Physiological Study of Visual and Non-Visual Effects of Light Exposure
Abstract
1. Introduction
2. Measurements of Physiological Responses
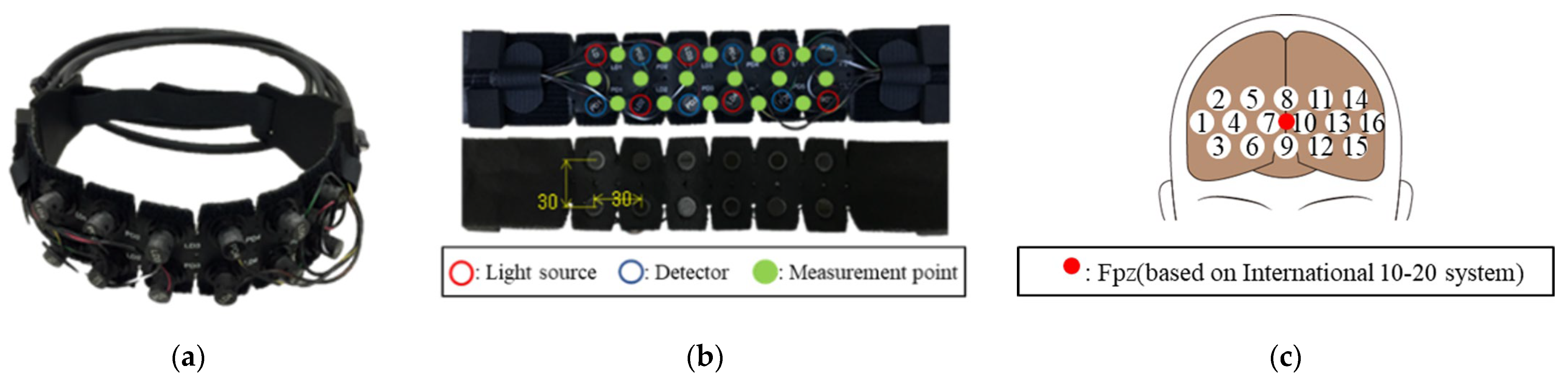
2.1. Brain Activity
2.2. Heart Rate Variability
2.3. Electrodermal Activity
3. Experiment on Visual and Non-Visual Effects of Light Exposure
3.1. Methods
- (I)
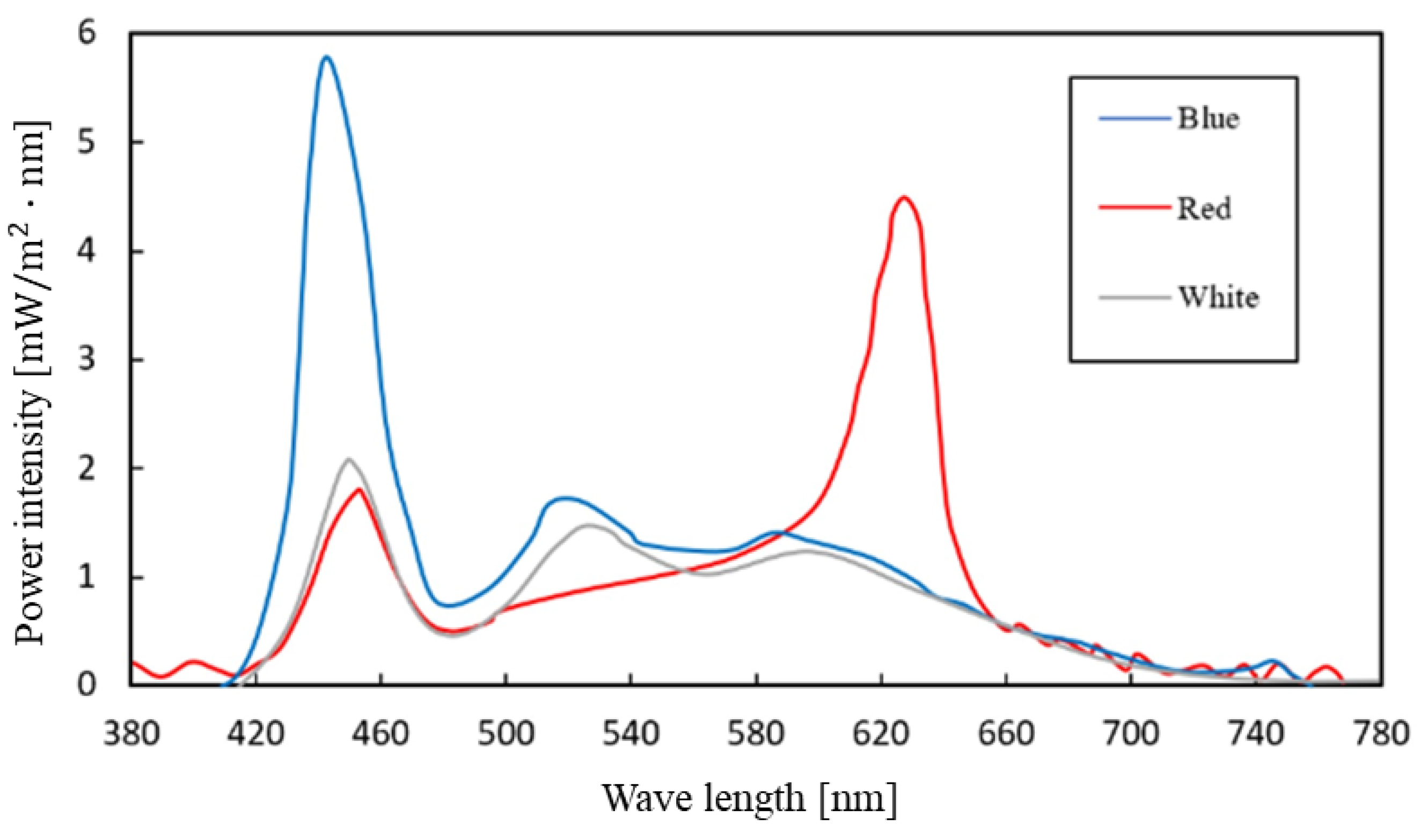
- Spectrum: According to a previous study [37] that investigated the spectral power of monochromatic blue light and the percentage of change in the suppression of melatonin secretion, the spectrum must have a short-wavelength component of at least 20 μW/cm2 to produce non-visual effects. In contrast, this study employed blue, red, and white (baseline condition) light. The light color spectra had to be difficult to perceive but sufficient to produce the visual effects. Spectra were measured with a C-7000 spectrometer (SECONIC, Tokyo, Japan) (Figure A1);
- (II)
- Illuminance: Illuminance had to be sufficiently high to affect arousal level but low enough not to induce a perceivable change in light color. According to a previous study [36] investigating the relationship between illuminance and arousal level (subjective arousal level using the percentage of suppressed melatonin secretion), the arousal level increased at around 70 lx (illuminance at the cornea) and reached an intermediate arousal level at 90–180 lx. Therefore, we set the illuminance to 90 lx at the corneal position;
- (III)
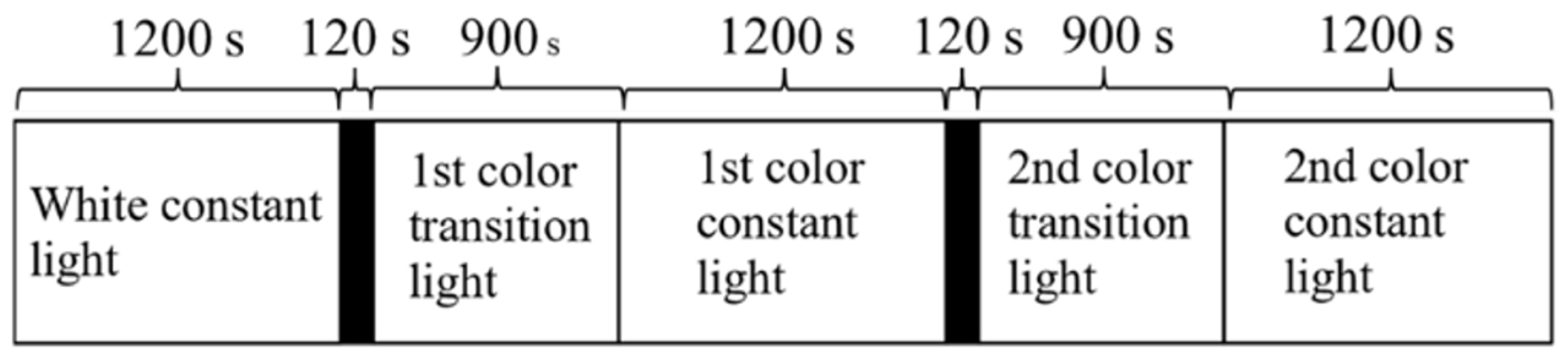
- Irradiation time period: The irradiation time period was set based on the following: (i) participants’ sleep habits; (ii) under appropriate lighting conditions, non-visual effects during the daytime may affect arousal level, performance, etc.; and (iii) irradiating light with a large short-wavelength component at night may lead to sleep disorders [38]. In this study, the irradiation time period was set as 10:00 to 17:00;
- (IV)
- Irradiation duration: Previous studies [6,16] indicated that non-visual effects could be observed after 12 min of irradiation. Additionally, the effect on arousal level was independent of the length of irradiation time if it was longer than 12 min. Therefore, we set the irradiation duration to 20 min in consideration of fatigue and to ensure a sufficient irradiation time for the onset of the non-visual effects;
- (V)
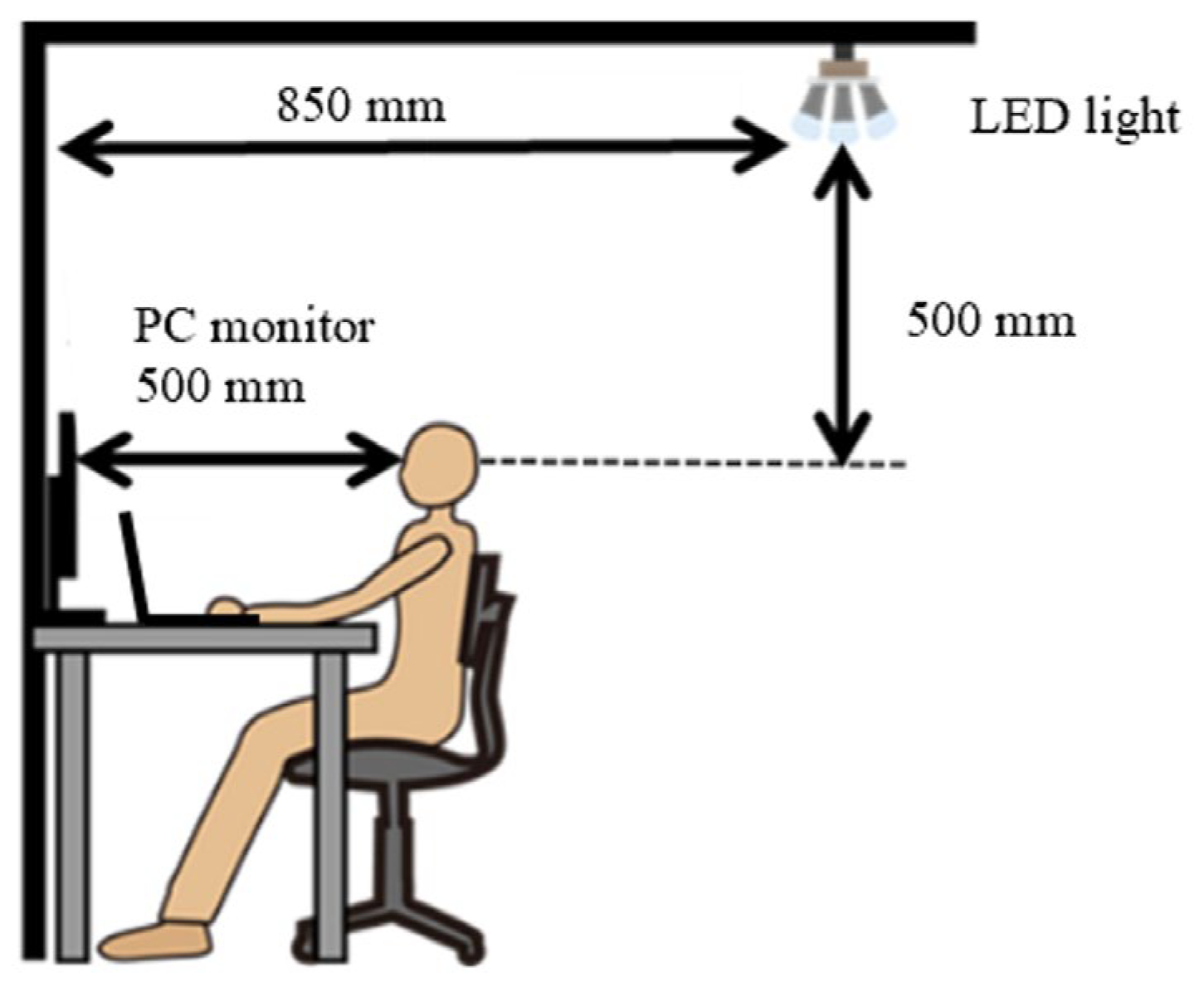
- Spatial distribution: Previous studies [39,40,41] revealed that non-visual effects depend on the retinal area irradiated by light. In this study, the light source was located behind the participant so that the entire visual field was illuminated through the wall (i.e., the entire retina was uniformly illuminated);
- (VI)
- Lighting environment: We focused on chromatic adaptation and change blindness to create an environment that made it difficult to perceive changes in light color. Chromatic adaptation is a characteristic where the sensitivity to a certain light color decreases as the color continues to be viewed [42]. In contrast, change blindness is a perceptual phenomenon in which an observer does not notice a change in a visual stimulus when they are focused or distracted by another visual stimulus [43]. On the basis of these two characteristics, the light color (spectrum) was changed gradually [44], and the participant undertook a task to distract them from the color change [45].
3.2. Results and Discussion
3.2.1. Light Color Perception
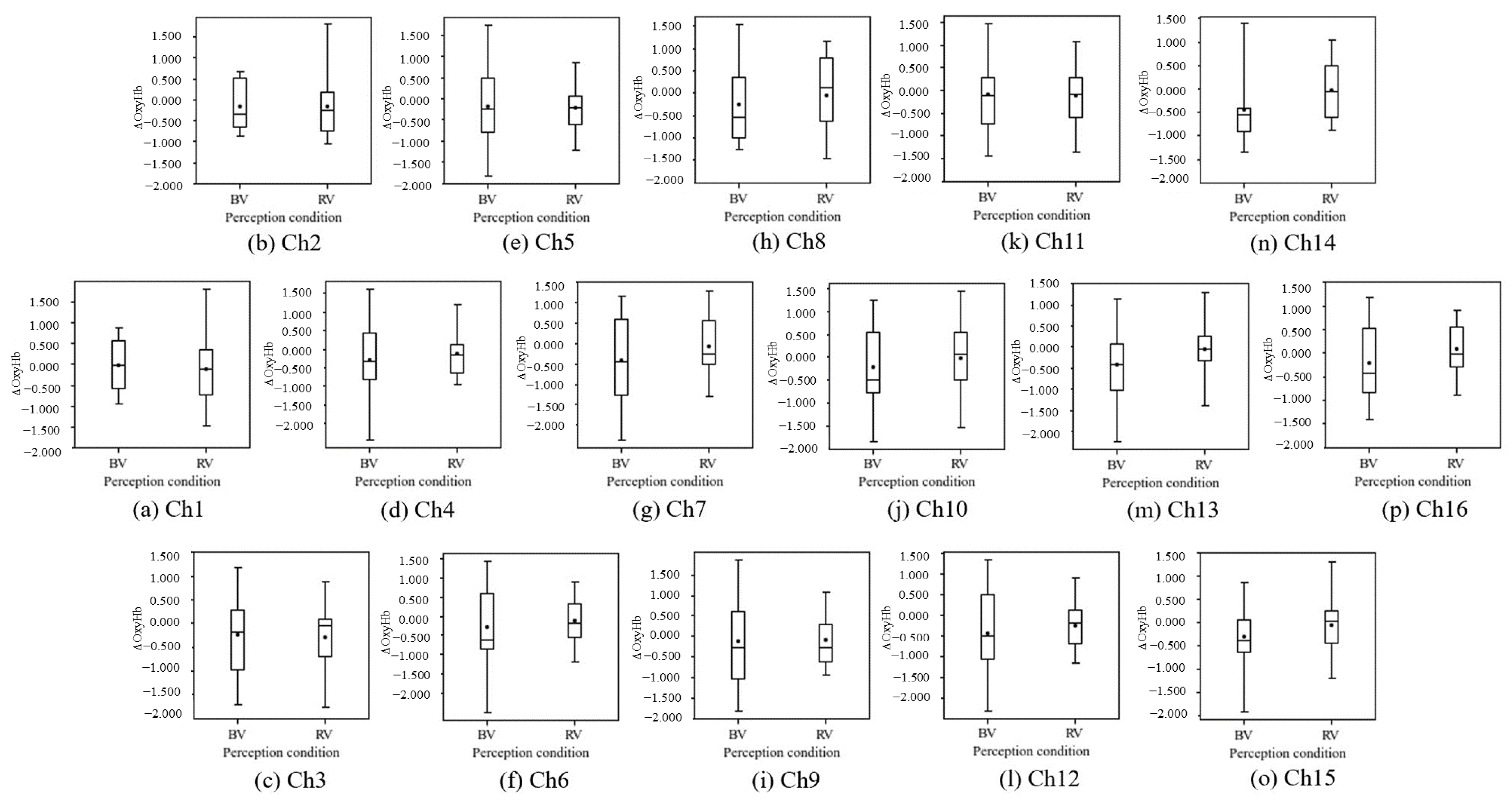
3.2.2. Brain Activity
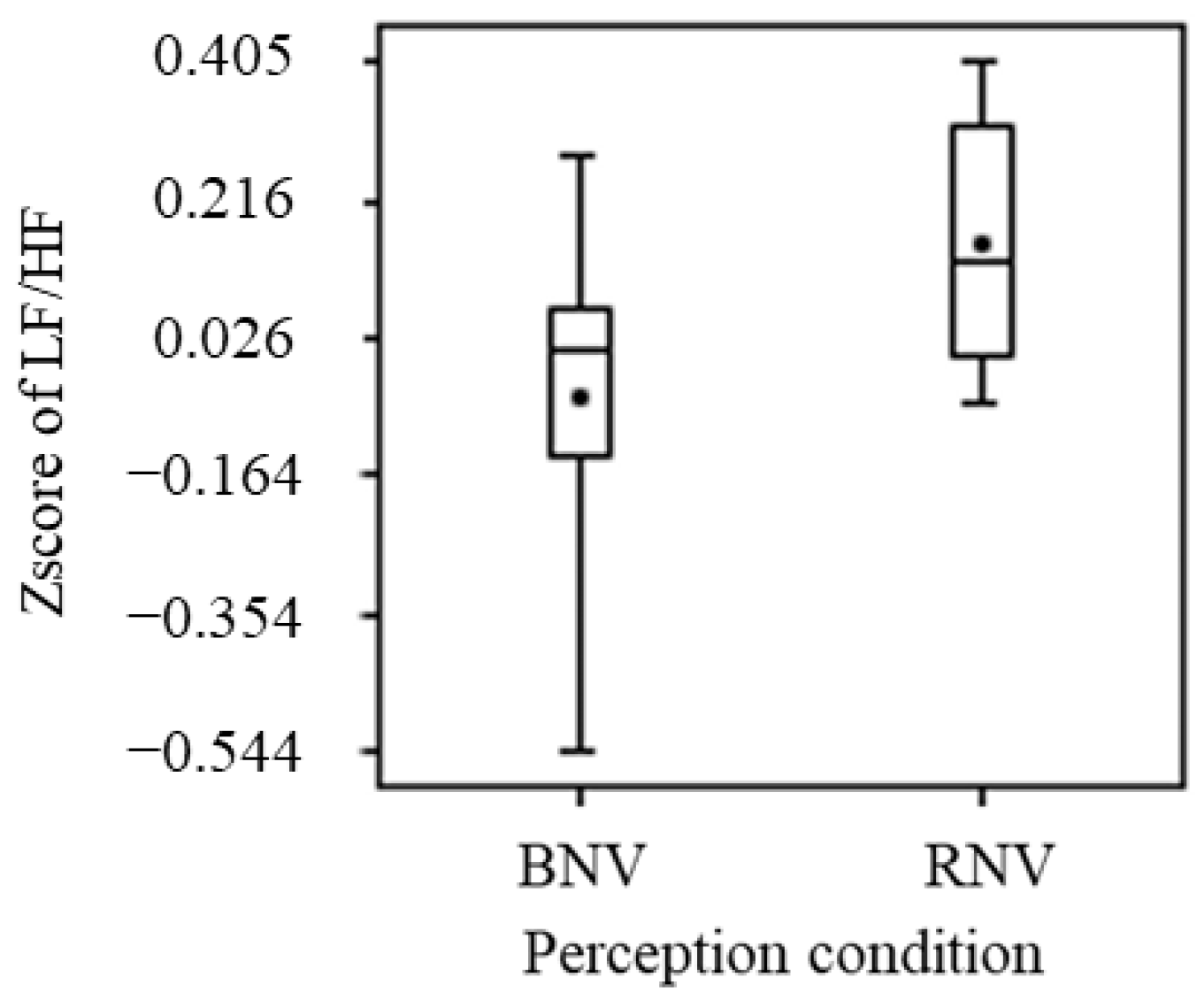
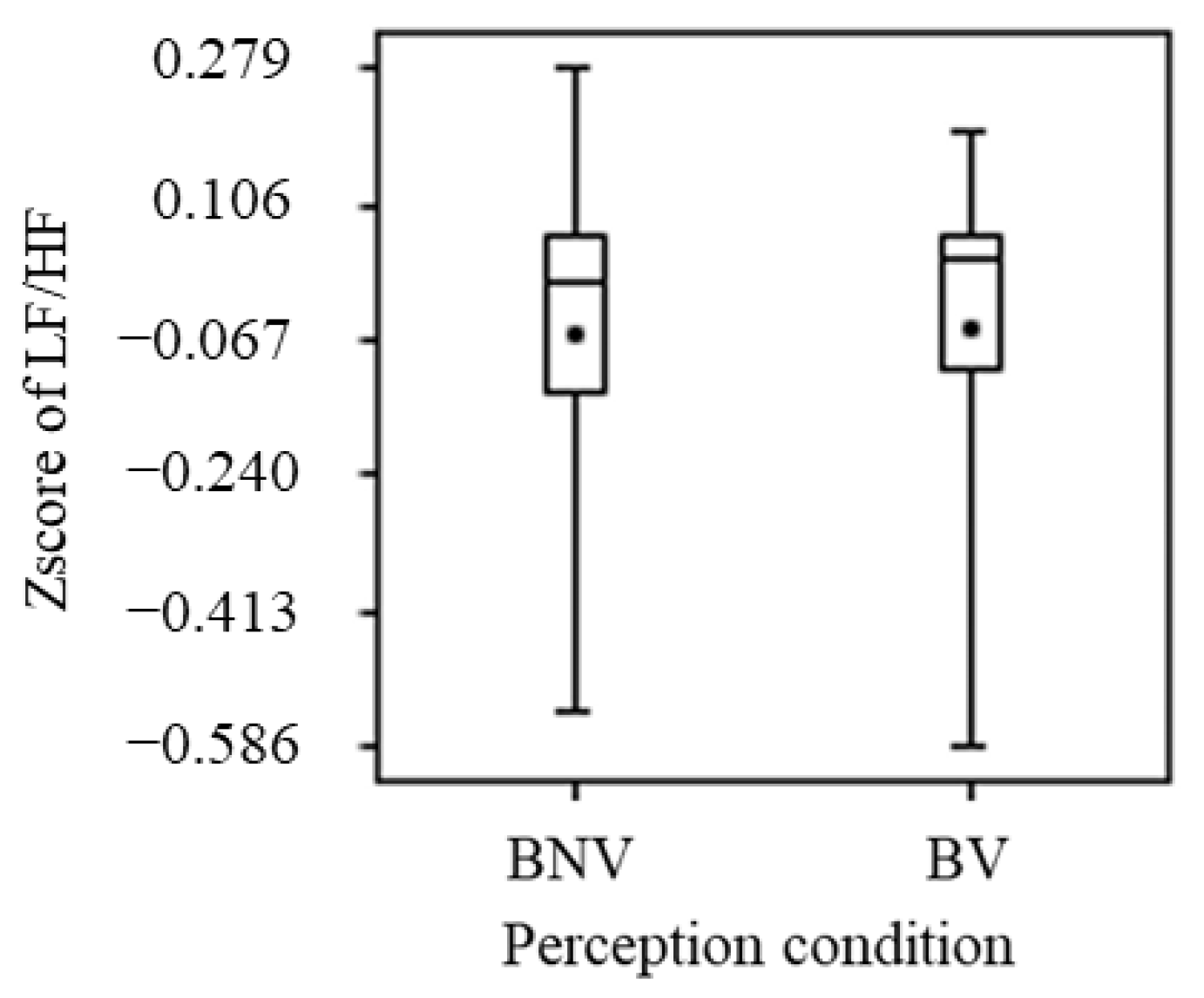
3.2.3. Heart Rate Variability
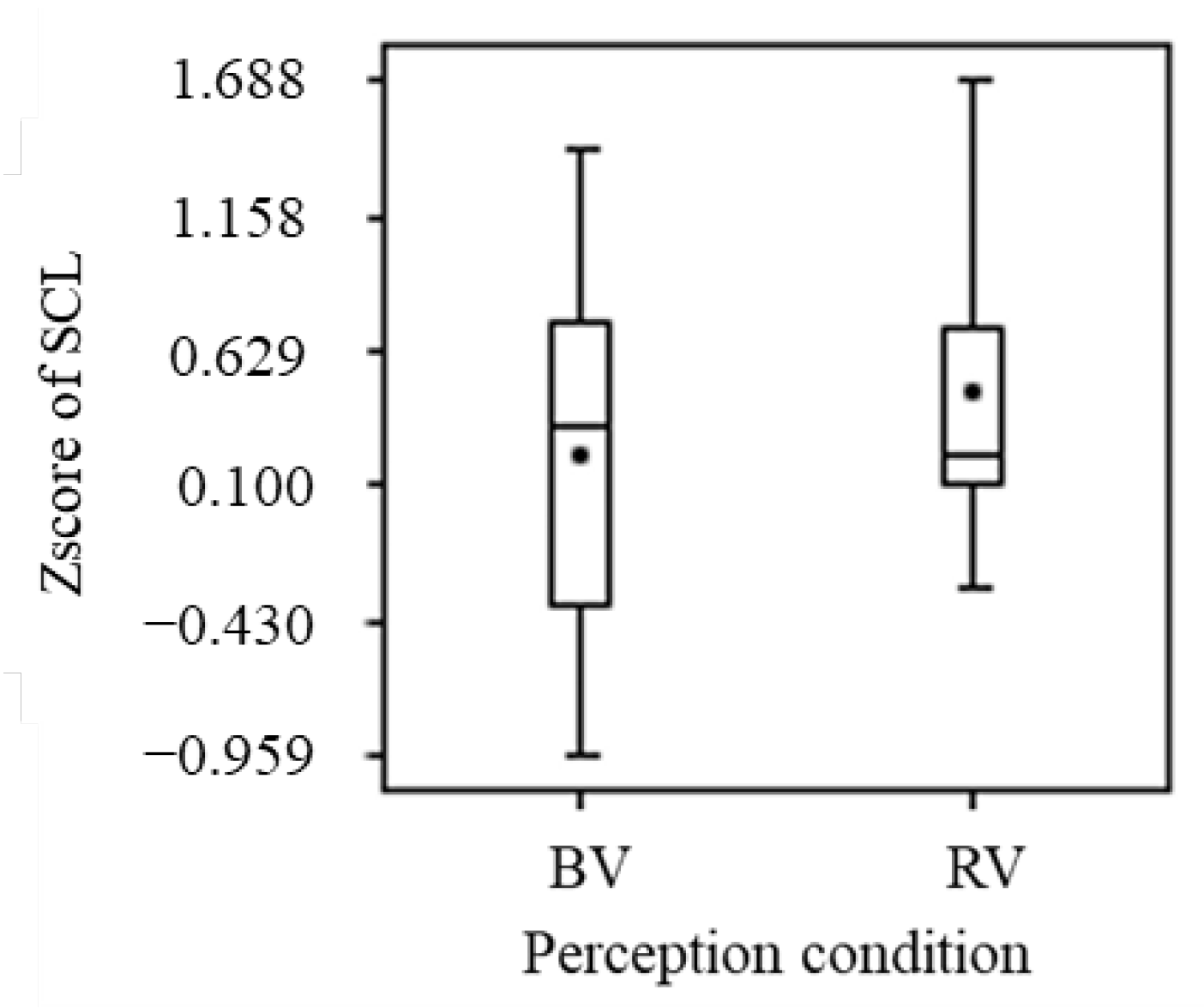
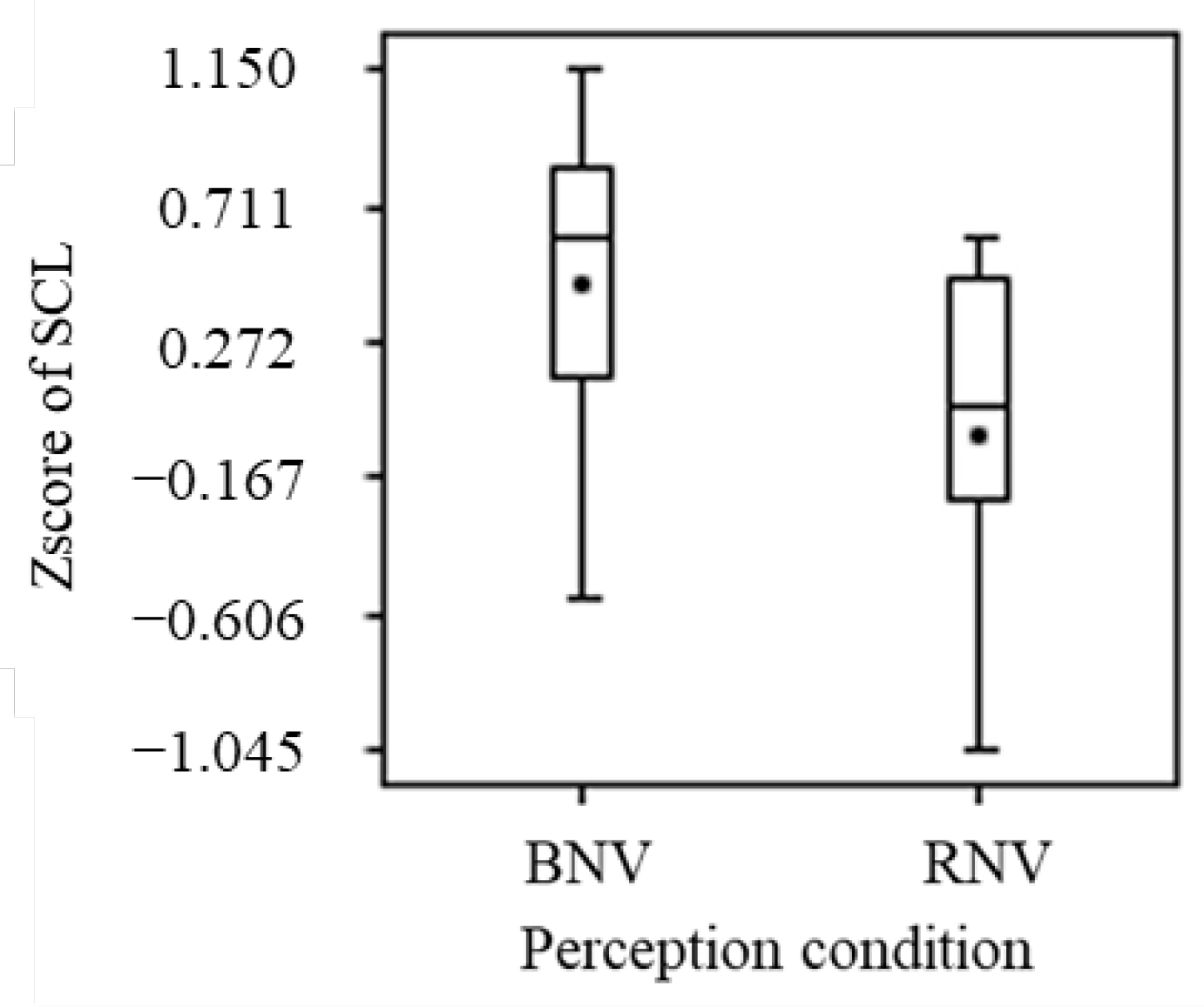
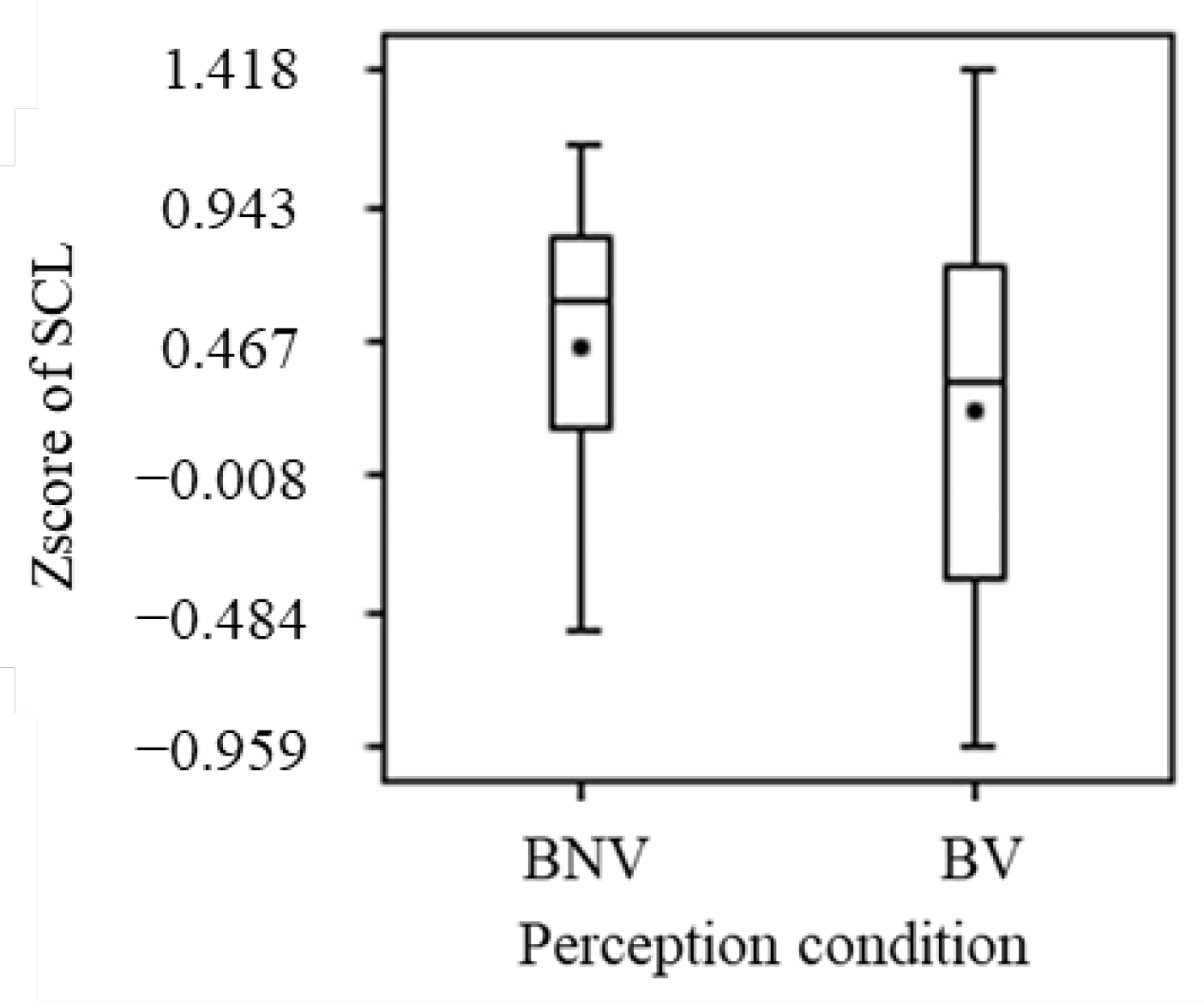
3.2.4. Electrodermal Activity
- This study set the red and blue light spectra to confirm the non-visual effects (i.e., the differences in the short-wavelength components of blue and red, especially from 446 nm to 477 nm). Simultaneously, to create an environment in which light color was not easily perceived, the long-wavelength component of red light was suppressed. Therefore, the red light used in this study may have been insufficient to induce visual effects;
- The participants were compared by grouping according to the presence or absence of visual effects based on their perceptual results. However, it is possible that individual differences, such as personal preference [2] for light color and light history (experience) [50], may have affected the comparison results. Therefore, an experimental method that could be used to obtain and compare the physiological response results of both effects for each light color within the same participant was necessary. Additionally, this study did not equalize the male–female ratio, which was the same as the conventional studies [8,9,12] that confirmed non-visual effects using physiological measurements. However, gender differences in color preference have been indicated [51,52,53] and could have affected the results. Aside from gender difference, color preference is affected by differences in personalities [54,55,56] and cultures/experiences [57,58,59,60] and by different times of day/seasons [56,61], etc. Therefore, these factors need to be considered to obtain an accurate analysis result;
- In this experiment, participants were assigned a free task using a PC or a book to prevent sleepiness. It is possible that differences regarding the task content may have affected the physiological response results. Therefore, it is necessary to consider tasks that can be conducted for a long period of time without making all participants feel sleepy.
4. Conclusions
- The brain activation (increase in ΔoxyHb) in some regions of the PFC (p < 0.05) confirmed the non-visual effects of blue light. A comparison of the mean values indicated that blue light activates the prefrontal cortex and increases sweating (increase in SCL), but the differences were not statistically significant;
- Although significant differences were not obtained for the visual effects of blue light, comparisons of the means showed tendencies toward a calming role for the prefrontal cortex and an inhibition of sweating;
- Although no significant differences were obtained for the visual effects of red light, comparisons of the means showed a tendency to enhance sweating.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Glossary
| BNV | Blue Non-visual |
| BV | Blue Visual |
| EDA | Electrodermal Activity |
| EEG | Electroencephalogram |
| fMRI | Functional Magnetic Resonance Imaging |
| HF | High frequency |
| LF | Low frequency |
| MEG | Magnetoencephalogram |
| NIRS | Near-infrared Spectroscopy |
| PC | Personal Computer |
| RNV | Red Non-visual |
| RRI | R–R Interval |
| RV | Red Visual |
| SCL | Skin Conductance Level |
| SD | Standard Deviation |
| ΔoxyHb | Change in Oxygenated Hemoglobin |
Appendix A

References
- Yasukouchi, A.; Ishibashi, K. Non-visual Effects of the Color Temperature of Fluorescent Lamps on Physiological Aspects in Humans. J. Physiol. Anthropol. Appl. Hum. Sci. 2005, 24, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, K.W.; Hustmyer, F.E. Effects of Four Psychological Primary Colors on GSR, Heart Rate and Respiration Rate. Percept. Mot. Skills 1974, 38, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Warthen, D.M.; Provencio, I. The Role of Intrinsically Photosensitive Retinal Ganglion Cells in Nonimage-forming Responses to Light. Eye Brain 2012, 4, 43–48. [Google Scholar]
- Wilson, G.D. Arousal Properties of Red versus Green. Percept. Mot. Skills 1966, 23, 947–949. [Google Scholar] [CrossRef]
- Mary, J.; Paul, G.; Justin, C.D. The Impact of Modulated Color Light on the Autonomic Nervous System. Adv. Mind Body Med. 2013, 27, 7–16. [Google Scholar]
- Chang, A.; Santhi, N.; Hilaire, M.S.; Gronfier, C.; Bradstreet, D.S.; Duffy, J.F.; Lockley, S.W.; Kronauer, R.E.; Czeisler, C.A. Human Responses to Bright Light of Different Durations. J. Physiol. 2012, 590, 3103–3112. [Google Scholar] [CrossRef]
- Lockley, S.W.; Evans, E.E.; Scheer, F.A.J.L.; Brainard, G.C.; Czeisler, C.A.; Aeschbach, D. Short-Wavelength Sensitivity for the Direct Effects of Light on Alertness, Vigilance, and the Waking Electroencephalogram in Humans. Sleep 2006, 29, 161–168. [Google Scholar]
- Cajochen, C.; Munch, M.; Kobialka, S.; Krauchi, K.; Steiner, R.; Oelhafen, P.; Orgul, S.; Wirz-Justice, A. High Sensitivity of Human Melatonin, Alertness, Thermoregulation, and Heart Rate to Short Wavelength Light. J. Clin. Endocrinol. Metab. 2005, 9, 1311–1316. [Google Scholar] [CrossRef]
- Rüger, M.; Gordijn, M.C.M.; Beersma, D.G.M.; Vries, B.; Daan, S. Time-of Day Dependent Effects of Bright Light Exposure on Human Psychophysiology: Comparison of Daytime and Nighttime Exposure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, 1419–1420. [Google Scholar] [CrossRef]
- Khademagha, P.; Aries, M.B.C.; Rosemann, A.L.P.; Loenen, E.J. Implementing Non-image-forming Effects of Light in the Built Environment: A review on what we need. Build. Environ. 2016, 108, 263–272. [Google Scholar] [CrossRef]
- Phipps-Nelson, J.; Redman, J.R.; Dijk, D.; Rajaratnam, S.M.W. Daytime Exposure to Bright Light, as Compared to Dim Light, Decreases Sleepiness and Improves Psychomotor Vigilance Performance. Sleep 2003, 26, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Chellappa, S.L.; Steiner, R.; Blattner, P.; Oelhafen, P.; Gotz, T.; Cajochen, C. Non-Visual Effects of Light on Melatonin, Alertness and Cognitive Performance: Can Blue-Enriched Light Keep Us Alert? PLoS ONE 2011, 6, e16429. [Google Scholar] [CrossRef]
- Berson, D.M.; Dunn, F.A.; Motoharu, T. Phototransduction by Retinal Ganglion Cells that Set the Circadion Clock. Science 2002, 295, 1070–1073. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.A.; Shapiro, C.M.; Wang, F.; Ainlay, H.; Kazmi, S.; Brown, T.J.; Casper, R.F. Effects of Filtering Visual Short Wavelengths During Nocturnal Shiftwork on Sleep and Performance. Chronobiol. Int. 2013, 30, 951–962. [Google Scholar] [CrossRef]
- Elliot, A.J.; Maier, M.A.; Moller, A.C.; Fiedman, R.; Meinhardt, J. Color and Psychological Functioning: The Effect of Red on Performance Attainment. J. Exp. Psychol. 2007, 136, 154–168. [Google Scholar] [CrossRef]
- Souman, J.L.; Tinga, A.M.; te Pas, S.F.; van Ee, R.; Vlaskamp, B.N.S. Acute Alerting Effects of Light: A Systematic Literature Review. Behav. Brain Res. 2018, 337, 228–239. [Google Scholar] [CrossRef]
- Takahashi, S. Psychological Study of Environmental Colors (1): Effects of Color Lighting on the Performance of Simple Task. J. Hum. Environ. Stud. 2005, 3, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, A.; Eto, K.; Shimada, H.A. Preliminary Analysis of Light Blue Backgrounds on the Scores of Web-based Tests Using NIRS. In Proceedings of the International Conference on Information Technology Based Higher Education and Training, Antalya, Turkey, 10–12 October 2013. [Google Scholar]
- Weinziri, J.; Heusser, P.; Nelle, M.; Wolf, U. Effects of Changes in Colored Light on Brain and Calf Muscle Blood Concentration and Oxygenation. Sci. World J. 2011, 11, 1216–1225. [Google Scholar] [CrossRef]
- Yamamoto, S.; Irikura, T. Physiological and Psychological Effects of Fluctuating Illumination on Heart Rate Variability in Concentration. J. Sci. Technol. Light. 2021, 45, 28–33. [Google Scholar] [CrossRef]
- Jing, L.; Yaru, Q.; Cheng, G.; Yanli, X.; Zhen, W.; Ruikang, Q. Lighting for Work: A Study on the Effect of Underground Low-light Environment on Miners’ Physiology. Environ. Sci. Pollut. Res. 2022, 29, 11644–11653. [Google Scholar]
- Vasung, L.; Abaci, T.E.; Ferradal, S.L.; Sutin, J. Exploring Early Human Brain Development with Structural and Physiological Neuroimaging. NeuroImage 2019, 187, 226–254. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, G.; Tanaka, M.; Horiuchi, T. Analysis of Harmony between Color and Fragrance in Lighting Environments by the Reaction of the Orbitofrontal Area. i-Perception 2022, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Akita, T. Effects of Illuminance and Color Temperature of a General Lighting System on Psychophysiology while Performing Paper and Computer Tasks. Build. Environ. 2022, 228, 109796. [Google Scholar] [CrossRef]
- Rodriguez, R.F.; Townsend, N.E.; Aughey, R.J.; Billaut, F. Influence of Averaging Method on Muscle Deoxygenation Interpretation during Repeated-sprint Exercise. Scand. J. Med. Sci. Sport. 2018, 28, 2263–2271. [Google Scholar] [CrossRef] [PubMed]
- Tsunashima, H.; Yanagisawa, K. Measurement of Brain Function of Car Driver Using Functional Near-Infrared Spectroscopy (fNIRS). Comput. Intell. Neurosci. 2009, 2009, 164958. [Google Scholar] [CrossRef]
- Yamada, T.; Umeyama, S.; Matsuda, K. Separation of fNIRS Signals into Functional and Systemic Components Based on Differences in Hemodynamic Modalities. PLoS ONE 2012, 7, e50271. [Google Scholar] [CrossRef]
- Tando, T.; Kaga, Y.; Kanemura, H.; Sugita, K.; Aihira, M. Developmental Changes in Frontal Lobe Function during a Verbal Fluency Task: A multi-channel near-infrared spectroscopy stud. Brain Dev. 2014, 36, 844–852. [Google Scholar] [CrossRef]
- Valera, B.; Dewailly, E.; Poirier, P. Impact of Mercury Exposure on Blood Pressure and Cardiac Autonomic Activity among Cree Adults. Environ. Res. 2011, 111, 1265–1270. [Google Scholar] [CrossRef]
- Dammen, L.; Finseth, T.T.; Mccurdy, B.H. Evoking stress reactivity in virtual reality: A Systematic Review and Meta-analysis. Neurosci. Biobehav. Rev. 2022, 138, 104709. [Google Scholar] [CrossRef]
- Bari, D.S.; Aldosky, H.; Tronstad, C.; Kalvoy, H.; Martinsen, O.G.; Martinsen, O.G. Electrodermal Responses to Discrete Stimuli Measured by Skin Conductance, Skin Potential, and Skin Susceptance. Ski. Res. Technol. 2018, 24, 108–116. [Google Scholar] [CrossRef]
- Woods, N.F.; Lentz, M.J.; Mitchell, E.S.; Kogan, H. Arousal and Stress Response across the Menstrual Cycle in Women with Three Perimenstrual Symptom Patterns. Res. Nurs. Health 1994, 17, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Janina, H.; Simon, S.; Claus, B. Examination of the Skin Conductance level (SCL) as an Index of the Activity of the Sympathetic Nervous System for Application the Continuous Blood Pressure Measurement. Int. J. Biomed. Eng. Sci. 2018, 5, 1–11. [Google Scholar]
- Garcia, M.E.; Ara, J.R.; Martin, J.; Almarcegui, C. Retinal and Optic Nerve Degeneration in Patients with Multiple Sclerosis Followed up for 5 Years. Ophthalmology 2017, 124, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, R.C. the Assessment and Analysis of Handedness: The Edinburgh Inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Cajochen, C.; Zeitzer, J.M.; Czeisler, C.A.; Dijk, D. Dose-response Relationship for Light Intensity and Ocular and Electroencephalographic Correlates of Human Alertness. Behav. Brain Res. 2000, 115, 75–83. [Google Scholar] [CrossRef]
- West, K.E.; Jablonski, M.R.; Warfield, B.; Cecil, K.S.; James, M.; Ayers, M.A.; Maida, J.; Bowen, C.; Sliney, D.H.; Rollag, M.D.; et al. Blue Light from Light-emitting Diodes Elicits a Dose-dependent Suppression of Melatonin in Humans. J. Appl. Physiol. 2011, 110, 619–626. [Google Scholar] [CrossRef]
- Wright, K.P.; McHill, A.W.; Birks, B.R.; Griffin, B.R.; Rusterholz, T.; Chinoy, E.D. Entrainment of the Human Circadian Clock to the Natural Light-Dark Cycle. Curr. Biol. 2013, 23, 1554–1558. [Google Scholar] [CrossRef]
- Glickman, G.; Hanifin, J.P.; Rollag, M.D.; Wang, J.; Cooper, H.; Brainard, G.C. Inferior Retinal Light Exposure is more Effective than Superior Retinal Exposure in Suppressing Melatonin in Humans. J. Biol. Rhythm. 2003, 18, 71–79. [Google Scholar] [CrossRef]
- Lasko, T.A.; Kripke, D.F.; Elliot, J.A. Melatonin Suppression by Illumination of Upper and Lower Visual Fields. J. Biol. Rhythm. 1999, 14, 122–125. [Google Scholar] [CrossRef]
- Visser, E.K.; Beersma, D.G.; Daan, S. Melatonin Suppression by Light in Humans is Maximal when the Nasal Part of the Retina is Illuminated. J. Biol. Rhythm. 1999, 14, 116–121. [Google Scholar] [CrossRef]
- Ikeda, M.; Pontawee, P.; Pichayada, K.; Aran, H. The Brain Adaptation to the Color of Illumination and not the Retinal Adaptation to the Color of Objects that Determines the Color Appearance of an Object in the Space. Opt. Rev. 2006, 13, 388–395. [Google Scholar] [CrossRef]
- Rensink, R.A. Change detection. Annu. Rev. Psychol. 2002, 53, 245–277. [Google Scholar] [CrossRef] [PubMed]
- Simons, D.J.; Franconeri, S.L.; Reimer, R.L. Change Blindness in the Absence of a Visual Disruption. Perception 2000, 29, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Simons, D.J.; Rensink, R.A. Change Blindness: Past, Present, and Future. Trends Cogn. Sci. 2005, 9, 16–20. [Google Scholar] [CrossRef]
- Qiu, L.; Zhong, Y.; Xie, Q.; He, Z.; Wang, X.; Chen, Y. Multi-Modal Integration of EEG-fNIRS for Characterization of Brain Activity Evoked by Preferred Music. Front. Neurorobot. 2022, 16, 823435. [Google Scholar] [CrossRef]
- Thom, E.C. The Discomfort Index. Weatherwise 1959, 12, 57–61. [Google Scholar] [CrossRef]
- Eckberg, D.L.; Kifle, Y.T.; Roberts, V.L. Phase Relationship between Normal Human Respiration and Baroreflex Responsiveness. J. Physiol. 1980, 304, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Huiberts, L.M.; Smolders, K.C.; De Kort, Y.A.W. Seasonal and Time-of-day Variations in Acute Non-image Forming Effects of Illuminance Level on Performance, Physiology, and Subjective Well-being. Chronobiol. Int. 2017, 34, 827–844. [Google Scholar] [CrossRef]
- Hebert, M.; Martin, S.K.; Lee, C.; Eastman, C.I. The Effects of Prior Light History on the Suppression of Melatonin by Light in Humans. J. Pineal Res. 2002, 33, 198–203. [Google Scholar] [CrossRef]
- Eysenck, H.J. A Critical and Experimental Study of Color Preference. Am. J. Psychol. 1941, 54, 385–391. [Google Scholar] [CrossRef]
- Hurlbert, A.; Owen, A. Biological, cultural, and developmental inflences on color preferences. In Handbook of Color Psychology; Cambridge University Press: Cambridge, UK, 2015; pp. 454–478. [Google Scholar]
- Valérie, B.; Sucharita, B.; Nijoo, D.; Mayukhini, P.; David, B. Gender Difference in Color Preference Across Cultures: An Archetypal Pattern Modulated by a Female Cultural Stereotype. Color Res. Appl. 2017, 43, 209–223. [Google Scholar]
- Charles, M.; Moyer, W.W. Correspondence of Self-referent Statements and Color Preference. Percept. Mot. Skills 1992, 74, 993–994. [Google Scholar] [CrossRef]
- Ireland, S.; Warren, Y.M.; Herringer, L.G. Anxiety and Color Saturation Preference. Percept. Mot. Skills 1992, 75, 545–546. [Google Scholar] [CrossRef]
- Zuber, I.; Ekehammar, B. Personality, Time of Day and Visual Perception: Preferences and Selective Attention. Personal. Individ. Differ. 1988, 9, 345–352. [Google Scholar] [CrossRef]
- Hurlbert, A.C.; Ling, Y.L. Biological Components of Sex Differences in Color Preference. Curr. Biol. 2007, 17, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Stephen, E.; Karen, B. An Ecological Valence Theory of Human Color Preference. Schloss 2010, 107, 8877–8882. [Google Scholar]
- Davis, J.T.M.; Robertson, E.; Lew-Levy, S.; Neldner, K.; Kapitany, R.; Nielsen, M.; Hines, M. Cultural Components of Sex Differences in Color Preference. Child Dev. 2021, 92, 1574–1589. [Google Scholar] [CrossRef]
- Taylor, C.; Clifford, A.; Franklin, A. Color Preferences Are Not Universal. J. Exp. Phychogy 2013, 142, 1015–1027. [Google Scholar] [CrossRef]
- Schloss, K.B.; Nelson, R.; Parker, L.; Heck, I.A.; Palmer, S.E. Seasonal Variations in Color Preference. Cogn. Sci. 2017, 41, 1589–1612. [Google Scholar] [CrossRef]







| Perception Condition | Participant Number |
|---|---|
| BV | 3, 4, 5, 6, 7, 8, 13, 16, 17, 19, 20, 21 |
| BNV | 1, 2, 9, 10, 11, 12, 14, 15, 18 |
| RV | 3, 4, 5, 6, 7, 8, 9, 10, 12, 13, 15, 16, 17, 18, 19, 20 |
| RNV | 1, 2, 11, 14, 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morioka, H.; Ozawa, H.; Kato, T. Physiological Study of Visual and Non-Visual Effects of Light Exposure. Appl. Sci. 2023, 13, 5785. https://doi.org/10.3390/app13095785
Morioka H, Ozawa H, Kato T. Physiological Study of Visual and Non-Visual Effects of Light Exposure. Applied Sciences. 2023; 13(9):5785. https://doi.org/10.3390/app13095785
Chicago/Turabian StyleMorioka, Haruki, Haruki Ozawa, and Takeo Kato. 2023. "Physiological Study of Visual and Non-Visual Effects of Light Exposure" Applied Sciences 13, no. 9: 5785. https://doi.org/10.3390/app13095785
APA StyleMorioka, H., Ozawa, H., & Kato, T. (2023). Physiological Study of Visual and Non-Visual Effects of Light Exposure. Applied Sciences, 13(9), 5785. https://doi.org/10.3390/app13095785






