Performance Qualification of Automatic System for Antineoplastic Preparation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Compounding and Formulation System
2.2. Quality Control Procedure
2.3. Media Fill Test
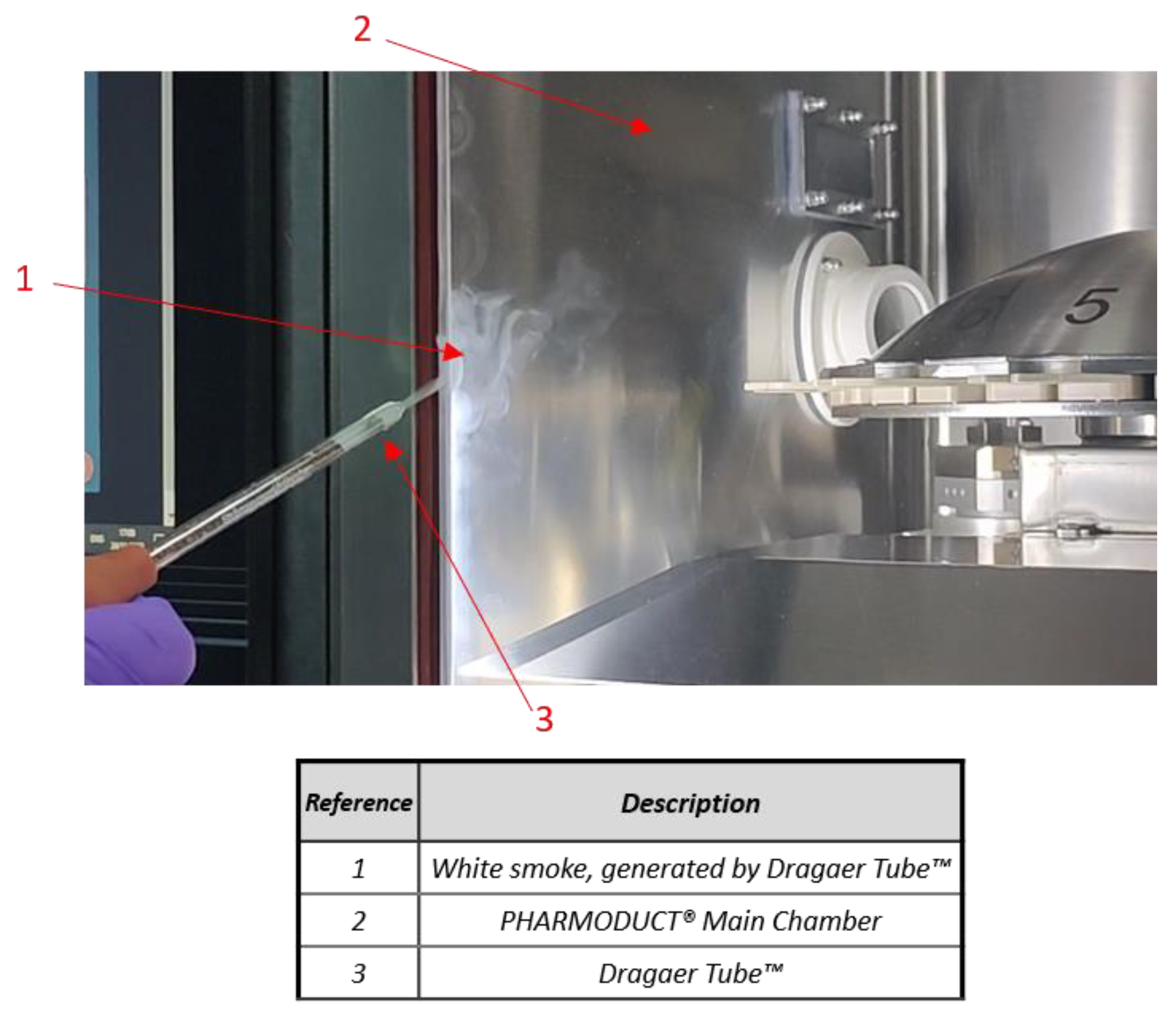
2.4. Differential Pressure of Main Chamber
2.5. Hourly Leak Rate (Tf)
- P is the pressure (initial = 1 and final = n) in pascals;
- T the temperature (initial = 1 and final = n) in kelvins;
- t is the duration of the test expressed in minutes.
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soumoy, L.; Hecq, J. Automated Compounding of Intravenous Therapy in European Countries: A Review in 2019. Pharm. Technol. Hosp. Pharm. 2019, 4, 51–57. [Google Scholar] [CrossRef]
- Geersing, T.H.; Franssen, E.J.F.; Pilesi, F.; Crul, M. Microbiological performance of a robotic system for aseptic compounding of cytostatic drugs. Eur. J. Pharm. Sci. 2019, 130, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Agalloco, J.; Akers, J.; Madsen, R. Aseptic processing: A review of current industry practice. Pharm. Technol. 2004, 28, 126. [Google Scholar]
- Fransman, W.; Vermeulen, R.; Kromhout, H. Dermal exposure to cyclophosphamide in hospitals during preparation, nursing and cleaning activities. Int. Arch. Occup. Environ. Health 2005, 78, 403–412. [Google Scholar] [CrossRef] [PubMed]
- McDiarmid, M.A.; Oliver, M.S.; Roth, T.S.; Rogers, B.; Escalante, C. Chromosome 5 and 7 abnormalities in oncology personnel handling anticancer drugs. J. Occup. Environ. Med. 2010, 52, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Fransman, W.; Kager, H.; Meijster, T.; Heederik, D.; Kromhout, H.; Portengen, L.; Blaauboer, B.J. Leukemia from dermal exposure to cyclophosphamide among nurses in the Netherlands: Quantitative assessment of the risk. Ann. Occup. Hyg. 2014, 58, 271–282. [Google Scholar] [PubMed]
- Hijiya, N.; Hudson, M.M.; Lensing, S.; Zacher, M.; Onciu, M.; Behm, F.G.; Razzouk, B.I.; Ribeiro, R.C.; Rubnitz, J.E.; Sandlund, J.T.; et al. Cumulative incidence of secondary neoplasms as a first event after childhood acute lymphoblastic leukemia. JAMA 2007, 297, 1207–1215. [Google Scholar] [CrossRef]
- Touzi, K.; Bussieres, J.F.; Langlois, E.; Lefebvre, M. Evaluation of surface contamination in a hospital hematology–oncology pharmacy. J. Oncol. Pharm. Pract. 2009, 15, 53–61. [Google Scholar] [CrossRef]
- Mason, H.J.; Blair, S.; Sams, C.; Jones, K.; Garfitt, S.J.; Cuschieri, M.J.; Baxter, P.J. Exposure to antineoplastic drugs in two UK hospital pharmacy units. Ann. Occup. Hyg. 2005, 49, 603–610. [Google Scholar]
- Sessink, P.J.; Wittenhorst, B.C.; Anzion, R.B.; Bos, R.P. Exposure of pharmacy technicians to antineoplastic agents: Reevaluation after additional protective measures. Arch. Environ. Health 1997, 52, 240–244. [Google Scholar] [CrossRef]
- Hall, A.L.; Demers, P.A.; Astrakianakis, G.; Ge, C.; Peters, C.E. Estimating national-level exposure to antineoplastic agents in the workplace: CAREX Canada findings and future research needs. Ann. Work Expo. Health 2017, 61, 656–658. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, J.J.; Lees, P.S.; McDiarmid, M.A. Exposure of hospital pharmacists and nurses to antineoplastic agents. J. Occup. Med. 1993, 35, 57–60. [Google Scholar] [PubMed]
- Cotteret, C.; Secretan, P.H.; Gilles-Afchain, L.; Rousseau, J.; Vidal, F.; Salguero-Hernandez, G.; Batista, J.; Valverde, V.; Guitton, J.; Cisternino, S.; et al. External contamination of antineoplastic drug vials: An occupational risk to consider. Eur. J. Hosp. Pharm. 2022, 29, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Gonzalez, C.G.; Herranz-Alonso, A.; Escudero-Vilaplana, V.; Ais-Larisgoitia, M.A.; Iglesias-Peinado, I.; Sanjurjo-Saez, M. Robotic dispensing improves patient safety, inventory management, and staff satisfaction in an outpatient hospital pharmacy. J. Eval. Clin. Pract. 2019, 25, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Krähenbühl-Melcher, A.; Schlienger, R.; Lampert, M.; Haschk, M.; Drewe, J.; Krähenbühl, S. Drug-related problems in hospitals: A review of the recent literature. Drug Saf. 2007, 30, 379–407. [Google Scholar] [CrossRef] [PubMed]
- Hänninen, K.; Ahtiainen, H.K.; Suvikas-Peltonen, E.M.; Tötterman, A.M. Automated unit dose dispensing systems producing individually packaged and labelled drugs for inpatients: A systematic review. Eur. J. Hosp. Pharm. 2023, 30, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.L.; Wang, T.; Zhu, J.Q.; Song, Y.J.; Gong, T.J.; Zou, L.K.; Liu, J.; Yan, J.F. Evaluation of external contamination on the vial surfaces of some hazardous drugs that commonly used in Chinese hospitals and comparison between environmental contamination generated during robotic compounding by IV: Dispensing robot vs. manual compounding in biological safety cabinet. J. Oncol. Pharm. Pract. 2022, 28, 1487–1498. [Google Scholar] [CrossRef]
- Connor, T.H.; DeBord, D.G.; Pretty, J.R.; Oliver, M.S.; Roth, T.S.; Lees, P.S.; Krieg, E.F., Jr.; Rogers, B.; Escalante, C.P.; Toennis, C.A.; et al. Evaluation of antineoplastic drug exposure of health care workers at three university-based US cancer centers. J. Occup. Environ. Med. 2010, 52, 1019–1027. [Google Scholar] [CrossRef]
- Davis, J.; McLauchlan, R.; Connor, T.H. Exposure to hazardous drugs in Healthcare: An issue that will not go away. J. Oncol. Pharm. Pract. 2011, 17, 9–13. [Google Scholar] [CrossRef]
- Perego, G.; Longobardo, G.; Baldisserotto, A.; Feliciani, M.; Fazio, M. Automated chemotherapy compounding: Process optimization for the preparation of admixture containing high-dose of cyclophosphamide. J. Oncol. Pharm. Pract. 2023, 29, 208–210. [Google Scholar] [CrossRef]
- Jobard, M.; Brandely-Piat, M.L.; Chast, F.; Batista, R. Qualification of a chemotherapy-compounding robot. J. Oncol. Pharm. Pract. 2020, 26, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, J.M.; Churchill, W.; Erickson, A.; Munz, K.; Schuur, J.D.; Salzberg, C.A.; Lewinski, D.; Shane, R.; Aazami, R.; Patka, J.; et al. Medication errors recovered by emergency department pharmacists. Ann. Emerg. Med. 2010, 55, 513–521. [Google Scholar] [CrossRef] [PubMed]
- ISO 14644-1:2015; (E) Clean Rooms and Associated Controlled Environments—Part 1: Classification of Air Cleanliness by Particle Concentration. International Organization for Standardization: Geneva, Switzerland, 1999.
- EudraLex—Volume 4—Good Manufacturing Practice (GMP) Guidelines, Annex 1 “Manufacture of Sterile Medicinal Products”. Available online: https://health.ec.europa.eu/system/files/2022-08/20220825_gmp-an1_en_0.pdf (accessed on 12 April 2023).
- Sterility. In European Pharmacopeia (Ph Eur), 11th ed.; Council of Europe: Strasbourg Cedex, France, 2023; Chapter 2.6.1.
- Bacterial Endotoxins. In European Pharmacopeia (Ph Eur), 11th ed.; Council of Europe: Strasbourg Cedex, France, 2023; Chapter 2.6.14.
- Batson, S.; Mitchell, S.A.; Lau, D.; Canobbio, M.; de Goede, A.; Singh, I.; Loesch, U. Automated compounding technology and workflow solutions for the preparation of chemotherapy: A systematic review. Eur. J. Hosp. Pharm. 2020, 27, 330–336. [Google Scholar] [CrossRef] [PubMed]
- ISO 10648-2:1994; Containment Enclosures—Part 2: Classification According to Leak Tightness and Associated Checking Methods. International Organization for Standardization: Geneva, Switzerland, 1994.
- Ranchon, F.; Salles, G.; Späth, H.M.; Schwiertz, V.; Vantard, N.; Parat, S.; Broussais, F.; You, B.; Tartas, S.; Souquet, P.J.; et al. Chemotherapeutic errors in hospitalised cancer patients: Attributable damage and extra costs. BMC Cancer 2011, 11, 478. [Google Scholar] [CrossRef]
- Sabatini, L.; Paolucci, D.; Marinelli, F.; Pianetti, A.; Sbaffo, M.; Bufarini, C.; Sisti, M. Microbiological validation of a robot for the sterile compounding of injectable non-hazardous medications in a hospital environment. Eur. J. Hosp. Pharm. 2020, 27, e63–e68. [Google Scholar] [CrossRef]
- EudraLex—Volume 4—Good Manufacturing Practice (GMP) Guidelines, Annex 15 “Qualification and Validation”. Available online: https://health.ec.europa.eu/system/files/2016-11/2015-10_annex15_0.pdf (accessed on 12 April 2023).


| Fluorouracil | |||||||
|---|---|---|---|---|---|---|---|
| DRUG | Diluent (NaCl 0.9%) | Total (Drug + Diluent) | Bag Name | Theoretical Concentration | |||
| RUN° 1 (High dosage) | Fluorouracil 1 | 6000 mg | 120 mL | 180 mL | 300 mL | Run1_1 | 20 mg/mL |
| Fluorouracil 2 | 6000 mg | 120 mL | 180 mL | 300 mL | Run1_2 | 20 mg/mL | |
| Fluorouracil 3 | 6000 mg | 120 mL | 180 mL | 300 mL | Run1_3 | 20 mg/mL | |
| Fluorouracil 4 | 6000 mg | 120 mL | 180 mL | 300 mL | Run1_4 | 20 mg/mL | |
| Fluorouracil 5 | 6000 mg | 120 mL | 180 mL | 300 mL | Run1_5 | 20 mg/mL | |
| RUN° 2 (Low dosage) | Fluorouracil 1 | 600 mg | 12 mL | 138 mL | 150 mL | Run2_1 | 4 mg/mL |
| Fluorouracil 2 | 600 mg | 12 mL | 138 mL | 150 mL | Run2_2 | 4 mg/mL | |
| Fluorouracil 3 | 600 mg | 12 mL | 138 mL | 150 mL | Run2_3 | 4 mg/mL | |
| Fluorouracil 4 | 600 mg | 12 mL | 138 mL | 150 mL | Run2_4 | 4 mg/mL | |
| Fluorouracil 5 | 600 mg | 12 mL | 138 mL | 150 mL | Run2_5 | 4 mg/mL | |
| RUN° 3 (Random dosage) | Fluorouracil 1 | 600 mg | 12 mL | 138 mL | 150 mL | Run3_1 | 4 mg/mL |
| Fluorouracil 2 | 1800 mg | 36 mL | 264 mL | 300 mL | Run3_2 | 6 mg/mL | |
| Fluorouracil 3 | 2400 mg | 48 mL | 252 mL | 300 mL | Run3_3 | 8 mg/mL | |
| Fluorouracil 4 | 3800 mg | 76 mL | 224 mL | 300 mL | Run3_4 | 12.7 mg/mL | |
| Fluorouracil 5 | 4200 mg | 84 mL | 216 mL | 300 mL | Run3_5 | 14 mg/mL | |
| Paclitaxel | |||||||
|---|---|---|---|---|---|---|---|
| DRUG | Diluent (NaCl 0.9%) | Total (Drug + Diluent) | Bag Name | Theoretical Concentration | |||
| RUN° 1 (High dosage) | Paclitaxel 1 | 350 mg | 58.33 mL | 441.67 mL | 500 mL | Run1_1 | 0.7 mg/mL |
| Paclitaxel 2 | 350 mg | 58.33 mL | 441.67 mL | 500 mL | Run1_2 | 0.7 mg/mL | |
| Paclitaxel 3 | 350 mg | 58.33 mL | 441.67 mL | 500 mL | Run1_3 | 0.7 mg/mL | |
| Paclitaxel 4 | 350 mg | 58.33 mL | 441.67 mL | 500 mL | Run1_4 | 0.7 mg/mL | |
| Paclitaxel 5 | 350 mg | 58.33 mL | 441.67 mL | 500 mL | Run1_5 | 0.7 mg/mL | |
| RUN° 2 (Low dosage) | Paclitaxel 1 | 75 mg | 12.5 mL | 237.5 mL | 250 mL | Run2_1 | 0.3 mg/mL |
| Paclitaxel 2 | 75 mg | 12.5 mL | 237.5 mL | 250 mL | Run2_2 | 0.3 mg/mL | |
| Paclitaxel 3 | 75 mg | 12.5 mL | 237.5 mL | 250 mL | Run2_3 | 0.3 mg/mL | |
| Paclitaxel 4 | 75 mg | 12.5 mL | 237.5 mL | 250 mL | Run2_4 | 0.3 mg/mL | |
| Paclitaxel 5 | 75 mg | 12.5 mL | 237.5 mL | 250 mL | Run2_5 | 0.3 mg/mL | |
| RUN° 3 (Random dosage) | Paclitaxel 1 | 75 mg | 12.5 mL | 237.5 mL | 250 mL | Run3_1 | 0.3 mg/mL |
| Paclitaxel 2 | 100 mg | 16.66 mL | 233.34 mL | 250 mL | Run3_2 | 0.4 mg/mL | |
| Paclitaxel 3 | 150 mg | 25 mL | 475 mL | 500 mL | Run3_3 | 0.3 mg/mL | |
| Paclitaxel 4 | 200 mg | 33.33 mL | 466.67 mL | 500 mL | Run3_4 | 0.4 mg/mL | |
| Paclitaxel 5 | 270 mg | 45 mL | 455 mL | 500 mL | Run3_5 | 0.54 mg/mL | |
| Cyclophosphamide | |||||||
|---|---|---|---|---|---|---|---|
| DRUG | Diluent (NaCl 0.9%) | Total (Drug + Diluent) | Bag Name | Theoretical Concentration | |||
| RUN° 1 (High dosage) | Cyclophosphamide 1 | 1500 mg | 75 mL | 175 mL | 250 mL | Run1_1 | 6 mg/mL |
| Cyclophosphamide 2 | 1500 mg | 75 mL | 175 mL | 250 mL | Run1_2 | 6 mg/mL | |
| Cyclophosphamide 3 | 1500 mg | 75 mL | 175 mL | 250 mL | Run1_3 | 6 mg/mL | |
| Cyclophosphamide 4 | 1500 mg | 75 mL | 175 mL | 250 mL | Run1_4 | 6 mg/mL | |
| Cyclophosphamide 5 | 1500 mg | 75 mL | 175 mL | 250 mL | Run1_5 | 6 mg/mL | |
| RUN° 2 (Low dosage) | Cyclophosphamide 1 | 600 mg | 30 mL | 220 mL | 250 mL | Run2_1 | 2.4 mg/mL |
| Cyclophosphamide 2 | 600 mg | 30 mL | 220 mL | 250 mL | Run2_2 | 2.4 mg/mL | |
| Cyclophosphamide 3 | 600 mg | 30 mL | 220 mL | 250 mL | Run2_3 | 2.4 mg/mL | |
| Cyclophosphamide 4 | 600 mg | 30 mL | 220 mL | 250 mL | Run2_4 | 2.4 mg/mL | |
| Cyclophosphamide 5 | 600 mg | 30 mL | 220 mL | 250 mL | Run2_5 | 2.4 mg/mL | |
| RUN° 3 (Random dosage) | Cyclophosphamide 1 | 600 mg | 30 mL | 220 mL | 250 mL | Run3_1 | 2.4 mg/mL |
| Cyclophosphamide 2 | 700 mg | 35 mL | 215 mL | 250 mL | Run3_2 | 2.8 mg/mL | |
| Cyclophosphamide 3 | 800 mg | 40 mL | 210 mL | 250 mL | Run3_3 | 3.2 mg/mL | |
| Cyclophosphamide 4 | 900 mg | 45 mL | 205 mL | 250 mL | Run3_4 | 3.6 mg/mL | |
| Cyclophosphamide 5 | 1000 mg | 50 mL | 200 mL | 250 mL | Run3_5 | 4 mg/mL | |
| Mass (m/z) | Formula | Species | Polarity | Analyte |
|---|---|---|---|---|
| 129.01058 | C4H3FN2O2 | −H | Negative | Fluorouracil |
| 261.03210 | C7H15Cl2N2O2P | +H | Positive | Cyclophosphamide |
| 286.10709 | C47H51NO14 | +H-C31H36O10 | Positive | Paclitaxel |
| DRUG | Theoretical Concentration | Found Concentration | Error |
|---|---|---|---|
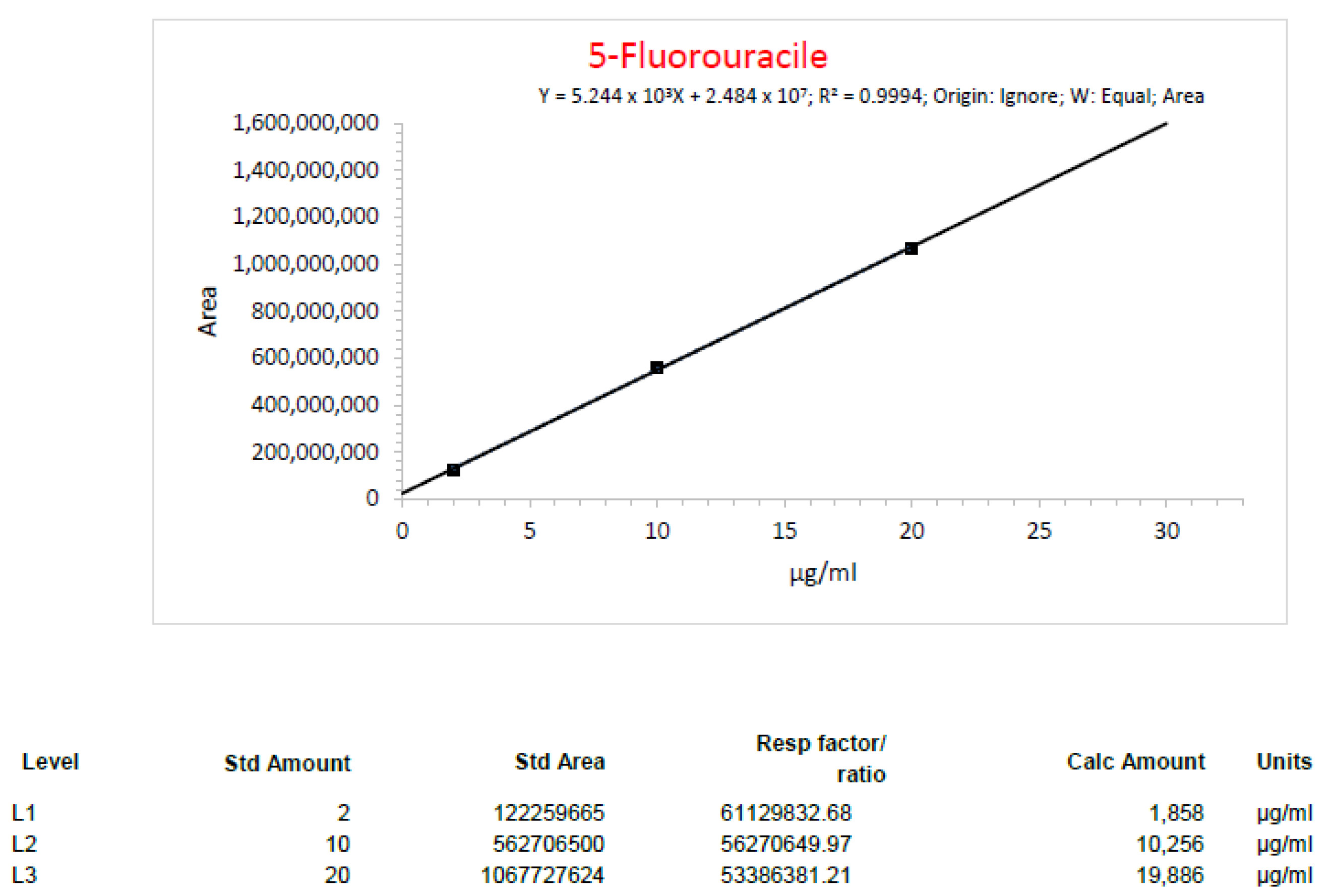
| Run1_1 5-Fluorouracil | 20 mg/mL | 19.45 mg/mL | 2.75% |
| Run1_2 5-Fluorouracil | 20 mg/mL | 20.75 mg/mL | 3.75% |
| Run2_3 5-Fluorouracil | 4 mg/mL | 3.71 mg/mL | 7.25% |
| Run2_4 5-Fluorouracil | 4 mg/mL | 3.70 mg/mL | 7.50% |
| Run3_1 5-Fluorouracil | 4 mg/mL | 3.83 mg/mL | 4.25% |
| Run3_3 5-Fluorouracil | 8 mg/mL | 7.67 mg/mL | 4.13% |
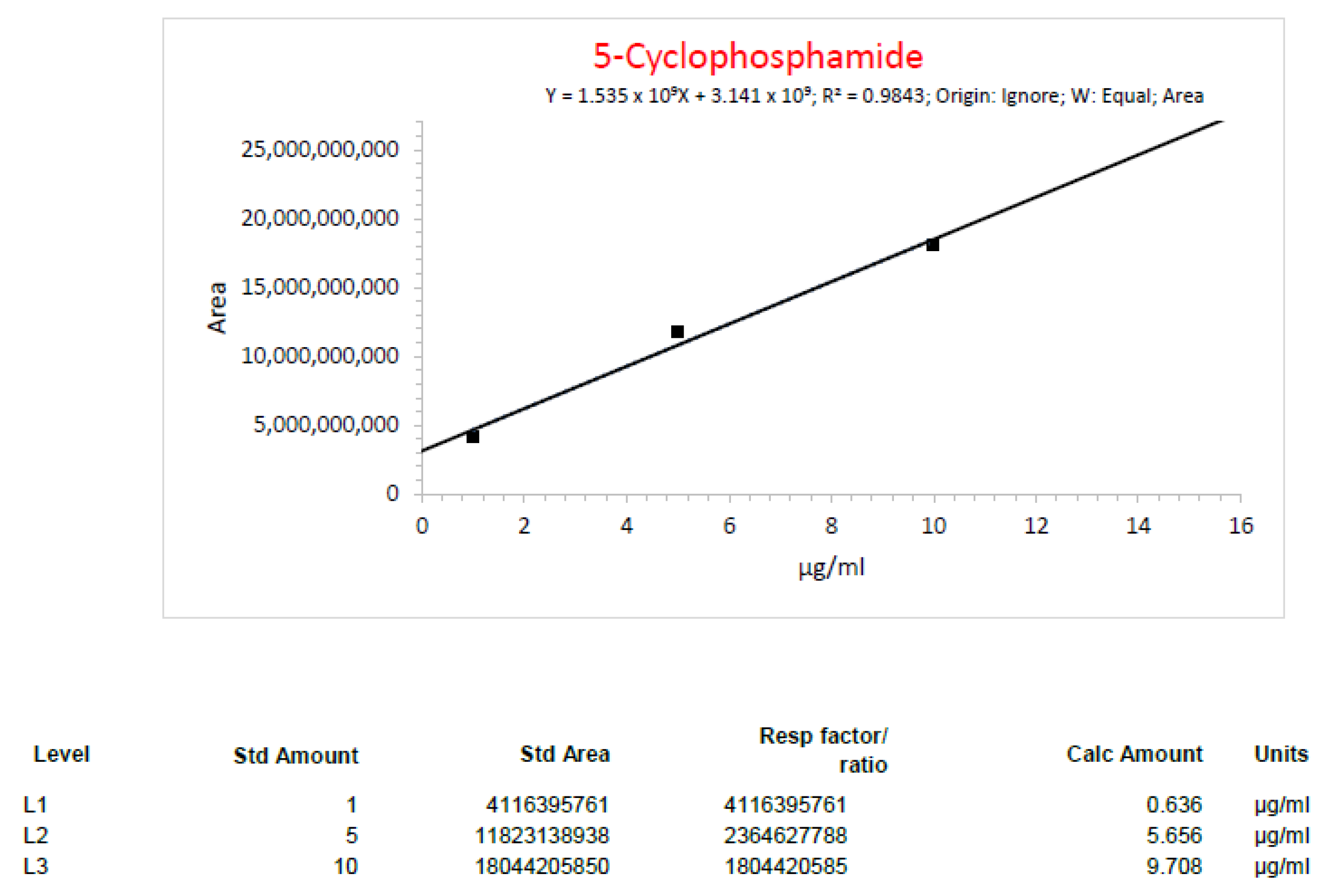
| Run1_1 Cyclophosphamide | 6 mg/mL | 6.36 mg/mL | 6% |
| Run1_2 Cyclophosphamide | 6 mg/mL | 6.26 mg/mL | 4.33% |
| Run2_3 Cyclophosphamide | 2.40 mg/mL | 2.43 mg/mL | 1.25% |
| Run2_4 Cyclophosphamide | 2.40 mg/mL | 2.24 mg/mL | 6.67% |
| Run3_1 Cyclophosphamide | 2.40 mg/mL | 2.25 mg/mL | 6.25% |
| Run3_3 Cyclophosphamide | 3.2 mg/mL | 3.44 mg/mL | 7.50% |
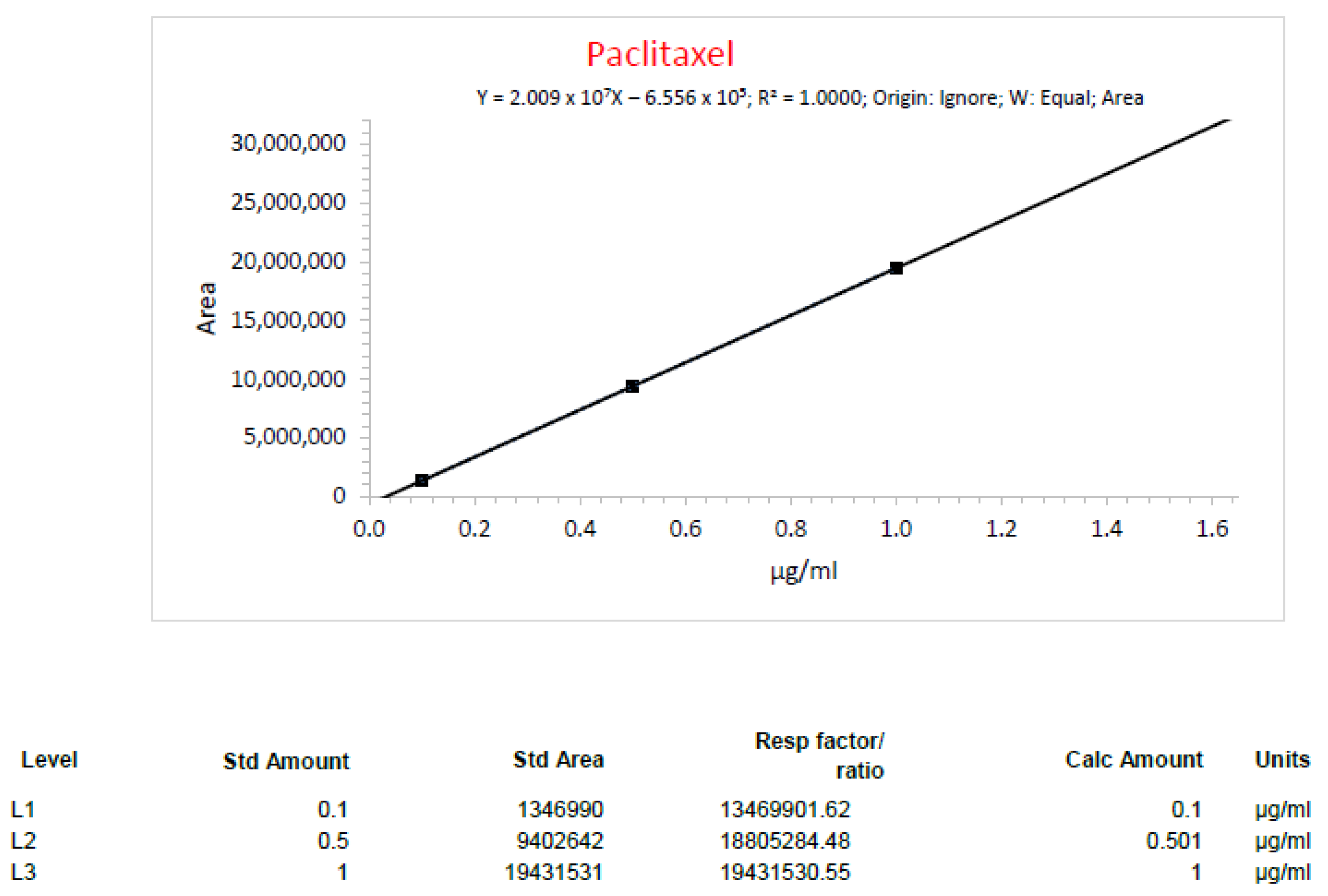
| Run1_1 Paclitaxel | 0.70 mg/mL | 0.68 mg/mL | 2.86% |
| Run1_2 Paclitaxel | 0.70 mg/mL | 0.68 mg/mL | 2.86% |
| Run2_3 Paclitaxel | 0.30 mg/mL | 0.32 mg/mL | 6.67% |
| Run2_4 Paclitaxel | 0.30 mg/mL | 0.29 mg/mL | 3.33% |
| Run3_1 Paclitaxel | 0.30 mg/mL | 0.32 mg/mL | 6.67% |
| Run3_3 Paclitaxel | 0.30 mg/mL | 0.31 mg/mL | 3.33% |
| T (min) | Relative Pressure (Pa) | Absolute Pressure (Pa) | Temperature (°K) | Tf (h−1) |
|---|---|---|---|---|
| 0 | −255.5 | 98,809.87 | 297.43 | / |
| 15 | −240.3 | 98,799.16 | 297.40 | / |
| 30 | −223.0 | 98,925.85 | 297.28 | / |
| 45 | −203.8 | 98,919.22 | 297.20 | / |
| 60 | −186.3 | 98,914.23 | 297.14 | 0.002033 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tesse, G.; Capasso, G.; Brattoli, S.; Tolomeo, A.; Dimiccoli, V.; Spartà, M.; Mazzotta, S.; Altieri, G.; Giannelli, A.; Ancona, D.; et al. Performance Qualification of Automatic System for Antineoplastic Preparation. Appl. Sci. 2024, 14, 106. https://doi.org/10.3390/app14010106
Tesse G, Capasso G, Brattoli S, Tolomeo A, Dimiccoli V, Spartà M, Mazzotta S, Altieri G, Giannelli A, Ancona D, et al. Performance Qualification of Automatic System for Antineoplastic Preparation. Applied Sciences. 2024; 14(1):106. https://doi.org/10.3390/app14010106
Chicago/Turabian StyleTesse, Giuseppe, Giuseppe Capasso, Stefano Brattoli, Anna Tolomeo, Vincenzo Dimiccoli, Marco Spartà, Stefano Mazzotta, Giuseppe Altieri, Anna Giannelli, Domenica Ancona, and et al. 2024. "Performance Qualification of Automatic System for Antineoplastic Preparation" Applied Sciences 14, no. 1: 106. https://doi.org/10.3390/app14010106






