Potential Effects of Photobiomodulation Therapy on Human Dental Pulp Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Sample Collection
2.2. Dental Pulp Stem Cell Culture and Expansion
2.3. Characterization of hDPSCs
2.3.1. Expression of MSC Surface Markers
2.3.2. Multi-Lineage Differentiation
2.4. Photobiomodulation Therapy
2.5. Cell Proliferation Assay (MTT Assay)
2.6. Cell Death (Apoptosis or Necrosis) Assay
2.7. Cell Cycle
2.8. Statistical Analysis
3. Results
3.1. Characterization of hDPSCs
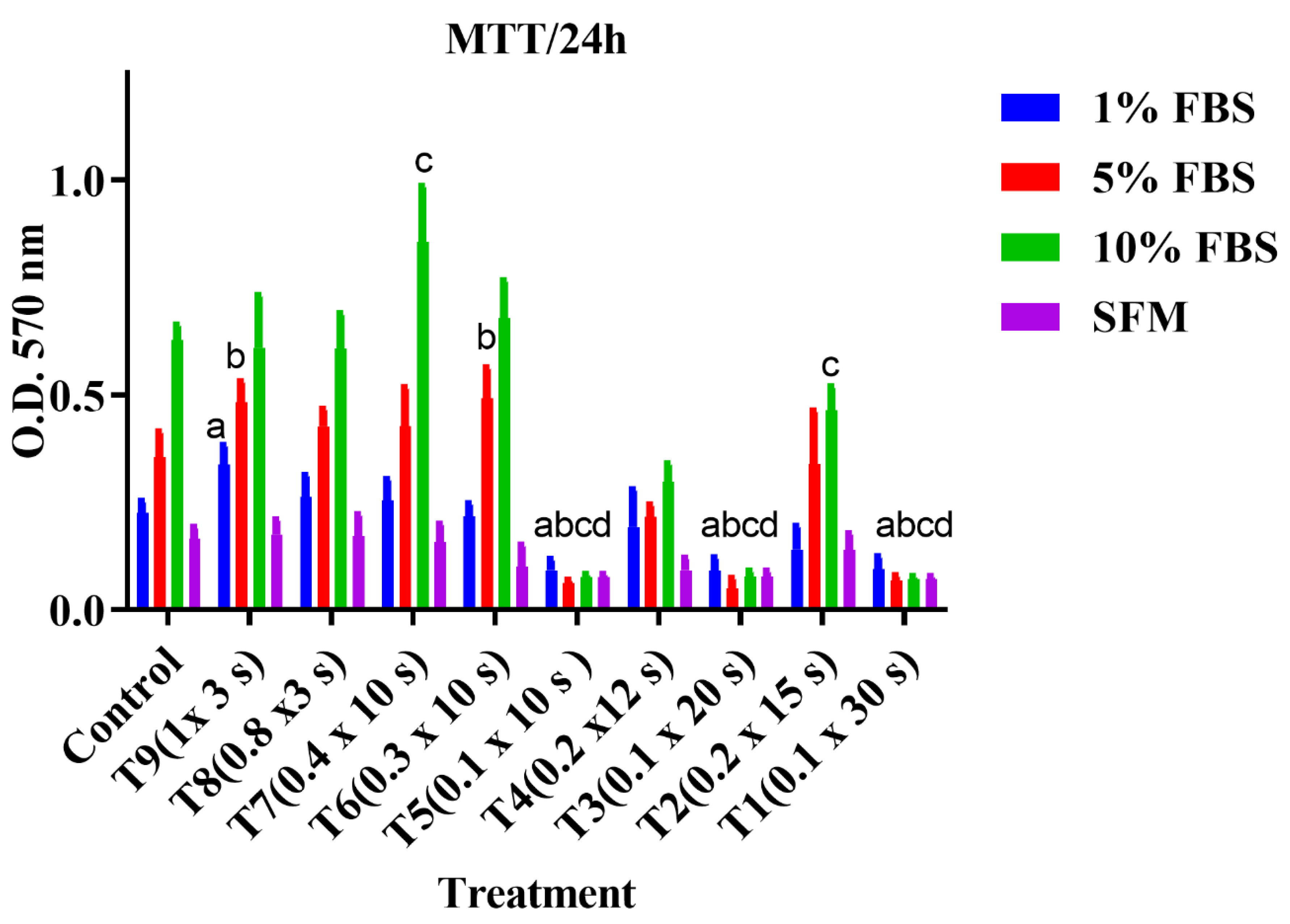
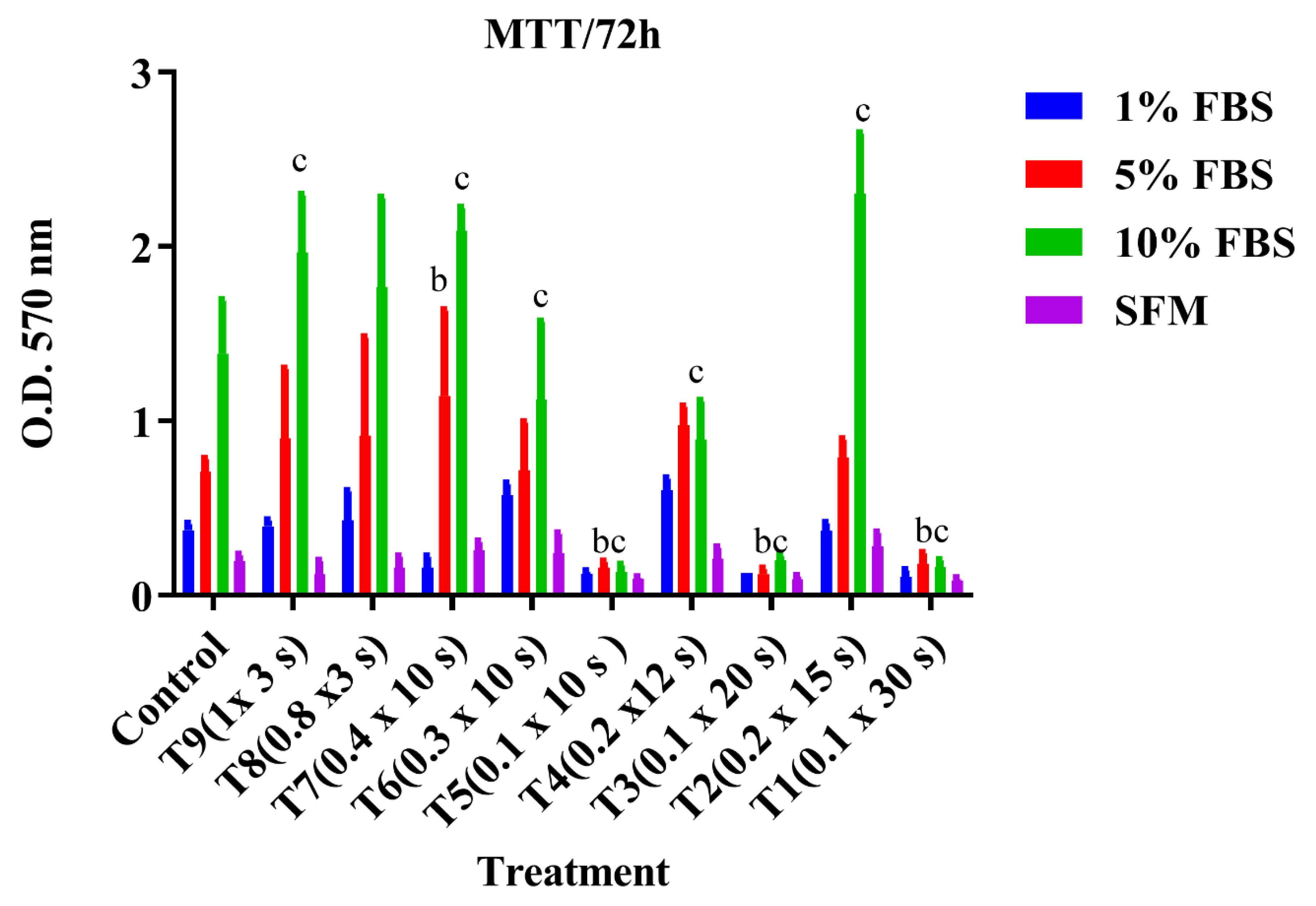
3.2. Cell Proliferation Assay (MTT)
3.3. Cell Death Analysis
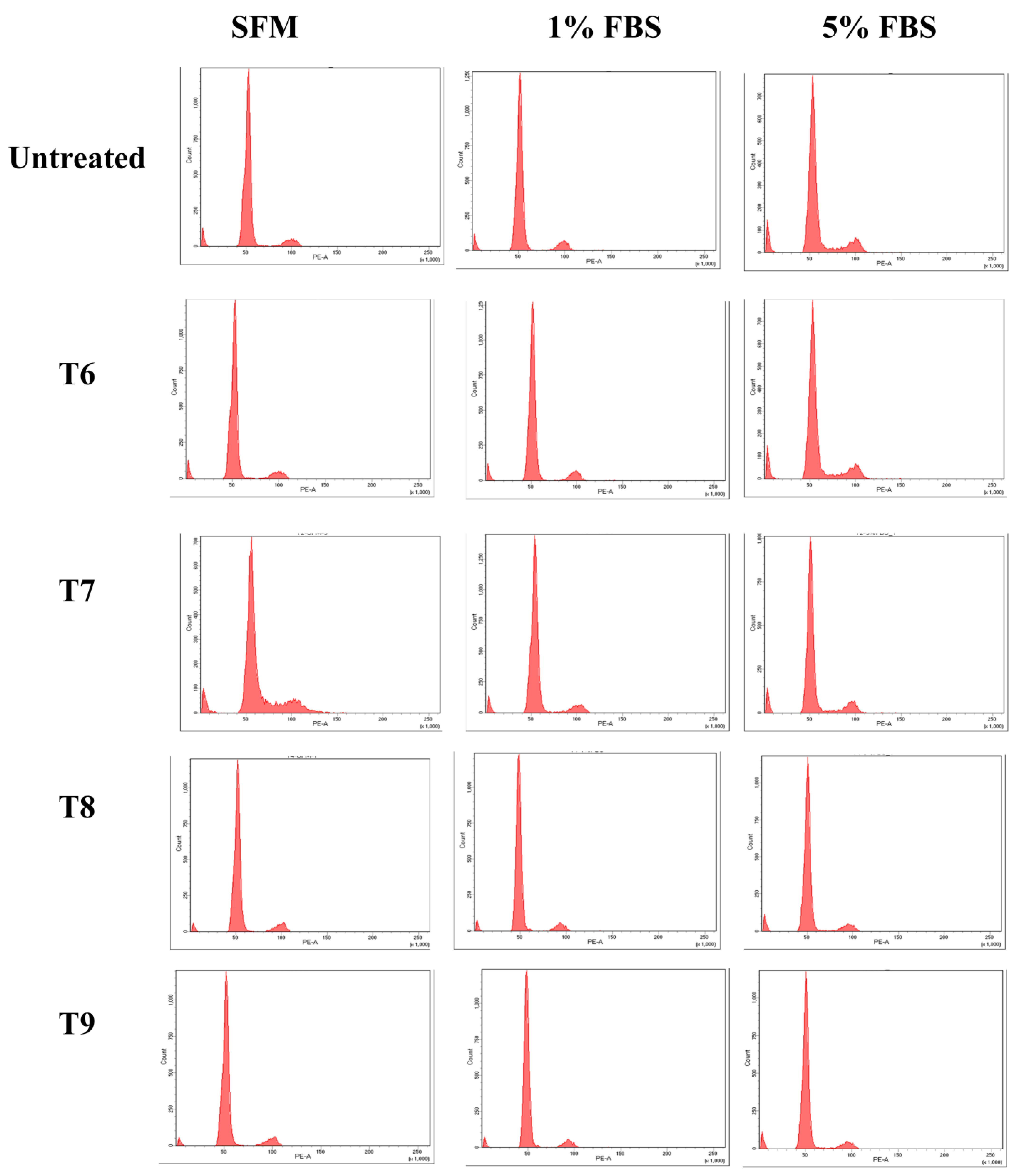
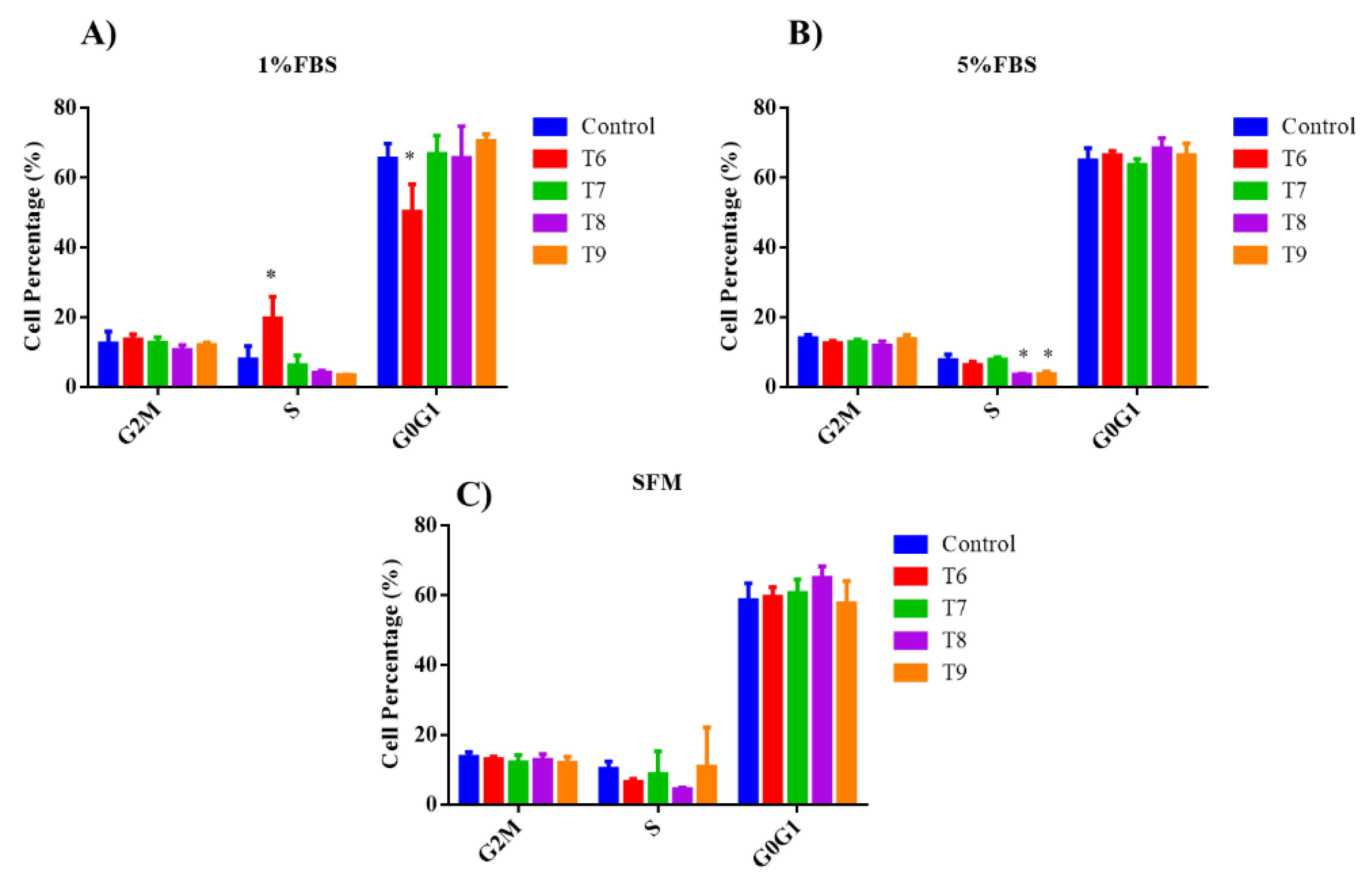
3.4. Cell Cycle
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tuby, H.; Maltz, L.; Oron, U. Low-level laser irradiation (LLLI) promotes proliferation of mesenchymal and cardiac stem cells in culture. Lasers Surg. Med. 2007, 39, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Arany, P.R.; Cho, A.; Hunt, T.D.; Sidhu, G.; Shin, K.; Hahm, E.; Huang, G.X.; Weaver, J.; Chen, A.C.-H.; Padwa, B.L.; et al. Photoactivation of endogenous latent transforming growth factor–β1 directs dental stem cell differentiation for regeneration. Sci. Transl. Med. 2014, 6, 238. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.-J. Dental pulp and dentin tissue engineering and regeneration–advancement and challenge. Front. Biosci. 2011, 3, 788. [Google Scholar] [CrossRef] [PubMed]
- Eduardo, F.D.P.; Bueno, D.F.; de Freitas, P.M.; Marques, M.M.; Passos-Bueno, M.R.; Eduardo, C.D.; Zatz, M. Stem cell proliferation under low-intensity laser irradiation: A preliminary study. Lasers Surg. Med. 2008, 40, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, S.; Huang, C.-C.; George, A. Extracellular matrix of dental pulp stem cells: Applications in pulp tissue engineering using somatic MSCs. Front. Physiol. 2016, 4, 395. [Google Scholar] [CrossRef]
- Marrelli, M.; Falisi, G.; Apicella, A.; Apicella, D.; Amantea, M.; Cielo, A.; Bonanome, L.; Palmieri, F.; Santacroce, L.; Giannini, S.; et al. The behavior of dental pulp stem cells on different types of innovative mesoporous and nanoporous silicon scaffolds with different functionalizations of the surfaces. J. Biol. Regul. Homeost. Agents 2015, 29, 991–997. [Google Scholar]
- Dannan, A. Dental-derived stem cells and whole tooth regeneration: An overview. J. Clin. Med. Res. 2009, 1, 63–71. [Google Scholar] [CrossRef]
- Kitamura, C.; Nishihara, T.; Terashita, M.; Tabata, Y.; Jimi, E.; Washio, A.; Hirata, S. Regeneration approaches for dental pulp and periapical tissues with growth factors, biomaterials, and laser irradiation. Polymers 2011, 3, 1776–1793. [Google Scholar] [CrossRef]
- Goldberg, M.; Smith, A.J. Cells and extracellular matrices of dentin and pulp: A biological basis for repair and tissue engineering. Crit. Rev. Oral Biol. Med. 2004, 15, 13–27. [Google Scholar] [CrossRef]
- Shen, C.C.; Yang, Y.C.; Chiao, M.T.; Chan, S.C.; Liu, B.S. Low-level laser stimulation on adipose-tissue-derived stem cell treatments for focal cerebral ischemia in rats. Evid. Based Complement. Altern. Med. 2013, 2013, 594906. [Google Scholar] [CrossRef]
- Khan, I.; Tang, E.; Arany, P. Molecular pathway of near-infrared laser phototoxicity involves ATF-4 orchestrated ER stress. Sci. Rep. 2015, 5, 10581. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Chen, A.C.; Carroll, J.D.; Hamblin, M.R. Biphasic dose response in low-level light therapy. Dose-Response 2009, 7, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Sharma, S.K.; Carroll, J.; Hamblin, M.R. Biphasic dose response in low-level light therapy–an update. Dose-Response 2011, 9, 602–618. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Arany, P. Photobiomodulation therapy promotes expansion of epithelial colony forming units. Photomed. Laser Surg. 2016, 34, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Tanikawa, D.Y.S.; Pinheiro, C.C.G.; Almeida, M.C.A.; Oliveira, C.R.; Coudry, R.D.; Rocha, D.L.; Bueno, D.F. Deciduous dental pulp stem cells for maxillary alveolar reconstruction in cleft lip and palate patients. Stem Cells Int. 2020, 2020, 6234167. [Google Scholar] [CrossRef] [PubMed]
- Jafar, H.; Abuarqoub, D.; Ababneh, N.; Hasan, M.; Al-Sotari, S.; Aslam, N.; Kailani, M.; Ammoush, M.; Shraideh, Z.; Awidi, A. HPL promotes osteogenic differentiation of stem cells in 3D scaffolds. PLoS ONE 2019, 14, e0215667. [Google Scholar] [CrossRef]
- Dominici, M.; Blanc, K.L.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Borzabadi-Farahani, A. Effect of low-level laser irradiation on proliferation of human dental mesenchymal stem cells; a systemic review. J. Photochem. Photobiol. B Biol. 2016, 162, 577–582. [Google Scholar] [CrossRef]
- Gholami, L.; Afshar, S.; Mahmoudi, R.; Arkian, A.A.; Parsamanesh, G.; Rad, M.R.; Baghaei, K. Evaluation of the effect of near-infra-red photobiomodulation on buccal fat pad-derived stem cells. Int. J. Dent. Oral Sci. 2020, 7, 960–967. [Google Scholar]
- Santos, L.T.O.; Santos, L.O.; Guedes, C.D. LASERTERAPIA NA ODONTOLOGIA: Efeitos e aplicabilidades. Sci. Gen. 2021, 2, 29–46. [Google Scholar]
- Keshri, G.K.; Kumar, G.; Sharmak, M.; Bora, K.; Kumar, B.; Gupta, A. Photobiomodulation effects of pulsed-NIR laser (810 nm) and LED (808±3 nm) with identical treatment regimen on burn wound healing: A quantitative label-free global proteomic approach. J. Photochem. Photobiol. 2021, 6, 100024. [Google Scholar] [CrossRef]
- de Freitas, L.F.; Hamblin, M.R. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 348–364. [Google Scholar] [CrossRef] [PubMed]
- Zein, R.; Selting, W.; Hamblin, M.R. Review of light parameters and photobiomodulation efficacy: Dive into complexity. J. Biomed. Opt. 2018, 23, 120901. [Google Scholar] [CrossRef] [PubMed]
- Kocherova, I.; Bryja, A.; Błochowiak, K.; Kaczmarek, M.; Stefańska, K.; Matys, J.; Grzech-Leśniak, K.; Dominiak, M.; Mozdziak, P.; Kempisty, B.; et al. Photobiomodulation with red and near-infrared light improves viability and modulates the expression of mesenchymal and apoptotic-related markers in human gingival fibroblasts. Materials 2021, 14, 3427. [Google Scholar] [CrossRef] [PubMed]
- Babuccu, C.; Keklikoğlu, N.; Baydoğan, M.; Kaynar, A. Cumulative effect of low-level laser therapy and low-intensity pulsed ultrasound on bone repair in rats. Int. J. Oral Maxillofac. Surg. 2014, 43, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Schimberg, A.S.; Heldens, G.T.N.; Klabbers, T.M.; Grunsven, A.C.v.E.-V.; Verdaasdonk, R.M.; Takes, R.P.; Wellenstein, D.J.; Broek, G.B.v.D. Thermal effects of CO2, KTP, and blue lasers with a flexible fiber delivery system on vocal folds. J. Voice 2022, in press. [CrossRef] [PubMed]
- El Gammal, Z.H.; Zaher, A.M.; El-Badri, N. Effect of low-level laser-treated mesenchymal stem cells on myocardial infarction. Lasers Med. Sci. 2017, 32, 1637–1646. [Google Scholar] [CrossRef]
- Scribante, A.; Gallo, S.; Pascadopoli, M.; Frani, M.; Butera, A. Ozonized gels vs chlorhexidine in non-surgical periodontal treatment: A randomized clinical trial. Oral Dis. 2023, in press. [CrossRef]
- Amini, F.; Rezvani, M.B.; Bakhtiari, R.; Ghomsheh, E.T. Effects of dental pulp stem cell preconditioning on osteogenesis using conditioned media of probiotics bacteria. Avicenna J. Med. Biotechnol. 2023, 15, 76–83. [Google Scholar] [CrossRef]



| Treatment Group | Power (W) | Time (s) | Energy (J) | Spot Size (cm2) | Irradiance (W/cm2) | Surface Area (cm2) (6 or 96-Well Plate) | Fluence (J/cm2) |
|---|---|---|---|---|---|---|---|
| T1 | 0.1 | 30 | 3 | 0.8 | 0.125 | 1.086 | 2.76 |
| T2 | 0.2 | 15 | 3 | 0.8 | 0.25 | 1.086 | 2.76 |
| T3 | 0.1 | 20 | 2 | 0.8 | 0.125 | 1.086 | 1.84 |
| T4 | 0.2 | 12 | 2.4 | 0.8 | 0.25 | 1.086 | 2.21 |
| T5 | 0.1 | 10 | 1 | 0.8 | 0.125 | 1.086 | 0.92 |
| T6 | 0.3 | 10 | 3 | 0.8 | 0.375 | 9.6 | 0.3125 |
| T7 | 0.4 | 10 | 4 | 0.8 | 0.5 | 9.6 | 0.416 |
| T8 | 0.8 | 3 | 2.4 | 0.8 | 1 | 9.6 | 0.25 |
| T9 | 1 | 3 | 3 | 0.8 | 1.25 | 9.6 | 0.3125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Asmar, A.A.; Abuarqoub, D.; Ababneh, N.; Jafar, H.; Zalloum, S.; Ismail, M.; Arany, P.; Awidi, A. Potential Effects of Photobiomodulation Therapy on Human Dental Pulp Stem Cells. Appl. Sci. 2024, 14, 124. https://doi.org/10.3390/app14010124
Al-Asmar AA, Abuarqoub D, Ababneh N, Jafar H, Zalloum S, Ismail M, Arany P, Awidi A. Potential Effects of Photobiomodulation Therapy on Human Dental Pulp Stem Cells. Applied Sciences. 2024; 14(1):124. https://doi.org/10.3390/app14010124
Chicago/Turabian StyleAl-Asmar, Ayah A., Duaa Abuarqoub, Nidaa Ababneh, Hanan Jafar, Suzan Zalloum, Mohammad Ismail, Praveen Arany, and Abdalla Awidi. 2024. "Potential Effects of Photobiomodulation Therapy on Human Dental Pulp Stem Cells" Applied Sciences 14, no. 1: 124. https://doi.org/10.3390/app14010124






