Novel Materials as Exogenous Carbon Sources for Denitrifying Biofilters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
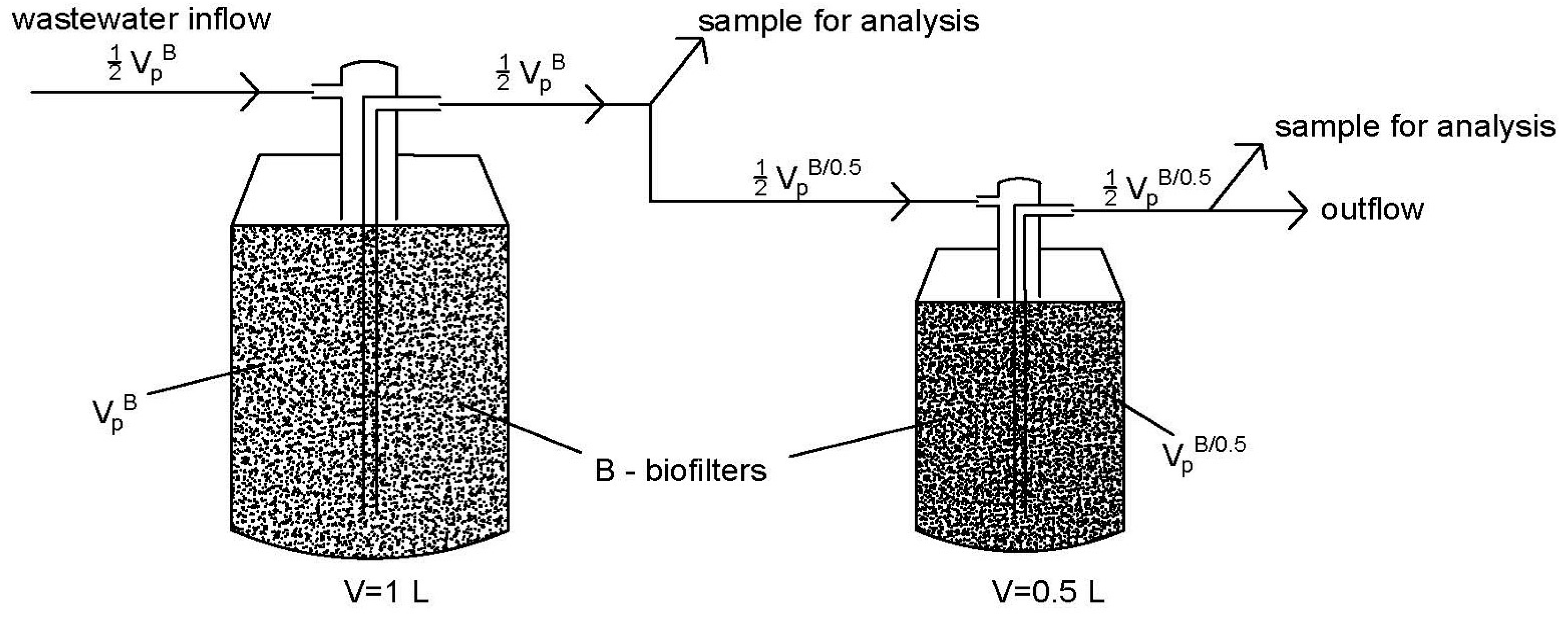
2.2. Experimental Station
2.3. Analytical Methods
2.4. Calculation Methods
- —total nitrogen in the influent [mg N/L],
- —total nitrogen in the treated effluent [mg N/L],
- —total phosphorus in the influent [mg P/L],
- —total phosphorus in the treated effluent [mg P/L].
2.5. Statistical Analysis
3. Results and Discussion
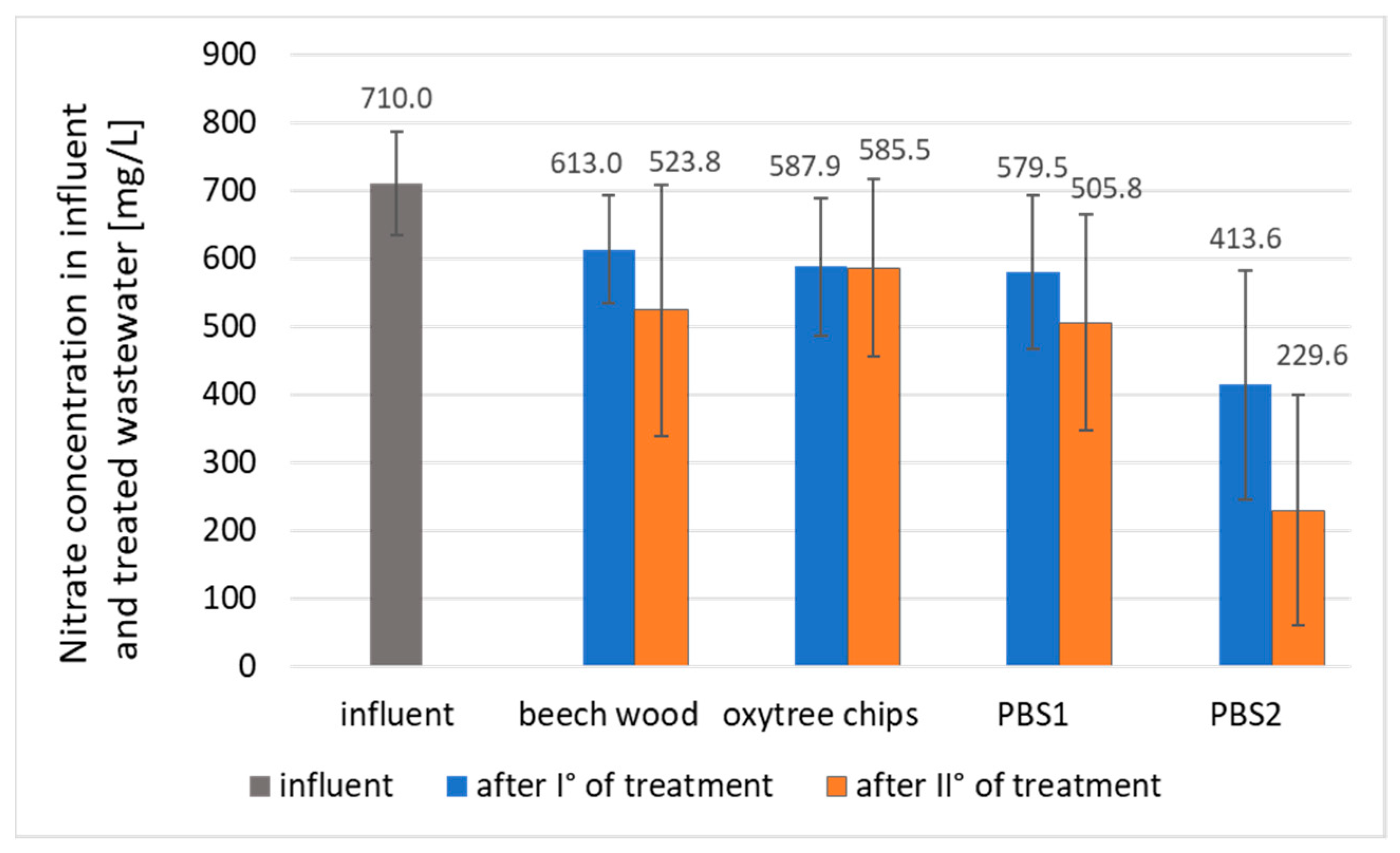
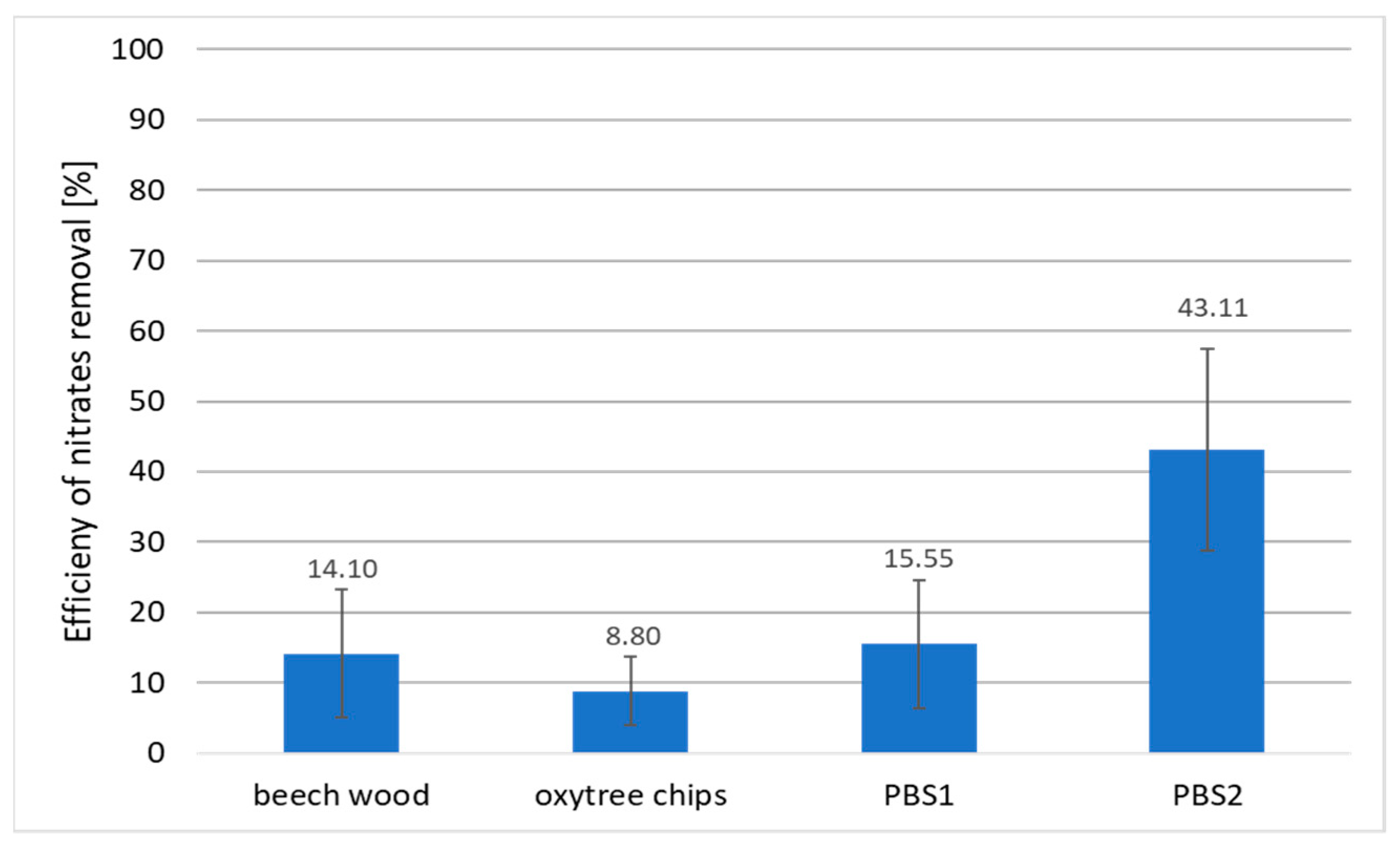
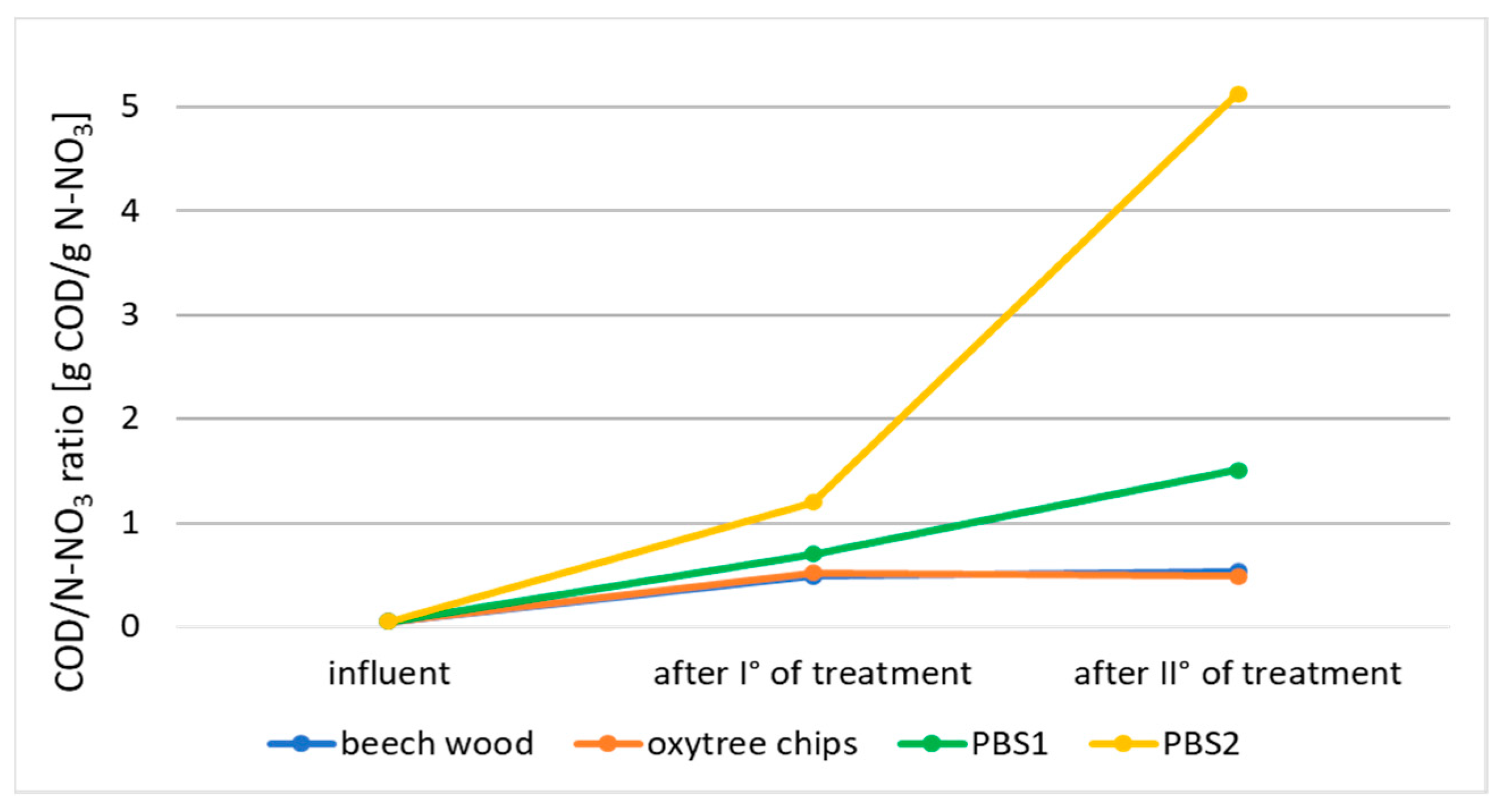
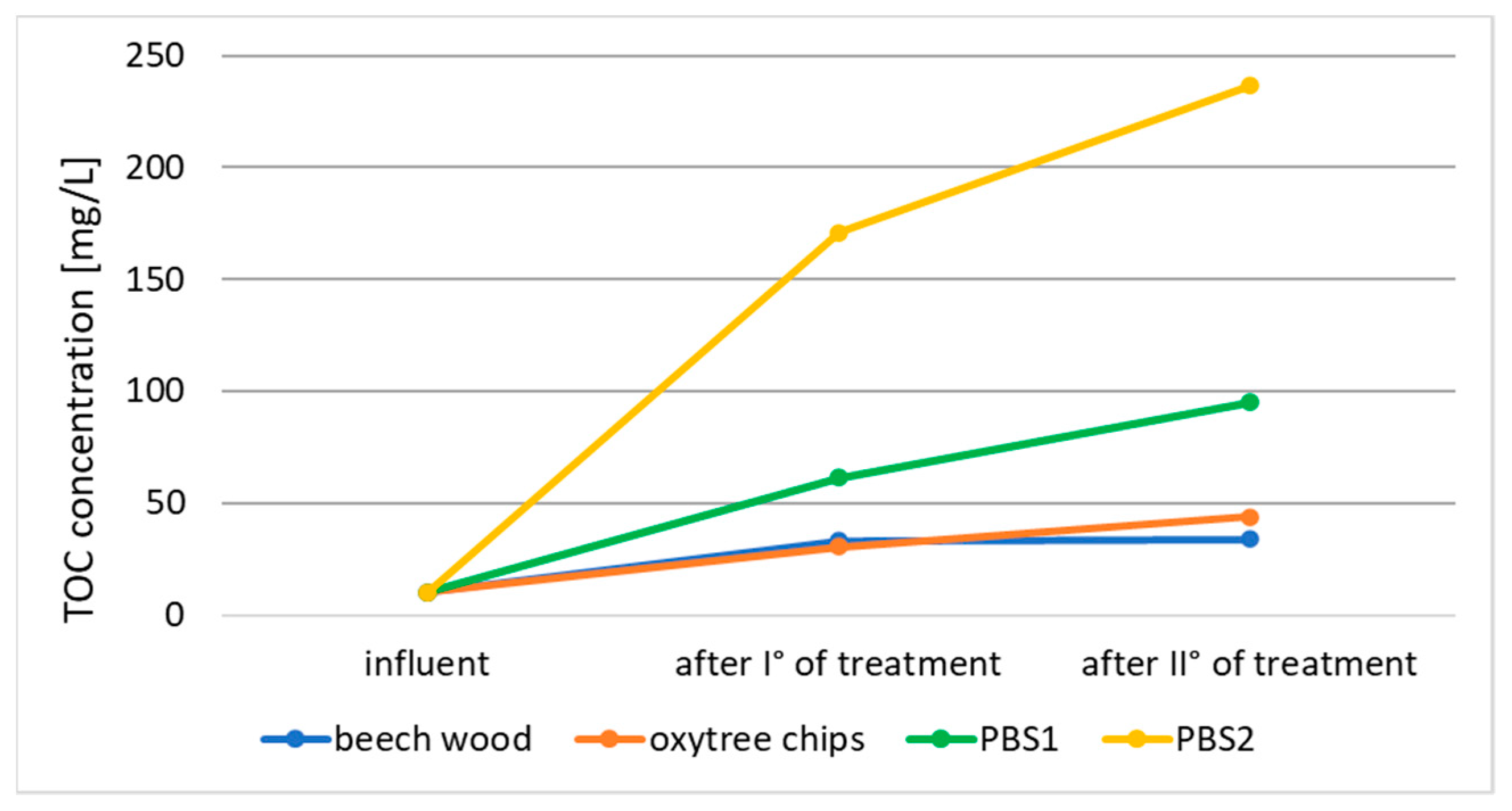
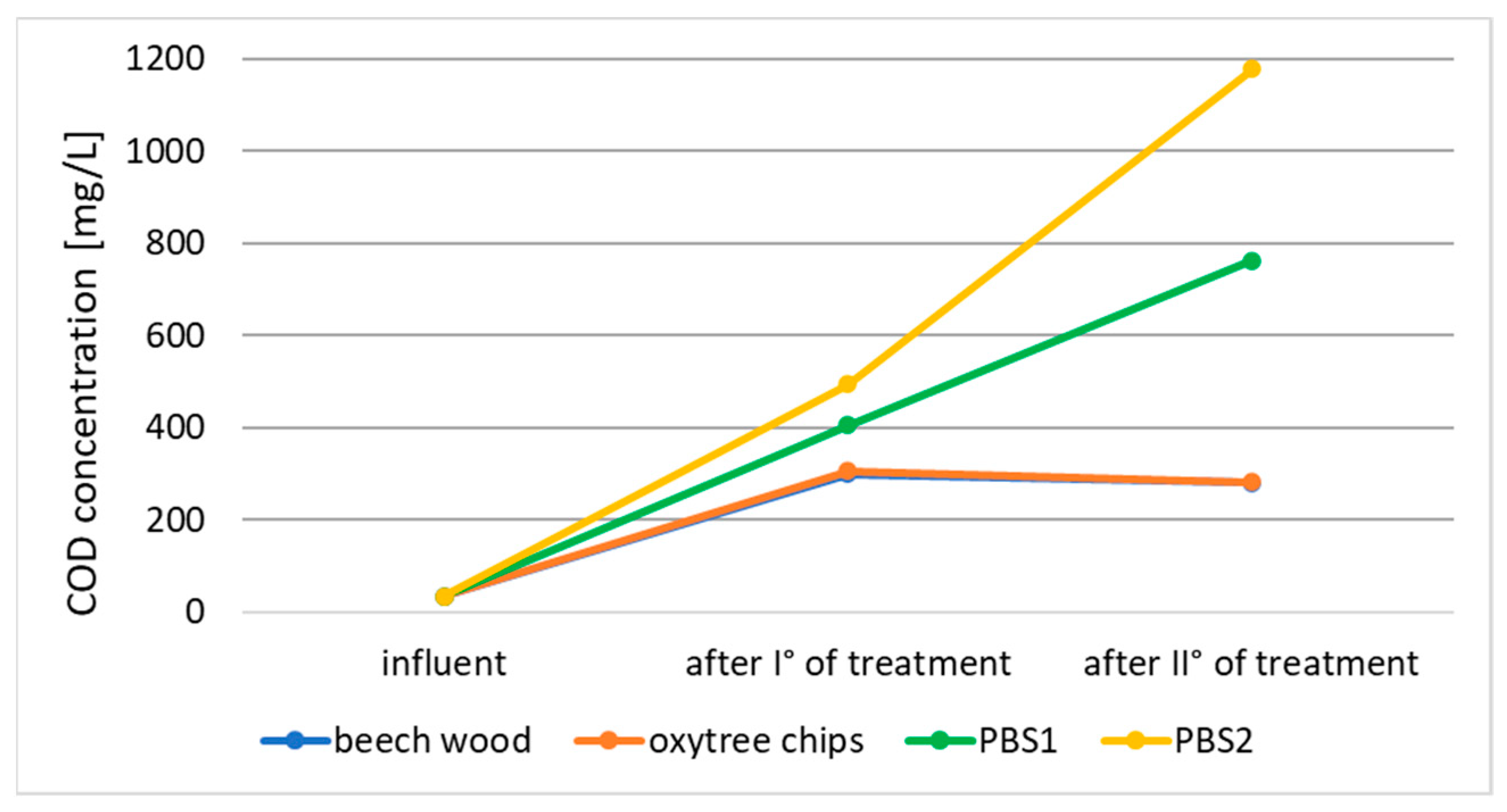
3.1. Nitrogen Removal
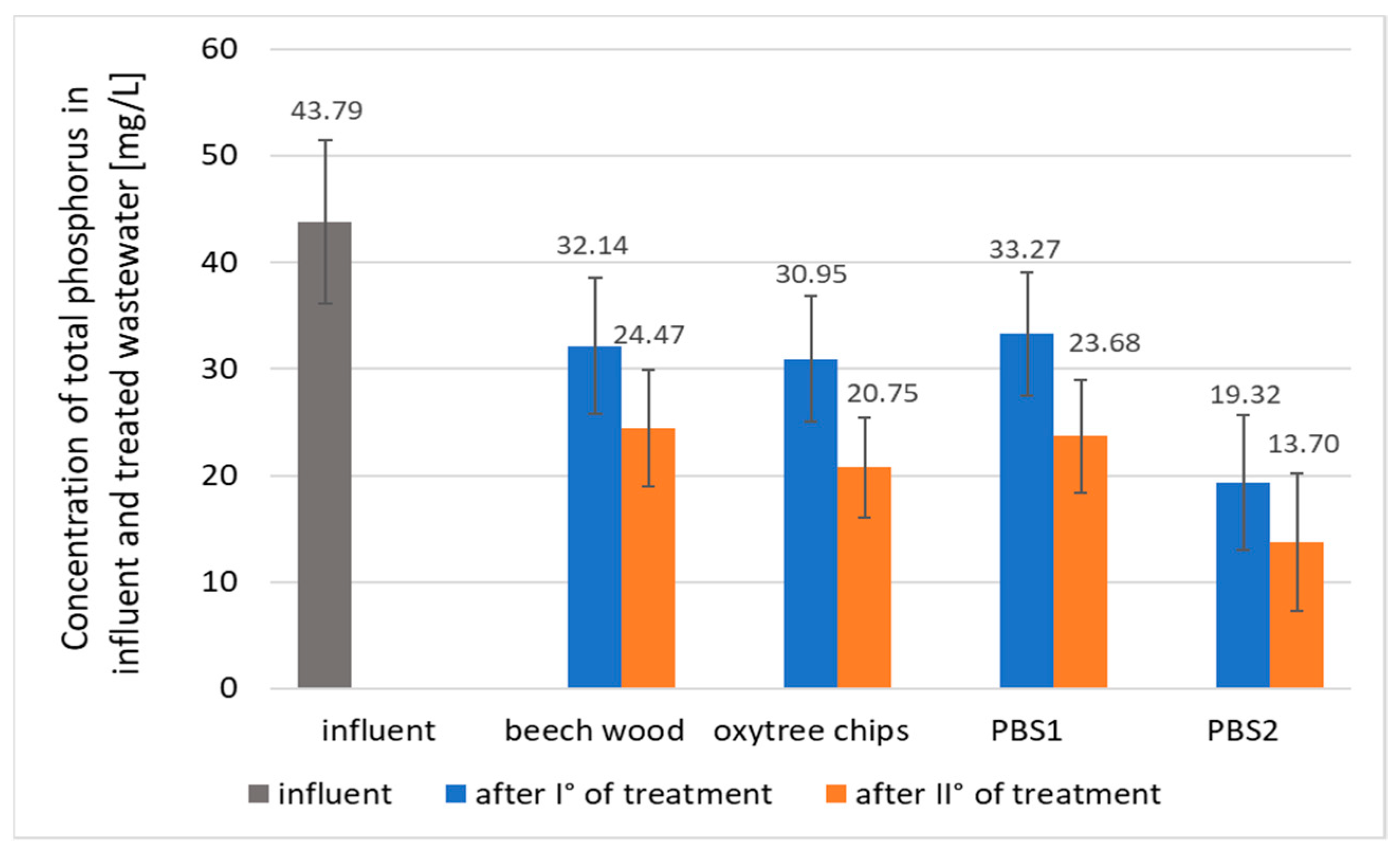
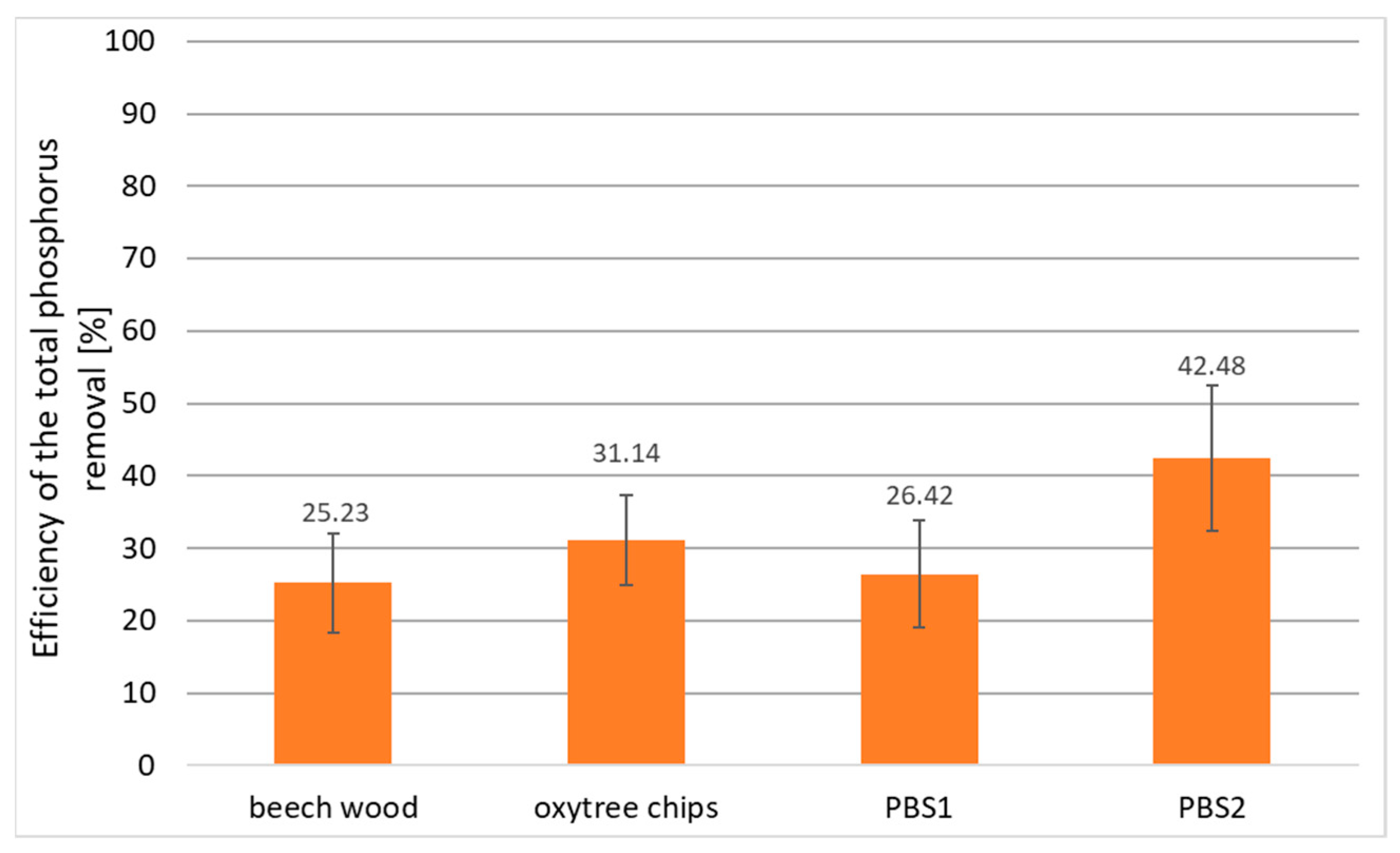
3.2. Phosphorus Removal
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bojarska, J.E.; Piłat, B.; Majewska, K.M.; Sobiechowska, D.A.; Narwojsz, A. Selected physical parameters and chemical compounds of different types of tomatoes. Czech J. Food Sci. 2020, 38, 28–35. [Google Scholar] [CrossRef]
- Ibrahim, A.; Elfaki, J. Impact of Irrigation with Saline Water on the Production of Tomato (Solanum lycopersicum) under Soilless and Traditional Techniques. Asian Soil. Res. J. 2020, 2, 1–14. [Google Scholar] [CrossRef]
- Komosa, A.; Markiewicz, B.; Kleiber, T.; Mieloszyk, E.; Mieloch, M. Yield and nutrient status of greenhouse tomato (Lycopersicon esculentum Mill.) grown in new and re-used rockwool, polyurethane, NFT and aeroponics. J. Elem. 2020, 25, 523–536. [Google Scholar] [CrossRef]
- Mielcarek, A.; Rodziewicz, J.; Janczukowicz, W.; Dobrowolski, A. Analysis of Wastewater Generated in Greenhouse Soilless Tomato Cultivation in Central Europe. Water 2019, 11, 2538. [Google Scholar] [CrossRef]
- Putra, P.A.; Yuliando, H. Soilless Culture System to Support Water Use Efficiency and Product Quality: A Review. Agric. Agric. Sci. Procedia 2015, 3, 283–288. [Google Scholar] [CrossRef]
- Askari-Khorasgani, O.; Pessarakli, M. Tomato (Solanum lycopersicum) culture in vermi-aquaponic systems: I. Cultural practices. J. Plant Nutr. 2020, 43, 1712–1725. [Google Scholar] [CrossRef]
- European Parliament. Regulation (EU) 2020/741 of the European Parliament and of the Council of 25 May 2020 on Minimum Requirements for Water Reuse; European Parliament: Brussels, Belgium, 2020. [Google Scholar]
- Metcalf & Eddy Inc. Wastewater Engineering: Treatment and Resource Recovery, 5th ed.; McGraw-Hill Professional: New York, NY, USA, 2014. [Google Scholar]
- Miksch, K.; Sikora, J. Wastewater Biotechnology; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2010. (In Polish) [Google Scholar]
- Ostrowska, K.; Janczukowicz, W.; Rodziewicz, J.; Mielcarek, A. The influence of the filtration process on the content of organic compounds in relations to the concentration of nutrients in dairy wastewater. Annu. Set Environ. Prot. 2013, 15, 1411–1425. [Google Scholar]
- Prystay, W.; Lo, K.V. Treatment of greenhouse wastewater using constructed wetlands. J. Environ. Sci. Health B 2001, 36, 341–353. [Google Scholar] [CrossRef]
- Liu, R.; Xia, L.; Liu, M.; Gao, Z.; Feng, J.; You, H.; Qu, W.; Xing, T.; Wang, J.; Zhao, Y. Influence of the carbon source concentration on the nitrate removal rate in groundwater. Environ. Technol. 2022, 43, 3355–3365. [Google Scholar] [CrossRef]
- Cao, S.; Wang, L.; Yan, W.; Zhou, Y. Primary sludge as solid carbon source for biological denitrification: System optimization at micro-level. Environ. Res. 2020, 191, 110160. [Google Scholar] [CrossRef]
- Schipper, L.A.; Robertson, W.D.; Gold, A.J.; Jaynes, D.B.; Cameron, S.C. Denitrifying bioreactors—An approach for reducing nitrate loads to receiving waters. Ecol. Eng. 2010, 36, 1532–1543. [Google Scholar] [CrossRef]
- Fu, X.; Hou, R.; Yang, P.; Qian, S.; Feng, Z.; Chen, Z.; Wang, F.; Yuan, R.; Chen, H.; Zhou, B. Application of external carbon source in heterotrophic denitrification of domestic sewage: A review. Sci. Total Environ. 2022, 817, 153061. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, N.; Feng, C.; Deng, Y. Insights into heterotrophic denitrification diversity in wastewater treatment systems: Progress and future prospects based on different carbon sources. Sci. Total Environ. 2021, 780, 146521. [Google Scholar] [CrossRef] [PubMed]
- Collivignarelli, M.C.; Abbà, A.; Caccamo, F.M.; Carnevale Miino, M.; Durante, A.; Bellazzi, S.; Baldi, M.; Bertanza, G. How to Produce an Alternative Carbon Source for Denitrification by Treating and Drastically Reducing Biological Sewage Sludge. Membranes 2021, 11, 977. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chu, L. Biological nitrate removal from water and wastewater by solid-phase denitrification process. Biotechnol. Adv. 2016, 34, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Putri, F.E.; Hung, T.C. Comparison of nutrient removal and biomass production between macrophytes and microalgae for treating artificial citrus nursery wastewater. J. Environ. Manag. 2020, 264, 110303. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, V.; Maltais-Landry, G.; Puigagut, J.; Chazarenc, F.; Brisson, J. Treatment of hydroponics wastewater using constructed wetlands in winter conditions. Water Air Soil Pollut. 2010, 212, 483–490. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, S.H.; Delaune, R.D.; Cho, J.S.; Heo, J.S.; Ok, Y.S.; Seo, D.C. Enhancement of nitrate removal in constructed wetlands utilizing a combined autotrophic and heterotrophic denitrification technology for treating hydroponic wastewater containing high nitrate and low organic carbon concentrations. Agric. Water Manag. 2015, 162, 1–14. [Google Scholar] [CrossRef]
- Kwon, M.J.; Hwang, Y.; Lee, J.; Ham, B.; Rahman, A.; Azam, H.; Yang, J.S. Waste nutrient solutions from full-scale open hydroponic cultivation: Dynamics of effluent quality and removal of nitrogen and phosphorus using a pilot-scale sequencing batch reactor. J. Environ. Manag. 2021, 281, 111893. [Google Scholar] [CrossRef]
- Zhong, H.; Cheng, Y.; Ahmad, Z.; Shao, Y.; Zhang, H.; Lu, Q.; Shim, H. Solid-phase denitrification for water remediation: Processes, limitations, and new aspects. Crit. Rev. Biotechnol. 2020, 40, 1113–1130. [Google Scholar] [CrossRef]
- Xue, Z.; Wang, C.; Cao, J.; Luo, J.; Feng, Q.; Fang, F.; Li, C.; Zhang, Q. An alternative carbon source withdrawn from anaerobic fermentation of soybean wastewater to improve the deep denitrification of tail water. Biochem. Eng. J. 2018, 132, 217–224. [Google Scholar] [CrossRef]
- von Ahnen, M.; Pedersen, P.B.; Dalsgaard, J. Performance of full-scale woodchip bioreactors treating effluents from commercial RAS. Aquac. Eng. 2018, 83, 130–137. [Google Scholar] [CrossRef]
- Oboodi, A.; Fathi, E.; Nezammahalleh, H. Development of a Denitrifying Biofilter with Short Start-up Period. J. Biol. Today’s World 2017, 6, 75–82. [Google Scholar] [CrossRef]
- Aalto, S.L.; Suurnäkki, S.; von Ahnen, M.; Siljanen, H.M.P.; Pedersen, P.B.; Tiirola, M. Nitrate removal microbiology in woodchip bioreactors: A case-study with full-scale bioreactors treating aquaculture effluents. Sci. Total Environ. 2020, 723, 138093. [Google Scholar] [CrossRef] [PubMed]
- Peterson, I.J.; Igielski, S.; Davis, A.P. Enhanced denitrification in bioretention using woodchips as an organic carbon source. J. Sustain. Water Built Environ. 2015, 1, 04015004. [Google Scholar] [CrossRef]
- Bortniak, M.; Sekutowski, T.R.; Zajączkowska, O.; Kucharski, M. Influence of the soil from Oxytree [Paulownia elongata S. Y. Hu × Paulownia fortunei (Seem.) Hemsl.] plantation on germination and initial growth of winter wheat and winter rape. Prog. Plant Prot. 2018, 58, 247–250. [Google Scholar] [CrossRef]
- ASTM D6866-10; Standard Test Methods for Determining the Biobased Content of Solid, Liquid, and Gaseous Samples Using Radiocarbon Analysis. ASTM: West Conshohocken, PA, USA.
- Regulation of the Minister of Maritime Economy and Inland Navigation of 12 July 2019 on Substances Particularly Harmful to the Aquatic Environment and Conditions to Be Met When Introducing Sewage into Waters or into the Ground, as Well as When Dischargin; Polish Parliament: Warsaw, Poland, 2019. (In Polish)
- Wu, W.; Yang, F.; Yang, L. Biological denitrification with a novel biodegradable polymer as carbon source and biofilm carrier. Bioresour. Technol. 2012, 118, 136–140. [Google Scholar] [CrossRef]
- Zhu, S.M.; Deng, Y.L.; Ruan, Y.J.; Guo, X.S.; Shi, M.M.; Shen, J.Z. Biological denitrification using poly(butylene succinate) as carbon source and biofilm carrier for recirculating aquaculture system effluent treatment. Bioresour. Technol. 2015, 192, 603–610. [Google Scholar] [CrossRef]
- Chu, L.; Wang, J. Denitrification performance and biofilm characteristics using biodegradable polymers PCL as carriers and carbon source. Chemosphere 2013, 91, 1310–1316. [Google Scholar] [CrossRef]
- Elefsiniotis, P.; Li, D. The effect of temperature and carbon source on denitrification using volatile fatty acids. Biochem. Eng. J. 2006, 28, 148–155. [Google Scholar] [CrossRef]
- Sadecka, Z.; Mazurkiewicz, M. Conditions of the Denitrification Process on the Example of the Treatment Plant in Kostrzyn-on-Odra; Uniwersytet Zielonogórski, Zeszyty Naukowe: Zielona Góra, Poland, 2011; Volume 142, pp. 35–43. (In Polish) [Google Scholar]
- Beaubien, A.; Hu, Y.; Bellahcen, D.; Urabin, V.; Chang, J. Monitoring metabolic activity of denitrification processes using gas production measurements. Water Res. 1995, 29, 2269–2274. [Google Scholar] [CrossRef]
- Janczukowicz, W.; Rodziewicz, J. Carbon Sources in Biological Removal Processes of Nitrogen and Phosphorus Compounds; Monografie Komitetu Inżynierii Środowiska Polskiej Akademii Nauk: Lublin, Poland, 2013; Volume 114. (In Polish) [Google Scholar]
- Li, C.; Wang, H.; Yan, G.; Dong, W.; Chu, Z.; Wang, H.; Chang, Y.; Ling, Y.; Zhang, Y. Initial carbon release characteristics, mechanisms and denitrification performance of a novel slow release carbon source. J. Environ. Sci. 2022, 118, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, L.; Yin, Z.; Cui, Z.; Qu, K.; Wang, D.; Wang, Z.; Zhu, S.; Cui, H. Comparative investigation on heterotrophic denitrification driven by different biodegradable polymers for nitrate removal in mariculture wastewater: Organic carbon release, denitrification performance, and microbial community. Front. Microbiol. 2023, 14, 1141362. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Li, L.; Liu, Q.; Xu, G.; Tan, H. Effect of dissolved oxygen on heterotrophic denitrification using poly(butylene succinate) as the carbon source and biofilm carrier. Bioresour. Technol. 2014, 171, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Wing, M.T.; Malone, R.F.; Rusch, K.A. Evaluation of polyhydroxybutyrate as a carbon source for recirculating aquaculture water denitrification. Aquac. Eng. 2012, 51, 36–43. [Google Scholar] [CrossRef]
- Ashok, V.; Hait, S. Remediation of nitrate-contaminated water by solid-phase denitrification process–A review. Environ. Sci. Pollut. Res. 2015, 22, 8075–8093. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.-C.; Bao, X.; Che, L.; Wu, Q.-L. Enhance biological nitrogen and phosphorus removal in wastewater treatment process by adding food waste fermentation liquid as external carbon source. Biochem. Eng. J. 2021, 165, 107811. [Google Scholar] [CrossRef]
- Wu, C.Y.; Peng, Y.Z.; Li, X.L.; Wang, S.Y. Effect of carbon source on biological nitrogen and phosphorus removal in an anaerobic-anoxic-oxic (A2O) process. J. Environ. Eng. 2010, 136, 1248–1254. [Google Scholar] [CrossRef]
- Sharrer, K.L.; Christianson, L.E.; Lepine, C.; Summerfelt, S.T. Modeling and mitigation of denitrification ‘woodchip’ bioreactor phosphorus releases during treatment of aquaculture wastewater. Ecol. Eng. 2016, 93, 135–143. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, X.; Feng, L. Effect of different types of electron acceptors on the anoxic phosphorus uptake activity of denitrifying phosphorus removing bacteria. Bioresour. Technol. 2010, 101, 1603–1610. [Google Scholar] [CrossRef]
- Hu, J.Y.; Ong, S.L.; Ng, W.J.; Lu, F.; Fan, X.J. A new method for characterizing denitrifying phosphorus removal bacteria by using three different types of electron acceptors. Water Res. 2003, 37, 3463–3471. [Google Scholar] [CrossRef] [PubMed]

| Indicators | Arithmetic Mean | Minimum Value | Maximum Value | Standard Deviation |
|---|---|---|---|---|
| pH | 6.37 | 6.20 | 6.63 | 0.13 |
| DO (dissolved oxygen) [mg O2/L] | 8.94 | 8.59 | 9.15 | 0.18 |
| Temperature [°C] | 22.20 | 21.00 | 23.50 | 0.77 |
| EC (electrical conductivity) [mS/cm] | 6.707 | 6.401 | 7.391 | 0.279 |
| TOC (total organic carbon) [mg/L] | 10.02 | 0.75 | 16.62 | 4.45 |
| COD [mg O2/L] | 34.96 | 25.60 | 49.20 | 7.91 |
| Nitrates [mg N/L] | 710.0 | 618.0 | 920.0 | 76.3 |
| Nitrites [mg N/L] | 0.042 | 0.020 | 0.076 | 0.019 |
| Ammonium nitrogen [mg N/L] | 0.115 | 0.028 | 0.446 | 0.122 |
| Total nitrogen [mg N/L] | 721.1 | 643.0 | 763.8 | 36.2 |
| Total phosphorus [mg P/L] | 43.79 | 34.40 | 57.80 | 7.62 |
| Temperature [°C] | pH | |||||||
|---|---|---|---|---|---|---|---|---|
| Arithmetic Mean | Minimum Value | Maximum Value | Standard Deviation | Arithmetic Mean | Minimum Value | Maximum Value | Standard Deviation | |
| Influent | 22.20 | 21.00 | 23.50 | 0.77 | 6.37 | 6.20 | 6.63 | 0.13 |
| Beech wood | 22.51 | 20.80 | 24.00 | 0.95 | 7.28 | 6.56 | 7.98 | 0.37 |
| Oxytree chips | 22.46 | 20.80 | 23.80 | 0.88 | 7.22 | 6.56 | 8.17 | 0.45 |
| PBS 1 | 22.49 | 20.90 | 23.90 | 0.90 | 7.06 | 6.03 | 7.98 | 0.65 |
| PBS 2 | 22.65 | 20.90 | 24.10 | 0.93 | 7.49 | 7.06 | 7.91 | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kłobukowska, K.; Rodziewicz, J.; Mielcarek, A.; Bryszewski, K.Ł.; Janczukowicz, W.; Bugajski, P.; Jóźwiakowski, K.; Operacz, A. Novel Materials as Exogenous Carbon Sources for Denitrifying Biofilters. Appl. Sci. 2024, 14, 176. https://doi.org/10.3390/app14010176
Kłobukowska K, Rodziewicz J, Mielcarek A, Bryszewski KŁ, Janczukowicz W, Bugajski P, Jóźwiakowski K, Operacz A. Novel Materials as Exogenous Carbon Sources for Denitrifying Biofilters. Applied Sciences. 2024; 14(1):176. https://doi.org/10.3390/app14010176
Chicago/Turabian StyleKłobukowska, Karolina, Joanna Rodziewicz, Artur Mielcarek, Kamil Łukasz Bryszewski, Wojciech Janczukowicz, Piotr Bugajski, Krzysztof Jóźwiakowski, and Agnieszka Operacz. 2024. "Novel Materials as Exogenous Carbon Sources for Denitrifying Biofilters" Applied Sciences 14, no. 1: 176. https://doi.org/10.3390/app14010176
APA StyleKłobukowska, K., Rodziewicz, J., Mielcarek, A., Bryszewski, K. Ł., Janczukowicz, W., Bugajski, P., Jóźwiakowski, K., & Operacz, A. (2024). Novel Materials as Exogenous Carbon Sources for Denitrifying Biofilters. Applied Sciences, 14(1), 176. https://doi.org/10.3390/app14010176












