Re-Thinking Table Salt Reduction in Bread with Halophyte Plant Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dried Sarcocornia and Other Ingredients
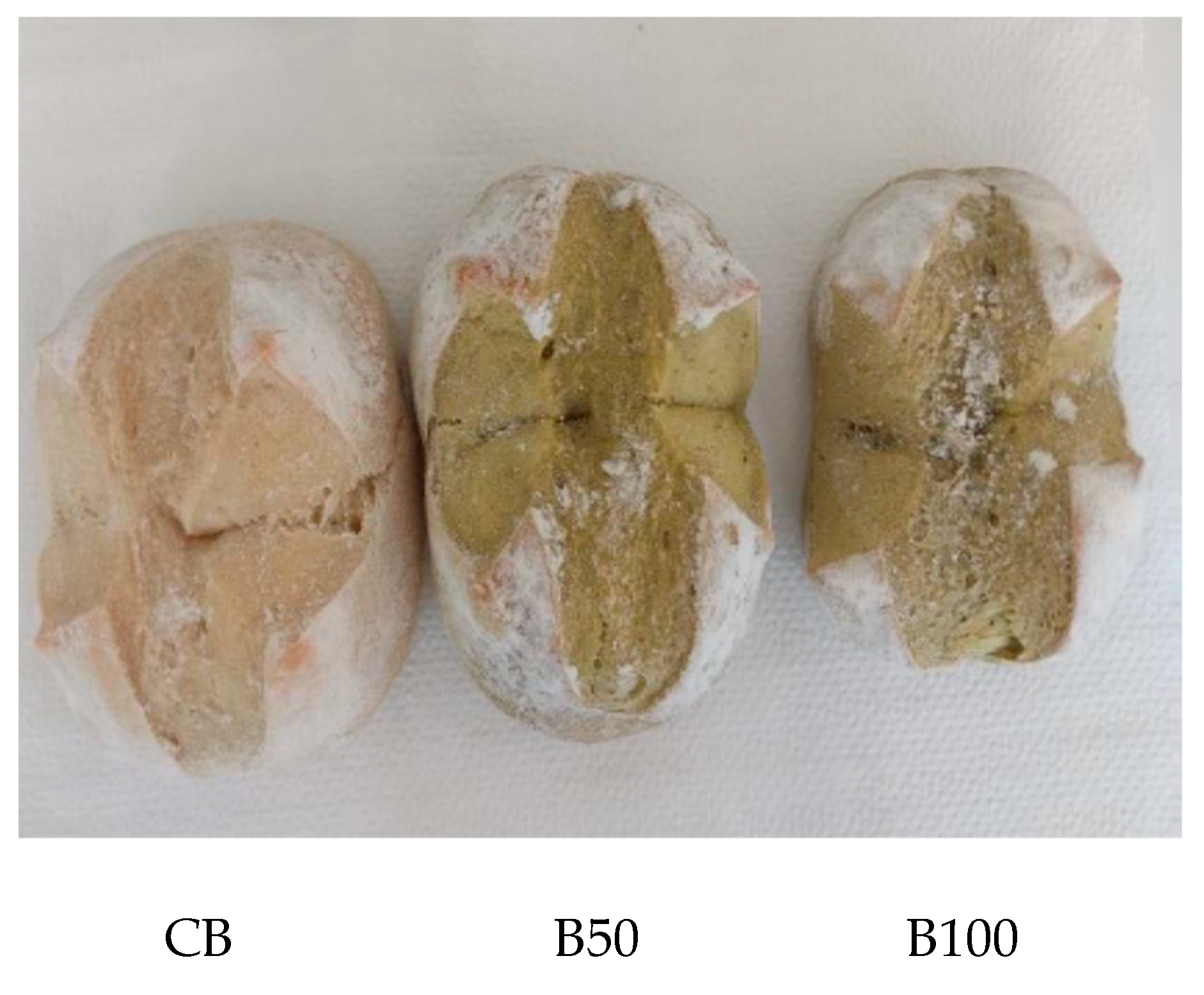
2.2. Bread Preparation
2.3. Bread Physico-Chemical Analysis
2.3.1. Total Baking Loss
2.3.2. Specific Volume
2.3.3. Color Evaluation
2.3.4. Texture Analysis
2.4. Microbiological Analysis
2.5. Proximate Composition of S. perennis and Bread
2.6. Sensory Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition of S. perennis
3.2. Bread Characterization
3.2.1. Baking Loss and Specific Volume
3.2.2. Color
3.2.3. Texture
3.2.4. Proximate Composition
3.2.5. Mineral Composition
3.2.6. Microbial Counts
3.2.7. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statsenko:, E.S.; Korneva, N.Y.; Pokotilo, O.V.; Litvinenko, O.V. Development of Technology for Producing Wheat Bread Enriched with Soy Ingredient. Food Sci. Technol. Int. 2023, 29, 97–104. [Google Scholar] [CrossRef]
- Adamczyk, G.; Ivanišová, E.; Kaszuba, J.; Bobel, I.; Khvostenko, K.; Chmiel, M.; Falendysh, N. Quality Assessment of Wheat Bread Incorporating Chia Seeds. Foods 2021, 10, 2376. [Google Scholar] [CrossRef]
- Barros, J.H.; Franco, C.M. Changes in Rheology, Quality, and Staling of White Breads Enriched with Medium-Polymerized Inulin. Food Sci. Technol. Int. 2022, 28, 32–39. [Google Scholar] [CrossRef]
- Bourekoua, H.; Gawlik-Dziki, U.; Różyło, R.; Zidoune, M.N.; Dziki, D. Acerola Fruit as a Natural Antioxidant Ingredient for Gluten-Free Bread: An Approach to Improve Bread Quality. Food Sci. Technol. Int. 2021, 27, 13–21. [Google Scholar] [CrossRef]
- Elkatry, H.O.; Ahmed, A.R.; El-Beltagi, H.S.; Mohamed, H.I.; Eshak, N.S. Biological Activities of Grape Seed By-Products and Their Potential Use as Natural Sources of Food Additives in the Production of Balady Bread. Foods 2022, 11, 1948. [Google Scholar] [CrossRef]
- Gallo, V.; Romano, A.; Ferranti, P.; D’Auria, G.; Masi, P. Properties and in Vitro Digestibility of a Bread Enriched with Lentil Flour at Different Leavening Times. Food Struct. 2022, 33, 100284. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, T.; Su, C.; Li, Q.; Yu, X. Fortification of Chinese Steamed Bread with Flaxseed Flour and Evaluation of Its Physicochemical and Sensory Properties. Food Chem. X 2022, 13, 100267. [Google Scholar] [CrossRef]
- Gumul, D.; Ziobro, R.; Korus, J.; Kruczek, M. Apple Pomace as a Source of Bioactive Polyphenol Compounds in Gluten-Free Breads. Antioxidants 2021, 10, 807. [Google Scholar] [CrossRef]
- Junejo, S.A.; Rashid, A.; Yang, L.; Xu, Y.; Kraithong, S.; Zhou, Y. Effects of Spinach Powder on the Physicochemical and Antioxidant Properties of Durum Wheat Bread. LWT 2021, 150, 112058. [Google Scholar] [CrossRef]
- Montevecchi, G.; Santunione, G.; Licciardello, F.; Köker, Ö.; Masino, F.; Antonelli, A. Enrichment of Wheat Flour with Spirulina. Evaluation of Thermal Damage to Essential Amino Acids during Bread Preparation. Food Res. Int. 2022, 157, 111357. [Google Scholar] [CrossRef]
- Pycia, K.; Ivanišová, E. Physicochemical and Antioxidant Properties of Wheat Bread Enriched with Hazelnuts and Walnuts. Foods 2020, 9, 1081. [Google Scholar] [CrossRef]
- Wójcik, M.; Różyło, R.; Schönlechner, R.; Matwijczuk, A.; Dziki, D. Low-Carbohydrate, High-Protein, and Gluten-Free Bread Supplemented with Poppy Seed Flour: Physicochemical, Sensory, and Spectroscopic Properties. Molecules 2022, 27, 1574. [Google Scholar] [CrossRef]
- Cacak-Pietrzak, G.; Różyło, R.; Dziki, D.; Gawlik-Dziki, U.; Sułek, A.; Biernacka, B. Cistus Incanus L. as an Innovative Functional Additive to Wheat Bread. Foods 2019, 8, 349. [Google Scholar] [CrossRef]
- Maietti, A.; Tedeschi, P.; Catani, M.; Stevanin, C.; Pasti, L.; Cavazzini, A.; Marchetti, N. Nutrient Composition and Antioxidant Performances of Bread-Making Products Enriched with Stinging Nettle (Urtica Dioica) Leaves. Foods 2021, 10, 938. [Google Scholar] [CrossRef]
- Webster, J.L.; Dunford, E.K.; Neal, B.C. A Systematic Survey of the Sodium Contents of Processed Foods. Am. J. Clin. Nutr. 2010, 91, 413–420. [Google Scholar] [CrossRef]
- Riis, N.L.; Lassen, A.D.; Bjoernsbo, K.; Toft, U.; Trolle, E. Dietary Effects of Introducing Salt-Reduced Bread with and without Dietary Counselling-A Cluster Randomized Controlled Trial. Nutrients 2022, 14, 3852. [Google Scholar] [CrossRef]
- WHO. Guideline: Sodium Intake for Adults and Children; World Health Organization (WHO): Geneva, Switzerland, 2012. [Google Scholar]
- WHO. Report of the Formal Meeting of Member States to Conclude the Work on the Comprehensive Global Monitoring Framework, Including Indicators, and a Set of Voluntary Global Targets for the Prevention and Control of Non-Communicable Diseases; World Health Organization (WHO): Geneva, Switzerland, 2012. [Google Scholar]
- Custódio, L.; Rodrigues, M.J.; Pereira, C.G.; Castañeda-Loaiza, V.; Fernandes, E.; Standing, D.; Neori, A.; Shpigel, M.; Sagi, M. A Review on Sarcocornia Species: Ethnopharmacology, Nutritional Properties, Phytochemistry, Biological Activities and Propagation. Foods 2021, 10, 2778. [Google Scholar] [CrossRef]
- Sánchez-Gavilán, I.; Ramírez Chueca, E.; de la Fuente García, V. Bioactive Compounds in Sarcocornia and Arthrocnemum, Two Wild Halophilic Genera from the Iberian Peninsula. Plants 2021, 10, 2218. [Google Scholar] [CrossRef]
- De La Fuente, V.; Rufo, L.; Rodríguez, N.; Sánchez-Mata, D.; Franco, A.; Amils, R. A Study of Sarcocornia, A.J. Scott (Chenopodiaceae) from Western Mediterranean Europe. Plant Biosyst. 2016, 150, 343–356. [Google Scholar] [CrossRef]
- Barroca, M.J.; Guiné, R.P.F.; Amado, A.M.; Ressurreição, S.; da Silva, A.M.; Marques, M.P.M.; de Carvalho, L.A.E.B. The Drying Process of Sarcocornia Perennis: Impact on Nutritional and Physico-Chemical Properties. J. Food Sci. Technol. 2020, 57, 4443–4458. [Google Scholar] [CrossRef]
- Clavel-Coibrié, E.; Sales, J.R.; da Silva, A.M.; Barroca, M.J.; Sousa, I.; Raymundo, A. Sarcocornia Perennis: A Salt Substitute in Savory Snacks. Foods 2021, 10, 3110. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Karkanis, A.; Martins, N.; Ferreira, I.C.F.R. Edible Halophytes of the Mediterranean Basin: Potential Candidates for Novel Food Products. Trends Food Sci. Technol. 2018, 74, 69–84. [Google Scholar] [CrossRef]
- Barreira, L.; Resek, E.; Rodrigues, M.J.; Rocha, M.I.; Pereira, H.; Bandarra, N.; da Silva, M.M.; Varela, J.; Custódio, L. Halophytes: Gourmet Food with Nutritional Health Benefits? J. Food Compos. Anal. 2017, 59, 35–42. [Google Scholar] [CrossRef]
- Gargouri, M.; Magné, C.; Dauvergne, X.; Ksouri, R.; El Feki, A.; Metges, M.-A.G.; Talarmin, H. Cytoprotective and Antioxidant Effects of the Edible Halophyte Sarcocornia Perennis L. (Swampfire) against Lead-Induced Toxicity in Renal Cells. Ecotoxicol. Environ. Saf. 2013, 95, 44–51. [Google Scholar] [CrossRef]
- Pereira, T.; Caldeira, A.T.; Caseiro, A.; Osório, N.; da Silva, A.M.; Barroca, M.J. Randomized Pilot Study on the Effects of Sarcocornia as a Salt Substitute in Arterial Blood Pressure and Vascular Function in Healthy Young Adults. Foods 2022, 11, 2888. [Google Scholar] [CrossRef]
- Lopes, M.; Cavaleiro, C.; Ramos, F. Sodium Reduction in Bread: A Role for Glasswort (Salicornia ramosissima J. Woods). Compr. Rev. Food Sci. Food Saf. 2017, 16, 1056–1071. [Google Scholar] [CrossRef]
- Toumi, O.; Conte, P.; Maria Gonçalves Moreira da Silva, A.; João Barroca, M.; Fadda, C. Use of Response Surface Methodology to Investigate the Effect of Sodium Chloride Substitution with Salicornia ramosissima Powder in Common Wheat Dough and Bread. J. Funct. Foods 2022, 99, 105349. [Google Scholar] [CrossRef]
- Pires, A.; Agreira, S.; Ressurreição, S.; Marques, J.; Guiné, R.; Barroca, M.J.; Moreira da Silva, A. Sea Purslane as an Emerging Food Crop: Nutritional and Biological Studies. Appl. Sci. 2021, 11, 7860. [Google Scholar] [CrossRef]
- Djordjević, M.; Šoronja-Simović, D.; Nikolić, I.; Djordjević, M.; Šereš, Z.; Milašinović-Šeremešić, M. Sugar Beet and Apple Fibres Coupled with Hydroxypropylmethylcellulose as Functional Ingredients in Gluten-Free Formulations: Rheological, Technological and Sensory Aspects. Food Chem. 2019, 295, 189–197. [Google Scholar] [CrossRef]
- Cunniff, P. (Ed.) Official Methods of Analysis of AOAC International, 16th ed.; 3rd rev.; AOAC International: Gaithersburg, MD, USA, 1997; ISBN 978-0-935584-54-7. [Google Scholar]
- European Union. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011; European Union: Brussels, Belgium, 2015. [Google Scholar]
- Kosová, K.; Práil, I.T.; Vítámvás, P. Protein Contribution to Plant Salinity Response and Tolerance Acquisition. Int. J. Mol. Sci. 2013, 14, 6757–6789. [Google Scholar] [CrossRef]
- Milić, D.; Luković, J.; Ninkov, J.; Zeremski-Škorić, T.; Zorić, L.; Vasin, J.; Milić, S. Heavy Metal Content in Halophytic Plants from Inland and Maritime Saline Areas. Cent. Eur. J. Biol. 2012, 7, 307–317. [Google Scholar] [CrossRef]
- Dunteman, A.; Yang, Y.; McKenzie, E.; Lee, Y.; Lee, S.-Y. Sodium Reduction Technologies Applied to Bread Products and Their Impact on Sensory Properties: A Review. Int. J. Food Sci. Technol. 2021, 56, 4396–4407. [Google Scholar] [CrossRef]
- Reißner, A.-M.; Wendt, J.; Zahn, S.; Rohm, H. Sodium-Chloride Reduction by Substitution with Potassium, Calcium and Magnesium Salts in Wheat Bread. LWT 2019, 108, 153–159. [Google Scholar] [CrossRef]
- Marco, E.R.; Navarro, J.L.; León, A.E.; Steffolani, M.E. Sodium Chloride Replacement by Potassium Chloride in Bread: Determination of Sensorial Potassium Threshold and Effect on Dough Properties and Breadmaking Quality. Int. J. Gastron. Food Sci. 2022, 27, 100486. [Google Scholar] [CrossRef]
- European Union. Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006; European Union: Brussels, Belgium, 2006. [Google Scholar]
- Levings, J.L.; Gunn, J.P. The Imbalance of Sodium and Potassium Intake: Implications for Dietetic Practice. J. Acad. Nutr. Diet. 2014, 114, 838–841. [Google Scholar] [CrossRef]
- Bertin, R.L.; Gonzaga, L.V.; Borges, G.d.S.C.; Azevedo, M.S.; Maltez, H.F.; Heller, M.; Micke, G.A.; Tavares, L.B.B.; Fett, R. Nutrient Composition and, Identification/Quantification of Major Phenolic Compounds in Sarcocornia Ambigua (Amaranthaceae) Using HPLC–ESI-MS/MS. Food Res. Int. 2014, 55, 404–411. [Google Scholar] [CrossRef]
- Saranraj, D.P. Microbial Spoilage of Bakery Products and Its Control by Preservatives. Int. J. Pharm. Biol. Arch. 2012, 3, 38–48. [Google Scholar]
- Ijah, U.J.J.; Auta, H.S.; Aduloju, M.O.; Aransiola, S.A. Microbiological, Nutritional, and Sensory Quality of Bread Produced from Wheat and Potato Flour Blends. Int. J. Food Sci. 2014, 2014, 671701. [Google Scholar] [CrossRef]
- Debonne, E.; De Leyn, I.; Verwaeren, J.; Moens, S.; Devlieghere, F.; Eeckhout, M.; Van Bockstaele, F. The Influence of Natural Oils of Blackcurrant, Black Cumin Seed, Thyme and Wheat Germ on Dough and Bread Technological and Microbiological Quality. LWT 2018, 93, 212–219. [Google Scholar] [CrossRef]
- Nanditha, B.; Prabhasankar, P. Antioxidants in Bakery Products: A Review. Crit. Rev. Food Sci. Nutr. 2009, 49, 1–27. [Google Scholar] [CrossRef]
- Nahar, N.; Madzuki, I.; Izzah, N.; Ab Karim, M.; Mohd Ghazali, H.; Karim, R. Bakery Science of Bread and the Effect of Salt Reduction on Quality: A Review. Borneo J. Sci. Technol. 2019, 1, 9–14. [Google Scholar]
- Gorman, M.; Moss, R.; Barker, S.; Falkeisen, A.; Knowles, S.; McSweeney, M.B. Consumer Perception of Salt-Reduced Bread with the Addition of Brown Seaweed Evaluated under Blinded and Informed Conditions. J. Sci. Food Agric. 2023, 103, 2337–2346. [Google Scholar] [CrossRef] [PubMed]
- Antúnez, L.; Giménez, A.; Vidal, L.; Ares, G. Partial Replacement of NaCl with KCl in Bread: Effect on Sensory Characteristics and Consumer Perception. J. Sens. Stud. 2018, 33, e12441. [Google Scholar] [CrossRef]
- Sinesio, F.; Raffo, A.; Peparaio, M.; Moneta, E.; Saggia Civitelli, E.; Narducci, V.; Turfani, V.; Ferrari Nicoli, S.; Carcea, M. Impact of Sodium Reduction Strategies on Volatile Compounds, Sensory Properties and Consumer Perception in Commercial Wheat Bread. Food Chem. 2019, 301, 125252. [Google Scholar] [CrossRef] [PubMed]
- Raffo, A.; Carcea, M.; Moneta, E.; Narducci, V.; Nicoli, S.; Peparaio, M.; Sinesio, F.; Turfani, V. Influence of Different Levels of Sodium Chloride and of a Reduced-Sodium Salt Substitute on Volatiles Formation and Sensory Quality of Wheat Bread. J. Cereal Sci. 2018, 79, 518–526. [Google Scholar] [CrossRef]






| Ingredients | CB | B50 | B100 |
| Wheat flour T65 (g) | 1000 | 1000 | 1000 |
| Water (mL) | 600 | 600 | 600 |
| Dough Strengthener | 13 | 13 | 13 |
| Sugar (g) | 11 | 11 | 11 |
| Dry baker’s yeast (g) | 7 | 7 | 7 |
| Salt (NaCl) (g) | 12 | - | - |
| S. perennis powder (g) | - | 30.85 | 61.7 |
| Biochemical Compounds (g/100 g) | |
| Moisture content | 6.42 ± 0.05 |
| Total lipids | 0.85 ± 0.03 |
| Protein | 11.28 ± 0.08 |
| Crude fiber | 9.84 ± 0.06 |
| Total dietary fiber | 23.18 ± 0.05 |
| Insoluble dietary fiber | 19.99 ± 0.03 |
| Soluble dietary fiber | 3.19 ± 0.03 |
| Carbohydrates | 14.79 ± 0.10 |
| Total ash | 33.84 ± 0.03 |
| Minerals (mg/100 g) | |
| Sodium (Na) | 7614.12 ± 49.78 |
| Potassium (K) | 1116.57 ± 11.84 |
| Calcium (Ca) | 275.81 ± 6.32 |
| Magnesium (Mg) | 510.80 ± 8.94 |
| Phosphor (P) | 144.22 ± 1.96 |
| Iron (Fe) | 11.54 ± 050 |
| Copper (Cu) | 1.09 ± 0.09 |
| Zinc (Zn) | 2.98 ± 0.08 |
| Manganese (Mn) | 2.97 ± 0.07 |
| CB | B50 | B100 | |
|---|---|---|---|
| Total baking loss (%) | 13.5 | 14.1 | 9.5 |
| Weight (g) | 71.2 ± 1.8 | 73.4 ± 1.8 | 76.42 ± 2.0 |
| Specific volume (cm3/g) | 3.24 | 3.50 | 3.66 |
| CB | B50 | B100 | |
|---|---|---|---|
| Moisture | 34.06 ± 0.07 c | 34.75 ± 0.10 b | 36.19 ± 0.10 a |
| Ash | 2.56 ± 0.01 b | 2.28 ± 0.02 c | 3.16 ± 0.03 a |
| Fat | 0.11 ± 0.01 b | 0.44 ± 0.03 a | 0.38 ± 0.05 a |
| Protein | 11.13 ± 0.18 b | 11.19 ± 0.02 b | 11.48 ± 0.05 a |
| Total Fibre | 2.15 ± 0.14 c | 4.40 ± 0.12 b | 6.83 ± 0.15 a |
| Fibre insoluble | 0.25 ± 0.08 b | 0.54 ± 0.06 b | 1.05 ± 0.12 a |
| Fibre soluble | 1.91 ± 0.06 c | 3.85 ± 0.18 b | 5.78 ± 0.03 a |
| Carbohydrates | 84.00 ± 0.35 | 81.70 ± 0.11 | 78.17 ± 0.23 |
| Energy (kcal/100 g) | 386.06 ± 0.21 a | 384.25 ± 0.01 b | 375.42 ± 0.16 c |
| Energy (J/100 g) | 1616.35 ± 0.86 a | 1608.75 ± 0.02 b | 1571.84 ± 0.69 c |
| Mineral | CB | B50 | B100 |
|---|---|---|---|
| Na | 613.40 ± 11.01 a | 339.01 ± 5.38 b | 643.65 ± 14.08 a |
| K | 152.43 ± 6.71 | 195.63 ± 2.54 | 243.71 ± 2.36 |
| Ca | 393.27 ± 15.29 b | 401.09 ± 1.90 b | 444.11 ± 4.96 a |
| P | 370.04 ± 7.53 b | 379.53 ± 5.97 a,b | 397.66 ± 1.71 a |
| Mg | 40.28 ± 4.75 b | 48.80 ± 1.18 b | 68.81 ± 5.03 a |
| Fe | 3.26 ± 0.02 b | 4.87 ± 0.23 a | 5.52 ± 0.27 a |
| Zn | 0.89 ± 0.01 b | 0.99 ± 0.02 a | 1.00 ± 0.02 a |
| Cu | 0.33 ± 0.01 b | 0.43 ± 0.02 a | 0.37 ± 0.01 b |
| Mn | 0.92 ± 0.02 b | 1.01 ± 0.05 a,b | 1.10 ± 0.05 a |
| TVBC-2d | YMC-2d | CC-2d | TVBC-4d | YMC-4d | CC-4d | ||
|---|---|---|---|---|---|---|---|
| (a) | CB | 2.9 × 102 | 7 × 101 | NG | 3.5 × 103 | 1.2 × 103 | NG |
| B50 | 3 × 102 | 9 × 101 | NG | 6.8 × 103 | 1.4 × 103 | NG | |
| B100 | 1.9 × 103 | 1.6 × 103 | NG | 7.36 × 104 | 1.84 × 104 | NG | |
| (b) | CB | 9.7 × 102 | 7.4 × 102 | NG | 4.06 × 104 | 1.32 × 104 | NG |
| B50 | 2.6 × 102 | 1.9 × 102 | NG | 5.28 × 104 | 4.1 × 104 | NG | |
| B100 | 2.4 × 103 | 2.4 × 103 | NG | 8.9 × 104 | 6.2 × 104 | NG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barroca, M.J.; Flores, C.; Ressurreição, S.; Guiné, R.; Osório, N.; Moreira da Silva, A. Re-Thinking Table Salt Reduction in Bread with Halophyte Plant Solutions. Appl. Sci. 2023, 13, 5342. https://doi.org/10.3390/app13095342
Barroca MJ, Flores C, Ressurreição S, Guiné R, Osório N, Moreira da Silva A. Re-Thinking Table Salt Reduction in Bread with Halophyte Plant Solutions. Applied Sciences. 2023; 13(9):5342. https://doi.org/10.3390/app13095342
Chicago/Turabian StyleBarroca, Maria João, Catarina Flores, Sandrine Ressurreição, Raquel Guiné, Nádia Osório, and Aida Moreira da Silva. 2023. "Re-Thinking Table Salt Reduction in Bread with Halophyte Plant Solutions" Applied Sciences 13, no. 9: 5342. https://doi.org/10.3390/app13095342
APA StyleBarroca, M. J., Flores, C., Ressurreição, S., Guiné, R., Osório, N., & Moreira da Silva, A. (2023). Re-Thinking Table Salt Reduction in Bread with Halophyte Plant Solutions. Applied Sciences, 13(9), 5342. https://doi.org/10.3390/app13095342









