Assessing Soil Nutrient Content and Mapping in Tropical Tamil Nadu, India, through Precursors IperSpettrale Della Mission Applicative Hyperspectral Spectroscopy
Abstract
:1. Introduction
- This study aims to critically analyse the application of PRISMA hyperspectral spectroscopy in the evaluation of soil nutrient levels within the tropical region of Tamil Nadu, India.
- To generate precise and high-quality soil nutrient maps utilizing PRISMA hyperspectral data, with the aim of offering significant insights for agricultural practices, land administration, and environmental research within the specified area.
- To explore the capabilities of hyperspectral remote sensing technology in enhancing the evaluation of soil nutrient levels. This advancement has the potential to contribute to sustainable land use planning and effective crop management, particularly in tropical regions.
- In this study, we want to establish a systematic approach for utilizing hyperspectral data in order to accurately estimate essential soil nutrient parameters, specifically nitrogen, phosphorus, and potassium. Our primary emphasis will be on achieving high levels of precision and dependability in the estimation process.
- This study aims to enhance the comprehension of remote sensing techniques’ application in treating soil nutrient deficiencies and variability in tropical soils. The findings of this research can have substantial consequences for improving agricultural production and promoting sustainable land use practices in Tamil Nadu, India.
2. Materials and Methods
2.1. Spectral Measurement and Pre-Treatment of Soil Samples
2.2. Selection of Spectral Variables
2.3. The Optimal Spectral Reflectance Variables for Soil Nutrient Concentrations
2.4. Calculation and Evaluation of Soil Nutrient Concentrations at Soil Reflectance Locations with Precision
3. Results and Discussion
3.1. AOC Concentration in Radhapuram, Tirunelveli District
3.2. Estimation of Soil Nutrient Content for Satellite PRISMA Hyperspectral Region
3.3. Methodology
3.3.1. Preprocessing Levels of PRISMA Hyperspectral Data
- Level 0: the raw binary files, comprising instrument data and cloud percentage, are included in the L0 data product.
- Level 1: the L1 data product contains radiometrically calibrated data for hyperspectral and panchromatic radiance cubes on the top of the atmospheric radiance image.
- Level 2: the L2 data product is further split into three different groups:
- -
- L2B: atmospheric calibration and geolocation of the L1 data product, i.e., atmospheric radiance at the bottom.
- -
- L2C: atmospheric calibration and geolocation of the L1 data product, i.e., bottom of atmospheric radiance including aerosol thickness and map of water vapour.
- -
- L2D: orthorectification of L2C with significantly less cloud coverage.
- Level 1 and Level 2 data are generated in the Hierarchical Data format release 5 (HDF5 or he5). In this work, we have used Level2D data to identify paddy production in a particular area.
3.3.2. File Format Conversion
3.3.3. PRISMA Dataset Descriptions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Soil Properties | Method Employed |
|---|---|
| Soil pH | Potentiometric method |
| Electric conductivity (mmhos/cm) | Conductivity bridge |
| Organic carbon (%) | Chromic acid wet digestion |
| Available phosphorus (kg ha) | Bray’s No.1, extractant (0.03 N ammonium fluoride + 0.025 N HCl) |
| Available potassium (kg ha) | NN NH4OAc extraction |
| Available calcium (mg kg) | NN NH4OAc versenate titration |
| Available magnesium (mg kg) | NN NH4OAc versenate titration |
| Available sulphur (mg kg) | DTPA extraction—AAS |
References
- Nocita, M.; Stevens, A.; Van Wesemael, B.; Aitkenhead, M.; Bachmann, M.; Barthès, B.; Dor, E.B.; Brown, D.J.; Clairotte, M.; Wetterlind, J.; et al. Soil spectroscopy: An alternative to wet chemistry for soil monitoring. Adv. Agron. 2015, 132, 139–159. [Google Scholar]
- Stenberg, B.; Rossel, R.A.V.; Mouazen, A.M.; Wetterlind, J. Visible and near-infrared spectroscopy in soil science. Adv. Agron. 2010, 107, 163–215. [Google Scholar]
- Ben-Dor, E.; Chabrillat, S.; Demattê, J.A.M.; Taylor, G.R.; Hill, J.; Whiting, M.L.; Sommer, S. Using imaging spectroscopy to study soil properties. Remote Sens. Environ. 2009, 113, S38–S55. [Google Scholar] [CrossRef]
- Hively, W.D.; McCarty, G.W.; Reeves, J.B.; Lang, M.W.; Oesterling, R.A.; Delwiche, S.R. Use of airborne hyperspectral imagery to map soil properties in tilled agricultural fields. Appl. Environ. Soil Sci. 2011, 2011, 358193. [Google Scholar] [CrossRef]
- Parikh, S.J.; Goyne, K.W.; Margenot, A.J.; Mukome, F.N.; Calderón, F.J. Soil chemical insights provided through vibrational spectroscopy. Adv. Agron. 2014, 126, 1–148. [Google Scholar]
- An, R.; Samiaappan, S.; Veni, S.; Worch, E.; Zhou, M. Airborne hyperspectral imagery for band selection using moth–flame metaheuristic optimization. J. Imaging 2022, 8, 126. [Google Scholar]
- Gao, H.Z.; Lu, Q.P. Near infrared spectral analysis and measuring system for primary nutrient of soil. Spectrosc. Spectr. Anal. 2011, 31, 1245–1249. [Google Scholar]
- Leone, A.P.; Viscarra-Rossel, R.A.; Amenta, P.; Buondonno, A. Prediction of soil properties with PLSR and vis-NIR spectroscopy: Application to mediterranean soils from Southern Italy. Curr. Anal. Chem. 2012, 8, 283–299. [Google Scholar] [CrossRef]
- Ramoelo, A.; Skidmore, A.K.; Cho, M.A.; Mathieu, R.; Heitkönig, I.M.A.; Dudeni-Tlhone, N.; Schlerf, M.; Prins, H.H.T. Nonlinear partial least square regression increases the estimation accuracy of grass nitrogen and phosphorus using in situ hyperspectral and environmental data. ISPRS J. Photogramm. Remote Sens. 2013, 82, 27–40. [Google Scholar] [CrossRef]
- Balabin, R.M.; Lomakina, E.I. Support vector machine regression (SVR/LS-SVM)—An alternative to neural networks (ANN) for analytical chemistry? Comparison of nonlinear methods on near infrared (NIR) spectroscopy data. Analyst 2011, 136, 1703–1712. [Google Scholar] [CrossRef]
- Balabin, R.M.; Smirnov, S.V. Interpolation and extrapolation problems of multivariate regression in analytical chemistry: Benchmarking the robusANess on near-infrared (NIR) spectroscopy data. Analyst 2012, 137, 1604–1610. [Google Scholar] [CrossRef] [PubMed]
- Li, D. Estimating the blood glucose of serum optical spectrum using support vector regression. In Proceedings of the Fourth International Conference on Photonics and Optical Engineering, Xi’an, China, 15–16 October 2020; International Society for Optics and Photonics: Bellingham, WA, USA, 2021; Volume 11761, p. 117611U. [Google Scholar]
- Walkley, A. An Examination of Methods for Determining Organic Carbon and Nitrogen in Soils1. (with One Text-figure). J. Agric. Sci. 1935, 25, 598–609. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, L.; Hu, Y.; Wang, G.; Wang, L.; Liu, Z. Prediction of soil nutrient contents using visible and near-infrared reflectance spectroscopy. ISPRS Int. J. Geo-Inf. 2019, 8, 437. [Google Scholar] [CrossRef]
- Yao, L.; Xu, M.; Liu, Y.; Niu, R.; Wu, X.; Song, Y. Estimating of heavy metal concentration in agricultural soils from hyperspectral satellite sensor imagery: Considering the sources and migration pathways of pollutants. Ecol. Indic. 2024, 158, 111416. [Google Scholar] [CrossRef]
- Morrissey, M.B.; Ruxton, G.D. Multiple regression is not multiple regressions: The meaning of multiple regression and the non-problem of collinearity. Philos. Theory Pract. Biol. 2018, 10, 3. [Google Scholar] [CrossRef]
- Ren, H.-Y.; Zhuang, D.F.; Singh, A.N.; Pan, J.-J.; Qiu, D.-S.; Shi, R.-H. Estimation of As and Cu contamination in agricultural soils around a mining area by reflectance spectroscopy: A case study. Pedosphere 2009, 19, 719–726. [Google Scholar]
- Wang, F.; Wang, F.; Zhang, Y.; Hu, J.; Huang, J.; Xie, J. Rice yield estimation using parcel-level relative spectral variables from UAV-based hyperspectral imagery. Front. Plant Sci. 2019, 10, 453. [Google Scholar] [CrossRef]
- Yaseen, Z.M.; Tran, M.T.; Kim, S.; Bakhshpoori, T.; Deo, R.C. Shear strength prediction of steel fiber reinforced concrete beam using hybrid intelligence models: A new approach. Eng. Struct. 2018, 177, 244–255. [Google Scholar] [CrossRef]
- Singh, S.; Sarma, K. Exploring Soil Spatial Variability with GIS, Remote Sensing, and Geostatistical Approach. J. Soil Plant Environ. 2023, 2, 79–99. [Google Scholar] [CrossRef]
- Loizzo, R.; Guarini, R.; Longo, F.; Scopa, T.; Formaro, R.; Facchinetti, C.; Varacalli, G. PRISMA: The Italian hyperspectral mission. In Proceedings of the IGARSS 2018–2018 IEEE International Geoscience and Remote Sensing Symposium, Valencia, Spain, 22–27 July 2018; pp. 175–178. [Google Scholar]
- Folkman, M.A.; Pearlman, J.; Liao, L.B.; Jarecke, P.J. EO-1/Hyperion hyperspectral imager design, development, characterization, and calibration. Hyperspectral Remote Sens. Land Atmos. 2001, 4151, 40–51. [Google Scholar]
- Hecker, C.; Van der Meijde, M.; van der Werff, H.; van der Meer, F.D. Assessing the influence of reference spectra on synthetic SAM classification results. IEEE Trans. Geosci. Remote Sens. 2018, 46, 4162–4172. [Google Scholar] [CrossRef]
- Guarini, R.; Loizzo, R.; Facchinetti, C.; Longo, F.; Ponticelli, B.; Faraci, M.; Di Nicolantonio, W. PRISMA hyperspectral mission products. In Proceedings of the IGARSS 2018–2018 IEEE International Geoscience and Remote Sensing Symposium, Valencia, Spain, 22–27 July 2018; pp. 179–182. [Google Scholar]
- Christian, B.; Krishnayya, N.S.R. Classification of tropical trees growing in a sanctuary using Hyperion (EO-1) and SAM algorithm. Curr. Sci. 2009, 96, 1601–1607. [Google Scholar]
- Zhang, X.; Li, P. Lithological mapping from hyperspectral data by improved use of spectral angle mapper. Int. J. Appl. Earth Obs. Geoinf. 2014, 31, 95–109. [Google Scholar] [CrossRef]
- Harris, A.T. Spectral mapping tools from the earth sciences applied to spectral microscopy data. Cytom. Part A J. Int. Soc. Anal. Cytol. 2006, 69, 872–879. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Veni, S.; Aravinth, J. Robust classification technique for hyperspectral images based on 3D-discrete wavelet transform. Remote Sens. 2021, 13, 1255. [Google Scholar]
- An, R.; Veni, S.; Geetha, P.; Subramoniam, S.R. Extended morphological profiles analysis of airborne hyperspectral image classification using machine learning algorithms. Int. J. Intell. Networks 2021, 2, 1–6. [Google Scholar]
- Aravinth, J.; Nath, B.; Subramanian, M.S.; Bulusu, R.V.; Monish, P. Machine learning based Detection of Zinc Mineralization North India using Hyperspectral Image Processing. In Proceedings of the 2019 International Conference on Communication and Electronics Systems (ICCES), Coimbatore, India, 17–19 July 2019; pp. 1777–1781. [Google Scholar]
- Zhong, L.; Yang, S.; Chu, X.; Sun, Z.; Li, J. Inversion of heavy metal copper content in soil-wheat systems using hyperspectral techniques and enrichment characteristics. Sci. Total. Environ. 2024, 907, 168104. [Google Scholar] [CrossRef]
- Mohan, D.; Aravinth, J.; Rajendran, S. Reconstruction of Compressed Hyperspectral Image Using SqueezeNet Coupled Dense Attentional Net. Remote Sens. 2023, 15, 2734. [Google Scholar] [CrossRef]
- Shanthi, T.; An, R.; Annapoorani, S.; Birundha, N. Analysis of Phonocardiogram Signal Using Deep Learning. In International Conference on Innovative Computing and Communications; Springer: Singapore, 2023; pp. 621–629. [Google Scholar]
- Pan, B.; Cai, S.; Zhao, M.; Cheng, H.; Yu, H.; Du, S.; Xie, F. Predicting the Surface Soil Texture of Cultivated Land via Hyperspectral Remote Sensing and Machine Learning: A Case Study in Jianghuai Hilly Area. Appl. Sci. 2023, 13, 9321. [Google Scholar] [CrossRef]
- Lawrence, R.; Labus, M. Early detection of Douglas-fir beetle infestation with subcanopy resolution hyperspectral imagery. West. J. Appl. For. 2003, 18, 202–206. [Google Scholar] [CrossRef]
- Golia, E.E.; Diakoloukas, V. Soil parameters affecting the levels of potentially harmful metals in Thessaly area, Greece: A robust quadratic regression approach of soil pollution prediction. Environ. Sci. Pollut. Res. 2022, 29, 29544–29561. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, S.R.; Anupallavi, S.; Premkumar, M.; Jeevanantham, V. Implementation of deep neural networks for classifying electroencephalogram signal using fractional S-transform for epileptic seizure detection. Int. J. Imaging Syst. Technol. 2021, 31, 895–908. [Google Scholar] [CrossRef]
- Bowker, D.E. Spectral Reflectances of Natural Targets for Use in Remote Sensing Studies; NASA: Washington, DC, USA, 1985; Volume 1139.
- Benesty, J.; Chen, J.; Huang, Y.; Cohen, I. Pearson correlation coefficient. In Noise Reduction in Speech Processing; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–4. [Google Scholar]
- Tsai, F.; Philpot, W. Derivative analysis of hyperspectral data. Remote Sens. Environ. 1998, 66, 41–51. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, H.; Shi, T.; Chen, Y.; Jiang, Q.; Linderman, M. Prediction of soil organic carbon sAOCk by laboratory spectral data and airborne hyperspectral images. Geoderma 2019, 337, 32–41. [Google Scholar] [CrossRef]
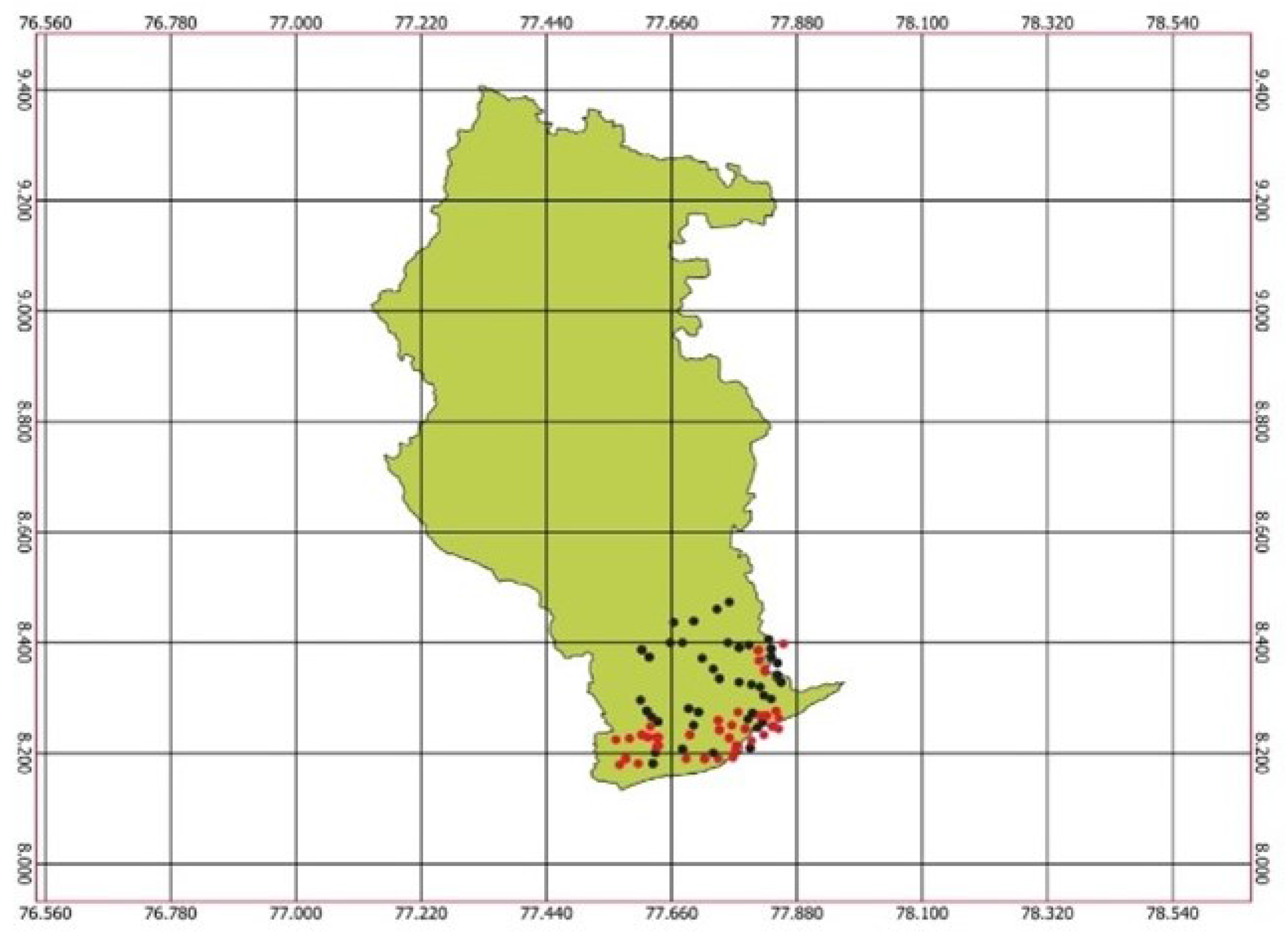
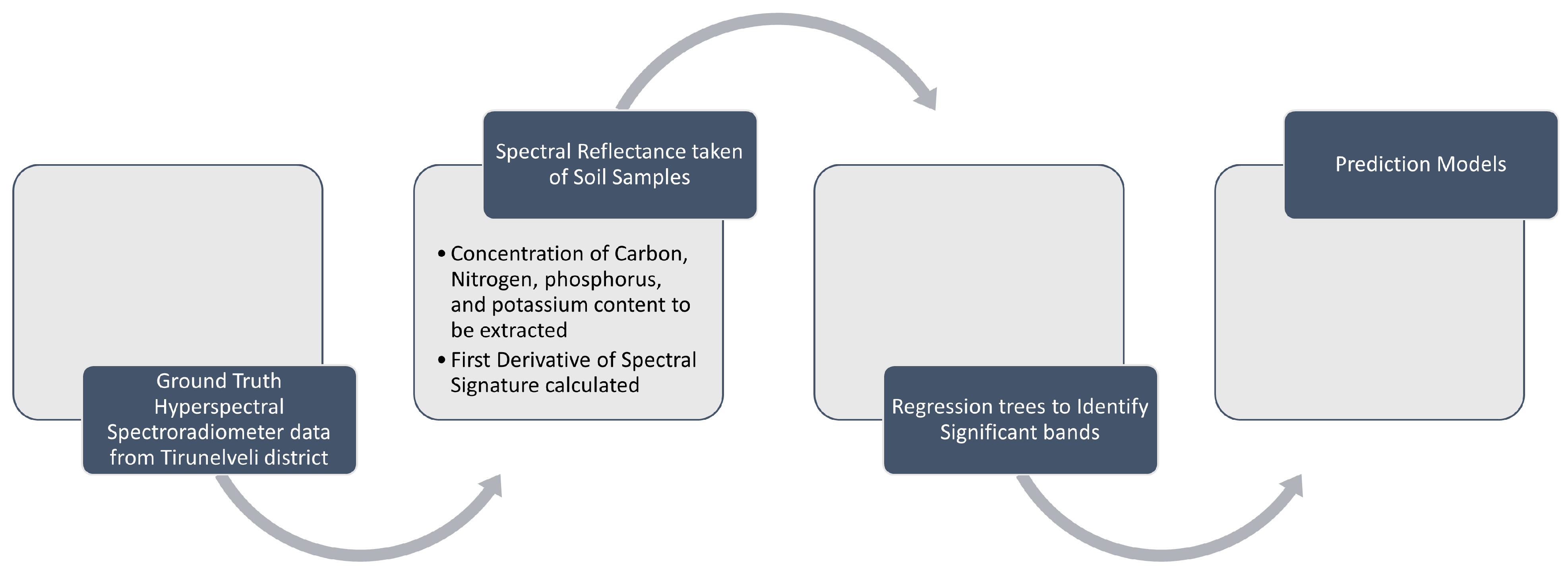

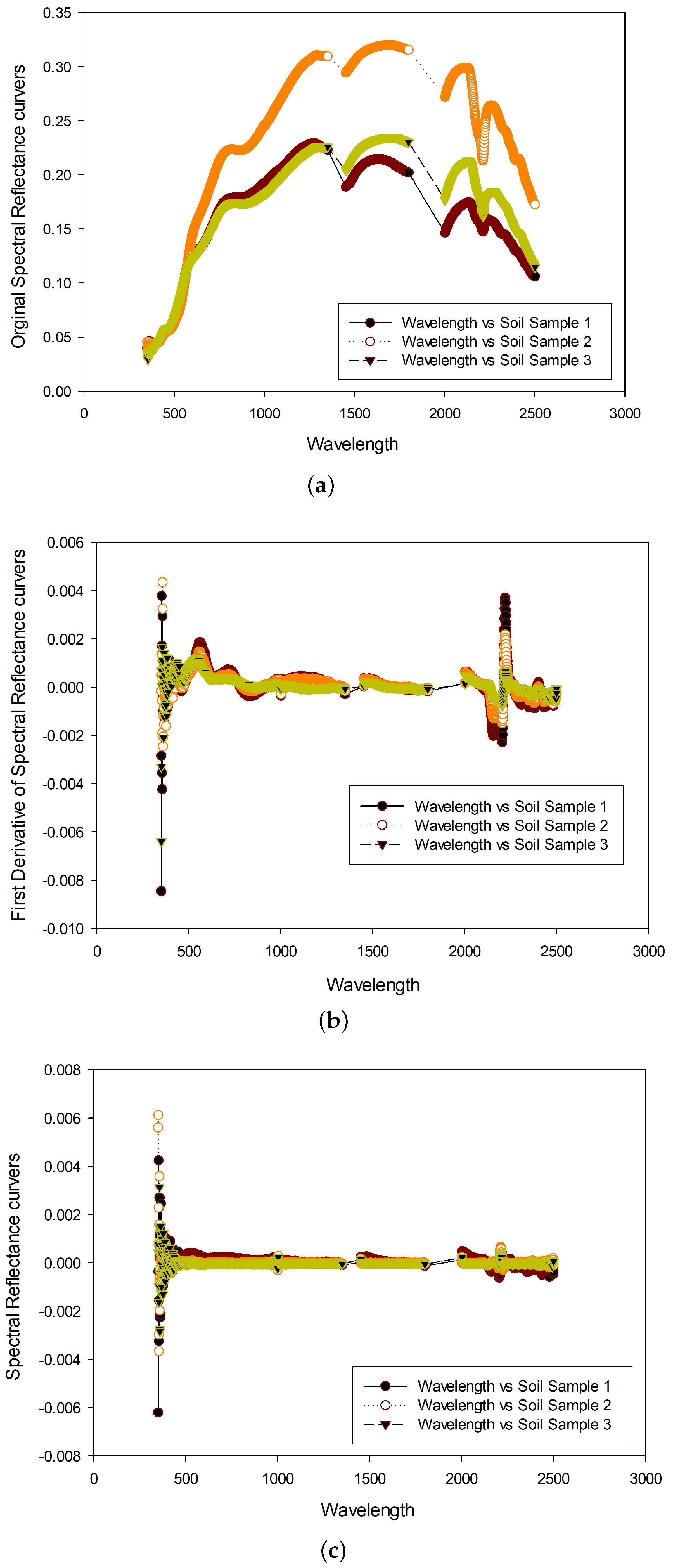
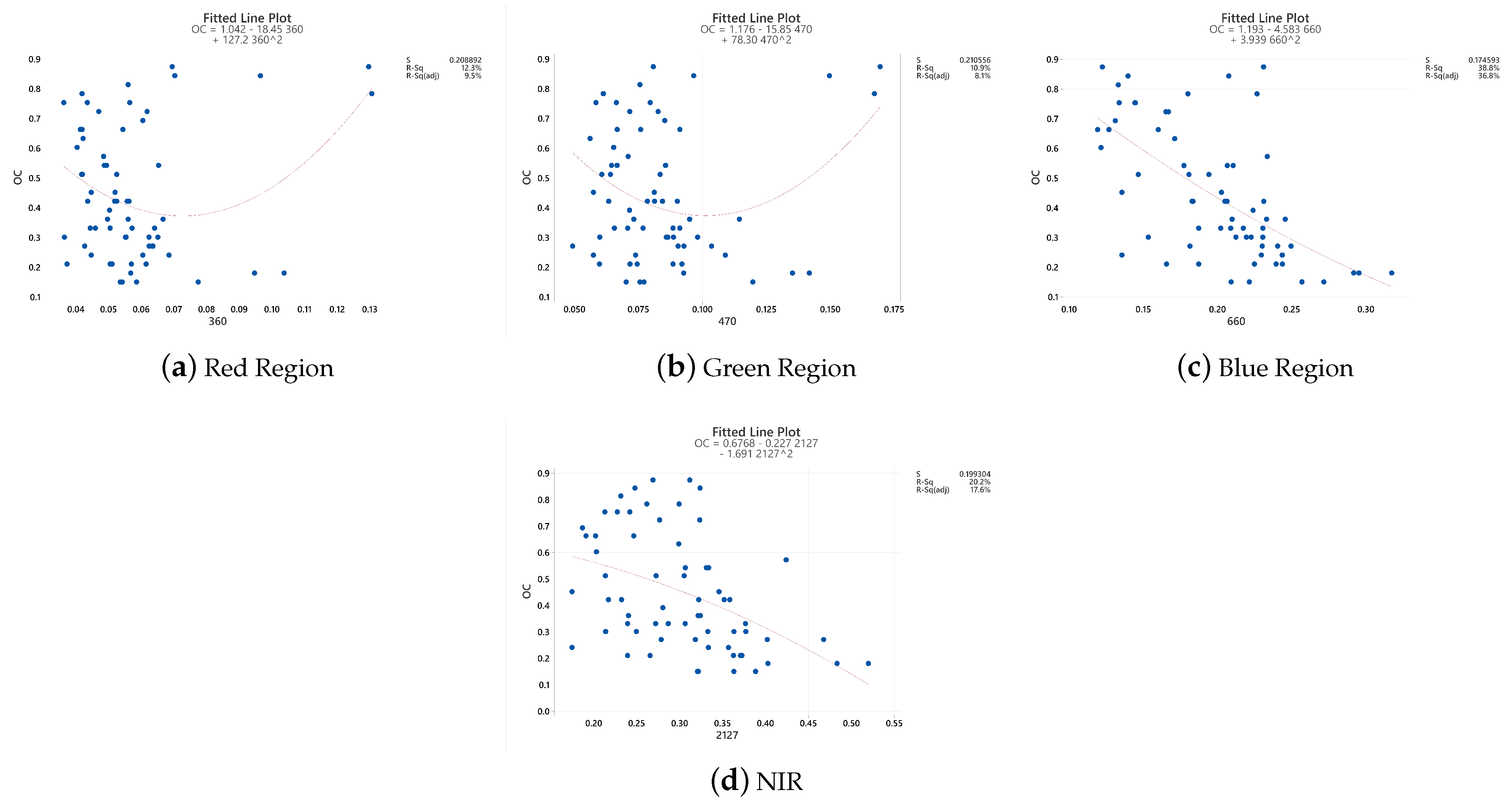

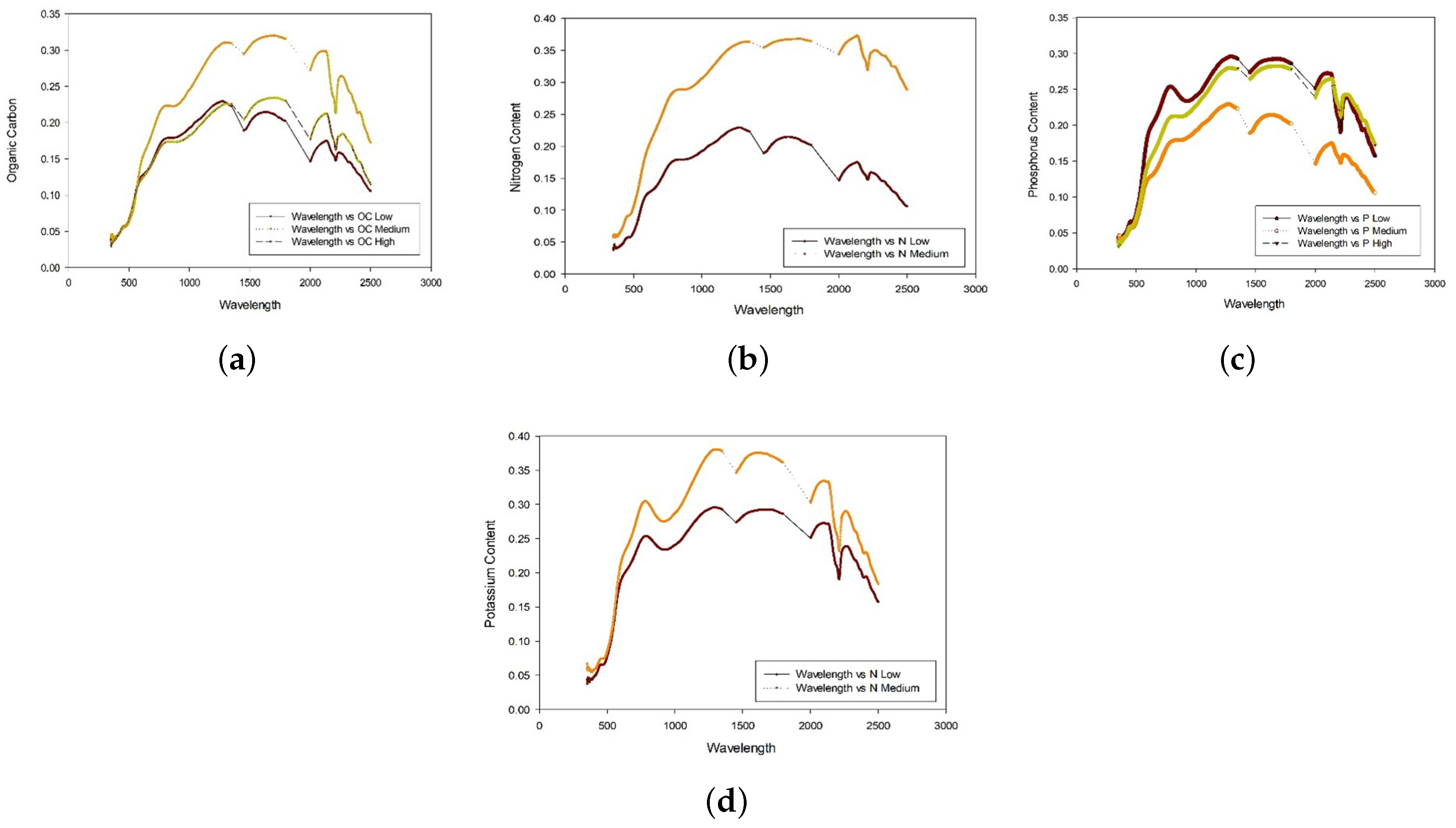

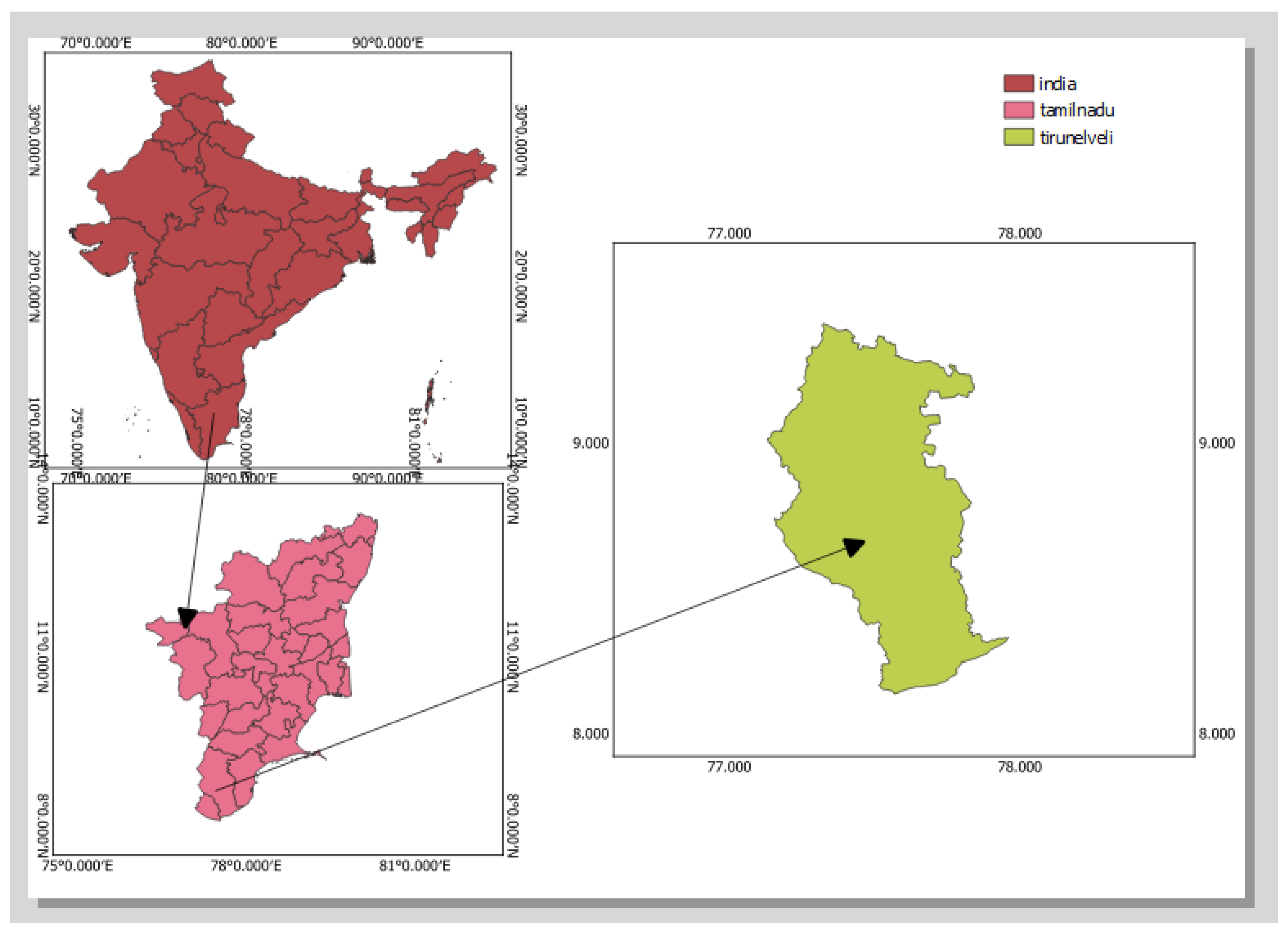
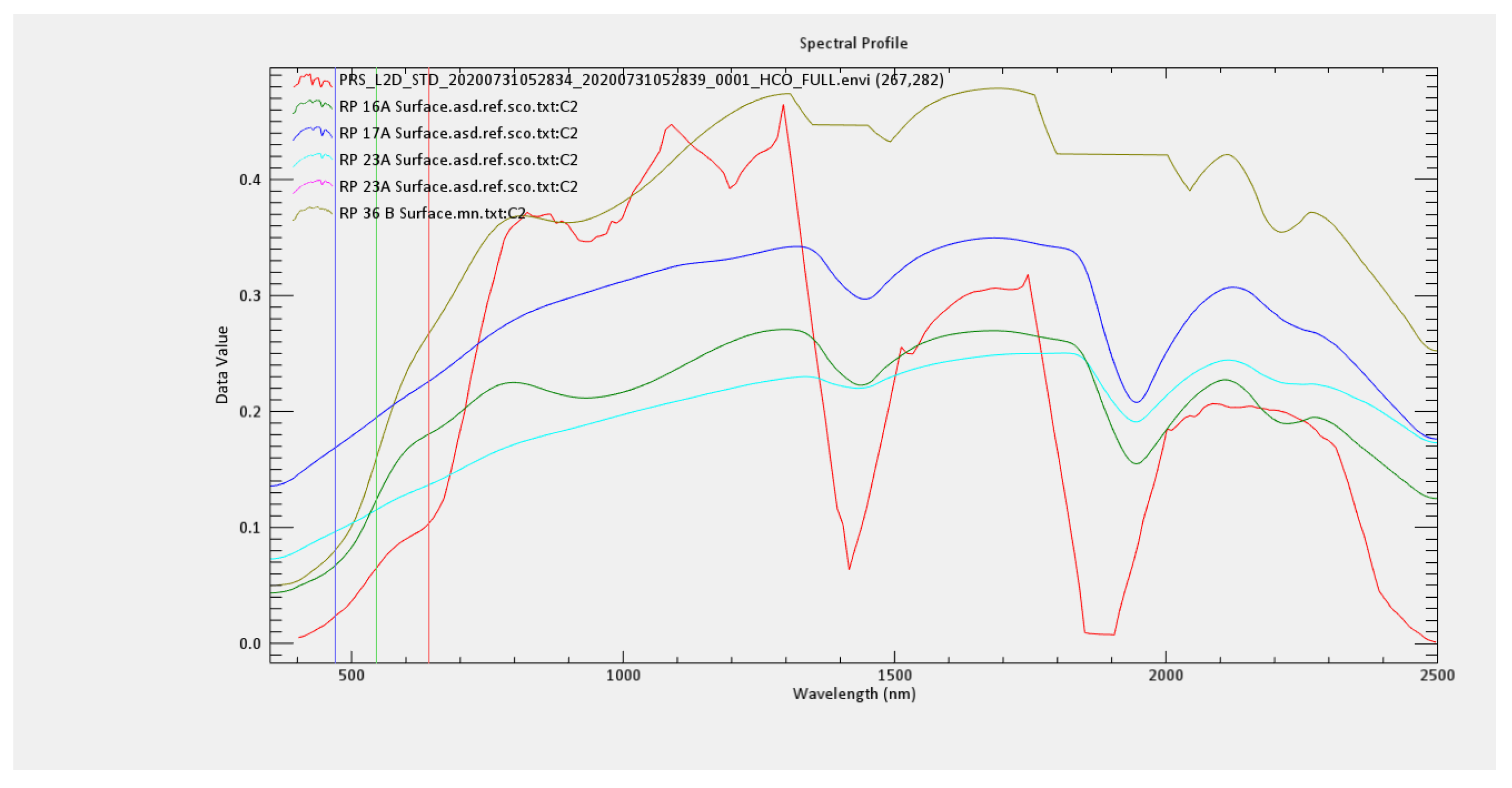

| Sample Code | OC | N | P | K |
|---|---|---|---|---|
| RP-10A | 0.150754 | 112 | 18.3904 | 272.272 |
| RP-10B | 0.150754 | 134.4 | 17.808 | 296.24 |
| RP-11A | 0.633166 | 224 | 51.9904 | 491.568 |
| RP-11B | 0.271357 | 168 | 43.1312 | 230.272 |
| RP-12A | 0.512563 | 179.2 | 21.4816 | 650.944 |
| RP-12B | 0.512563 | 179.2 | 18.48 | 463.344 |
| RP-13A | 0.572864 | 156.8 | 71.3328 | 484.176 |
| RP-13B | 0.211055 | 134.4 | 37.6992 | 169.344 |
| RP-14A | 0.663317 | 235.2 | 28.1456 | 498.288 |
| RP-14B | 0.603015 | 235.2 | 27.3168 | 475.888 |
| RP-15A | 0.331658 | 224 | 25.424 | 478.352 |
| RP-15B | 0.693467 | 224 | 36.0304 | 276.864 |
| RP-16A | 0.422111 | 156.8 | 17.36 | 474.32 |
| RP-16B | 0.723618 | 179.2 | 22.3664 | 520.24 |
| RP-17A | 0.874372 | 224 | 24.3264 | 793.408 |
| Soil Chemical Nutrient | Dataset | Mean | Min | Max | STD | CV (%) |
|---|---|---|---|---|---|---|
| AOC | All | 0.44 | 0.87 | 0.15 | 0.22 | 49.47 |
| Train | 0.51 | 0.15 | 0.87 | 0.21 | 40.38 | |
| Test | 0.27 | 0.15 | 0.69 | 0.12 | 45.63 | |
| AN | All | 190.23 | 257.60 | 112.00 | 36.69 | 19.29 |
| Train | 197.46 | 134.40 | 257.60 | 30.72 | 15.56 | |
| Test | 164.08 | 112.00 | 224.00 | 36.21 | 22.07 | |
| AP | All | 24.95 | 71.33 | 10.25 | 12.23 | 49.00 |
| Train | 25.48 | 10.84 | 71.33 | 13.40 | 52.58 | |
| Test | 22.54 | 11.35 | 43.13 | 8.85 | 39.27 | |
| AK | All | 437.50 | 1313.42 | 127.57 | 251.59 | 57.51 |
| Train | 528.93 | 291.98 | 1313.42 | 234.98 | 44.42 | |
| Test | 202.08 | 127.57 | 276.86 | 50.66 | 25.07 |
| Soil Nutrient | Selected Spectral Variables | Pearson Correlation Coefficients |
|---|---|---|
| AOC | R353, R1640, FD642, FD1676, SD392, SD1245 | −0.34 **, 0.26 **, 0.47 **, 0.35 **, 0.34 **, −0.26 ** |
| AN | R1256, FD796, FD1122, FD2132, SD556 | −0.23 **, 0.25 **,0.36 **, 0.45 **, 0.43 ** |
| AP | R2245, R2486, FD1545, FD1702, SD657 | 0.14 **, 0.50 **, 0.45 **, −0.26 **, 0.32 ** |
| AK | R1498, FD542, SD1635, SD2456 | 0.24 **, 0.47 **, −0.36 **, 0.33 **, 0.28 ** |
| Datasets | R-Squared (R2) | Root Mean Squared Error (RMSE) | Mean Squared Error (MSE) | Mean Absolute Percent Error (MAPE) | |
|---|---|---|---|---|---|
| AOC | Train | 100.00% | 0 | 0 | 0 |
| Test | 97.50% | 0.124 | 0.0154 | 0.04 | |
| AN | Train | 99.99% | 0.0005 | 0 | 0.0026 |
| Test | 99.98% | 0.0007 | 0 | 0.0039 | |
| AP | Train | 51.02% | 8.4924 | 72.1203 | 0.2286 |
| Test | 49.93% | 8.5864 | 73.7256 | 0.2294 | |
| AK | Train | 26.72% | 213.7068 | 45,670.578 | 0.3795 |
| Test | 25.06% | 216.123 | 46,709.154 | 0.3865 |
| Optimization Algorithms | Running Time | Important Parameter Involved | Convergence for Hyperspectral Band Selection | Iteration for Obtaining Global Minimum | Accuracy for Selecting Optimal Relevant Hyperspectral Bands |
|---|---|---|---|---|---|
| PSO [5] | Low | Learning factor, number of dimensions, range of particles, Vmax max change of particle velocity | Moderate | 1100 | High |
| GWO [5] | Moderate | Population size, mutation, and crossover probability | Slow | 450 | Moderate |
| CSO [5] | High | Number of nests, Levy flight solution | Fast | 400 | Moderate |
| FPO [5] | Very High | Number of flowers | Very Fast | 150 | Very High |
| Optimization Techniques | RMSE | R-Squared | MSE | MAE |
|---|---|---|---|---|
| PSO | 0.152 | 0.890 | 0.023 | 0.124 |
| CSO | 0.136 | 0.910 | 0.019 | 0.116 |
| GWO | 0.154 | 0.870 | 0.024 | 0.129 |
| MFO | 0.106 | 0.840 | 0.011 | 0.086 |
| Hybrid Optimization | 0.182 | 0.970 | 0.033 | 0.157 |
| Product Name | PRS_L2D_STD_20201211052010_ 20201211052014_0001.HE5 | Product Id | PRS_L2D_STD |
|---|---|---|---|
| Processor Version | 02.04 | L1 Processor Version | - |
| Processor Name | L2D | Processing Time | 2021-08-11T04:56:29.000749 |
| Processing Level | 2D | Acquisition Type | EARTH OBSERVATION |
| Acquisition Size | 30 KM | Acquisition Purpose | NOT SPECIAL PRODUCT |
| Number of Bands (VNIR + SWIR) | 234 | Image Id | 15549 |
| Product Start Time | 2020-12-11T05:20:10.524999 | Product Stop Time | 2020-12-11T05:20:14.830690 |
| Sun Zenith Angle | 39.602° | View Zenith Angle | 15.724° |
| DN Reflectance Soil Sample | Number of Points | Actual | Percentage Accuracy |
|---|---|---|---|
| RP16A | 662,577 | 662,577 | 47.2648 |
| RP17A | 589,432 | 1,252,009 | 89.3118 |
| RP23A | 149,615 | 1,401,624 | 99.9846 |
| RP36B | 5 | 1,401,629 | 99.9849 |
| RP8A | 207 | 1,401,836 | 99.9997 |
| RP98 | 4 | 1,401,840 | 100.0000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raju, A.; Subramoniam, R. Assessing Soil Nutrient Content and Mapping in Tropical Tamil Nadu, India, through Precursors IperSpettrale Della Mission Applicative Hyperspectral Spectroscopy. Appl. Sci. 2024, 14, 186. https://doi.org/10.3390/app14010186
Raju A, Subramoniam R. Assessing Soil Nutrient Content and Mapping in Tropical Tamil Nadu, India, through Precursors IperSpettrale Della Mission Applicative Hyperspectral Spectroscopy. Applied Sciences. 2024; 14(1):186. https://doi.org/10.3390/app14010186
Chicago/Turabian StyleRaju, Anand, and Rama Subramoniam. 2024. "Assessing Soil Nutrient Content and Mapping in Tropical Tamil Nadu, India, through Precursors IperSpettrale Della Mission Applicative Hyperspectral Spectroscopy" Applied Sciences 14, no. 1: 186. https://doi.org/10.3390/app14010186






