Innovative Surface-Enhanced Raman Spectroscopy Method as a Fast Tool to Assess the Oxidation of Lipids in Ground Pork
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Samples
2.3. Films
2.4. Sample Preparation
2.5. Extraction of Lipids
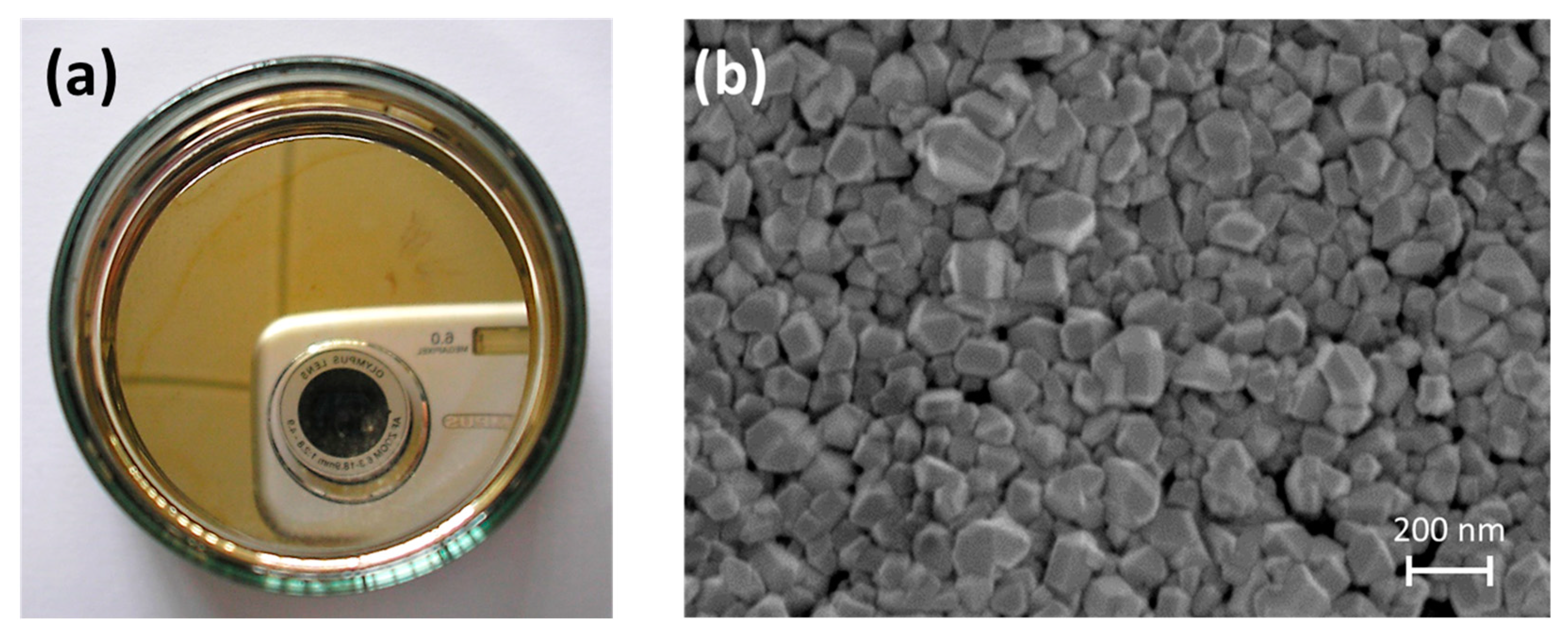
2.6. SERS Substrate
2.7. Equipment
2.8. Cooling System for Improved Raman Measurement
2.9. TBARS
2.10. Optical Images of Samples
2.11. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, J.; Xiong, Y.L. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: A review. Meat Sci. 2016, 120, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Nethra, P.V.; Sunooj, K.V.; Aaliya, B.; Navaf, M.; Akhila, P.P.; Sudheesh, C.; Mir, S.A.; Shijin, A.; George, J. Critical factors affecting the shelf life of packaged fresh red meat—A review. Meas. Food 2023, 10, 100086. [Google Scholar] [CrossRef]
- Pujol, A.; Ospina-E, J.C.; Alvarez, H.; Muñoz, D.A. Myoglobin content and oxidative status to understand meat products’ color: Phenomenological based model. J. Food Eng. 2023, 348, 111439. [Google Scholar] [CrossRef]
- Faustman, C.; Sun, Q.; Mancini, R.; Suman, S.P. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci. 2010, 86, 86–94. [Google Scholar] [CrossRef]
- Jia, W.; Di, C.; Shi, L. Applications of lipidomics in goat meat products: Biomarkers, structure, nutrition interface and future perspectives. J. Proteom. 2023, 270, 104753. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 10, 429. [Google Scholar] [CrossRef]
- Jung, S.; Nam, K.C.; Jo, C. Detection of malondialdehyde in processed meat products without interference from the ingredients. Food Chem. 2016, 209, 90–94. [Google Scholar] [CrossRef]
- Sobsey, C.A.; Han, J.; Lin, K.; Swardfager, W.; Levitt, A.; Borchers, C.H. Development and evaluation of a liquid chromatography–mass spectrometry method for rapid, accurate quantitation of malondialdehyde in human plasma. J. Chromatogr. B 2016, 1029–1030, 205–212. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef]
- Camo, J.; Beltran, J.A.; Roncales, P. Extension of the display life of lamb with an antioxidant active packaging. Meat Sci. 2008, 80, 1086–1091. [Google Scholar] [CrossRef]
- Camo, J.; Lores, A.; Djenane, D.; Beltran, J.A.; Roncales, P. Display life of beef packaged with an antioxidant active film as a function of the concentration of oregano extract. Meat Sci. 2011, 88, 174–178. [Google Scholar] [CrossRef]
- Djenane, D.; Sanchez-Escalante, A.; Beltran, J.A.; Roncales, P. Extension of the shelf life of beef steaks packaged in a modified atmosphere by treatment with rosemary and displayed under UV-free lighting. Meat Sci. 2003, 64, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Nerin, C.; Tovar, L.; Djenane, D.; Camo, J.; Salafranca, J.; Beltran, J.A.; Roncales, P. Stabilisation of beef meat by a new active packaging containing natural antioxidants. J. Agric. Food Chem. 2006, 54, 7840–7846. [Google Scholar] [CrossRef]
- Nerin, C.; Tovar, L.; Salafranca, J. Behaviour of a new antioxidant active film versus oxidizable model compounds. J. Food Eng. 2008, 84, 313–320. [Google Scholar] [CrossRef]
- Atares, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar]
- Bolumar, T.; LaPeña, D.; Skibsted, L.H.; Orlien, V. Rosemary and oxygen scavenger in active packaging for prevention of high-pressure induced lipid oxidation in pork patties. Food Packag. Shelf Life 2016, 7, 26–33. [Google Scholar] [CrossRef]
- Contini, C.; Alvarez, R.; O’Sullivan, M.; Dowling, D.P.; Gargan, S.O.; Monahan, F.J. Effect of an active packaging with citrus extract on lipid oxidation and sensory quality of cooked turkey meat. Meat Sci. 2014, 96, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Rizzolo, A.; Bianchi, G.; Povolo, M.; Migliori, C.A.; Contarini, G.; Pelizzola, V.; Cattaneo, T.M.P. Volatile compound composition and antioxidant activity of cooked ham slices packed in propolis-based active packaging. Food Packag. Shelf Life 2016, 8, 41–49. [Google Scholar] [CrossRef]
- de Barros Santos, E.; Sigoli, F.A.; Mazali, I.O. Metallic Cu nanoparticles dispersed into porous glass: A simple green chemistry approach to prepare SERS substrates. Mater. Lett. 2013, 108, 172–175. [Google Scholar] [CrossRef]
- Kruszewski, S.; Cyrankiewicz, M. Aggregated silver sols as SERS substrates. Acta Phys. Pol. A 2012, 121, A68–A74. [Google Scholar] [CrossRef]
- Sharma, B.; Frontiera, R.R.; Henry, A.I.; Ringe, E.; Van Duyne, R.P. SERS: Materials, applications, and the future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Yao, W.; Sun, Y.; Xie, Y.; Wang, S.; Ji, L.; Wang, H.; Qian, H. Development and evaluation of a surface-enhanced Raman scattering (SERS) method for the detection of the antioxidant butylated hydroxyanisole. Eur. Food Res. Technol. 2011, 233, 835–840. [Google Scholar] [CrossRef]
- Sadeghi-Jorabchi, H.; Wilson, R.H.; Belton, P.S.; Edwards-Webb, J.D.; Coxon, D.T. Quantitative analysis of oils and fats by Fourier transform Raman spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1991, 47, 1449–1458. [Google Scholar] [CrossRef]
- Fowler, S.M.; Ponnampalam, E.N.; Schmidt, H.; Wynn, P.; Hopkins, D.L. Prediction of intramuscular fat content and major fatty acid groups of lamb M. longissimus lumborum using Raman spectroscopy. Meat Sci. 2015, 110, 70–75. [Google Scholar] [CrossRef]
- Gouvinhas, I.; Machado, N.; Carvalho, T.; de Almeida, J.M.M.M.; Barros, A.I.R.N.A. Short wavelength Raman spectroscopy applied to the discrimination and characterisation of three cultivars of extra virgin olive oils in different maturation stages. Talanta 2015, 132, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Herrero, A.M.; Ruiz-Capillas, C.; Jimenez-Colmenero, F.; Carmona, P. Raman spectroscopic study of structural changes upon chilling storage of frankfurters containing olive oil bulking agents as fat replacers. J. Agric. Food Chem. 2014, 62, 5963–5971. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lopez, E.; Sanchez-Rodriguez, M.I.; Marinas, A.; Marinas, J.M.; Urbano, F.J.; Caridad, J.M.; Moalem, M. Chemometric study of Andalusian extra virgin olive oils Raman spectra: Qualitative and quantitative information. Talanta 2016, 156–157, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Li, Y.; Du, S.; Geng, Y.; Su, M.; Liu, H. Raman spectroscopy for rapid fingerprint analysis of meat quality and security: Principles, progress and prospects. Food Res. Int. 2022, 161, 111805. [Google Scholar] [CrossRef]
- Robert, C.; Jessep, W.; Sutton, J.J.; Hicks, T.M.; Loeffen, M.; Farouk, M.; Ward, J.F.; Bain, W.E.; Craigie, C.R.; Fraser-Miller, S.J.; et al. Evaluating low- mid- and high-level fusion strategies for combining Raman and infrared spectroscopy for quality assessment of red meat. Food Chem. 2021, 361, 130154. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Driver, M.; Decker, E.; He, L.L. Lipid and lipid oxidation analysis using surface enhanced Raman spectroscopy (SERS) coupled with silver dendrites. Food Res. Int. 2014, 58, 1–6. [Google Scholar] [CrossRef]
- Weldon, M.K.; Morris, M.D.; Harris, A.B.; Stoll, J.K. Surface enhanced Raman spectroscopic monitor of P-acnes lipid hydrolysis in vitro. J. Lipid Res. 1998, 39, 1896–1899. [Google Scholar] [CrossRef]
- Hamdan, M.N.; Mansor, W.; Radzol, A.R.M.; Umut, I.; Mahmud, A.R.; Beeran Kutty, S. Principal Component Analysis of SERS-Based Chicken, Beef and Pork Fats. Int. J. Emerg. Technol. Adv. Eng. 2022, 12, 144–151. [Google Scholar] [CrossRef]
- Pezo, D.; Wrona, M.; Rodriguez-Lafuente, A.; Nerin, C. A sulphuric acid-impregnated silica gel clean-up procedure for the determination of n-alkanes migration from paraffin based paper packaging into cheddar cheese. Food Chem. 2012, 134, 405–411. [Google Scholar] [CrossRef]
- von Liebig, J. On silvering glass. Philos. Mag. Ser. 4 1868, 35, 146–147. [Google Scholar] [CrossRef]
- Wrona, M.; Salafranca, J.; Rocchia, M.; Nerin, C. Application of SERS to the determination of butylated hydroxyanisole in edible and essential oils. Spectroscopy 2015, 30, 40–45. [Google Scholar]
- Pfalzgraf, A.; Frigg, M.; Steinhart, H. Tocopherol contents and lipid oxidation in pork muscle and adipose tissue during storage. J. Agric. Food Chem. 1995, 43, 1339–1342. [Google Scholar] [CrossRef]
- Xiao, C.; Chen, Z.; Zhang, D.; Xiao, W.; Qing, M.; Liu, X. Research on the temperature effect characteristics of SERS enhancement factor. Optik 2016, 127, 9926–9931. [Google Scholar] [CrossRef]
- de Moraes Crizel, T.; Haas Costa, T.M.; de Oliveira Rios, A.; Hickmann Flores, S. Valorisation of food-grade industrial waste in the obtaining active biodegradable films for packaging. Ind. Crops Prod. 2016, 87, 218–228. [Google Scholar] [CrossRef]
- Yan, X.; Cheng, M.; Wang, Y.; Zhao, P.; Wang, K.; Wang, Y.; Wang, X.; Wang, J. Evaluation of film packaging containing mesoporous nanosilica and oregano essential oil for postharvest preservation of mushrooms (Agaricus bisporus). Postharvest Biol. Techn. 2023, 198, 112263. [Google Scholar] [CrossRef]
- Muriel-Galet, V.; Cran, M.J.; Bigger, S.W.; Hernandez-Muñoz, P.; Gavara, R. Antioxidant and antimicrobial properties of ethylene vinyl alcohol copolymer films based on the release of oregano essential oil and green tea extract components. J. Food Eng. 2015, 149, 9–16. [Google Scholar] [CrossRef]
- Gherardi, R.; Becerril, R.; Nerin, C.; Bosetti, O. Development of a multilayer antimicrobial packaging material for tomato puree using an innovative technology. LWT-Food Sci. Technol. 2016, 72, 361–367. [Google Scholar] [CrossRef]
- Pezo, D.; Salafranca, J.; Nerin, C. Development of an automatic multiple dynamic hollow fibre liquid-phase microextraction procedure for specific migration analysis of new active food packagings containing essential oils. J. Chromatogr. A 2007, 1174, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, R.; Krepker, M.; Nitzan, N.; Vaxman, A.; Segal, E. Active packaging containing encapsulated carvacrol for control of postharvest decay. Postharvest Biol. Technol. 2016, 118, 175–182. [Google Scholar] [CrossRef]
- Baeten, V.; Hourant, P.; Morales, M.T.; Aparicio, R. Oil and fat classification by FT-Raman spectroscopy. J. Agric. Food Chem. 1998, 46, 2638–2646. [Google Scholar] [CrossRef]
- Lv, M.Y.; Zhang, X.; Ren, H.R.; Liu, L.; Zhao, Y.M.; Wang, Z.; Wu, Z.L.; Liu, L.M.; Xu, H.J. A rapid method to authenticate vegetable oils through surface-enhanced Raman scattering. Sci. Rep. 2016, 6, 23405. [Google Scholar] [CrossRef] [PubMed]
- Muik, B.; Lendl, B.; Molina-Diaz, A.; Ayora-Cañada, M.J. Direct monitoring of lipid oxidation in edible oils by Fourier transform Raman spectroscopy. Chem. Phys. Lipids 2005, 134, 173–182. [Google Scholar] [CrossRef]
- Czamara, K.; Majzner, K.; Pacia, M.Z.; Kochan, K.; Kaczor, A.; Baranska, M. Raman spectroscopy of lipids: A review. J. Raman Spectrosc. 2015, 46, 4–20. [Google Scholar] [CrossRef]
- Carmona, M.A.; Lafont, F.; Jimenez-Sanchidrian, C.; Ruiz, J.R. Raman spectroscopy study of edible oils and determination of the oxidative stability at frying temperatures. Eur. J. Lipid Sci. Technol. 2014, 116, 1451–1456. [Google Scholar] [CrossRef]
- Rzhevskii, A. Basic Aspects of Experimental Design in Raman Microscopy. Spectroscopy 2016, 31, 40–45. [Google Scholar]
- Irie, M.; Swatland, H.J. Assessment of porcine fat quality by fiber-optic spectrophotometry. Asian Australas. J. Anim. Sci. 1992, 5, 753–756. [Google Scholar] [CrossRef]
- Araujo de Vizcarrondo, C.; Carrillo de Padilla, F.; Martin, E. Fatty acid composition of beef, pork, and poultry fresh cuts, and some of their processed products. Arch. Latinoam. Nutr. 1998, 48, 354–358. [Google Scholar] [PubMed]
- Fidanza, F.; Keys, A.; Anderson, J.T. Density of body fat in man and other mammals. J. Appl. Physiol. 1953, 6, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Le Ru, E.C.; Blackie, E.; Meyer, M.; Etchegoin, P. Surface enhanced Raman scattering enhancement factors: A comprehensive study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]
- Hakonen, A.; Svedendahl, M.; Ogier, R.; Yang, Z.J.; Lodewijks, K.; Verre, R.; Shegai, T.; Andersson, P.O.; Käll, M. Dimer-on-mirror SERS substrates with attogram sensitivity fabricated by colloidal lithography. Nanoscale 2015, 7, 9405–9410. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Y.; Wang, Y.; Zhang, K.; Lai, Y. Robust and reliable detection of malondialdehyde in biological samples via microprobe-triggered surface-enhanced Raman spectroscopy. Microchem. J. 2022, 181, 107815. [Google Scholar] [CrossRef]






| Raman Shift [cm−1] | Functional Group |
|---|---|
| 3008 | C–H stretching of C=C–H |
| 2850 | C–H stretching ν(C–H) in –CH3 and –CH2– |
| 1655 | stretching of C=C bonds |
| 1439 | scissoring deformation δ(CH2) of –CH2– |
| 1301 | in-plane C=C–H deformation |
| 1266 | in-phase –CH2– twisting |
| Day | RCU% (LDPE) | RCU% (Active Packaging) |
|---|---|---|
| 0 | 0 ± 0 | 0 ± 0 |
| 7 | 19 ± 3 | 1 ± 0 |
| 9 | 51 ± 9 | 30 ± 3 |
| 11 | 66 ± 9 | 44 ± 5 |
| 14 | 71 ± 12 | 51 ± 10 |
| 16 | 74 ± 14 | 49 ± 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wrona, M.; Lours, J.; Salafranca, J.; Joly, C.; Nerín, C. Innovative Surface-Enhanced Raman Spectroscopy Method as a Fast Tool to Assess the Oxidation of Lipids in Ground Pork. Appl. Sci. 2023, 13, 5533. https://doi.org/10.3390/app13095533
Wrona M, Lours J, Salafranca J, Joly C, Nerín C. Innovative Surface-Enhanced Raman Spectroscopy Method as a Fast Tool to Assess the Oxidation of Lipids in Ground Pork. Applied Sciences. 2023; 13(9):5533. https://doi.org/10.3390/app13095533
Chicago/Turabian StyleWrona, Magdalena, Juliette Lours, Jesús Salafranca, Catherine Joly, and Cristina Nerín. 2023. "Innovative Surface-Enhanced Raman Spectroscopy Method as a Fast Tool to Assess the Oxidation of Lipids in Ground Pork" Applied Sciences 13, no. 9: 5533. https://doi.org/10.3390/app13095533
APA StyleWrona, M., Lours, J., Salafranca, J., Joly, C., & Nerín, C. (2023). Innovative Surface-Enhanced Raman Spectroscopy Method as a Fast Tool to Assess the Oxidation of Lipids in Ground Pork. Applied Sciences, 13(9), 5533. https://doi.org/10.3390/app13095533







