Abstract
Cookie consumption can change the serum level of oxidized low-density lipoprotein (oxLDL) and oxLDL receptors, both playing important roles in the pathogenesis of atherosclerosis and cardiovascular diseases. This study investigated the nutritional value and the antioxidant activity of whole grain cookies in which 24% of the cocoa powder was substituted with grape and aronia pomace and were further coated with edible films enriched with grape seed extract (GAP with KGAE) as well as the effects of their consumption on the serum level of oxLDL receptors in women. The proximate composition, mineral content, antioxidant activity, and starch digestibility in vitro of experimental and control cookies were determined. A group of 12–13 healthy women (median age 36) consumed 45 g of GAP with KGAE or commercial cookies for 10 days. The results showed that GAP and KGAE cookies had increased flavonoid content (22%) and antioxidant potential (27–73%) compared to the control. The content of slowly digestible starch prevailed over rapidly digestible starch. The serum concentrations of the oxLDL receptors between the test and control groups were similar. We can conclude that the moderate consumption of whole grain cookies with fruit by-products does not lead to the formation of oxLDL receptors in healthy women.
1. Introduction
The excessive consumption of cookies that are high in sugar and saturated fat can lead to chronic non-communicable diseases such as cardiovascular disease (CVD) and diabetes type II [1]. CVD is a leading cause of premature death and disability in Europe, according to the World Health Organization [2]. Cookie consumption changes the blood concentration of glucose, but also triglycerides, low-density lipoprotein (LDL), and high-density lipoprotein [1]. This alone does not cause CVD because LDL must first be oxidized. If there is an imbalance between radical formation (production of reactive oxygen species) and radical removal (action of antioxidants), oxidized low-density lipoprotein (oxLDL) can form [3]. The newly formed oxLDL triggers the onset of atherosclerosis in the following steps: increased expression of adhesion molecules on vascular endothelial cells, the attraction of leukocytes to endothelial cells, the sequestration of leukocytes into the intimal layer, macrophage activation, the release of cytokines and reactive oxygen species (ROS), and plaque formation [4]. In addition, the lectin-like receptor-1 for oxLDL plays an important role in triggering atherosclerosis since different actions commence when oxLDL binds to the oxLDL receptor in different cell types. This binding increases the absorption of oxLDL in vascular smooth muscle cells (VSMCs) and macrophages and promotes the production of foam cells. Moreover, it induces apoptosis in VSMCs and contributes to endothelial activation [5]. However, to our knowledge, no study investigated the effect of cookie consumption on the formation of oxLDL receptors in humans.
Polyphenols and other antioxidants in human nutrition represent a valuable nutritional tool in preventing CVD, diabetes, osteoporosis, and cancer [6]. Polyphenols counteract the oxidation of LDL and the subsequent deposition of atherosclerotic material in tissues [7]. Epidemiological studies have shown that polyphenols present in berries [8], cocoa [9] and red wine [10] are strong antioxidants that can slower CVD progression.
Recently, fruit-by products have become interesting for the recovery of their antioxidants. Aronia or chokeberry (Aronia melanocarpa) is the richest source of polyphenols among berry fruits [11], with a very high content of procyanidins, anthocyanidins, and phenolic acids, while flavonols are present in lower amounts [12]. Aronia is mostly used for juice production, in which aronia pomace remains a by-product. In addition to their high antioxidant capacity, the main polyphenolic constituents of aronia also possess anti-inflammatory, anticancer, antimicrobial, antiviral, antidiabetic, antiatherosclerotic, antihypertensive, antiplatelet, and anti-inflammatory properties [13]. Grapes (Vitis vinifera), are one of the most widely grown crops worldwide [14] and are used for wine or juice production. The remaining grape pomace and seeds contain significant amounts of dietary fiber with high antioxidant activity due to the natural presence of polyphenols and other bioactive compounds, indicating the potential use of this sustainable resource as a food or beverage ingredient [15,16,17,18]. In our previous studies, we found that up to 24% of cocoa powder can be successfully replaced in cookie recipes with a mixture of grape and aronia pomace (GAP) without affecting their sensory acceptability [18].
Polysaccharide-based edible films are a form of biodegradable food packaging [19] that not only fulfill the role of protection but also reinforce the mechanical strength and enhance the phenolic content of a food [20]. Edible films are used for various food categories, including baked goods [20,21]. Previously, we found that the application of an edible film based on chitosan and gum arabic enriched with grape seed extract (KGAE) positively affects cookies’ shelf life [20]. Although there is great interest in the health benefits of edible films enriched with bioactive compounds, the detailed nutritional profile of coated cookies is missing.
The aim of the present study was to investigate the effects of replacing cocoa powder with fruit by-products and the application of edible film on the nutritional value, antioxidant activity, and starch digestibility of cookies. The second goal of this study was to investigate the effects of cookies consumption on oxLDL receptors in healthy women.
2. Materials and Methods
2.1. Materials for Preparation of Cookies and Edible Films
Fine rolled oats (Crownfield, Germany) with a protein content of 13.5% and whole spelt flour (Siladi, Croatia) with a protein content of 12% were purchased at a local market. Margarine (containing 30% butter) (70% fat) and cocoa powder (20% fat) were purchased from Zvijezda and Kraš (Zagreb, Croatia), respectively. Dried red grape pomace (Vitis vinifera L., Frankovka and Syrah varieties, with seeds, containing 9.2% fat) and aronia pomace (Aronia melanocarpa L., without seeds, containing 4.0% fat) were obtained as by-products from local juice producers (Davorka Šipek, Natkrižovljan, Cestica municipality, and Tomislav Jurendić, Koprivnica, Croatia). Dried grape and aronia pomaces (7 g) were ground in a laboratory ball mill (Cryomill, Retsch, Golling an der Salzach, Austria) with 12 steel balls (10 mm diameter) for 3 min in a 50-mL stainless steel container at a vibration frequency of 30 Hz to achieve the median diameter of the 50th percentile (36 ± 5 µm) similar as cocoa powder (41 ± 3 µm) [18].
The edible film was prepared using chitosan (France Chitin, Orange, France, type 652, Mw 165 kDa, DA > 85%), gum arabic (GA) (Enologica vasons. p.a., San Pietro in Carino, Italy) and MegaNatural Gold grape seed extract (GSE) donated by Polyphenolics (Madera, CA, USA; the total phenolic content was 90%, expressed as mg of gallic acid equivalent per 100 g). GSE was stored in its original packaging at −18 °C. A film-forming solution (FFS) was prepared using acetic acid (Merck, Darmstadt, Germany) and purified water.
2.2. Cookies and Edible Film Preparation
Three types of cookies were prepared according to Molnar et al. [20]: control cocoa cookies without fruit pomace in the recipe (CC), cookies enriched with grape and aronia pomace (GAP), and cookies with grape and aronia pomace covered with edible film (GAP with KGAE). The recipe for CC contained (with a percentage of spelt flour and oat flakes combined weight in brackets): fine rolled oats 300 g, whole spelt flour 252 g, margarine 200 g (36.2%), brown sugar 135 g (24.5%), cocoa powder 48 g (8.7%), vanilla sugar 35 g (6.3%), tap water 20 g (3.6%), salt 4 g (0.7%), baking powder 3 g (0.5%), and sodium bicarbonate 3 g (0.5%). In GAP and GAP with KGAE, 11 g (23.6%) of cocoa powder was replaced with a mixture of grape pomace (8.2 g which is 17.5% of cocoa) and aronia pomace (2.8 g which is 6.1% of cocoa) [18].
The FFS, consisting of chitosan, GA and GSE, was prepared as described previously by Molnar et al. [21]. First, chitosan powder was dissolved in 1% (v/v) aqueous acetic solution while GA powder was dissolved in distilled water to obtain 5% (w/v) solutions. Then, GSE (1 g/L) was added to the GA solution and mixed for 40 min on a magnetic stirrer. Finally, the GA containing GSE and the chitosan solutions were mixed in a 50:50 ratio to obtain an active FFS (KGAE). After spraying KGAE over the GAP cookies, they were dried for 30 min at 80 °C in an oven [20].
CC, GAP, and GAP with KGAE were used for nutritional and biochemical analyses as well as for the determination of starch digestibility, while GAP with KGAE was used in the intervention study along with commercial cookies.
Commercial cookies (Cioccograno, Mulino Bianco, Barilla) purchased at the local market contained 22 g of fat of which 10 g were saturated fatty acids, 60.4 g of carbohydrates of which 23.4 g were sugars, 7.6 g of protein, 6.3 g of dietary fiber and 0.43 g of salt per 100 g.
2.3. Determination of Proximate Composition of Cookies
Ash content was determined after incineration at 550 °C according to the AACC 08-01 method [22]. Moisture content was determined gravimetrically using a moisture analyzer (PMB53 Adam Equipment, Oxford, CT, USA). Protein content was analyzed according to the standard Kjeldahl method [23] and the conversion factor 6.25 was used for the calculation. Fat content was analyzed according to ISO 6492:1999 [24] and using the Foss Soxtec TM 8000 extraction system and the Foss Hydrotec TM 8000 Hydrolasys system (Foss, Hilleroed, Denmark), while the methyl esters of fatty acids [saturated (SFA), monounsaturated (MUFA) and polyunsaturated (PUFA)] were determined in accordance with ISO 12966-4:2015 [25] using an Agilent 7890B gas chromatograph (Santa Clara, CA, USA). Crude fiber content was measured using the FIBERTEC 2010 and M6 (Foss Analytical AB, Höganäs, Sweden). The method is based on successive steps of chemical treatments to dissolve the non-fiber components and the final determination of the residue obtained. The determination of mineral content was performed according to the European standards EN 14084:2003 [26] and EN 15763:2010 [27]. The determination of ash, protein and fat content was performed in duplicate, while the determination of crude fiber and minerals was performed in triplicate for each cookie sample.
2.4. In Vitro Starch Digestibility
Starch digestibility was studied in vitro using a slightly modified method of Englyst [28], which directly correlates with the glycemic response [29]. The method is based on the measurement of glucose released from a test food during a time-limited incubation with digestive enzymes under controlled conditions and allows the determination of free glucose (FG), rapidly available glucose (RAG), rapidly digestible starch (RDS), slowly digestible starch (SDS), resistant starch (RS), and total starch (TS). Heat-stable amylase (A3306) used to determine total starch, as well as pancreatin (P7547), amyloglucosidase (A7095), and invertase (I4504) used for in vitro hydrolysis of starch, were purchased from Sigma Aldrich Co. (St. Luis, MO, USA). The absorbance of the standards and samples was measured at 490 nm using a microplate reader (VICTOR X3, PerkinElmer, Shelton, CT, USA). The following formulas were used to calculate TS, RDS, SDS, RS, and RAG:
where G20 represents glucose released after 20 min, G120 represents glucose released after 120 min, At is the absorbance of the test solution, Vt is the total volume of the test solution, C is the concentration of standard (mg glucose/mL), D is the dilution factor, As is the absorbance of standard and Wt is the weight (in mg) of the sample taken for analysis.
TS = (TG − FG) × 0.9
RDS = (G20 − FG) × 0.9
SDS = (G120 − G20) × 0.9
RS = (TG − G120) × 0.9
RAG = At × Vt × C × D/As × Wt × 100
2.5. Determination of Flavonoid Content and Antioxidant Activities
The extract was prepared as described by Molnar et al. [20]. It was used for the measurement of flavonoid content and antioxidant activities in triplicate using a UV 1600PC spectrophotometer (VWR International, Leuven, Belgium).
For the determination of flavonoid content, 2 mL of the extract, 1.5 mL of 96% ethanol, 0.1 mL of 10% aluminum chloride, 0.1 mL of 1 M potassium acetate, and 100 μL of distilled water were pipetted into a cuvette. A blank sample was prepared in the same manner, using the extraction solvent instead of the extract and distilled water instead of 10% aluminum chloride. After 30 min, the absorbance was measured at 415 nm. The six-point calibration curve (0.02–0.10 µmol/mL) was prepared using a rutin standard solution.
For the ferric reducing antioxidant power (FRAP) assay, 300 µL of the extract was mixed with 200 µL of methanol and acetone solution (50:50, v/v) and 2 mL of the FRAP reagent (temperate at 37 °C) was immediately added. The absorbance was measured at 593 nm. Radical scavenging activity was determined using 2,2-diphenyl-1-picrylhydrazyl (DPPH). In a microcuvette, 150 µL of the extract was mixed with 0.95 mL of a 0.06 mM DPPH solution. After standing in the dark for 30 min, absorbance was measured at 517 nm. For the 2,2′-Azinobis-(3-ethylbenzthiazolin-6-sulfonic acid) (ABTS) assay, 150 µL of the extract was mixed well with 2 mL of ABTS+ and shaken. The extraction solvent was used for the blank samples. The absorbance was measured after 6 min with a blank sample (extraction solvent instead of extract) at 734 nm. The five-point standard calibration curves (0.02–0.10 µmol/mL) were prepared for FRAP, DPPH, and ABTS antioxidant tests using a Trolox solution (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid).
2.6. Determination of oxLDL
The study was designed as a randomized controlled trial. It was carried out in May 2021, at the University Hospital Center Sestre Milosrdnice, the Department of Clinical Chemistry (Zagreb, Croatia).
2.6.1. Subjects
A cohort of 25 women (aged 23–60) was randomly divided into two groups. The control group included 12 women who consumed 45 g of commercial cookies (Cioccograno, Mulino Bianco, and Barilla). The test group included 13 women who consumed 45 g of GAP with KGAE. Participants were advised to eat 30 g of cookies at breakfast and 15 g of cookies between meals daily for 10 days. Study participants were nonsmokers, did not suffer from chronic diseases, were not taking medications to treat cardiovascular diseases, and had not taken dietary supplements for at least three months prior to the start of the study. Subjects who were pregnant, had a body mass index (BMI) of >30 kg/m2, or were vegetarians were excluded because these factors affect oxidative stress, which would affect the results of the study. In addition, subjects who consumed more than 500 mL of flavonoid-rich foods (tea, coffee, cocoa, fruit juices) were excluded, as determined by a food intake frequency questionnaire. Subjects suffering from allergies to any of the ingredients were also excluded. All subjects were asked to maintain their usual lifestyle and to report any illness if it occurred during the study. All participants signed an informed consent form to participate in this study.
2.6.2. Anthropometric and Biochemical Parameters
Assessment of participants included anthropometric measurements (body height (BH), bodyweight (BW), and waist and hip circumference), dietary methods (Food Frequency Questionnaire (FFQ)), and biochemical methods (determination of glucose, triglycerides, total cholesterol, HDL and LDL cholesterol, oxLDL receptor, and iron in blood/serum). Anthropometric measurements were performed at baseline to calculate body mass index (BMI = BW (kg)/BH2 (m)), and waist-to-hip ratio (WHR). All necessary information about the study participants (age, level of education, number of people in the household, alcohol, medications, dietary supplements, allergy status, frequency of consumption of food prepared outside the home, and level of physical activity) was provided in the first section of the FFQ. The reference period for data collection was the month preceding the participation.
Dietary intake was followed using the modified validated FFQ [30]. Foods were divided into nine categories (cereals, fruits, vegetables, dairy products, legumes, fats, nuts and seeds, sweets, and beverages). A Likert scale was used to determine frequencies and the following responses were possible: always (every day—5), often (three to five times a week—4), sometimes (once a week—3), rarely (once or twice a month—2), and never (1).
All biochemical parameters were measured at the beginning of the study in the fasting state, while the oxLDL receptor was also measured 2 h after consumption of cookies, and after 10 days of the intervention. Glucose, iron, and lipid profile concentrations were measured with the Abbott Architect c8000 (Abbott Laboratories, Chicago, IL, USA) using the manufacturer’s original reagents. The oxidized LDL receptor was determined using an enzyme-linked immunosorbent assay (ELISA) kit for lectin-like oxidized LDL receptor 1 (LOX1) (SEB859Hu 96) (CLOUD-CLONE CORP., Houston, TX, USA) according to the manufacturer’s instructions. Briefly, the method is a double antibody sandwich ELISA on wells precoated with a LOX1-specific antibody. After incubation of the standards and samples with the pre-coated wells, the next step was to add avidin conjugated to horseradish peroxidase followed by biotin-conjugated antibodies to each well. When the 3,3′5,5′-Tetramethylbenzidine substrate is added, the color of the wells changes only in the presence of LOX1. Sulfuric acid stops the reaction. The concentration of LOX1 in the samples is proportional to the color intensity measured at 450 nm. The detection range of the method is 12.5–800 pg/mL. The ELISA was performed manually, with automated washing steps on the Hydroflex Microplate Washer, and absorbance was measured on Infinite F50 Readers (Tecan Ltd., Männedorf, Switzerland).
The data collected from the subjects were kept confidential. Moreover, the names of the subjects were encrypted during the processing of the sample and the analysis of the results and were known only to the principal investigator. At the end of the study, all personal data and biological materials were disposed of appropriately. Approval for this research was granted by the Ethics Committee of the University Hospital Center Sestre Milosrdnice (251-29-11-21-01-9) and the Ethics Committee of the Faculty of Medicine, University of Zagreb (380-59-10106-21-111/122).
2.7. Data Analysis
Statistical differences between chemical composition, antioxidant level, and starch digestibility were assessed using one-way analysis of variance (ANOVA) and Tukey’s multiple comparison tests. Results of the FFQ are expressed as a median with 25th and 75th percentile using a box plot obtained in Statistica 10 (StatSoft, Tulsa, OK, USA). Biochemical data were analyzed using the Mann-Whitney test or Fisher’s exact test and the Spearman correlation coefficient. The nonparametric paired test (Wilcoxon’s signed-rank test) was used to compare differences before and after the intervention within the same group. Xlstat-Pro (win) 7.5.3 GraphPad Prism 8.4.3 was used for statistical analysis (Addinsoft, New York, NY, USA). A significance level of p < 0.05 was considered in all statistical analyses.
3. Results and Discussion
3.1. Proximate Composition of Cookies
The nutritional profile of CC, GAP, and GAP with KGAE is shown in Table 1. The cookies differed slightly in fat, saturated fat, protein, crude fiber, TS, glucose, and mineral content, suggesting that fruit by-products (grape and aronia pomace) can be successfully used as a partial substitute for cocoa powder.

Table 1.
Proximate composition, starch digestibility in vitro, minerals, and antioxidant activity (FRAP, ABTS and DPPH) of different types of cookies.
In all types of cookies, SDS prevailed over RDS. Lower RDS content and higher SDS content directly correlate with a lower glycemic response [29,31]. The amount of SDS was 1.3- to 2-fold higher in our cookies compared with the results of Garsetti et al. [31], suggesting that their consumption might lead to longer satiety and that the cookies might have a medium to low glycemic index.
A significantly higher RDS (19%) was observed in GAP with KGAE than in CC. This could be due to the spraying of water-based edible film over cookies and the subsequent drying process. Starch in cookies is only partly gelatinized, but heating in the presence of water leads to a disruption of the inter- and intramolecular hydrogen bonds between starch chains, causing the chains to separate and become more available to digestive enzymes [32]. In contrast to our results, Diao et al. [33] found that the addition of 3% and 6% chitosan lowered the digestion rate of waxy maize starch by reducing amylase-driven hydrolysis reactions. Here, the chitosan was applied on the cookie surface in the form of an edible film. Similarly, Bae et al. [34] found that the addition of up to 2% of GA effectively reduces in vitro starch digestibility by lowering RDS content while increasing the RS content of Segoami rice noodles. However, since our results showed that cookies containing GAP with KGAE had the highest RDS, it may be that a thicker layer of edible film must be used to represent an appropriate choice for lowering the glycemic index of the final product. In addition to fiber, RS has a positive effect on the natural flora of the gastrointestinal tract [35] and was present in all three cookie varieties. In both of our modified cookies, GAP and GAP with KGAE, the RS content increased, although the difference was insignificant from CC.
The mineral composition differed between the cookies. The iron content was slightly higher in GAP than in the other two samples, probably due to the addition of red grape pomace. According to the literature, iron content in cocoa powder is about 25 mg/100 g [36]; in dry aronia pomace, it ranges from 7.5 to 8.6 mg/100 g [12]; whereas in red grape pomace, it ranges from 117 to 398 mg/100 g of dry weight, depending on the grape variety [37]. The concentrations of calcium and magnesium were higher in CC (13% and 27% respectively) than in GAP with KGAE, whereas GAP content was in between. This could be due to the fact that cocoa is naturally abundant in calcium and magnesium, which could reduce the risk of hypertension and atherosclerosis [36,38]. On the other hand, CC had also the highest sodium content, which is disadvantageous in the risk of CVD [2,38]. It is interesting to note that GAP with KGAE was lower in each mineral analyzed than the GAP sample. It could be due to the presence of GA in edible film, which has been reported to be effective in the removal of boron [39]. Therefore, future studies should investigate the effect of edible films on the bioaccessibility of essential minerals in foods.
The flavonoid content was the highest in GAP with KGAE, which was 21% higher compared with CC. This agrees with the total phenolic content measured in our previous study [20]. The CC showed the lowest antioxidant potential, which indicated the relevance of using fruit by-products in cookies. The FRAP antioxidant capacity of aronia pomace is 52.2 ± 0.2 mmol TE/100 g on a dry weight basis [40]. In grape pomace, it ranges from 110 to 530 µmol TE/g [41]. In comparison, cocoa powders have FRAP antioxidant activity from 110–454 µmol TE/g [42], of which 54% is lost in baking [43]. Foods with higher amounts of polyphenols (such as anthocyanins, flavonols, and phenolic acids) have a greater ability to reduce Fe and scavenge both DPPH and ABTS [42]. In this study, GAP with KGAE showed the highest antioxidant potential in every antioxidant assay, which depending on the test was 27–73% higher compared to CC, and even 5–9% higher than GAP. Similarly, Pawłowska et al. [44] found an increased antioxidant activity (36% in ABTS and 83% in DPPH) in muffins in which cocoa powder was completely replaced with carob powder (5% at flour basis). We showed an additional benefit of applying an edible film with GSE as a source of antioxidants since the FFS contained phenolics at a concentration of 0.71 g GAE/L [20]. Our results agree with the study of Cádiz-Gurrea et al. [45] who showed that GSE shows a stronger antioxidant capacity and a higher phenolic and flavan-3-ol content than cocoa.
Due to the highest antioxidant potential and flavonoid content, GAP and KGAE were further used for the intervention study in comparison with the commercial cookies of similar proximate composition.
3.2. Baseline Characteristics
Characteristics and concentrations of biochemical parameters at baseline for the control and test groups are shown in Table 2 and the results of the FFQ are shown in Figure 1.

Table 2.
Baseline characteristics of the subjects at the beginning of the study (with p value between the control and test group).
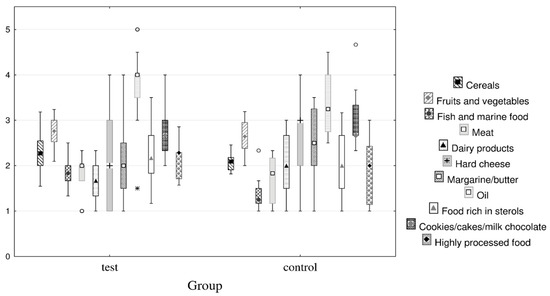
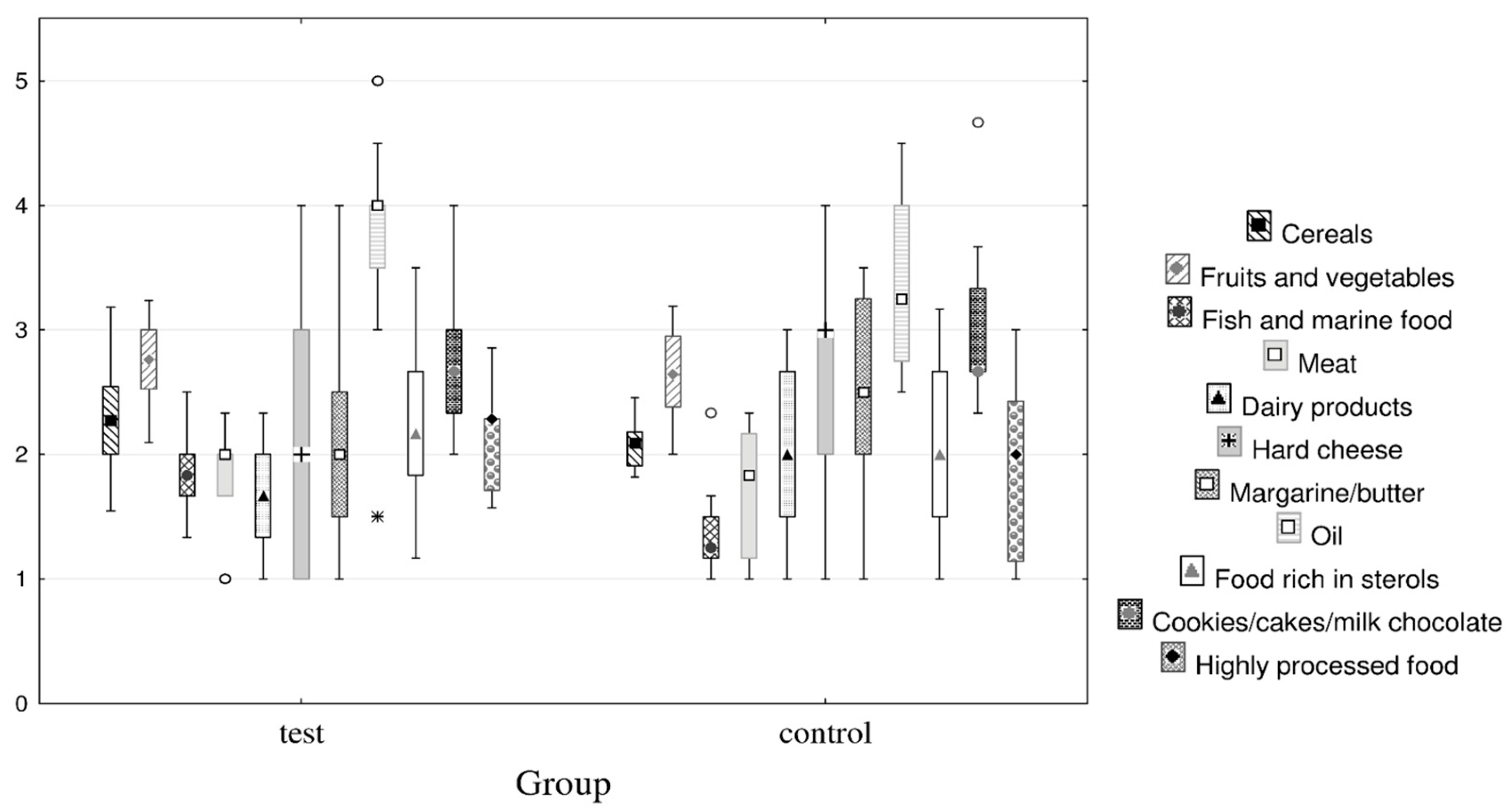
Figure 1.
Box-Whisker plot of food frequency questionnaire by the control and test group (median with 25th and 75th percentile (Box value) and non-outlier range (Whisker value); markers ° denote outliers and * extreme).
The participants in the study had normal BMI. Most of the women, particularly in the test group, were physically active and consumed dietary supplements and alcohol (p = 0.041). This can be explained by the fact that many women probably exercise and take supplements to achieve the socially prescribed ideal body image [46]. Most of the biochemical parameters did not differ at baseline. The only statistically significant difference was observed in the higher concentration of HDL cholesterol in the test group (p = 0.029), which could be due to their higher consumption of fish and marine products compared to the control group (Figure 1). However, the concentrations for both groups were within the reference interval, so this difference was not considered clinically relevant.
Food-item consumption frequencies are on a 1 to 5 Likert scale (5—every day; 4—three to five times a week; 3—once a week; 2—one to two times a month; 1—never).
The diet of participants contained items from each food group. The consumption of biscuits/cake/milk chocolate (including some extremes) was higher while the consumption of fish and marine products was lower in the control group than in the test group.
3.3. Oxidized LDL Receptor
The concentration of oxLDL receptors did not statistically differ between the test or control group at the beginning or the end of the study (mean 0.41 ng/mL and 0.39 ng/mL, respectively; p = 0.462) (Table 3).

Table 3.
Concentration of oxidized low-density lipoprotein (oxLDL) receptors in control and test group at the beginning of the study and after intervention.
There was no statistically significant predictor of change in concentration of the oxLDL receptors at day 10 in either group (Table 4), which was confirmed by the study results.

Table 4.
Logistic regression analysis of the prediction of the fall in oxidized low-density lipoprotein (oxLDL) receptor concentration on the 10th day of study.
Similar to our results, Pokimica et al. [47] found 100 mL/day of aronia juice (chokeberry) at a high or low polyphenol dose during the 8-week intervention had no positive effect on the change in oxLDL levels. A similar randomized, double-blind trial of forty-four patients who had suffered myocardial infarction found that oxLDL levels in the intervention group decreased significantly by 29% after 6 weeks of oral intake of aronia flavonoid extract [48]. It should be noted that this study was performed on patients after myocardial infarction who received concomitant statin therapy. In addition, several studies reported the potential of hydroxytyrosol-enriched biscuits for lowering oxidized LDL levels in humans [49,50]. Nevertheless, it must be considered that the above studies observed the effect of chokeberry extract or grape pomace only on oxLDL and not on the oxLDL receptor. Moreover, the putative benefits of increased consumption of polyphenols have limited bioavailability and the mechanisms by which these compounds may modulate lipid metabolism should be further investigated. Here, we can conclude that whole grain cookies can be safely consumed in moderate amounts without the risk of increasing oxLDL in healthy women.
3.4. Oxidized LDL Receptor and Waist Circumference
In our study, we observed an inverse correlation between waist circumference and oxLDL receptor concentration in the control group on the 10th day of the study (r = −0.67; p = 0.034) (Table 5), but not in the test group. A possible reason for this could be a wider range of waist circumference in the test group.

Table 5.
Correlation of concentration of oxidized low-density lipoprotein (oxLDL) receptor with anthropometric parameters in control and test group.
Sikora et al. [51] conducted a study to investigate the effects of two-month supplementation with aronia extract on angiotensin-converting enzyme activity in patients with metabolic syndrome. They showed a slight decrease in BMI (30.9 to 30.4 kg/m2; p < 0.001) and a moderate decrease in waist circumference (95–93.7 cm; p = 0.001) in the obese population compared to the control group, which showed a sharp increase in both parameters. Several other studies investigating the effects of 300–500 mg of aronia extract per day on overweight/obese patients with metabolic syndrome found no association between oxLDL and waist circumference or change in waist circumference alone [52,53]. In our study, we did not observe a correlation between BMI and oxLDL receptors, which may be because our participants had normal BMI.
3.5. Oxidized LDL Receptor and Serum Iron
We observed quite contradictory results regarding the relationship between serum iron and oxLDL receptor levels (Table 6).

Table 6.
Correlation of oxidized low-density lipoprotein (oxLDL) receptor concentration with biochemical parameters in the control and test groups.
The oxLDL receptor had a significant direct relationship with serum iron levels (r = 0.69; p = 0.012) and iron saturation (r = 0.61; p = 0.037) in the control group and a significant inverse relationship with serum iron levels (r = −0.62; p = 0.022) and iron saturation (r = −0.47; p = 0.105) in the tested group at day 10. This could be due to the fact that the control group had a lower serum iron concentration at baseline. Whether this was a coincidence remains to be determined, but it is known that aronia pomace has an indirect effect on increasing blood iron levels [54]. Jakovljevic et al. [53] demonstrated that oral supplementation with aronia extract (0.45 mL/kg/day) for four weeks in combination with different diets (high fat vs. standard) resulted in a significant increase in serum iron levels in rats regardless of diet. In contrast to our results, Brouwers et al. [55] found in their study that serum ferritin concentration and haptoglobin phenotype (Hp) were independently associated with circulating oxLDL levels in males. On the other hand, D’Amelio et al. [56] showed in their study that the association depends on iron-related genes, especially haptoglobin phenotypes. The Hp of the individual has antioxidant properties and determines the availability of iron to the cells. It could be that the Hp was not uniform in our subjects and thus contributed to the different results. A recent study that examined the relationship between iron stress and oxidative damage in patients with metabolic syndrome found a strong association between serum ferritin and oxLDL levels. In fact, people with elevated ferritin and oxLDL levels had a higher risk of metabolic syndrome [57]. In addition, there is evidence that some polyphenols may affect the bioavailability of iron, which in turn may increase the risk of developing cardiovascular disease, especially in higher-risk populations [58].
Our study has some possible limitations. The size of the groups studied was small, and they were only composed of healthy women. Furthermore, we were not able to monitor the dietary intake of our participants during the intervention, although participants were instructed not to change their dietary habits and lifestyle significantly. In addition, a longer intervention period is required.
4. Conclusions
In this study, we investigated the nutritional profile, starch digestibility, and antioxidant potential of whole grain cookies with fruit by-products and edible film enriched with grape seed extract as well as the concentration of oxidized LDL receptors after their consumption by women. We found that the partial replacement of cocoa powder with grape and aronia pomace and the use of an edible film did not negatively affect the nutritional profile or starch digestibility of the cookies but contributed to their flavonoid content and antioxidant potential. Our results showed that moderate cookie consumption does not increase the concentrations of oxidized LDL receptors in healthy women. Nevertheless, our study provides information about a possible relationship between oxidized LDL receptor concentration and waist circumference. In addition, our results provide evidence for the potential preventive effect of whole grain cookies on cardiovascular disease prevention. Further studies with more participants and a longer intervention period need to be conducted to confirm the efficacy of this potential prevention. The improved antioxidant properties of the cookies suggest that fruit by-products and edible films should be further investigated for potential applications in the development of functional foods.
Author Contributions
Conceptualization, D.M., D.N. and N.N.G.; Methodology, D.M., D.N., L.V., J.K., M.M. and N.N.G.; Formal analysis, D.M., D.N. and N.N.G.; Investigation, D.M., D.N., M.Š. (Mario Štefanović), L.V., J.K., M.M. and N.N.G.; Resources, D.N., L.V. and N.N.G.; Writing—Original Draft Preparation, D.M.; Writing-Review and Editing, D.N., M.Š. (Mario Štefanović), L.V., J.K., M.M., M.Š. (Mario Ščetar) and N.N.G.; Supervision, D.N. and N.N.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the University Hospital Center Sestre Milosrdnice (251-29-11-21-01-9, 8 April 2021) and the Ethics Committee of the Faculty of Medicine, University of Zagreb (380-59-10106-21-111/122, 19 May 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to thank Steve Kupina (Mega Natural Gold) for providing grape seed extract and Davorka Šipek for providing grape pomace. The authors also thank Tanja Dinić, Anamarija Ilijaš and Sample Control laboratory for great help and assistance with physicochemical analyzes.
Conflicts of Interest
The authors declare no conflict of interest.
References
- DiNicolantonio, J.J.; Lucan, S.C.; O’Keefe, J.H. The evidence for saturated fat and for sugar related to coronary heart disease. Prog. Cardiovasc. Dis. 2016, 58, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart disease and stroke statistics-2019 update: A report from the American Heart Association. Circ. J. 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- Leopold, J.A.; Loscalzo, J. Oxidative mechanisms and atherothrombotic cardiovascular disease. Drug Discov. 2008, 5, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Obermayer, G.; Afonyushkin, T.; Binder, C.J. Oxidized low-density lipoprotein in inflammation-driven thrombosis. J. Thromb. 2018, 16, 418–428. [Google Scholar] [CrossRef]
- Poznyak, V.A.; Nikiforov, G.N.; Markin, M.A.; Kashirskikh, A.D.; Myasoedova, A.V.; Gerasimova, V.E.; Orekhov, N.V. Overview of oxLDL and its impact on cardiovascular health: Focus on atherosclerosis. Front. Pharmacol. 2021, 11, 613780. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef] [PubMed]
- Marrugat, J.; Covas, M.I.; Fitó, M.; Schröder, H.; Miró-Casas, E.; Gimeno, E.; López-Sabater, M.C.; de la Torre, R.; Farré, M. Effects of differing phenolic content in dietary olive oils on lipids and LDL oxidation. Eur. J. Nutr. 2004, 43, 140–147. [Google Scholar] [CrossRef]
- Li, S.H.; Zhao, P.; Tian, H.B.; Chen, L.H.; Cui, L.Q. Effect of grape polyphenols on blood pressure: A meta-analysis of randomized controlled trials. PLoS ONE 2015, 10, e0137665. [Google Scholar] [CrossRef]
- Jumar, A.; Schmieder, R.E. Cocoa flavanol cardiovascular effects beyond blood pressure reduction. J. Clin. Hypertens. 2016, 18, 352–358. [Google Scholar] [CrossRef]
- Cosmi, F.; Di Giulio, P.; Masson, S.; Finzi, A.; Marfisi, R.M.; Cosmi, D.; Scarano, M.; Tognoni, G.; Maggioni, A.P.; Porcu, M.; et al. Regular wine consumption in chronic heart failure impact on outcomes, quality of life, and circulating biomarkers. Circ. Heart Fail. 2015, 8, 428–437. [Google Scholar] [CrossRef]
- Jakobek, L.; Šeruga, M.; Medvidović-Kosanović, M.; Novak, I. Antioxidant activity and polyphenols of Aronia in comparison to other berry species. Agric. Conspec. Sci. 2007, 72, 301–306. [Google Scholar]
- Jurendić, T.; Ščetar, M. Aronia melanocarpa products and by-products for health and nutrition: A review. Antioxidants 2021, 10, 1052. [Google Scholar] [CrossRef]
- Jurikova, T.; Mlcek, J.; Skrovankova, S.; Sumczynski, D.; Sochor, J.; Hlavacova, I.; Snopek, L.; Orsavová, J. Fruits of black chokeberry aronia melanocarpa in the prevention of chronic diseases. Molecules 2017, 22, 944. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO). 2018. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 14 October 2022).
- Acan, B.G.; Kilicli, M.; Bursa, K.; Toker, O.S.; Palabiyik, I.; Gulcu, M.; Yaman, M.; Gunes, R.; Konar, N. Effect of grape pomace usage in chocolate spread formulation on textural, rheological and digestibility properties. LWT 2021, 138, 110451. [Google Scholar] [CrossRef]
- Vilela, A.; Cruz, I.; Oliveira, I.; Pinto, A.; Pinto, T. Sensory and nutraceutical properties of infusions prepared with grape pomace and edible-coated dried–minced grapes. Coatings 2022, 12, 443. [Google Scholar] [CrossRef]
- Lončarević, I.; Petrović, J.; Teslić, N.; Nikolić, I.; Maravić, N.; Pajin, B.; Pavlić, B. Cocoa spread with grape seed oil and encapsulated grape seed extract: Impact on physical properties, sensory characteristics and polyphenol content. Foods 2022, 11, 2730. [Google Scholar] [CrossRef]
- Molnar, D.; Novotni, D.; Krisch, J.; Bosiljkov, T.; Ščetar, M. The optimisation of biscuit formulation with grape and aronia pomace powders as cocoa substitutes. Croat. J. Food Technol. Biotechnol. Nutr. 2020, 15, 38–44. [Google Scholar] [CrossRef]
- Kumar, N. Polysaccharide-based component and their relevance in edible film/coating: A review. Nutr. Food Sci. 2019, 49, 793–823. [Google Scholar] [CrossRef]
- Molnar, D.; Novotni, D.; Kurek, M.; Galić, K.; Iveković, D.; Bionda, H.; Ščetar, M. Characteristics of edible films enriched with fruit by-products and their application on cookies. Food Hydrocoll. 2023, 135, 108191. [Google Scholar] [CrossRef]
- Mouzakitis, C.K.; Sereti, V.; Matsakidou, A.; Kotsiou, K.; Biliaderis, C.G.; Lazaridou, A. Physicochemical properties of zein-based edible films and coatings for extending wheat bread shelf life. Food Hydrocoll. 2022, 132, 107856. [Google Scholar] [CrossRef]
- American Association of Cereal Chemists (AACC). Approved Methods of the American Association of Cereal Chemists International, 11th ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 2000; Available online: https://www.cerealsgrains.org/resources/Methods/Pages/default.aspx (accessed on 26 September 2022).
- ISO 1871:2009; Food and Feed Products—General Guidelines for the Determination of Nitrogen by the Kjeldahl Method. International Organization for Standardization: Geneva, Switzerland, 2009. Available online: https://www.iso.org/standard/41320.html (accessed on 15 September 2022).
- ISO 6492:1999; Animal Feeding Stuffs—Determination of Fat Content. International Organization for Standardization: Geneva, Switzerland, 1999. Available online: https://www.iso.org/standard/12865.html (accessed on 15 September 2022).
- ISO 12966-4:2015; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 4: Determination by Capillary Gas Chromatography Method. International Organization for Standardization: Geneva, Switzerland, 2015. Available online: https://www.iso.org/standard/63503.html (accessed on 15 September 2022).
- EN 14084:2003; Foodstuffs. Determination of Trace Elements. Determination of Lead, Cadmium, Zinc, Copper and Iron by Atomic Absorption Spectrometry (AAS) after Microwave Digestion. European Standard: Plzen, Czech Republic, 2003. Available online: https://standards.iteh.ai/catalog/standards/cen/5a1ec234-434f-42a0-8447-b5c00aee9bae/en-14082-2003 (accessed on 4 October 2022).
- EN 15763:2010; Foodstuffs. Determination of Trace Elements. Determination of Arsenic, Cadmium, Mercury and Lead in Foodstuffs by Inductively Coupled Plasma Mass Spectrometry (ICP–MS) after Pressure Digestion. European Standard: Plzen, Czech Republic, 2010. Available online: https://standards.iteh.ai/catalog/standards/sist/44d9ee33-afaf-49df-b541-f6b4b28e97e6/sist-en-15763-2010 (accessed on 4 October 2022).
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar] [PubMed]
- Vujić, L.; Čepo, D.V.; Dragojević, I.V. Impact of dietetic tea biscuits formulation on starch digestibility and selected nutritional and sensory characteristics. LWT–Food Sci. Technol. 2014, 62, 647–653. [Google Scholar] [CrossRef]
- Babić, D.; Sindik, J.; Missoni, S. Development and validation of a self-administered food frequency questionnaire to assess habitual dietary intake and quality of diet in healthy adults in the Republic of Croatia. Coll. Antropol. 2014, 38, 1017–1026. Available online: https://hrcak.srce.hr/128218 (accessed on 4 April 2021).
- Garsetti, M.; Vinoy, S.; Lang, V.; Holt, S.; Loyer, S.; Brand-Miller, J.C. The glycemic and insulinemic index of plain sweet biscuits: Relationships to in vitro starch digestibility. J. Am. Coll. Nutr. 2005, 24, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J.; Lim, H.S.; Lim, S.T. Effect of partial gelatinization and retrogradation on the enzymatic digestion of waxy rice starch. J. Cereal Sci. 2006, 43, 353–359. [Google Scholar] [CrossRef]
- Diao, Y.; Sia, X.; Shanga, W.; Zhoua, Z.; Wanga, Z.; Zhenga, P.; Strappeb, P.; Blanchard, C. Effect of interactions between starch and chitosan on waxy maize starch physicochemical and digestion properties. CYTA J. Food. 2017, 15, 327–335. [Google Scholar] [CrossRef]
- Bae, I.Y.; Oh, I.K.; Jung, D.S.; Lee, H.G. Influence of arabic gum on in vitro starch digestibility and noodle-making quality of segoami. Int. J. Biol. Macromol. 2019, 125, 668–673. [Google Scholar] [CrossRef]
- Liu, X.; Martin, D.A.; Valdez, J.C.; Sudakaran, S.; Rey, F.; Bolling, B.W. Aronia berry polyphenols have matrix-dependent effects on the gut microbiota. Food Chem. 2021, 359, 129831. [Google Scholar] [CrossRef]
- Cinquanta, L.; Di Cesare, C.; Manoni, R.; Piano, A.; Roberti, P.; Salvatori, G. Mineral essential elements for nutrition in different chocolate products. Int. J. Food Sci. Nutr. 2016, 67, 773–778. [Google Scholar] [CrossRef]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape pomace valorization: A systematic review and meta-analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef]
- Steinberg, F.M.; Bearden, M.M.; Keen, C.L. Cocoa and chocolate flavonoids: Implications for cardiovascular health. J. Am. Diet. Assoc. 2003, 103, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Duran, H.; Yavuz, E.; Sismanoglu, T.; Senkal, B.F. Functionalization of gum arabic including glycoprotein and polysaccharides for the removal of boron. Carbohydr. Polym. 2019, 226, 115139. [Google Scholar] [CrossRef]
- Oszmiański, J.; Lachowicz, S. Effect of the production of dried fruits and juice from chokeberry (Aronia melanocarpa L.) on the content and antioxidative activity of bioactive compounds. Molecules 2016, 21, 1098. [Google Scholar] [CrossRef] [PubMed]
- Ayuda-Durán, B.; González-Manzano, S.; Gil-Sánchez, I.; Moreno-Arribas, M.V.; Bartolomé, B.; Sanz-Buenhombre, M.; Guadarrama, A.; Santos-Buelga, C.; González-Paramás, A.M. Antioxidant characterization and biological effects of grape pomace extracts supplementation in Caenorhabditis elegans. Foods 2019, 8, 75. [Google Scholar] [CrossRef]
- Todorovic, V.; Milenkovic, M.; Vidovic, B.; Todorovic, Z.; Sobajic, S. Correlation between antimicrobial, antioxidant activity, and polyphenols of alkalized/nonalkalized cocoa powders. J. Food Sci. 2017, 82, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Stahl, L.; Miller, K.B.; Apgar, J.; Sweigart, D.S.; Stuart, D.A.; McHale, N.; Ou, B.; Kondo, M.; Hurst, W.J. Preservation of cocoa antioxidant activity, total polyphenols, flavan-3-ols, and procyanidin content in foods prepared with cocoa powder. J. Food Sci. 2009, 74, C456–C461. [Google Scholar] [CrossRef] [PubMed]
- Pawłowska, K.; Kuligowski, M.; Jasińska-Kuligowska, I.; Kidoń, M.; Siger, A.; Rudzińska, M.; Nowak, J. Effect of replacing cocoa powder by carob powder in the muffins on sensory and physicochemical properties. Plant Foods Hum. Nutr. 2018, 73, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Cádiz-Gurrea, M.L.; Borrás-Linares, I.; Lozano-Sánchez, J.; Joven, J.; Fernández-Arroyo, S.; Segura-Carretero, A. Cocoa and grape seed byproducts as a source of antioxidant and anti-inflammatory proanthocyanidins. Int. J. Mol. Sci. 2017, 18, 376. [Google Scholar] [CrossRef]
- Klein, K.M. Why Don’t I Look Like Her? The Impact of Social Media on Female Body Image. Senior Thesis, Claremont Colleges, Claremont, CA, USA, 2013. Available online: http://scholarship.claremont.edu/cmc_theses/720 (accessed on 10 November 2022).
- Pokimica, B.; García-Conesa, M.T.; Zec, M.; Debeljak-Martačić, J.; Ranković, S.; Vidović, N.; Petrović-Oggiano, G.; Konić-Ristić, A.; Glibetić, M. Chokeberry juice containing polyphenols does not affect cholesterol or blood pressure but modifies the composition of plasma phospholipids fatty acids in individuals at cardiovascular risk. Nutrients 2019, 11, 850. [Google Scholar] [CrossRef]
- Naruszewicz, M.; Łaniewska, I.; Millo, B.; Dłużniewski, M. Combination therapy of statin with flavonoids rich extract from chokeberry fruits enhanced reduction in cardiovascular risk markers in patients after myocardial infraction (MI). Atherosclerosis 2007, 194, e179–e184. [Google Scholar] [CrossRef]
- Mateos, R.; Martínez-López, S.; Arévalo, G.B.; Amigo-Benavent, M.; Sarriá, B.; Bravo-Clemente, L. Hydroxytyrosol in functional hydroxytyrosol-enriched biscuits is highly bioavailable and decreases oxidised low density lipoprotein levels in humans. Food Chem. 2016, 205, 248–256. [Google Scholar] [CrossRef]
- Conterno, L.; Martinelli, F.; Tamburini, M.; Fava, F.; Mancini, A.; Sordo, M.; Conterno, L.; Martinelli, F.; Tamburini, M.; Fava, F.; et al. Measuring the impact of olive pomace enriched biscuits on the gut microbiota and its metabolic activity in mildly hypercholesterolaemic subjects. Eur. J. Nutr. 2019, 58, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Sikora, J.; Broncel, M.; Mikiciuk-Olasik, E. Aronia melanocarpa Elliot reduces the activity of angiotensin I-converting enzyme—In vitro and ex vivo studies. Oxid. Med. Cell. Longev. 2014, 2014, 739721. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Vance, T.; Kim, B.; Lee, S.G.; Caceres, C.; Wang, Y.; Hubert, P.A.; Li, J.-Y.; Chun, O.K.; Bolling, B.W. Aronia berry polyphenol consumption reduces plasma total and low-density lipoprotein cholesterol in former smokers without lowering biomarkers of inflammation and oxidative stress: A randomized controlled trial. Nutr. Res. 2017, 37, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Jakovljevic, V.; Milic, P.; Bradic, J.; Jeremic, J.; Zivkovic, V.; Srejovic, I.; Nikolic Turnic, T.; Milosavljevic, I.; Jeremic, N.; Bolevich, S.; et al. Standardized Aronia melanocarpa extract as novel supplement against metabolic syndrome: A rat model. Int. J. Mol. Sci. 2018, 20, 6. [Google Scholar] [CrossRef] [PubMed]
- Broncel, M.; Kozirog, M.; Duchnowicz, P.; Koter-Michalak, M.; Sikora, J.; Chojnowska-Jezierska, J. Aronia melanocarpa extract reduces blood pressure, serum endothelin, lipid, and oxidative stress marker levels in patients with metabolic syndrome. Med. Sci. Monit. 2010, 16, CR28–CR34. [Google Scholar] [PubMed]
- Brouwers, A.; Langlois, M.; Delanghe, J.; Billiet, J.; De Buyzere, M.; Vercaemst, R.; Rietzschel, E.; Bernard, D.; Blaton, V. Oxidized low-density lipoprotein, iron stores, and haptoglobin polymorphism. Atherosclerosis 2004, 176, 189–195. [Google Scholar] [CrossRef]
- D’Amelio, P.; Cristofaro, M.A.; Tamone, C.; Morra, E.; Di Bella, S.; Isaia, G.; Grimaldi, A.; Gennero, L.; Gariboldi, A.; Ponzetto, A.; et al. Role of iron metabolism and oxidative damage in postmenopausal bone loss. Bone 2008, 43, 1010–1015. [Google Scholar] [CrossRef]
- Leiva, E.; Mujica, V.; Sepúlveda, P.; Guzmán, L.; Núñez, S.; Orrego, R.; Palomo, I.; Andrews, M.; Arredondo, M.A. High levels of iron status and oxidative stress in patients with metabolic syndrome. Biol. Trace Elem. Res. 2013, 151, 1–8. [Google Scholar] [CrossRef]
- Lesjak, M.; Hoque, R.; Balesaria, S.; Skinner, V.; Debnam, E.S.; Srai, S.K.S.; Sharp, P.A. Quercetin inhibits intestinal iron absorption and ferroprotein transporter expression in vivo and in vitro. PLoS ONE 2014, 9, e102900. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).