] [CrossRef]
- Todd, K.H.; Funk, J.P. The minimum clinically important difference in physician-assigned visual analog pain scores. Acad. Emerg. Med. 1996, 3, 142–146. [Google Scholar] [CrossRef]
- Bird, S.B.; Dickson, E.W. Clinically significant changes in pain along the visual analog scale. Ann. Emerg. Med. 2001, 38, 639–643. [Google Scholar] [CrossRef]
- Simons, D.; Travell, J.; Simons, L. Dolor Y Disfunción Miofascial. El Manual de Los Puntos Gatillo. Volumen 1. Mitad Superior Del Cuerpo, 2nd ed.; Medica Panamericana: Madrid, Spain, 2002. [Google Scholar]
- Gerwin, R.D.; Shannon, S.; Hong, C.Z.; Hubbard, D.; Gevirtz, R. Interrater reliability in myofascial trigger point examination. Pain 1997, 69, 65–73. [Google Scholar] [CrossRef]
- Fischer, A.A. Pressure algometry over normal muscles. Standard values, validity and reproducibility of pressure threshold. Pain 1987, 30, 115–126. [Google Scholar] [CrossRef]
- Silva, R.d.S.; Conti, P.C.R.; Lauris, J.R.P.; da Silva, R.O.F.; Pegoraro, L.F. Pressure pain threshold in the detection of masticatory myofascial pain: An algometer-based study. J. Orofac. Pain 2005, 19, 318–324. [Google Scholar]
- do Nascimento, J.D.S.; Alburquerque-Sendín, F.; Vigolvino, L.P.; de Oliveira, W.F.; de Oliveira Sousa, C. Absolute and Relative Reliability of Pressure Pain Threshold Assessments in the Shoulder Muscles of Participants with and without Unilateral Subacromial Impingement Syndrome. J. Manip. Physiol. Ther. 2020, 43, 57–67. [Google Scholar] [CrossRef]
- Scott, B.; D’Souza, J.; Perinparajah, N.; Lowe, D.; Rogers, S.N. Longitudinal evaluation of restricted mouth opening (trismus) in patients following primary surgery for oral and oropharyngeal squamous cell carcinoma. Br. J. Oral Maxillofac. Surg. 2011, 49, 106–111. [Google Scholar] [CrossRef]
- Chantaracherd, P.; John, M.T.; Hodges, J.S.; Schiffman, E.L. Temporomandibular Joint Disorders’ Impact on Pain, Function, and Disability. J. Dent. Res. 2015, 94, 79–86. [Google Scholar] [CrossRef]
- Armijo-Olivo, S.; Fuentes, J.; Major, P.W.; Warren, S.; Thie, N.M.R.; Magee, D.J. The association between neck disability and jaw disability. J. Oral Rehabil. 2010, 37, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Buesa-Bárez, J.M.; Martín-Ares, M.; Martínez-Rodríguez, N.; Barona-Dorado, C.; Sanz-Alonso, J.; Cortés-Bretón-Brinkmann, J.; Martínez-González, J.M. Masseter and temporalis muscle electromyography findings after lower third molar extraction. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e92–e97. [Google Scholar] [CrossRef]
- Skyba, D.A.; Radhakrishnan, R.; Sluka, K.A. Characterization of a method for measuring primary hyperalgesia of deep somatic tissue. J. Pain 2005, 6, 41–47. [Google Scholar] [CrossRef]
- Moraes, M.; Naclerio-Homem, M.; Nascimento, R.; Oliveira Amorim, J.; Raldi, F. Analysis of masseter and temporal muscles during surgical extraction of impacted third molars. Gen. Dent. 2015, 63, e23-7. [Google Scholar]
- Damasceno, Y.S.S.; Espinosa, D.G.; Normando, D. Is the extraction of third molars a risk factor for the temporomandibular disorders? A systematic review. Clin. Oral Investig. 2020, 24, 3325–3334. [Google Scholar] [CrossRef]
- Armijo-Olivo, S.; Magee, D.J.; Parfitt, M.; Major, P.; Thie, N.M.R. The association between the cervical spine, the stomatognathic system, and craniofacial pain: A critical review. J. Orofac. Pain 2006, 20, 271–287. [Google Scholar]
- Campos López, A.; De-Miguel, E.E.; Malo-Urriés, M.; Acedo, T.C. Mouth opening, jaw disability, neck disability, pressure pain thresholds, and myofascial trigger points in patients with disc displacement with reduction: A descriptive and comparative study. CRANIO-J. Craniomandib. Pract. 2021. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Moreno, B.G.D.; Maluf, S.A.; Marques, A.P.; Crivello-Júnior, O. Clinical and quality-of-life assessment among women with temporomandibular disorder. Rev. Bras. Fisioter. 2009, 13, 210–214. [Google Scholar] [CrossRef]
- de Oliveira, N.; Marques, A.E.Z.S.; Pucci, R.L.A.; Sousa, É.A.; Ribeiro, F.T.; Navega, F.R.F.; Pedroni, C.R. Pain threshold in the masticatory and cervical muscles in different types of temporomandibular disorders. Man. Ther. Posturology Rehabil. J. 2016, 14, 1–4. [Google Scholar] [CrossRef]
- Bendsten, L. Central sensitization in tension-type headache--possible pathophysiological mechanisms. Cephalalgia 2000, 20, 486–508. [Google Scholar]
- Bouloux, G.F.; Steed, M.B.; Perciaccante, V.J. Complications of Third Molar Surgery. Oral Maxillofac. Surg. Clin. N. Am. 2007, 19, 117–128. [Google Scholar] [CrossRef]
- McNeely, M.L.; Armijo Olivo, S.; Magee, D.J. A systematic review of the effectiveness of physical therapy interventions for temporomandibular disorders. Phys. Ther. 2006, 86, 710–725. [Google Scholar] [CrossRef]
- Medlicott, M.S.; Harris, S.R. A Systematic Review of the Effectiveness of Exercise, Manual Training, and Biofeedback in the Management of Temporomandibular. Phys. Ther. 2006, 86, 955–973. [Google Scholar] [CrossRef]
- Lee, R.; Slevin, N.; Musgrove, B.; Swindell, R.; Molassiotis, A. Prediction of post-treatment trismus in head and neck cancer patients. Br. J. Oral Maxillofac. Surg. 2012, 50, 328–332. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).