Numerical Study of Steam–CO2 Mixture Condensation over a Flat Plate Based on the Solubility of CO2
Abstract
:1. Introduction
2. Boundary Layer Models of Steam–CO2 Mixture Condensation
2.1. Steam–CO2 Condensation Model Based on CO2 as an NCG
2.2. Model Modification Considering CO2 Solubility in the Condensate
2.3. Numerical Method
3. Model Verification
3.1. Comparing the Results with Numerical Data and Existing Experimental Data
3.2. Data Trends of Steam–CO2 Mixture Condensation
4. Results and Discussion
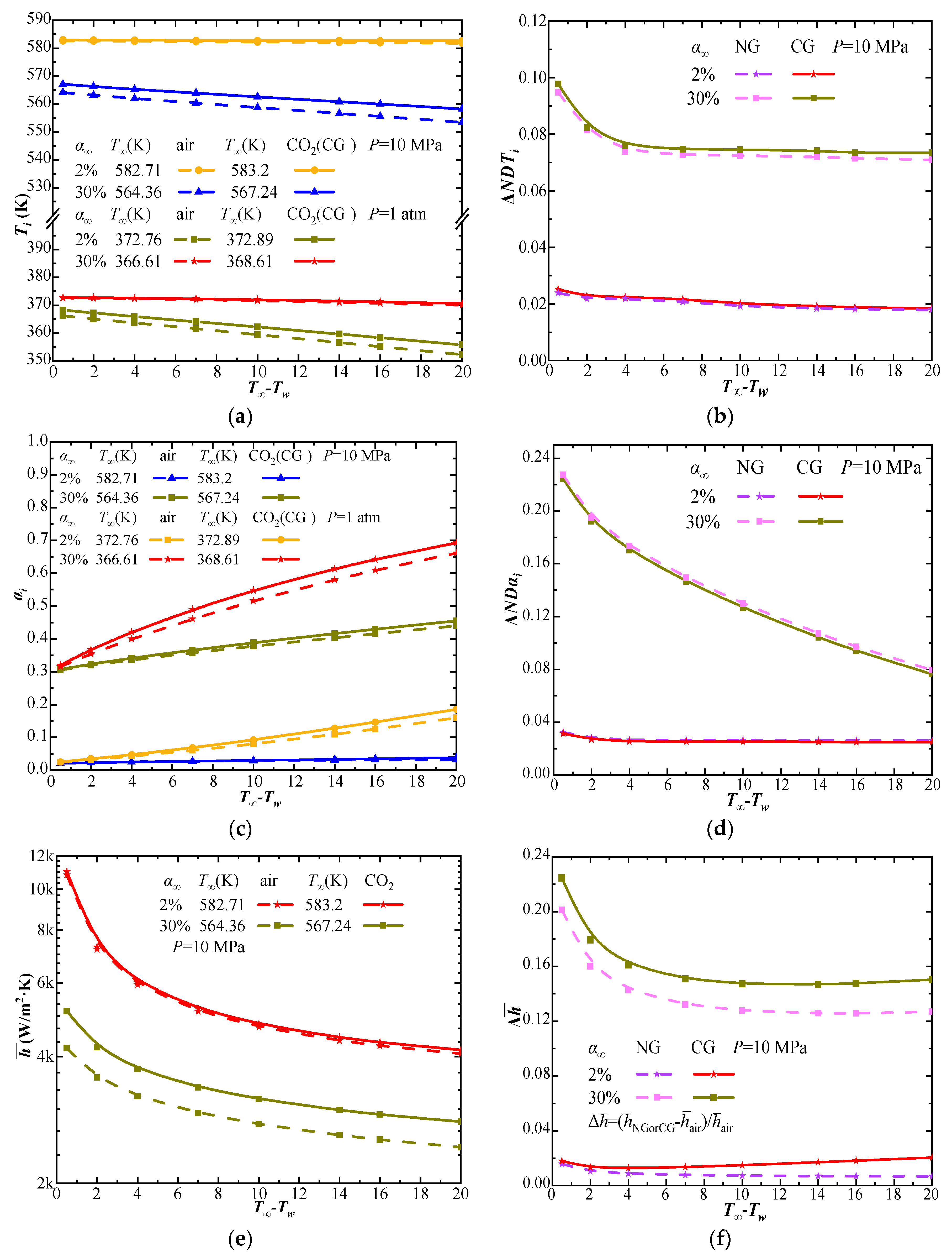
4.1. The Effect of CO2 Solubility on Steam–CO2 Mixture Condensation
4.2. Comparison of the Steam–CO2 Mixture Condensation Features with Steam–Air
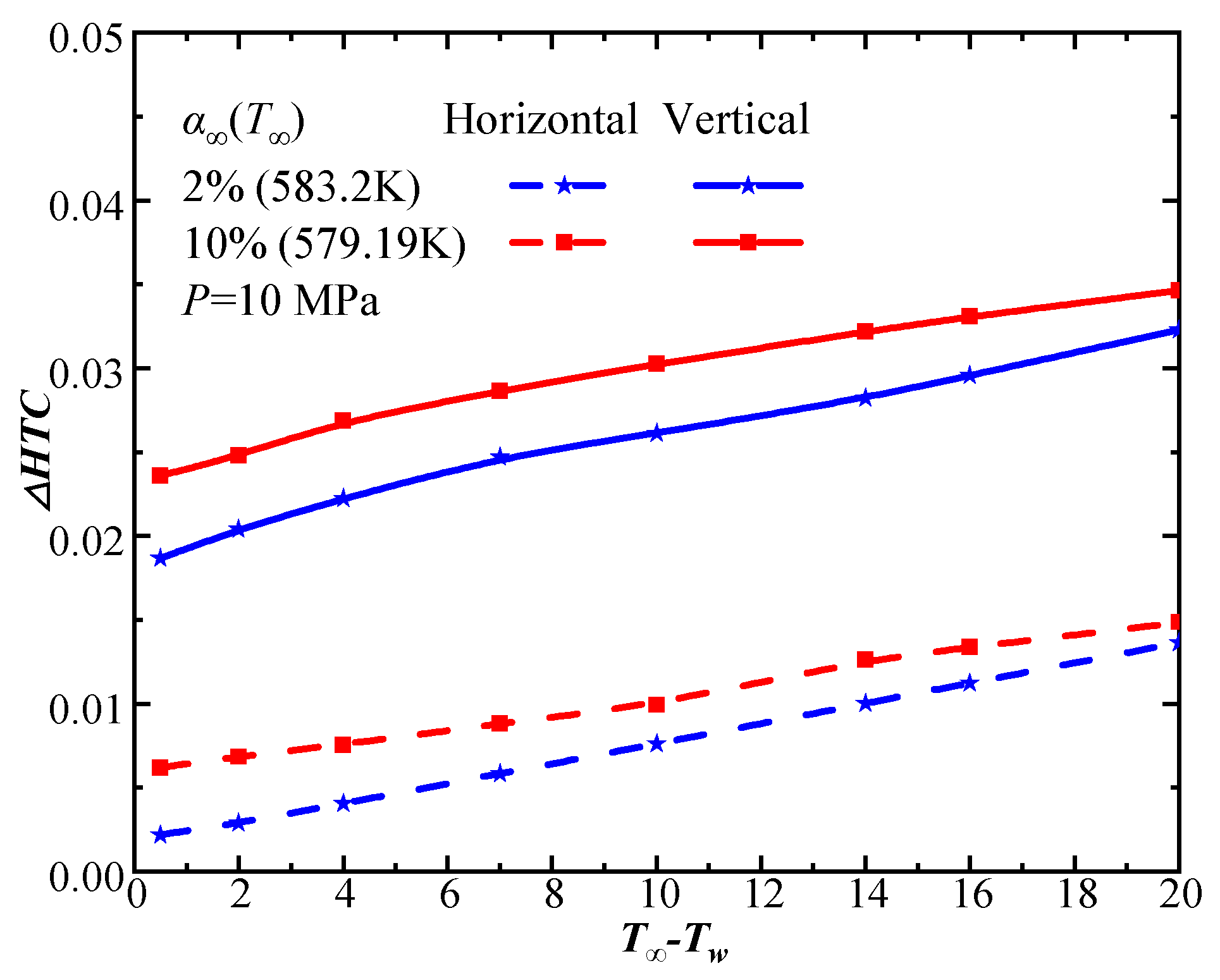
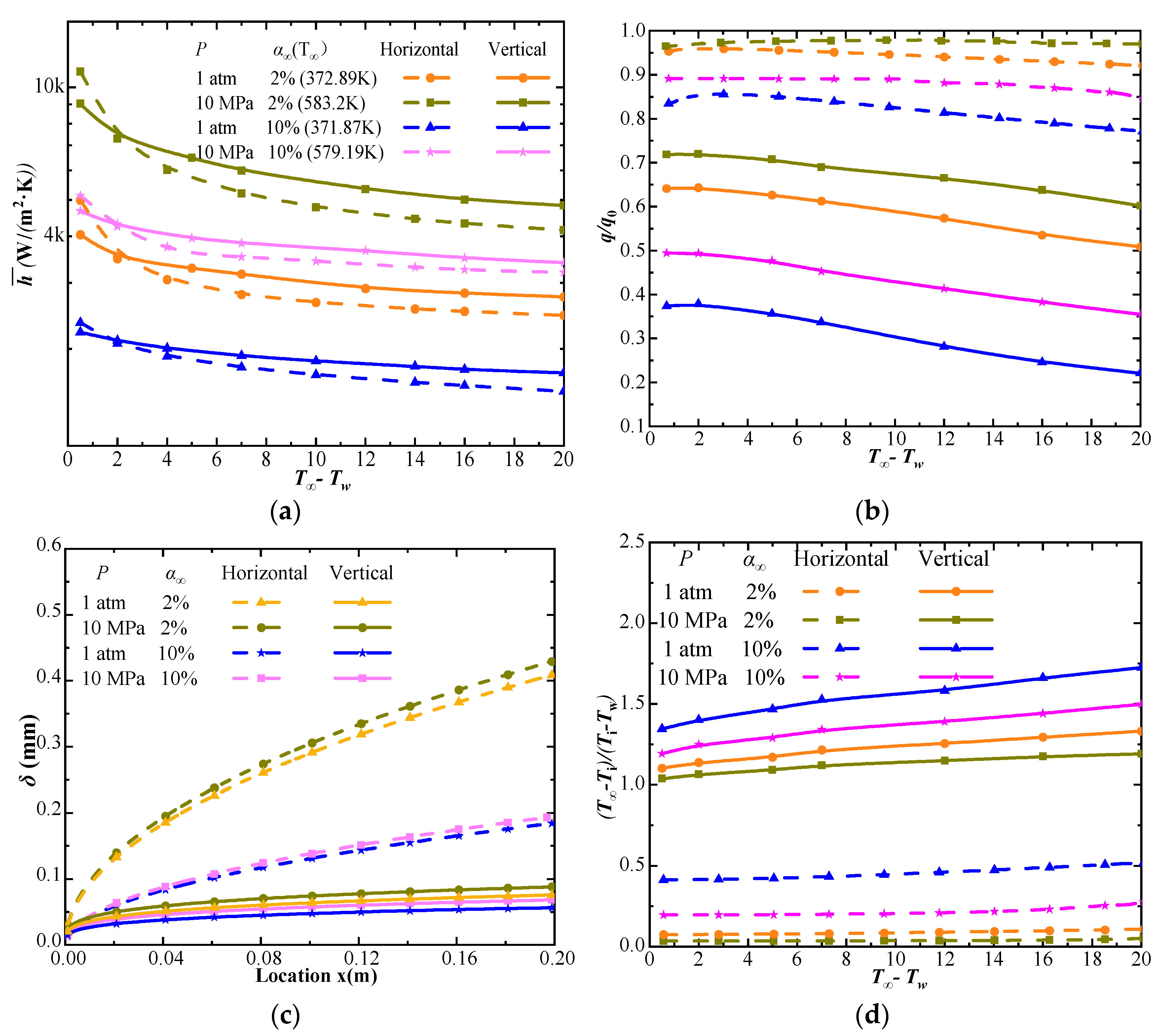
4.3. Comparison of Steam–CO2 Mixture Condensation Features on Horizontal and Vertical Plates
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| c | constant |
| CP | specific heat (J/(kg·K)) |
| D | diffusion coefficient (m2/s) |
| E | solution enthalpy (KJ/mol) |
| F, f | dimensionless function of η and |
| g | gravity (m/s2) |
| average heat transfer coefficient (W/(m2·K)) | |
| hfg | latent heat of condensation (J/kg) |
| hso | solution enthalpy of CO2 (kJ/kg) |
| hx | local heat transfer coefficient (W/(m2·K)) |
| j | diffusive mass flux (kg/(m2·s)) |
| L | length of the plate (m) |
| m | condensation mass flux (kg/(m2·s)) |
| M | molar mass (g/mol or kg/mol) |
| P | pressure (Pa) |
| Par | parameter depending on T and P |
| Pr | Prandtl number |
| Psi | steam partial pressure (Pa) |
| q | heat flux (W/m2) |
| R | fugacity coefficient of CO2 |
| S | solubility of CO2 (kg/kg water or g/kg water) |
| Sc | Schmidt number |
| T | temperature (K) |
| u, v | velocity (m/s) |
| U | inlet velocity (m/s) |
| x, y | direction or coordinate (m) |
| Greek symbols | |
| α | mass fraction of CO2 |
| δ | film thickness (mm or m) |
| η | dimensionless liquid coordinate |
| θ | dimensionless temperature |
| μ | dynamic viscosity (Pa·s) |
| ξ | dimensionless gas coordinate |
| ν | kinematic viscosity (m/s2) |
| ρ | density (kg/m3) |
| φ | concentration function |
| ψ | stream function |
| Acronyms | |
| CG | condensable gas model |
| ΔHTC | average HTC deviation |
| ΔNDTi | deviation in non-dimensional Ti |
| ΔNDWi | deviation in non-dimensional Wi |
| NCG | non-condensable gas |
| NG | non-condensable gas model |
| Subscripts | |
| cal | iterative calculation value |
| i | liquid and gas interface |
| l | liquid phase |
| w | wall |
| v | gas phase |
| x | local parameter |
| ∞ | infinity |
| 0 | pure steam |
References
- Mualim, A.; Huda, H.; Altway, A.; Sutikno, J.P.; Handogo, P. Evaluation of multiple time carbon capture and storage network with capital-carbon trade-off. J. Clean. Prod. 2021, 291, 125710. [Google Scholar] [CrossRef]
- Freire, A.L.; Jose, H.J.; Moreira, R.F.P.M. Potential applications for geopolymers in carbon capture and storage. Int. J. Greenh. Gas Control. 2022, 118, 103687. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Contribution of Working Group I. Contribution to the Sixth Assessment Report, the Physical Science Basis; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Chen, Y.P.; Zhu, Z.L.; Wu, J.F.; Yang, S.F.; Zhang, B.H. A novel LNG/CO2 combustion gas and steam mixture cycle with energy storage and CO2 capture. Energy 2017, 120, 128–137. [Google Scholar] [CrossRef]
- Zhao, W.J.; Chen, Y.P.; Wu, J.F.; Zhu, Z.L.; Wang, J. Study on CO2 capture upgrading of existing coal fired power plants with gas-H2O mixture cycle and supercritical water coal gasification. Int. J. Greenh. Gas Control. 2021, 112, 103482. [Google Scholar] [CrossRef]
- High, M.; Patzschke, C.F.; Zheng, L.Y.; Zeng, D.W.; Gavalda-Diaz, O.; Ding, N.; Chien, K.H.H.; Zhang, Z.L.; Wilson, G.E.; Berenov, A.V.; et al. Precursor engineering of hydrotalcite-derived redox sorbents for reversible and stable thermochemical oxygen storage. Nat. Commun. 2022, 13, 5109. [Google Scholar] [CrossRef]
- Yi, Q.J.; Tian, M.C.; Yan, W.J.; Qu, X.H.; Chen, X.B. Visualization study of the influence of non-condensable gas on steam condensation heat transfer. Appl. Therm. Eng. 2016, 106, 13–21. [Google Scholar] [CrossRef]
- Kuhn, S.Z.; Schrock, V.E.; Peterson, P.F. An investigation of condensation from steam-gas mixtures flowing downward inside a vertical tube. Nucl. Eng. Des. 1997, 177, 53–69. [Google Scholar] [CrossRef]
- Rose, J.W. Approximate equations for forced-convection condensation in the presence of a non-condensing gas on a flat-plate and horizontal tube. Int. J. Heat Mass Transf. 1980, 23, 539–546. [Google Scholar] [CrossRef]
- Huhtiniemi, I.K.; Corradini, M.L. Condensation in the presence of noncondensable gases. Nucl. Eng. Des. 1993, 141, 429–446. [Google Scholar] [CrossRef]
- Kang, H.C.; Kim, M.H. Characteristics of film condensation of supersaturated steam-air mixture on a flat plate. Int. J. Multiph. Flow 1999, 25, 1601–1618. [Google Scholar] [CrossRef]
- Aldiwany, H.K.; Rose, J.W. Free convection film condensation of steam in the presence of non-condensing gases. Int. J. Heat Mass Transf. 1973, 16, 1359–1369. [Google Scholar] [CrossRef]
- Morrison, J.N.A.; Deans, J. Augmentation of steam condensation heat transfer by addition of ammonia. Int. J. Heat Mass Transf. 1997, 40, 765–772. [Google Scholar] [CrossRef]
- Sparrow, E.M.; Yang, J.W.; Scott, C.J. Free convection in an air-water vapor boundary layer. Int. J. Heat Mass Transf. 1966, 9, 53. [Google Scholar] [CrossRef]
- Sparrow, E.M.; Minkowycz, W.J.; Saddy, M. Forced convection condensation in the presence of noncondensables and interfacial resistance. Int. J. Heat Mass Transf. 1967, 10, 1829–1845. [Google Scholar] [CrossRef]
- Minkowycz, W.J.; Sparrow, E.M. Condensation heat transfer in the presence of noncondensables, interfacial resistance, superheating, variable properties, and diffusion. Int. J. Heat Mass Transf. 1966, 9, 1125–1144. [Google Scholar] [CrossRef]
- Millsaps, K.; Pohlhausen, K. The Laminar-free-convective-heat transfer from the outer surface of a vertical circular cylinder. J. Aeronaut. Sci. 1958, 25, 357–360. [Google Scholar] [CrossRef]
- Popiel, C.O. Free convection heat transfer from vertical slender cylinders: A review. Heat Transf. Eng. 2008, 29, 521–536. [Google Scholar] [CrossRef]
- Angelino, M.; Boghi, A.; Gori, F. Numerical solution of three-dimensional rectangular submerged jets with the evidence of the undisturbed region of flow. Numer. Heat Transf. Part A 2016, 70, 815–830. [Google Scholar] [CrossRef]
- Boghi, A.; Venuta, I.D.; Gori, F. Passive scalar diffusion in the near field region of turbulent rectangular submerged free jets. Int. J. Heat Mass Transf. 2017, 112, 1017–1031. [Google Scholar] [CrossRef]
- Boghi, A.; Angelino, M.; Gori, F. Numerical Evidence of an Undisturbed Region of Flow in Turbulent Rectangular Submerged Free Jet. Numer. Heat Transf. 2016, 70, 14–29. [Google Scholar] [CrossRef]
- Hossein, S.; Nima, A.; Ali, J. Effects of Applying Brand-New Designs on the Performance of PEM Fuel Cell and Water Flooding Phenomena. Iran. J. Chem. Chem. Eng.-Int. Engl. Ed. 2022, 41, 618–635. [Google Scholar]
- Dehbi, A.; Janasza, F.; Bell, B. Prediction of steam condensation in the presence of noncondensable gases using a CFD-based approach. Nucl. Eng. Des. 2013, 258, 199–210. [Google Scholar] [CrossRef]
- Li, J.-D. CFD simulation of water vapor condensation in the presence of non-condensable gas in vertical cylindrical condensers. Int. J. Heat Mass Transf. 2013, 57, 708–721. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, R.; Das, U.J. Viscoelastic effects on the three- Dimensional hydrodynamic flow past a vertical porous plate. Int. J. Heat Technol. Assoc. 2013, 31, 1–8. [Google Scholar]
- Ge, M.H.; Zhao, J.; Wang, S.X. Experimental investigation of steam condensation with high concentration CO2 on a horizontal tube. Appl. Therm. Eng. 2013, 61, 334–343. [Google Scholar] [CrossRef]
- Ge, M.H.; Wang, S.X.; Zhao, J.; Zhao, Y.L.; Liu, L.S. Condensation of steam with high CO2 concentration on a vertical plate. Exp. Therm. Fluid Sci. 2016, 75, 147–155. [Google Scholar] [CrossRef]
- Lu, J.H.; Cao, H.S.; Li, J.M. Experimental study of condensation heat transfer of steam in the presence of non-condensable gas CO2 on a horizontal tube at sub-atmospheric pressure. Exp. Therm. Fluid Sci. 2019, 105, 278–288. [Google Scholar] [CrossRef]
- Takami, K.M.; Mahmoudi, J.; Time, R.W. A simulated H2O/CO2 condenser design for oxy-fuel CO2 capture process. Energy Procedia 2009, 1, 1443–1450. [Google Scholar] [CrossRef]
- Lu, J.H.; Ren, K.X.; Tang, J.J. Numerical simulations of laminar film condensation of H2O/Air or H2O/CO2 on a vertical plate. Heat Transf. Eng. 2022, 44, 720–733. [Google Scholar] [CrossRef]
- Han, J.M.; Shin, H.Y.; Min, B.M. Measurement and correlation of high pressure phase behavior of carbon dioxide+water system. Ind. Eng. Chem. Eng. Data 2009, 15, 212–216. [Google Scholar] [CrossRef]
- Lucile, F.; Cezac, P.; Contamine, F.; Serin, J.P.; Houssin, D.; Arpentinier, P. Solubility of Carbon Dioxide in Water and Aqueous Solution Containing Sodium Hydroxide at Temperatures from (293.15 to 393.15) K and Pressure up to 5 MPa: Experimental Measurements. J. Chem. Eng. Data 2012, 57, 784–789. [Google Scholar] [CrossRef]
- Hou, S.X.; Maitland, G.C.; Trusler, J.P.M. Measurement and modeling of the phase behavior of the (carbon dioxide+water) mixture at temperature from 298.15 K to 448.15 K. J. Supercrit. Fluids 2013, 73, 87–96. [Google Scholar] [CrossRef]
- Meyer, C.W.; Harvey, A.H. Dew-point measurements for water in compressed carbon dioxide. AICHE J. 2015, 61, 2913–2925. [Google Scholar] [CrossRef]
- Mao, S.D.; Zhang, D.H.; Li, Y.Q.; Liu, N.Q. An improved model for calculating CO2 solubility in aqueous NaCl solutions and the application to CO2-H2O-NaCl fluid inclusions. Chem. Geol. 2013, 347, 43–58. [Google Scholar] [CrossRef]
- Liu, G.Q.; Ma, L.X. Chemical Physics Data; Chemical Industry Press: Beijing, China, 2002; pp. 84–85. [Google Scholar]
- Duan, Z.H.; Sun, R.; Zhu, C.; Chou, I.M. An improved model for the calculation of CO2 solubility in aqueous solutions containing Na+, K+, Ca2+, Mg2+, Cl−, SO42−. Mar. Chem. 2006, 98, 131–139. [Google Scholar] [CrossRef]
- Valtz, A.; Chapoy, A.; Coquelet, C.; Paricaud, P.; Richon, D. Vapor-liquid equilibria in the carbon dioxide–water system, measurement and modelling from 278.2 to 318.2 K. Fluid Phase Equilibria 2004, 226, 333–344. [Google Scholar] [CrossRef]
- Fronk, B.M.; Garimella, S. Condensation of ammonia and high-temperature-glide zeotropic ammonia/water mixtures in minichannels-Part II: Heat transfer models. Int. J. Heat Mass Transf. 2016, 101, 1357–1373. [Google Scholar] [CrossRef]
- Wang, X.Y.; Ming, Z.Y. Measurements of gas enthalpy of dissolution. China Acad. J. Electron. Publ. House 1987, 1, 62–64. [Google Scholar]
- Koschel, D.; Coxam, J.Y.; Rodier, L.; Majer, V. Enthalpy and solubility data of CO2 in water and NaCl(aq) at conditions of interest for geological sequestration. Fluid Phase Equilibria 2006, 247, 107–120. [Google Scholar] [CrossRef]
- NIST. NIST Reference Fluid Thermodynamic and Transport Properties-REFPROP (Version 9.1); NIST Stand Ref Database 2007:23; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2013.
- Othmer, D.F. The condensation of steam. Ind. Eng. Chem. 1929, 21, 576–583. [Google Scholar] [CrossRef]



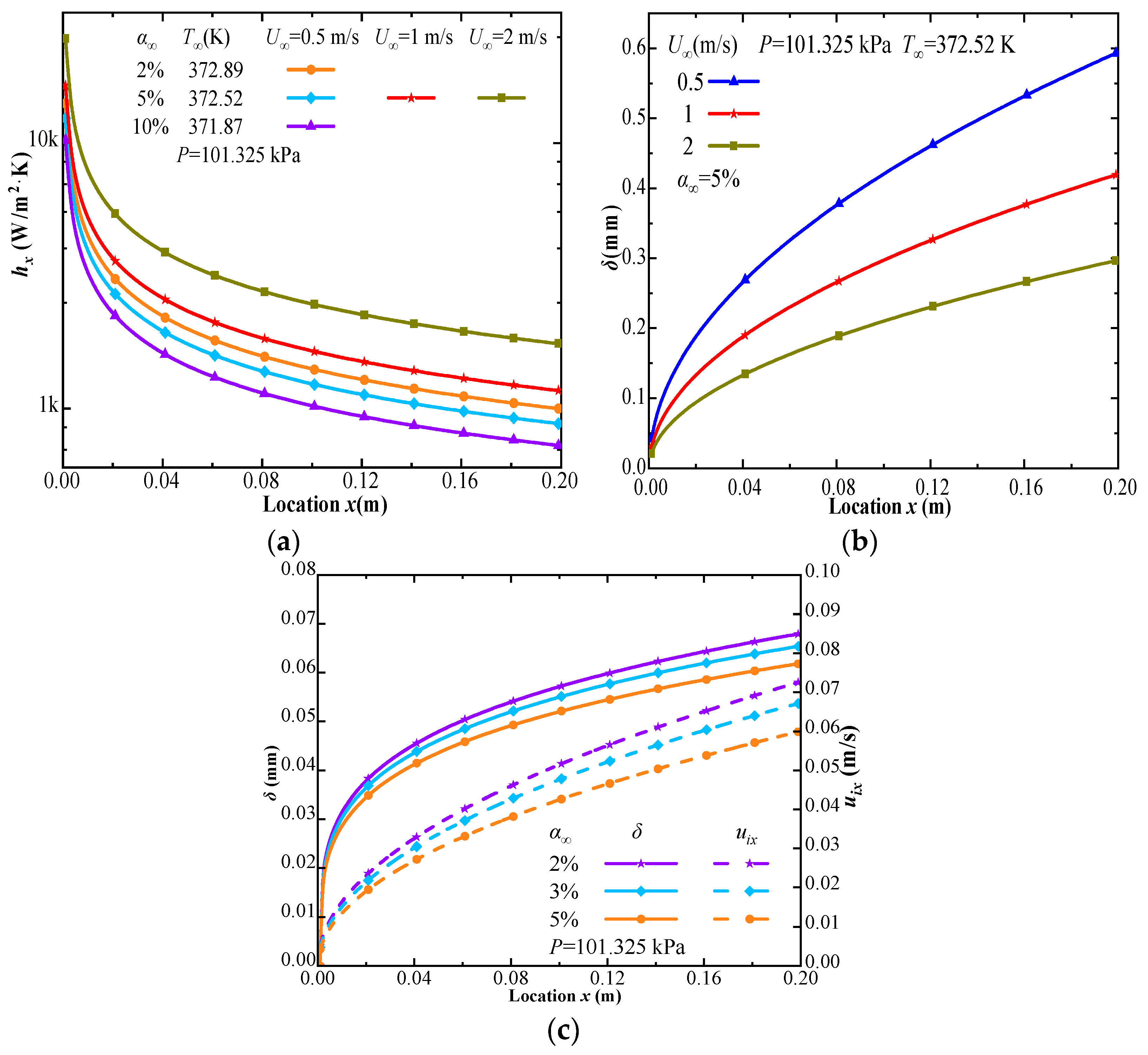
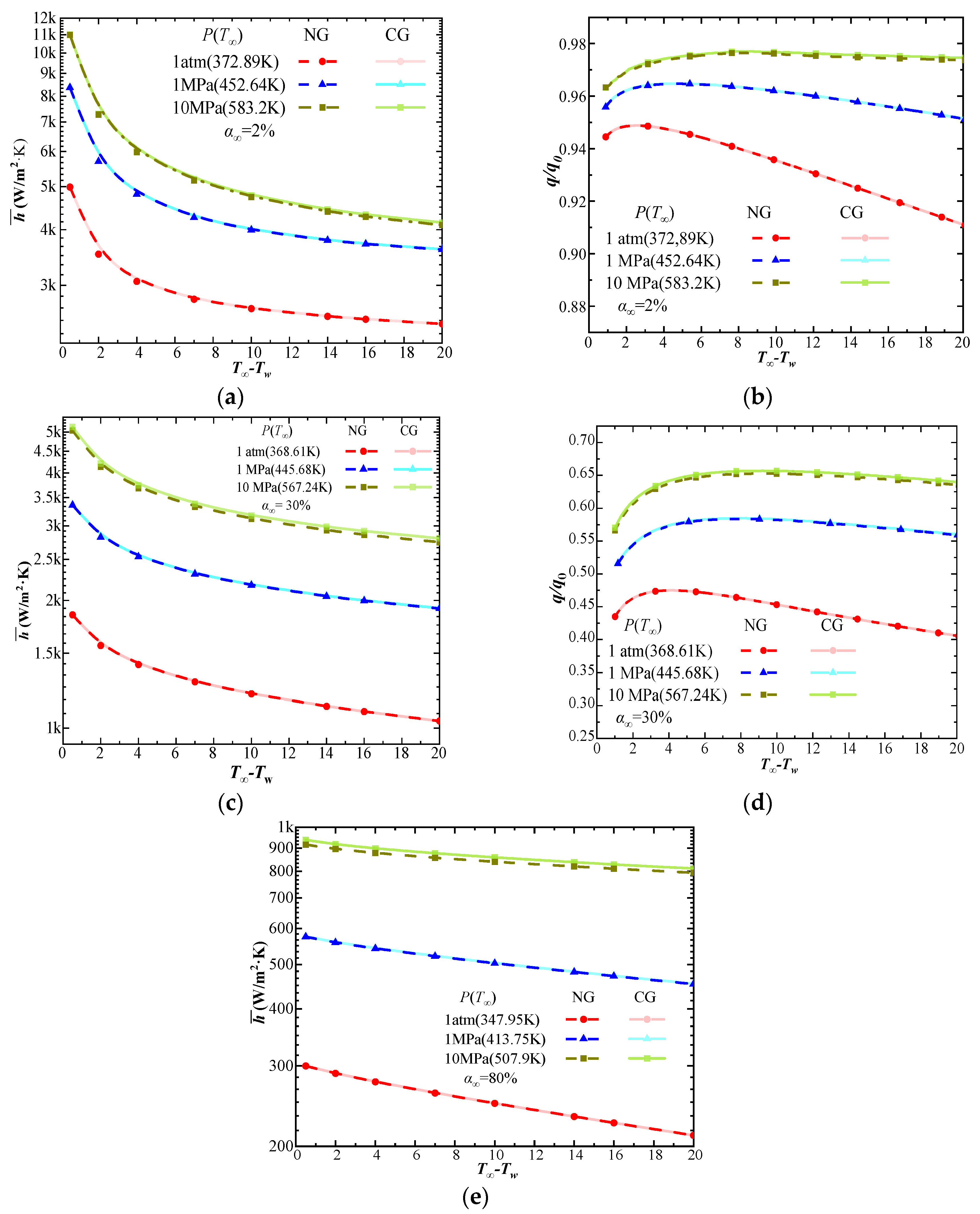
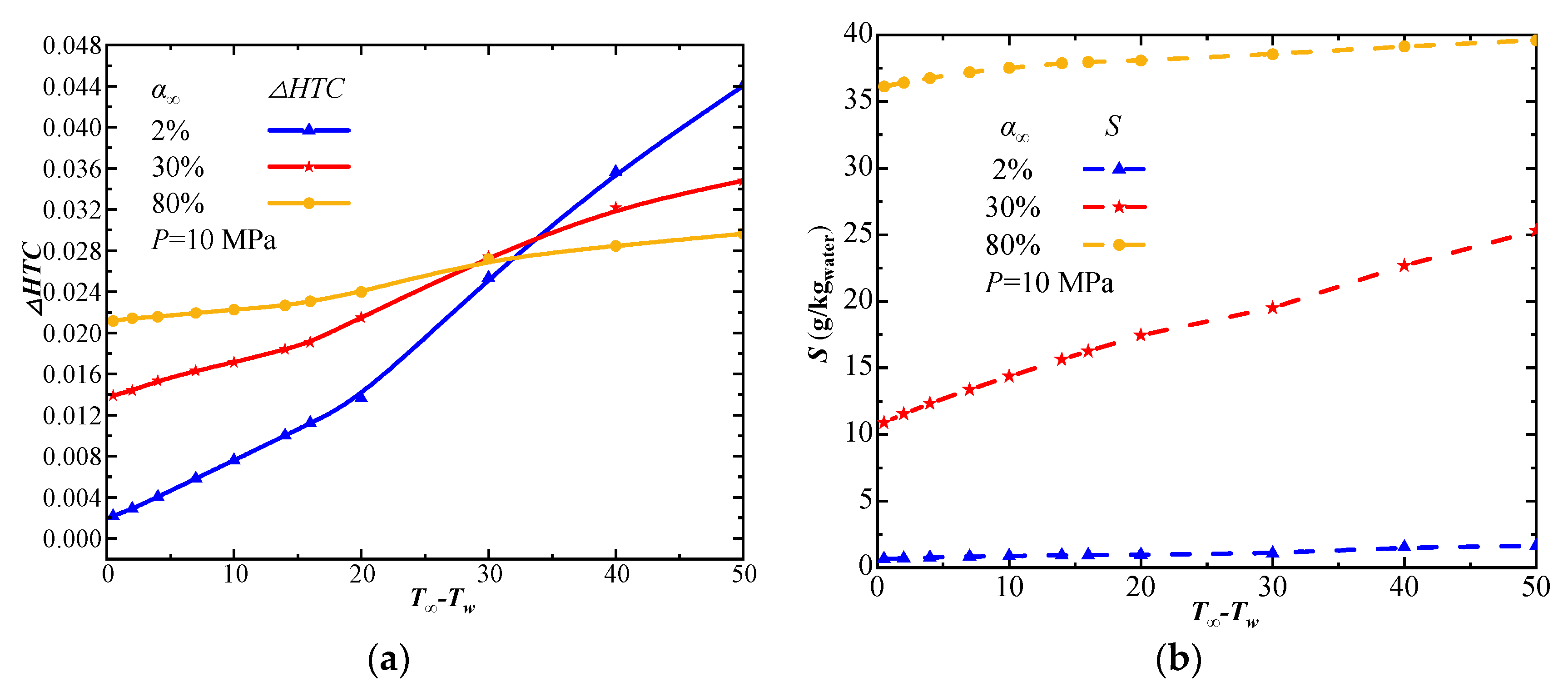
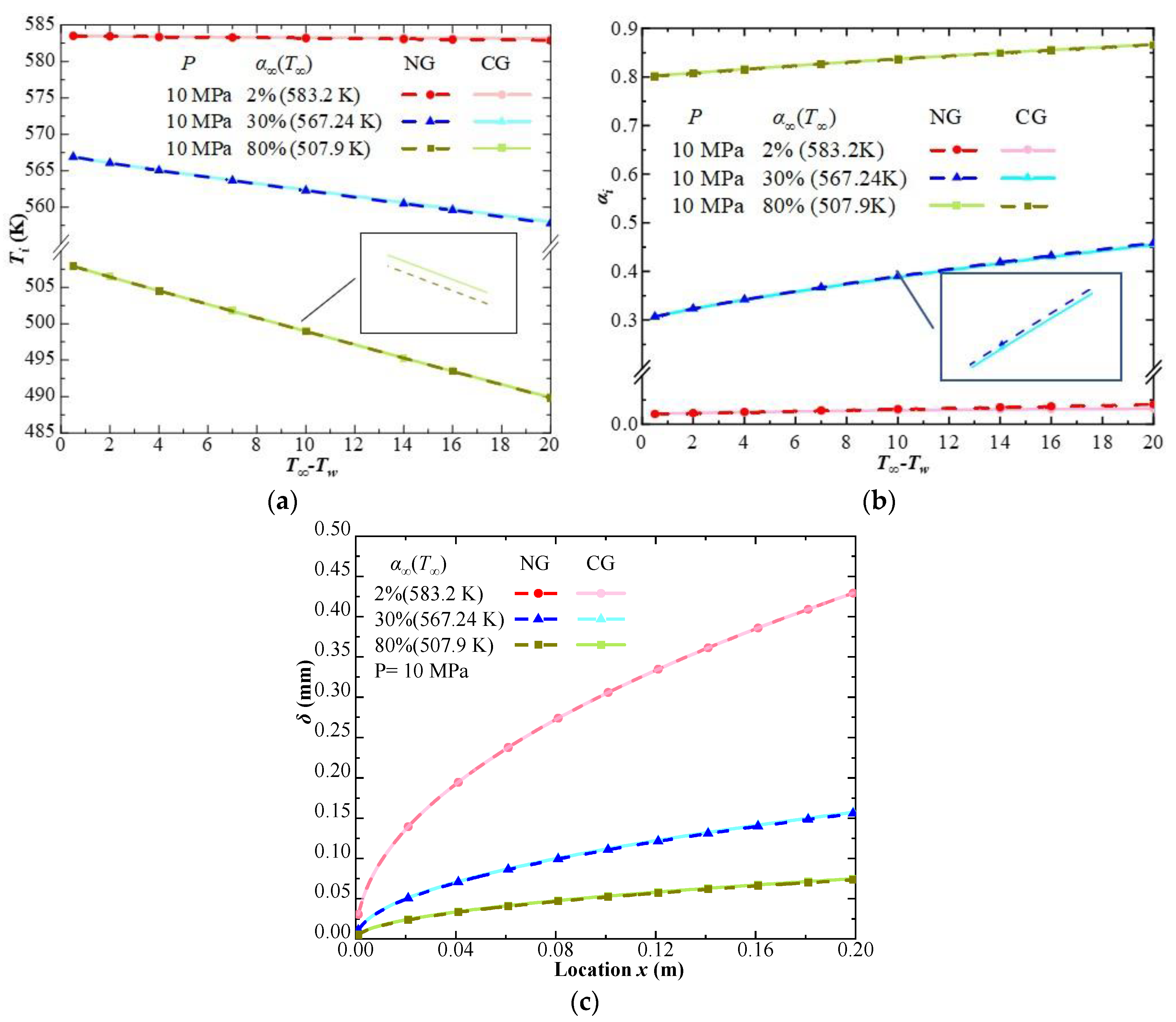
| Phase | Liquid | Gas | ||
|---|---|---|---|---|
| Plate | Horizontal | Vertical | Horizontal | Vertical |
| Continuity | (1a) | (1b) | ||
| Momentum conservation | (2a) | (2b) | (2c) | (2d) |
| Energy conservation or mass conservation | (3a) | (3b) | ||
| Plate | Phase | Derivation and Solution |
|---|---|---|
| Horizontal | Liquid | |
| Gas | ||
| Vertical | Liquid | |
| Gas |
| Pressure (MPa) | Temperature (K) | Solution Enthalpy (kJ/mol) | Solution Enthalpy (kJ/kg), hso | hso/hfg | hso∙S/hfg |
|---|---|---|---|---|---|
| 0.101325 | 298.15 | 19.75 [40] | 448.864 | 0.1838 | 0.0002698 |
| 2.06 | 323.1 | 14.8 [41] | 336.364 | 0.1412 | 0.002223 |
| 5.10 | 323.1 | 13.1 [41] | 297.727 | 0.1250 | 0.004401 |
| 10.53 | 323.1 | 7.5 [41] | 170.455 | 0.07155 | 0.003569 |
| 5.05 | 373.1 | 6.3 [41] | 143.182 | 0.06345 | 0.001235 |
| 10.08 | 373.1 | 4.6 [41] | 104.545 | 0.04633 | 0.001561 |
| Numerical Results | |||||
|---|---|---|---|---|---|
| Sparrow [15] | 0.1 | 0.36867 | 0.036827 | 1.3577 | 0.90510 |
| 0.2 | 0.40612 | 0.099244 | 1.0076 | 0.82332 | |
| 0.4 | 0.48325 | 0.25733 | 0.7772 | 0.69002 | |
| 0.6 | 0.56300 | 0.43799 | 0.6849 | 0.58654 | |
| 1 | 0.72887 | 0.82825 | 0.6037 | 0.43803 | |
| 2 | 1.16943 | 1.8495 | 0.5407 | 0.24075 | |
| This work | 0.1 | 0.36865 | 0.036828 | 1.3577 | 0.90509 |
| 0.2 | 0.40609 | 0.099247 | 1.0076 | 0.82330 | |
| 0.4 | 0.48321 | 0.25734 | 0.7772 | 0.68998 | |
| 0.6 | 0.5629 | 0.43800 | 0.6849 | 0.58648 | |
| 1 | 0.72880 | 0.82829 | 0.6038 | 0.43794 | |
| 2 | 1.16929 | 1.8496 | 0.5408 | 0.24064 |
| T∞ (K) | α∞ | P (kPa) | q/q0 [43] | q/q0 of This Work | Deviation |
|---|---|---|---|---|---|
| 383.15 | 0.017 | 145 | 0.4456 | 0.3857 | 13.44% |
| 383.15 | 0.0311 | 146.3 | 0.3255 | 0.3057 | 6.08% |
| 383.15 | 0.0457 | 147.75 | 0.2642 | 0.2479 | 6.17% |
| 373.15 | 0.0546 | 105.13 | 0.2377 | 0.2028 | 14.68% |
| 373.15 | 0.0226 | 102.91 | 0.3623 | 0.3375 | 6.85% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, B.; Jiang, Y.; Gu, H.; Chen, Y.; Wu, J. Numerical Study of Steam–CO2 Mixture Condensation over a Flat Plate Based on the Solubility of CO2. Appl. Sci. 2023, 13, 5747. https://doi.org/10.3390/app13095747
Jiang B, Jiang Y, Gu H, Chen Y, Wu J. Numerical Study of Steam–CO2 Mixture Condensation over a Flat Plate Based on the Solubility of CO2. Applied Sciences. 2023; 13(9):5747. https://doi.org/10.3390/app13095747
Chicago/Turabian StyleJiang, Bingran, Yi’ao Jiang, Huaduo Gu, Yaping Chen, and Jiafeng Wu. 2023. "Numerical Study of Steam–CO2 Mixture Condensation over a Flat Plate Based on the Solubility of CO2" Applied Sciences 13, no. 9: 5747. https://doi.org/10.3390/app13095747
APA StyleJiang, B., Jiang, Y., Gu, H., Chen, Y., & Wu, J. (2023). Numerical Study of Steam–CO2 Mixture Condensation over a Flat Plate Based on the Solubility of CO2. Applied Sciences, 13(9), 5747. https://doi.org/10.3390/app13095747








