Variable Responses to a Marine Heat Wave in Five Fringing Reefs of Southern Taiwan
Abstract
1. Introduction
2. Materials and Methods
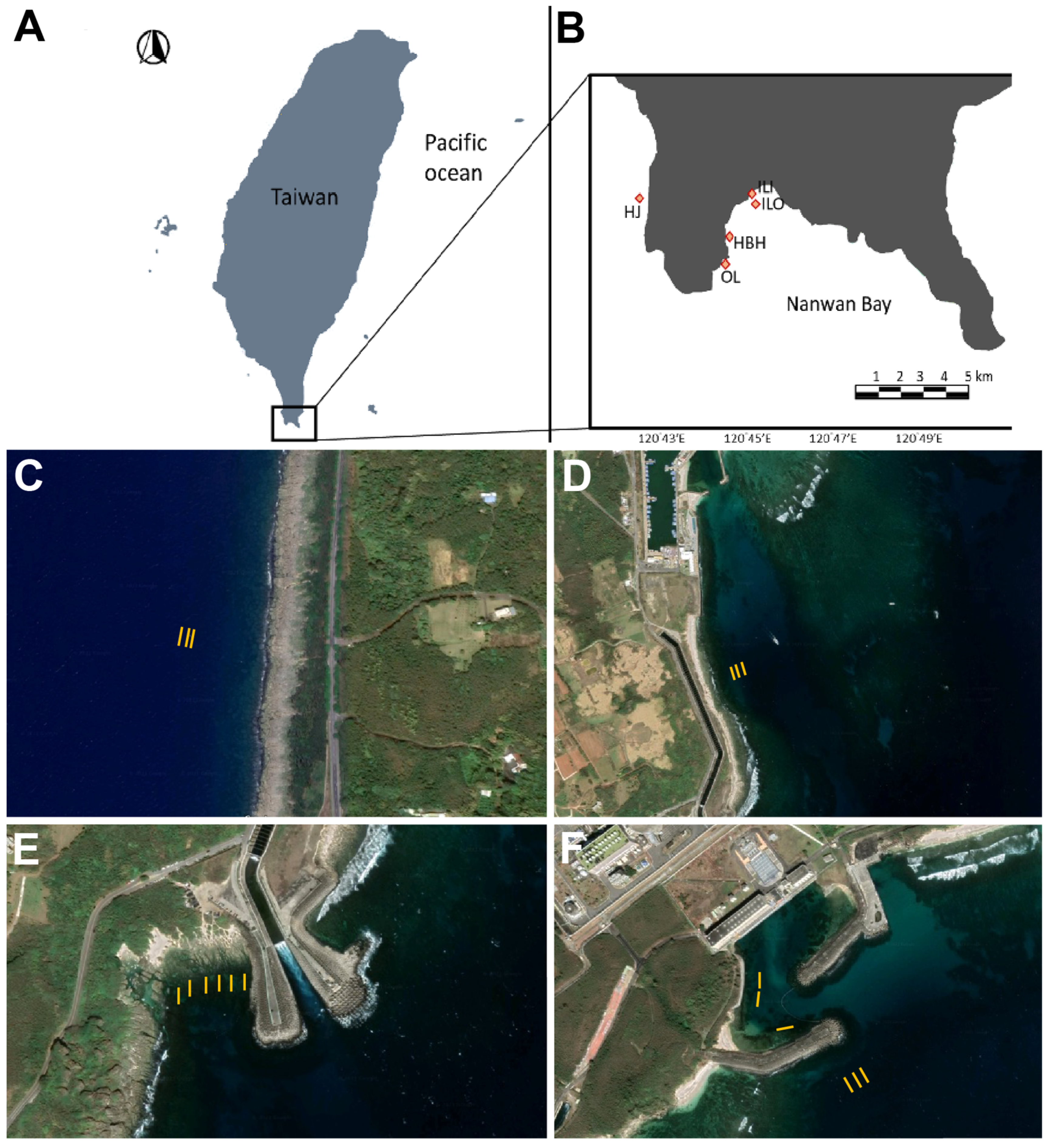
2.1. Study Sites and Benthic Surveys
| Site Name | Hejie | Houbihu | Outlet | Inlet-Inside | Inlet-Outside |
|---|---|---|---|---|---|
| Site abbreviation | HJ | HBH | OL | ILI | ILO |
| Latitude | 21°57′22.0′′ N | 21°56′17.7′′ N | 21°55′54.8′′ N | 21°57′16.8′′ N | 21°57′11.8′′ N |
| Longitude | 120°42′36.3′′ E | 120°44′46.1′′ E | 120°44′42.0′′ E | 120°45′13.9′′ E | 120°45′20.6′′ E |
| Location (Figure 1A) | West Peninsula | Nanwan Bay | Nanwan Bay | Nanwan Bay | Nanwan Bay |
| Upwelling? | No | Yes | Yes | Yes | Yes |
| Thermal effluent? | No | No | Yes | No | No |
| Depth (m) | 3 | 3 | 3 | 3 | 7 |
| Fringing reef type | Natural | Natural | Artificial | Artificial | Natural |
| Most common coral genera in April 2020 (pre-bleaching; % of total benthos) | Montipora (14%) Pocillopora (12%) Favites (4%) | Seriatopora (14%) Millepora (10%) Montipora (2%) | Montipora (24%) Favites (7%) Millepora (5%) | Acropora (38%) Pocillopora (3%) Montipora (2%) | Montipora (11%) Acropora (6%) Favites (3%) |
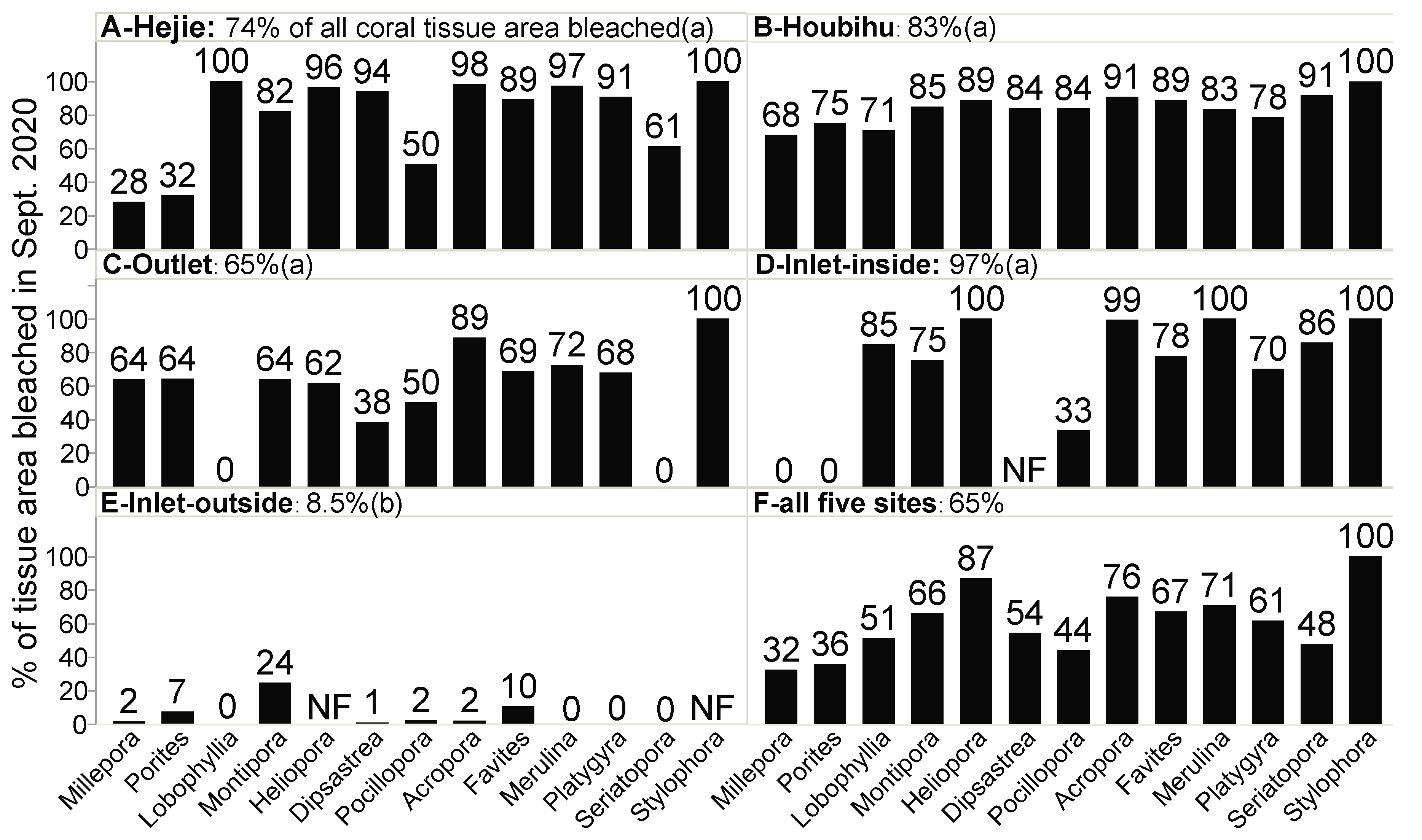
| Top three thermally susceptible coral genera (% tissue area bleached in September 2020) | Stylophora (100%) Lobophyllia (100%) Acropora (98%) | Stylophora (100%) Seriatopora (91%) Acropora (91%) | Stylophora (100%) Acropora (89%) Merulina (72%) | Merulina (100%) Stylophora (100%) Heliopora (100%) | Montipora (24%) Favites (10%) Porites (7%) |
| Top three thermally susceptible coral genera (% decrease in cover: April 2020 to September 2021) Figure S3 | Goniastrea (100%) Phymastrea (100%) Stylophora (94%) | Astreopora (100%) Goniastrea (100%) Merulina (100%) | Stylophora (100%) Psammocora (100%) Seriatopora (100%) a | Acanthastrea (100%) Heliopora (100%) Millepora (100%) | Fungia (100%) Leptoseris (100%) Pavona (100%) b |
| Emerged on reef post-bleaching (exhaustive list) Figure S3 c | Diploastrea Pachyseris Psammocora Turbinaria | Echinophyllia Turbinaria | Mycedium | Psammocora Tubastrea | Diploastrea Euphyllia Psammocora |
| Conservation action(s) proposed herein | Establish MPA | Establish MPA & control pollution from nearby harbor | Restrict number of visitors to limit physical damage | Establish MPA | Establish MPA |
| Site Name | HJ | HBH | OL | ILI | ILO | Site Effect | Temp. Effect |
|---|---|---|---|---|---|---|---|
| MMM-temp. (°C) | 28.6 | 28.3 | 29.3 | 28.0 | 28.4 | p < 0.0001 | NA |
| MMM-time window analyzed | 2010–2012 | 2010–2012 | 2010–2012 | 2010–2012 | 2013–2014 | NA | NA |
| Warmest month | Sept | Sept | Aug | Sept | Jul | NA | NA |
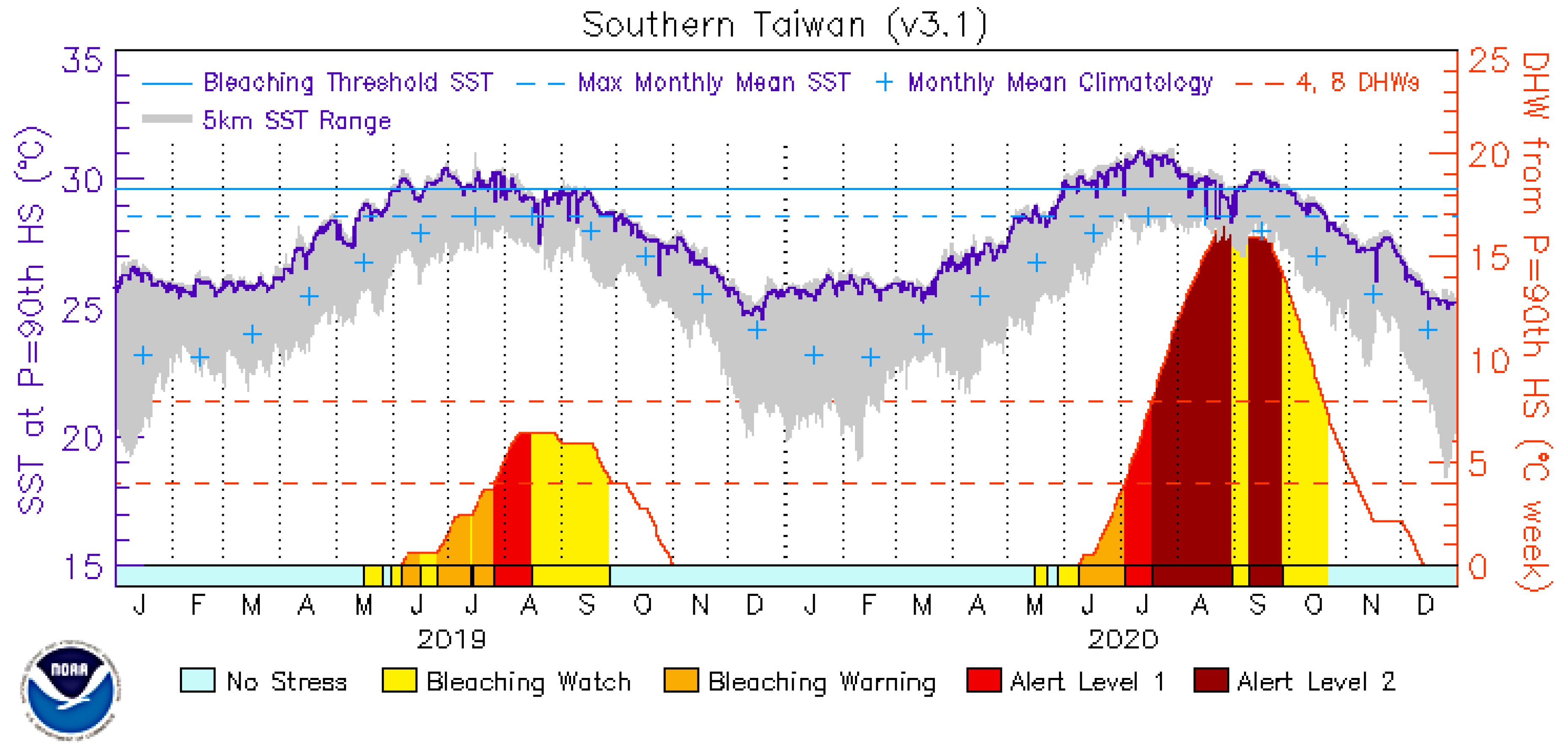
| 2020 DHWs (NOAA’s Coral Reef Watch; Figure 2) | 16 | 16 | 16 | 16 | 16 | NS | NA |
| 2020 DHWs (in situ measurements) a | 16.3 | 17.0 | 16.9 | 19.9 | 1.9 | p < 0.0001 | NA |
| 2020 DHDs (in situ measurements) b | 101 | 104 | 108 | 120 | 13 | p < 0.0001 | NA |
| Mean annual temp. (°C) | 27.0 | 26.5 | 27.2 | 26.5 | 25.9 | p < 0.0001 | NA |
| Mean monthly temp. range (max. minus min. °C) | 3.8 | 5.5 | 7.2 | 6.1 | 6.8 | p < 0.0001 | NA |
| Mean temp. in warmest month of 2020 (°C) | 30.3 | 29.9 | 31.1 | 29.8 | 28.7 | p < 0.0001 | NA |
| Max. hourly mean seawater temp. in 2020 (°C) | 31.8 | 32.2 | 35.6 | 32.7 | 31.3 | p < 0.0001 | NA |
| Summed time above 30 °C in 2020 (days) | 47 | 33 | 78 | 25 | 7 | p < 0.0001 | NA |
| # days in 2020 with mean temp. above 30 °C | 42 | 32 | 72 | 22 | 2 | p < 0.0001 | NA |
| Heat accrual interval (2020) | 3 Jun–1 Oct | 11 Jun–1 Oct | 27 Mar–14 Oct | 13 Jun–3 Oct | 12 Jun–16 Sept | NA | NA |
| Coral cover before bleaching event (%) b | 47 | 37 | 55 | 44 | 36 | NS | NA |
| Coral cover after bleaching event (%)-Dec 2020 b | 22▼ | 7.5▼ | 22▼ | 11▼ | 48 | p < 0.0001 | p < 0.0001 |
| Coral cover after bleaching event (%)-Sept 2021 b | 28▼ | 18▼ | 51 | 46 | 52 | p < 0.01 | p < 0.001 |
| % hard coral cover increase/decrease (18 months) | −36%▼ | −49%▼ | −7% | +7% | +44% | p < 0.0001 | p < 0.0001 |
| % of all corals that were bleached in Sept 2020 (Figure 4) c | 74 | 83 | 65 | 97 | 8.5 | p < 0.001 | p < 0.0001 |
| Mean bleaching percentage across genera d | 78 | 84 | 57 | 64 | 3.7 | p < 0.001 | p < 0.0001 |
| # genera present: Apr 2020➔Sept 2021 | 22➔24 | 21➔20 | 22➔17 | 17➔16 | 28➔26 | p < 0.001 | NS |
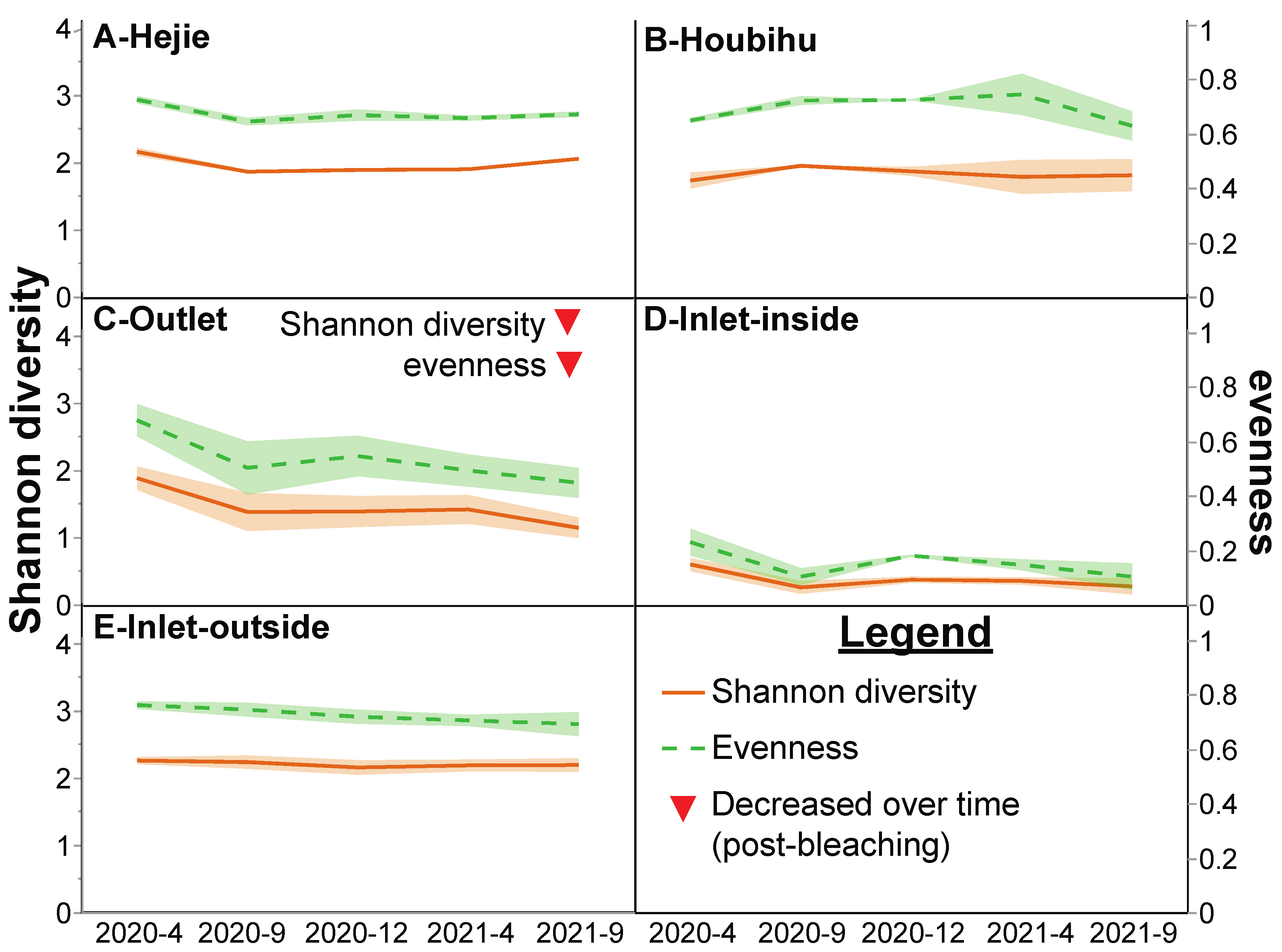
| Shannon diversity: April 2020➔Sept 2021 | 2.2➔2.0 | 1.7➔1.8 | 1.9➔1.1▼ | 0.6➔0.3 | 2.3➔2.2 | p < 0.001 | NS |
| Evenness: April 2020➔Sept 2021 | 0.7➔0.7 | 0.6➔0.6 | 0.7➔0.4▼ | 0.2➔0.1 | 0.8➔0.7 | p < 0.001 | NS |
| Coral/algae ratio: April 2020➔Sept 2021 | 1.4➔0.6▼ | 1.5➔0.7▼ | 2.9➔3.1▲ | 4.0➔2.6▼ | 1.6➔3.1▲ | p < 0.0001 | NS |

| Effect | Wilks’ Lambda | Approx./ Exact F | p | Greatest Contributor to Variation (% Variation Explained) |
|---|---|---|---|---|
| All five sites-83 benthic groupings, 4 MDS dimensions, and MDS stress of 0.08 (n = 86 data points) | ||||
| site | 0.02 | 26.87 | <0.0001 | Foliose Montipora spp. cover significantly higher at OL (27%) |
| month | 0.08 | 13.56 | <0.0001 | Encrusting macroalgae significantly more common in Apr. (both years; 27%) |
| site × month | 0.05 | 4.21 | <0.0001 | Foliose Montipora spp. cover significantly higher at OL in all months (21%) |
| depth | 0.21 | 3.70 | <0.01 | Sarcophyton sp. abundance was significantly higher at 7 m (43%) |
| depth × month | 0.66 | 2.01 | 0.01 | CCA on hard substrate significantly more abundant at 3 m in 2021 (22%) |
| type | 0.31 | 5.65 | <0.001 | Significantly higher abundance of Lobophyton sp. on natural reefs (16%) |
| type × month | 0.67 | 1.90 | 0.02 | |
| upwelling | 0.42 | 4.50 | <0.0001 | Pocillopora spp. significantly more abundant on the non-upwelling reef (69%) |
| upwelling × month | 0.93 | 0.34 | 0.99 | |
| transect (site × month) | 0.41 | 0.09 | 1.00 | |
| Hejie (HJ; shallow, natural, non-upwelling reef)-71 benthic groupings, 2 MDS dimensions, and MDS stress of 0.06 (n = 15) | ||||
| month | 0.002 | 45.29 | <0.0001 | Significantly more bleached soft corals in December 2020 (11%) |
| transect | 0.98 | 0.04 | 1.00 | |
| transect (month) | 0.89 | 0.02 | 1.00 | |
| Houbihu (HBH; shallow, natural, upwelling reef)-66 benthic groupings, 3 MDS dimensions, and MDS stress of 0.05 (n = 15) | ||||
| month | 0.002 | 16.15 | <0.0001 | Significantly more foliose Montipora spp. in April and September 2020 (13%) |
| transect | 0.87 | 0.24 | 0.96 | |
| transect (month) | 0.48 | 0.06 | 1.00 | |
| Outlet (OL; shallow, artificial, effluent-infused, upwelling reef)-61 benthic groupings, 3 MDS dimensions, and MDS stress of 0.09 (n = 25) | ||||
| month | 0.05 | 7.97 | <0.0001 | Bleached Montipora spp. significantly more common in Sept. 2020 (14%) |
| transect | 0.70 | 0.43 | 0.96 | |
| transect (month) | 0.10 | 0.13 | 1.00 | |
| Inlet-inside (ILI; shallow, artificial, cooler upwelling reef)-61 benthic groupings, 2 dimensions, and MDS stress of 0.09 (n = 15) | ||||
| month | 0.06 | 6.74 | <0.001 | More hard substrate (abiotic) in April. (both years; 16%) |
| transect | 0.66 | 1.29 | 0.30 | |
| transect (month) | 0.43 | 0.16 | 1.00 | |
| Inlet-outside (ILO; deep, natural, upwelling reef)-69 benthic groupings, 4 MDS dimensions, and MDS stress of 0.07 (n = 15) | ||||
| month | 0.001 | 7.11 | <0.001 | More CCA on hard substrate in 2021 vs. 2020 (14%) |
| transect | 0.05 | 4.32 | 0.02 | |
| transect (month) | 0.08 | 0.14 | 1.00 | |
2.2. Image Analysis
2.3. Statistical Analyses
2.4. Seawater Temperature
3. Results
3.1. Temperature
3.2. Benthic Community Changes in Response to the 2020 Marine Heatwave
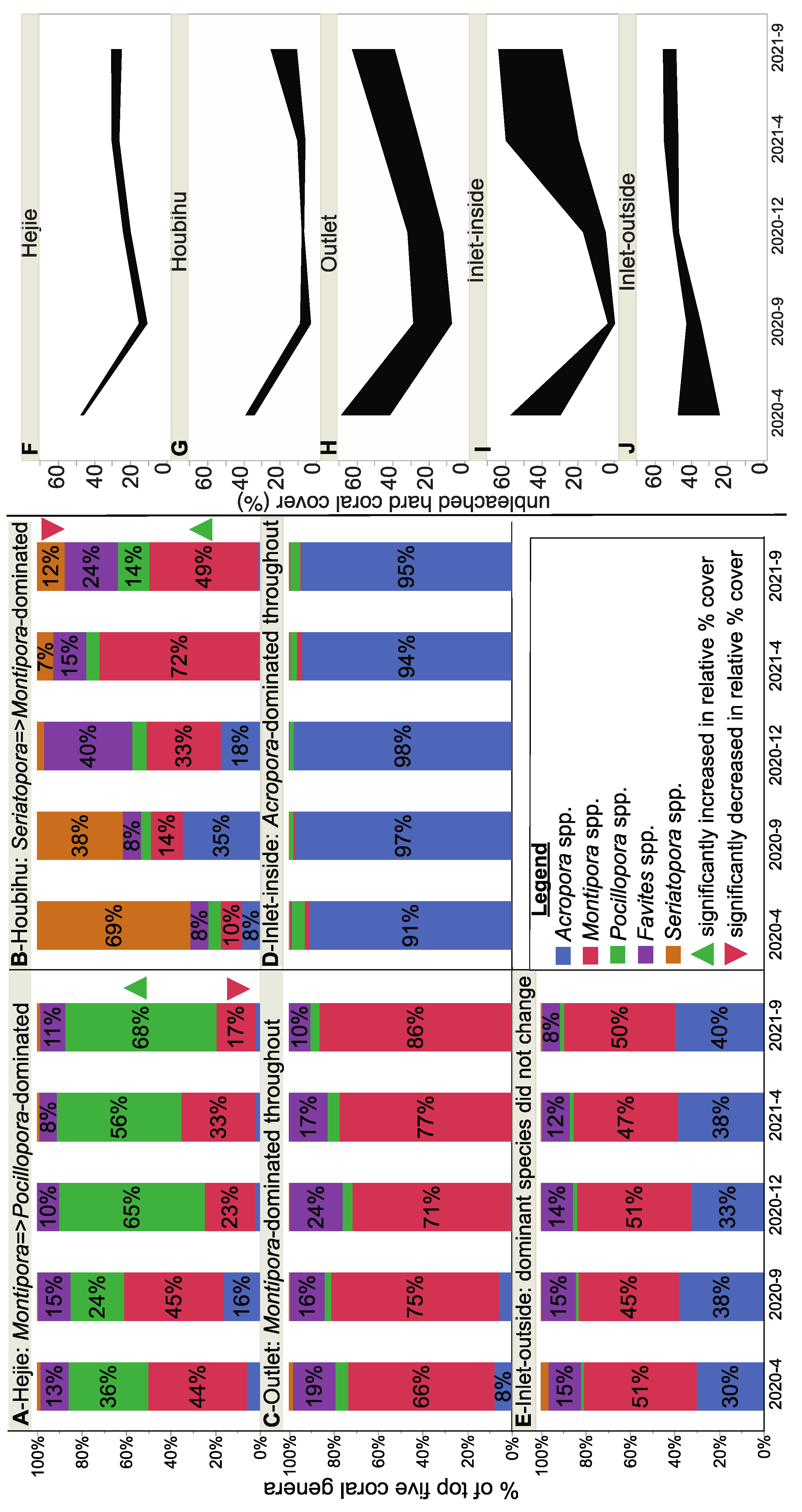
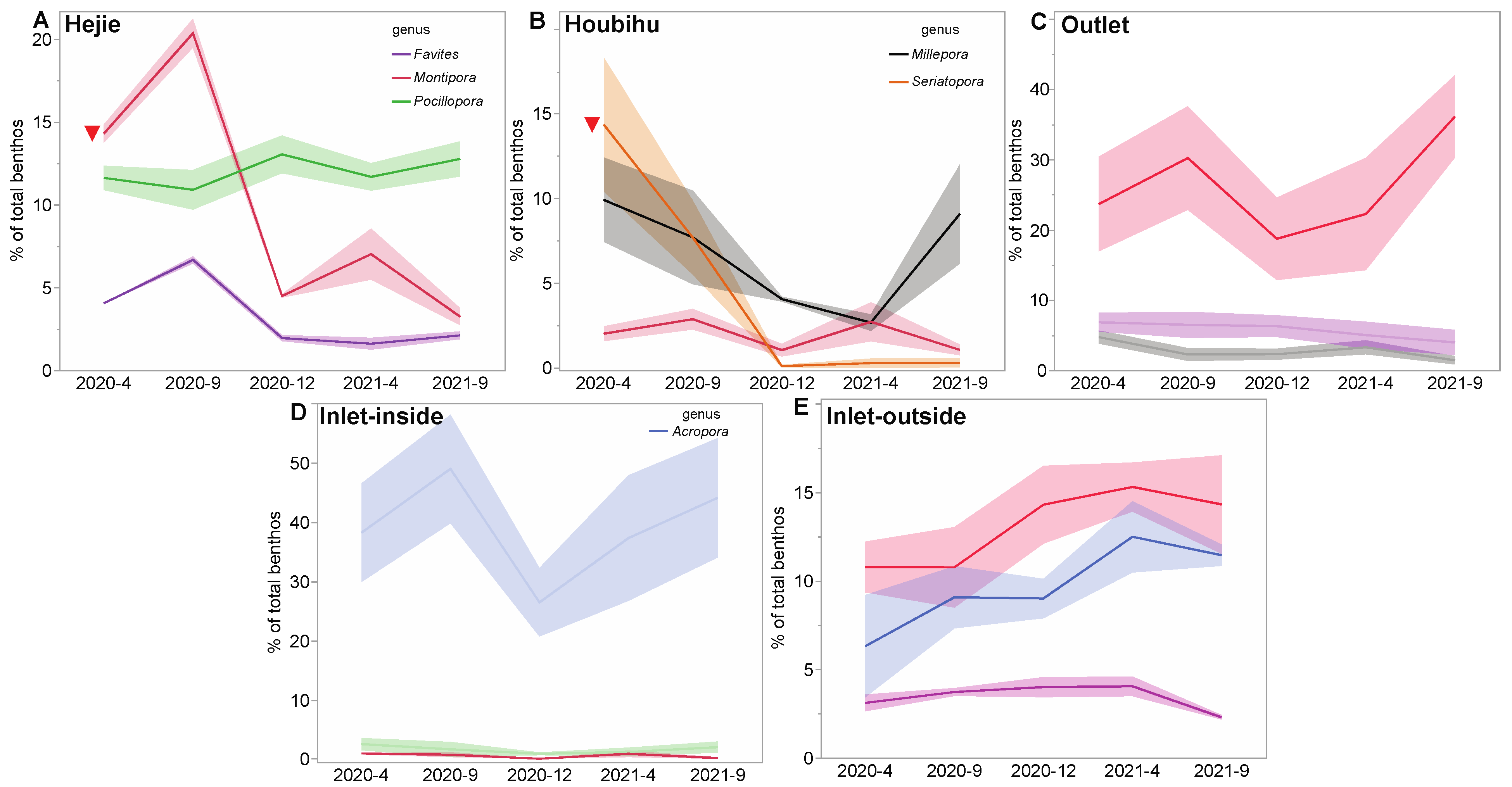
3.3. Changes in Dominant Reef Coral Genera
3.3.1. Hejie (HJ)
3.3.2. Houbihu (HBH)
3.3.3. Outlet (OL)
3.3.4. Inlet-Inside (ILI)
3.3.5. Inlet-Outside (ILO)
3.4. Inter-Site Analysis
4. Discussion
5. Conservation Implications and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mayfield, A.B.; Chen, C.S. A coral transcriptome in the Anthropocene as an “alternative stable state. ” Platax 2020, 17, 1–26. [Google Scholar]
- Hughes, T.P.; Kerry, J.T.; Álvarez-Noriega, M.; Álvarez-Romero, J.G.; Anderson, K.D.; Baird, A.H.; Babcock, R.C.; Beger, M.; Bellwood, D.R.; Berkelmans, R.; et al. Global warming and recurrent mass bleaching of corals. Nature 2017, 543, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Eakin, C.M.; Sweatman, H.P.A.; Brainard, R.E. The 2014–2017 global-scale coral bleaching event: Insights and impacts. Coral Reefs 2019, 38, 539–545. [Google Scholar] [CrossRef][Green Version]
- Woodhead, A.J.; Hicks, C.C.; Norström, A.V.; Williams, G.J.; Graham, N.A.J. Coral reef ecosystem services in the Anthropocene. Funct. Ecol. 2019, 33, 1023–1034. [Google Scholar] [CrossRef][Green Version]
- Goreau, T.J.F.; Hayes, R.L. Global warming triggers coral reef bleaching tipping point. AMBIO 2021, 50, 1137–1140. [Google Scholar] [CrossRef]
- Anthony, K.R.N.; Helmstedt, K.; Bay, L.K.; Fidelman, P.; Hussey, K.E.; Lundgren, P.; Mead, D.; McLeod, I.M.; Mumby, P.J.; Newlands, M.; et al. Interventions to help coral reefs under global change—a complex decision challenge. PLoS ONE 2020, 15, e0236399. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Mayfield, A.B.; Fan, T.-Y. Effects of feeding on the physiological performance of the stony coral Pocillopora acuta. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Mayfield, A.B.; Tsai, S.; Lin, C. The Coral Hospital. Biopreserv. Biobank. 2019, 17, 355–369. [Google Scholar] [CrossRef]
- Mayfield, A.B.; Chan, P.-H.; Putnam, H.M.; Chen, C.-S.; Fan, T.-Y. The effects of a variable temperature regime on the physiology of the reef-building coral Seriatopora hystrix: Results from a laboratory-based reciprocal transplant. J. Exp. Biol. 2012, 215, 4183–4195. [Google Scholar] [CrossRef][Green Version]
- Enochs, I.C.; Formel, N.; Manzello, D.; Morris, J.; Mayfield, A.; Boyd, A.; Kolodziej, G.; Adams, G.; Hendee, J. Coral persistence despite extreme periodic pH fluctuations at a volcanically acidified Caribbean reef. Coral Reefs 2020, 39, 523–528. [Google Scholar] [CrossRef]
- Cruz-García, R.; Rodríguez-Troncoso, A.P.; Zaragoza, F.A.R.; Mayfield, A.; Cupul-Magaña, A. Ephemeral effects of El Niño–Southern Oscillation events on an eastern tropical Pacific coral community. Mar. Freshw. Res. 2020, 71, 1259. [Google Scholar] [CrossRef]
- Oliver, T.A.; Palumbi, S.R. Do fluctuating temperature environments elevate coral thermal tolerance? Coral Reefs 2011, 30, 429–440. [Google Scholar] [CrossRef]
- Safaie, A.; Silbiger, N.J.; McClanahan, T.R.; Pawlak, G.; Barshis, D.J.; Hench, J.L.; Rogers, J.S.; Williams, G.J.; Davis, K.A. High frequency temperature variability reduces the risk of coral bleaching. Nat. Commun. 2018, 9, 1671. [Google Scholar] [CrossRef][Green Version]
- Schmidt, G.M.; Wall, M.; Taylor, M.; Jantzen, C.; Richter, C. Large-amplitude internal waves sustain coral health during thermal stress. Coral Reefs 2016, 35, 869–881. [Google Scholar] [CrossRef][Green Version]
- Carter, A.L.; Meriwether, A.; Wilson, W.; Bello, M.; Hoyos-Padilla, E.M.; Inall, M.E.; Ketchum, J.T.; Schurer, A.; Tudhope, A.W. Assessing opportunities to support coral reef climate change refugia in MPAs: A case study at the Revillagigedo Archipelago. Mar. Policy 2020, 112, 103769. [Google Scholar] [CrossRef]
- Wen, C.K.-C.; Chen, K.-S.; Hsieh, H.J.; Hsu, C.-M.; Chen, C.A. High coral cover and subsequent high fish richness on mature breakwaters in Taiwan. Mar. Pollut. Bull. 2013, 72, 55–63. [Google Scholar] [CrossRef]
- Burt, J.A.; Camp, E.F.; Enochs, I.C.; Johansen, J.L.; Morgan, K.M.; Riegl, B.; Hoey, A.S. Insights from extreme coral reefs in a changing world. Coral Reefs 2020, 39, 495–507. [Google Scholar] [CrossRef]
- Quimpo, T.J.R.; Requilme, J.N.C.; Gomez, E.J.; Sayco, S.L.G.; Tolentino, M.P.S.; Cabaitan, P.C. Low coral bleaching prevalence at the Bolinao-Anda Reef Complex, northwestern Philippines during the 2016 thermal stress event. Mar. Pollut. Bull. 2020, 160, 111567. [Google Scholar] [CrossRef]
- Ng, C.S.L.; Chan, Y.K.S.; Nguyen, N.T.H.; Kikuzawa, Y.P.; Sam, S.Q.; Toh, T.C.; Mock, A.Y.J.; Chou, L.M.; Huang, D. Coral community composition and carbonate production in an urbanized seascape. Mar. Environ. Res. 2021, 168, 105322. [Google Scholar] [CrossRef]
- Lee, I.H.; Fan, T.Y.; Ko, D.S.; Fu, K.H. Temporal variations of daily temperature minima in coral reefs in Nanwan Bay, southern Taiwan. Sci. Rep. 2020, 10, 8656. [Google Scholar] [CrossRef]
- Hung, T.-C.; Huang, C.-C.; Shao, K.-T. Ecological survey of coastal water adjacent to nuclear power plants in Taiwan. Chem. Ecol. 1998, 15, 129–142. [Google Scholar] [CrossRef]
- UNEP. Coral Bleaching Futures: Downscaled Projections of Bleaching Conditions for the World’s Coral Reefs, Implications of Climate Policy and Management Responses; United Nations Environment Programme: Nairobi, Kenya, 2017. [Google Scholar]
- Ribas-Deulofeu, L.; Denis, V.; De Palmas, S.; Kuo, C.-Y.; Hsieh, H.J.; Chen, C.A. Structure of benthic communities along the Taiwan latitudinal gradient. PLoS ONE 2016, 11, e0160601. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Keshavmurthy, S.; Kuo, C.-Y.; Huang, Y.-Y.; Carballo-Bolaños, R.; Meng, P.-J.; Wang, J.-T.; Chen, C.A. Coral reef resilience in Taiwan: Lessons from long-term ecological research on the coral reefs of Kenting National Park (Taiwan). J. Mar. Sci. Eng. 2019, 7, 388. [Google Scholar] [CrossRef][Green Version]
- Bang, A.H.Y.; Kuo, C.-Y.; Wen, C.K.-C.; Cherh, K.-L.; Ho, M.-J.; Cheng, N.-Y.; Chen, Y.-C.; Chen, C.A. Quantifying coral reef resilience to climate change and human development: An evaluation of multiple empirical frameworks. Front. Mar. Sci. 2021, 7, 610306. [Google Scholar] [CrossRef]
- Mo, S.; Chen, T.; Chen, Z.; Zhang, W.; Li, S. Marine heatwaves impair the thermal refugia potential of marginal reefs in the northern South China Sea. Sci. Total. Environ. 2022, 825, 154100. [Google Scholar] [CrossRef]
- Hsu, P.C.; Lee, H.J.; Zheng, Q.; Lai, J.W.; Su, F.C.; Ho, C.R. Tide-induced periodic sea surface temperature drops in the coral reef area of Nanwan Bay, southern Taiwan. J. Geophys. Res. Oceans 2020, 125, 5226. [Google Scholar] [CrossRef]
- Beijbom, O.; Edmunds, P.J.; Roelfsema, C.; Smith, J.; Kline, D.I.; Neal, B.P.; Dunlap, M.J.; Moriarty, V.; Fan, T.Y.; Tan, C.J.; et al. Towards automated annotation of benthic survey images: Variability of human experts and operational modes of automation. PLoS ONE 2015, 10, e0130312. [Google Scholar] [CrossRef]
- Burt, J.A.; Paparella, F.; Al-Mansoori, N.; Al-Mansoori, A.; Al-Jailani, H. Causes and consequences of the 2017 coral bleaching event in the southern Persian/Arabian Gulf. Coral Reefs 2019, 38, 567–589. [Google Scholar] [CrossRef]
- Liu, G.; Heron, S.F.; Eakin, C.M.; Muller-Karger, F.E.; Vega-Rodriguez, M.; Guild, L.S.; De La Cour, J.L.; Geiger, E.F.; Skirving, W.J.; Burgess, T.F.R.; et al. Reef-scale thermal stress monitoring of coral ecosystems: New 5-km global products from NOAA Coral Reef Watch. Remote. Sens. 2014, 6, 11579–11606. [Google Scholar] [CrossRef][Green Version]
- Wyatt, A.S.J.; Leichter, J.J.; Toth, L.T.; Miyajima, T.; Aronson, R.B.; Nagata, T. Heat accumulation on coral reefs mitigated by internal waves. Nat. Geosci. 2019, 13, 28–34. [Google Scholar] [CrossRef]
- Mayfield, A.B.; Chen, M.-N.; Meng, P.-J.; Lin, H.-J.; Chen, C.-S.; Liu, P.-J. The physiological response of the reef coral Pocillopora damicornis to elevated temperature: Results from coral reef mesocosm experiments in Southern Taiwan. Mar. Environ. Res. 2013, 86, 1–11. [Google Scholar] [CrossRef]
- Mayfield, A.B.; Fan, T.-Y.; Chen, C.-S. Physiological acclimation to elevated temperature in a reef-building coral from an upwelling environment. Coral Reefs 2013, 32, 909–921. [Google Scholar] [CrossRef]
- Putman, H.M.; Edmunds, P.J.; Fan, T.Y. Effect of a fluctuating thermal regime on adult reef corals and their larvae. Invert. Biol. 2010, 129, 199–209. [Google Scholar]
- Putnam, H.M.; Gates, R.D. Preconditioning in the reef-building coral Pocillopora damicornis and the potential for trans-generational acclimatization in coral larvae under future climate change conditions. J. Exp. Biol. 2015, 218, 2365–2372. [Google Scholar] [CrossRef][Green Version]
- Tang, P.C.; Hsu, C.M.; Kuo, C.Y.; Chen, C.A. An unexpectedly high Acropora species diversity at the inlet of a nuclear power plant within Kenting National Park, southern Taiwan. Zool. Stud. 2010, 49, 71. [Google Scholar]
- Ho, M.-J.; Hsu, C.-M.; Chen, C.A. Wall of orange cup coral, Tubastraea coccinea, at the inlet breakwaters of a nuclear power plant, southern Taiwan. Mar. Biodivers. 2016, 47, 163–164. [Google Scholar] [CrossRef]
- Ribas-Deulofeu, L.; Denis, V.; Château, P.-A.; Chen, C.A. Impacts of heat stress and storm events on the benthic communities of Kenting National Park (Taiwan). PeerJ 2021, 9, e11744. [Google Scholar] [CrossRef]
- Muko, S.; Suzuki, G.; Saito, M.; Nakamura, T.; Nadaoka, K. Transitions in coral communities over 17 years in the Sekisei Lagoon and adjacent reef areas in Okinawa, Japan. Ecol. Res. 2019, 34, 524–534. [Google Scholar] [CrossRef]
- Mayfield, A.B.; Chen, Y.H.; Dai, C.F.; Chen, C.S. The effects of temperature on gene expression in the Indo-Pacific reef-building coral Seriatopora hystrix: Insight from aquarium studies in Southern Taiwan. Int. J. Mar. Sci. 2014, 4, 1–23. [Google Scholar]
- Turner, N.R.; Renegar, D.A. Petroleum hydrocarbon toxicity to corals: A review. Mar. Pollut. Bull. 2017, 119, 1–16. [Google Scholar] [CrossRef]
- Guzman, H.M.; Kaiser, S.; Weil, E. Assessing the long-term effects of a catastrophic oil spill on subtidal coral reef communities off the Caribbean coast of Panama (1985–2017). Mar. Biodivers. 2020, 50, 1–19. [Google Scholar] [CrossRef]
- Chen, C.-C.; Hsieh, H.-Y.; Mayfield, A.B.; Chang, C.-M.; Wang, J.-T.; Meng, P.-J. The key impact on water quality of coral reefs in Kenting National Park. J. Mar. Sci. Eng. 2022, 10, 270. [Google Scholar] [CrossRef]
- Chen, C.-C.; Shiah, F.; Lee, H.; Li, K.; Meng, P.; Kao, S.; Tseng, Y.; Chung, C. Phytoplankton and bacterioplankton biomass, production and turnover in a semi-enclosed embayment with spring tide induced upwelling. Mar. Ecol. Prog. Ser. 2005, 304, 91–100. [Google Scholar] [CrossRef]
- Fox, M.D.; Williams, G.J.; Johnson, M.D.; Radice, V.Z.; Zgliczynski, B.J.; Kelly, E.L.; Rohwer, F.L.; Sandin, S.A.; Smith, J.E. Gradients in primary production predict trophic strategies of mixotrophic corals across spatial scales. Curr. Biol. 2018, 28, 3355–3363. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sully, S.; Burkepile, D.E.; Donovan, M.K.; Hodgson, G.; van Woesik, R. A global analysis of coral bleaching over the past two decades. Nat. Commun. 2019, 10, 1–5. [Google Scholar] [CrossRef][Green Version]
- Mayfield, A.B.; Wang, L.-H.; Tang, P.-C.; Fan, T.-Y.; Hsiao, Y.-Y.; Tsai, C.-L.; Chen, C.-S. Assessing the impacts of experimentally elevated temperature on the biological composition and molecular chaperone gene expression of a reef coral. PLoS ONE 2011, 6, e26529. [Google Scholar] [CrossRef][Green Version]
- Keshavmurthy, S.; Hsu, C.-M.; Kuo, C.-Y.; Meng, P.-J.; Wang, J.-T.; Chen, C.A. Symbiont communities and host genetic structure of the brain coral Platygyra verweyi, at the outlet of a nuclear power plant and adjacent areas. Mol. Ecol. 2012, 21, 4393–4407. [Google Scholar] [CrossRef]
- Keshavmurthy, S.; Meng, P.J.; Wang, J.T.; Kuo, C.Y.; Yang, S.Y.; Hsu, C.M.; Gan, C.-H.; Dai, C.-F.; Chen, C.A. Can resistant coral-Symbiodinium associations enable coral communities to survive climate change? A study of a site exposed to long-term hot water input. Peer. J. 2014, 2, e327. [Google Scholar] [CrossRef][Green Version]
- Torda, G.; Donelson, J.M.; Aranda, M.; Barshis, D.J.; Bay, L.; Berumen, M.L.; Bourne, D.G.; Cantin, N.; Foret, S.; Matz, M.; et al. Rapid adaptive responses to climate change in corals. Nat. Clim. Chang. 2017, 7, 627–636. [Google Scholar] [CrossRef][Green Version]
- Liu, P.-J.; Meng, P.-J.; Liu, L.-L.; Wang, J.-T.; Leu, M.-Y. Impacts of human activities on coral reef ecosystems of southern Taiwan: A long-term study. Mar. Pollut. Bull. 2012, 64, 1129–1135. [Google Scholar] [CrossRef]
- Asaad, I.; Lundquist, C.J.; Erdmann, M.V.; Van Hooidonk, R.; Costello, M.J. Designating spatial priorities for marine biodiversity conservation in the Coral Triangle. Front. Mar. Sci. 2018, 5, 400. [Google Scholar] [CrossRef]
- Dang, V.D.H.; Cheung, P.-Y.; Fong, C.-L.; Mulla, A.J.; Shiu, J.-H.; Lin, C.-H.; Nozawa, Y. Sea urchins play an increasingly important role for coral resilience across reefs in Taiwan. Front. Mar. Sci. 2020, 7, 581945. [Google Scholar] [CrossRef]
- Nozawa, Y.; Lin, C.-H.; Meng, P.-J. Sea urchins (diadematids) promote coral recovery via recruitment on Taiwanese reefs. Coral Reefs 2020, 39, 1199–1207. [Google Scholar] [CrossRef]
- Tkachenko, K.S.; Soong, K. Protection of habitat types: A case study of the effectiveness of a small marine reserve and impacts of different habitats on the diversity and abundance of coral reef fishes. Zool Stud. 2010, 49, 195–210. [Google Scholar]
- Camp, E.F.; Schoepf, V.; Mumby, P.J.; Hardtke, L.A.; Rodolfo-Metalpa, R.; Smith, D.J.; Suggett, D.J. The future of coral reefs subject to rapid climate change: Lessons from natural extreme environments. Front. Mar. Sci. 2018, 5. [Google Scholar] [CrossRef][Green Version]
- Beyan, C.; Boom, B.J.; Liefhebber, J.M.P.; Shao, K.-T.; Fisher, R.B. Natural swimming speed of Dascyllus reticulatus increases with water temperature. ICES J. Mar. Sci. 2015, 72, 2506–2511. [Google Scholar] [CrossRef][Green Version]
- Barott, K.L.; Huffmyer, A.S.; Davidson, J.M.; Lenz, E.A.; Matsuda, S.B.; Hancock, J.R.; Innis, T.; Drury, C.; Putnam, H.M.; Gates, R.D. Coral bleaching response is unaltered following acclimatization to reefs with distinct environmental conditions. Proc. Natl. Acad. Sci. USA 2021, 118, e2025435118. [Google Scholar] [CrossRef]
- McClanahan, T.; Darling, E.; Maina, J.; Muthiga, N.; D’agata, S.; Leblond, J.; Arthur, R.; Jupiter, S.; Wilson, S.; Mangubhai, S.; et al. Highly variable taxa-specific coral bleaching responses to thermal stresses. Mar. Ecol. Prog. Ser. 2020, 648, 135–151. [Google Scholar] [CrossRef]
- Hsu, C.-M.; De Palmas, S.; Kuo, C.-Y.; Denis, V.; Chen, C.A. Identification of scleractinian coral recruits using fluorescent censusing and DNA barcoding techniques. PLoS ONE 2014, 9, e107366. [Google Scholar] [CrossRef]
- Fan, T.-Y.; Hsieh, Y.-C.; Lin, K.-H.; Kuo, F.-W.; Soong, K.; McRae, C.J.; Edmunds, P.J.; Fang, L.-S. Plasticity in lunar timing of larval release of two brooding pocilloporid corals in an internal tide-induced upwelling reef. Mar. Ecol. Prog. Ser. 2017, 569, 117–127. [Google Scholar] [CrossRef][Green Version]
- Hughes, T.P.; Anderson, K.D.; Connolly, S.R.; Heron, S.F.; Kerry, J.T.; Lough, J.M.; Baird, A.H.; Baum, J.K.; Berumen, M.L.; Bridge, T.C.; et al. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 2018, 359, 80–83. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pisapia, C.; Burn, D.; Pratchett, M.S. Changes in the population and community structure of corals during recent disturbances (February 2016-October 2017) on Maldivian coral reefs. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morikawa, M.K.; Palumbi, S.R. Using naturally occurring climate resilient corals to construct bleaching-resistant nurseries. Proc. Natl. Acad. Sci. USA 2019, 116, 10586–10591. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barton, J.; Willis, B.L.; Hutson, K.S. Coral propagation: A review of techniques for ornamental trade and reef restoration. Rev. Aquac. 2015, 9, 238–256. [Google Scholar] [CrossRef]
- van Oppen, M.J.H.; Oliver, J.K.; Putnam, H.M.; Gates, R.D. Building coral reef resilience through assisted evolution. Proc. Natl. Acad. Sci. USA 2015, 112, 2307–2313. [Google Scholar] [CrossRef][Green Version]
- Schoepf, V.; Carrion, S.A.; Pfeifer, S.M.; Naugle, M.; Dugal, L.; Bruyn, J.; McCulloch, M.T. Stress-resistant corals may not acclimatize to ocean warming but maintain heat tolerance under cooler temperatures. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef][Green Version]
- McRae, C.J.; Huang, W.-B.; Fan, T.-Y.; Côté, I.M. Effects of thermal conditioning on the performance of Pocillopora acuta adult coral colonies and their offspring. Coral Reefs 2021, 40, 1491–1503. [Google Scholar] [CrossRef]
- Shaver, E.C.; Courtney, C.A.; West, J.M.; Maynard, J.; Hein, M.; Wagner, C.; Philibotte, J.; MacGowan, P.; McLeod, I.; Boström-Einarsson, L.; et al. A Manager’s Guide to Coral Reef Restoration Planning and Design; NOAA Coral Reef Conservation Program, NOAA Technical Memorandum CRCP 36; National Oceanic and Atmospheric Administration: Washington, DC, USA, 2020; 128p.
- Soong, K.; Chen, T.-A. Coral transplantation: Regeneration and growth of Acropora fragments in a nursery. Restor. Ecol. 2003, 11, 62–71. [Google Scholar] [CrossRef]
- Rinkevich, B. Ecological engineering approaches in coral reef restoration. ICES J. Mar. Sci. 2020, 78, 410–420. [Google Scholar] [CrossRef][Green Version]
- Mayfield, A.B. Machine-learning-based proteomic predictive modeling with thermally-challenged Caribbean reef corals. Diversity 2022, 14, 33. [Google Scholar] [CrossRef]
- Mayfield, A.B.; Dempsey, A.C.; Chen, C.-S.; Lin, C. Expediting the search for climate-resilient reef corals in the Coral Triangle with artificial intelligence. Appl. Sci. 2022, 12, 12955. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Z.-M.; Mayfield, A.B.; Fan, T.-Y. Variable Responses to a Marine Heat Wave in Five Fringing Reefs of Southern Taiwan. Appl. Sci. 2023, 13, 5554. https://doi.org/10.3390/app13095554
Ye Z-M, Mayfield AB, Fan T-Y. Variable Responses to a Marine Heat Wave in Five Fringing Reefs of Southern Taiwan. Applied Sciences. 2023; 13(9):5554. https://doi.org/10.3390/app13095554
Chicago/Turabian StyleYe, Zong-Min, Anderson B. Mayfield, and Tung-Yung Fan. 2023. "Variable Responses to a Marine Heat Wave in Five Fringing Reefs of Southern Taiwan" Applied Sciences 13, no. 9: 5554. https://doi.org/10.3390/app13095554
APA StyleYe, Z.-M., Mayfield, A. B., & Fan, T.-Y. (2023). Variable Responses to a Marine Heat Wave in Five Fringing Reefs of Southern Taiwan. Applied Sciences, 13(9), 5554. https://doi.org/10.3390/app13095554







