Estimation of Cervical Spinal Loading and Internal Motion at Adjacent Segments after C5–C6 Fusion Using a Musculoskeletal Multi-Body Dynamics Model during the Head Flexion–Extension Movement
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Normal Cervical Spine Model
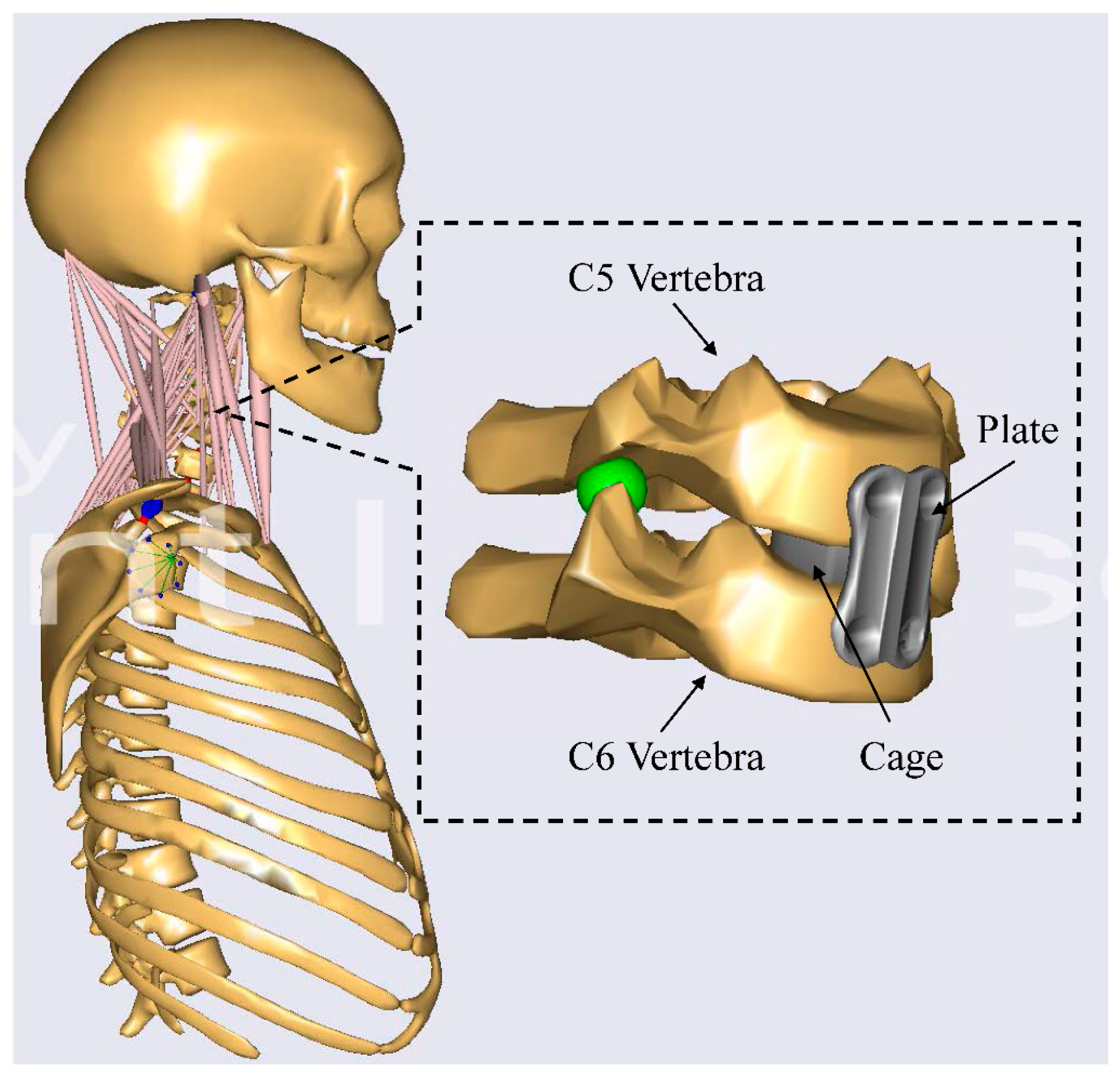
2.2. The Cervical Spinal Fusion Model
2.3. Inverse Dynamics Analysis
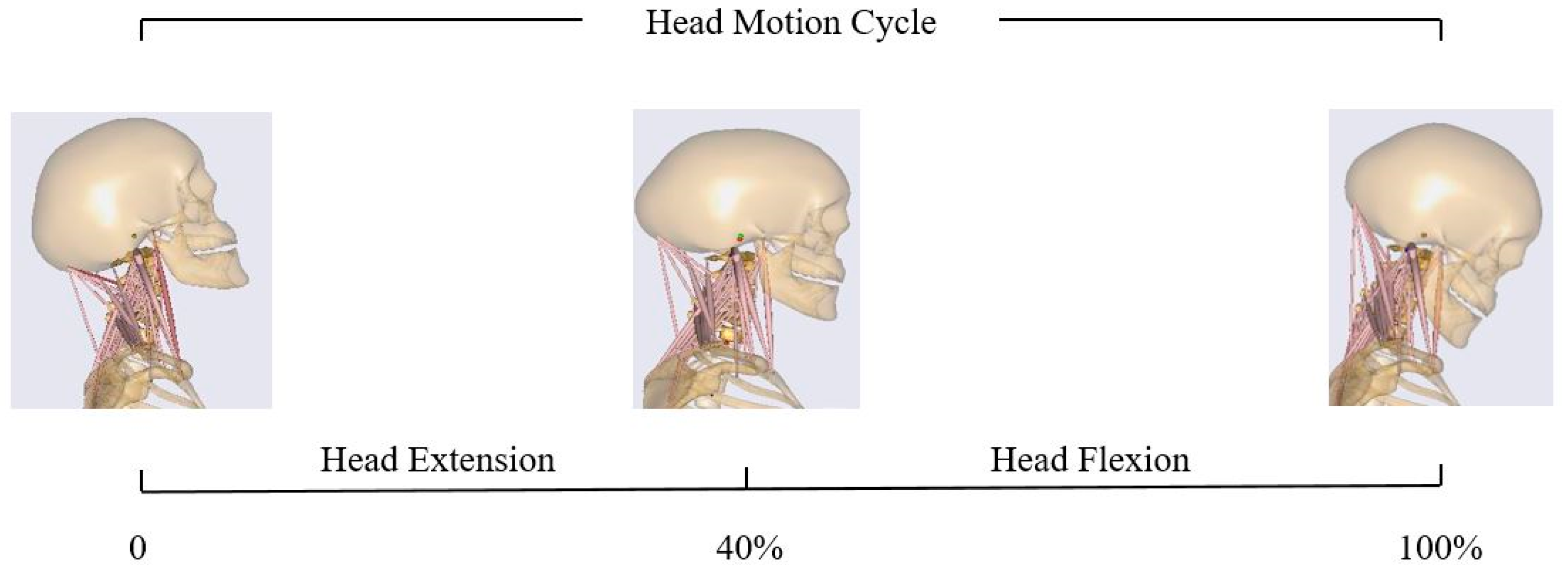
2.4. Flexion–Extension Simulation
3. Results
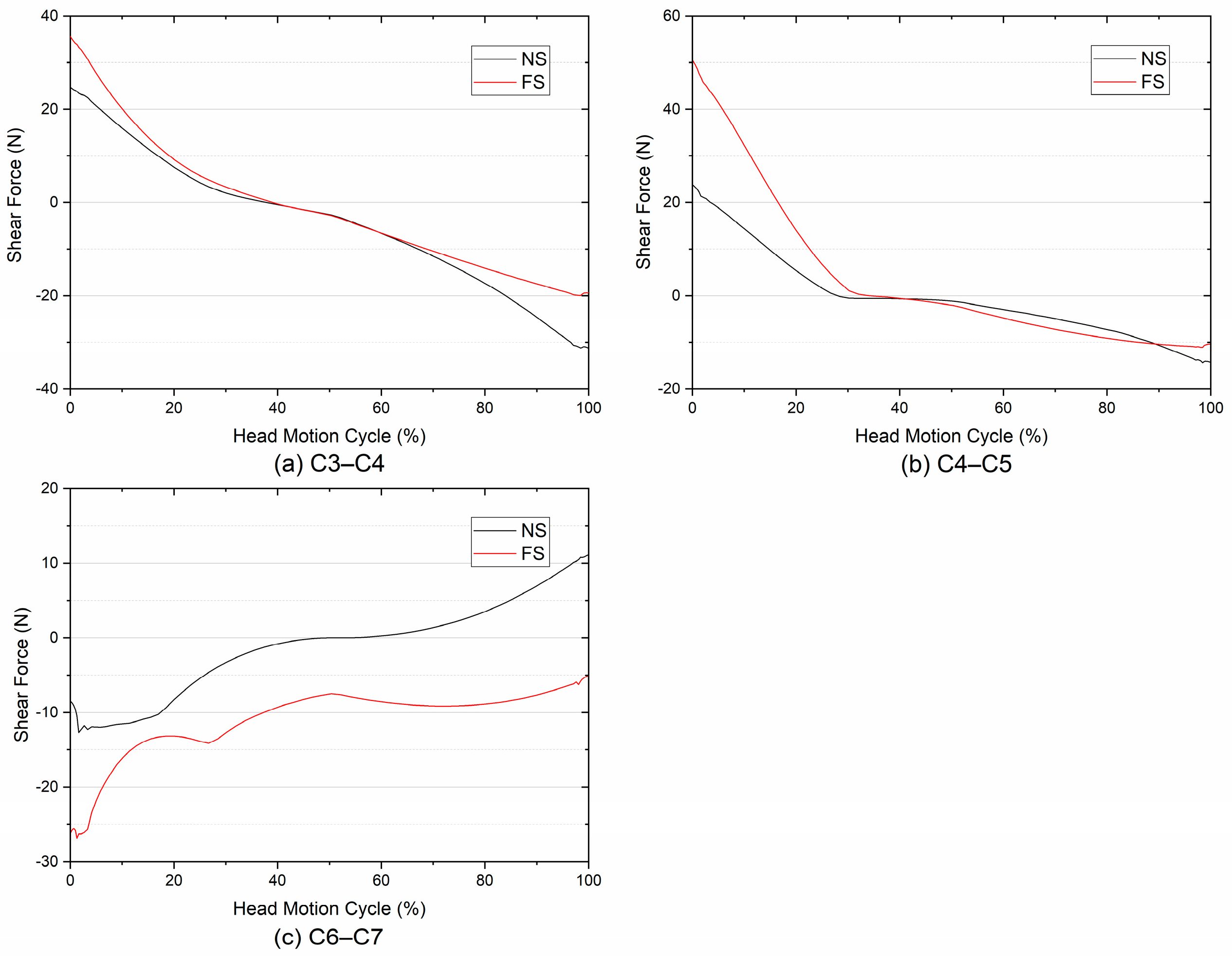
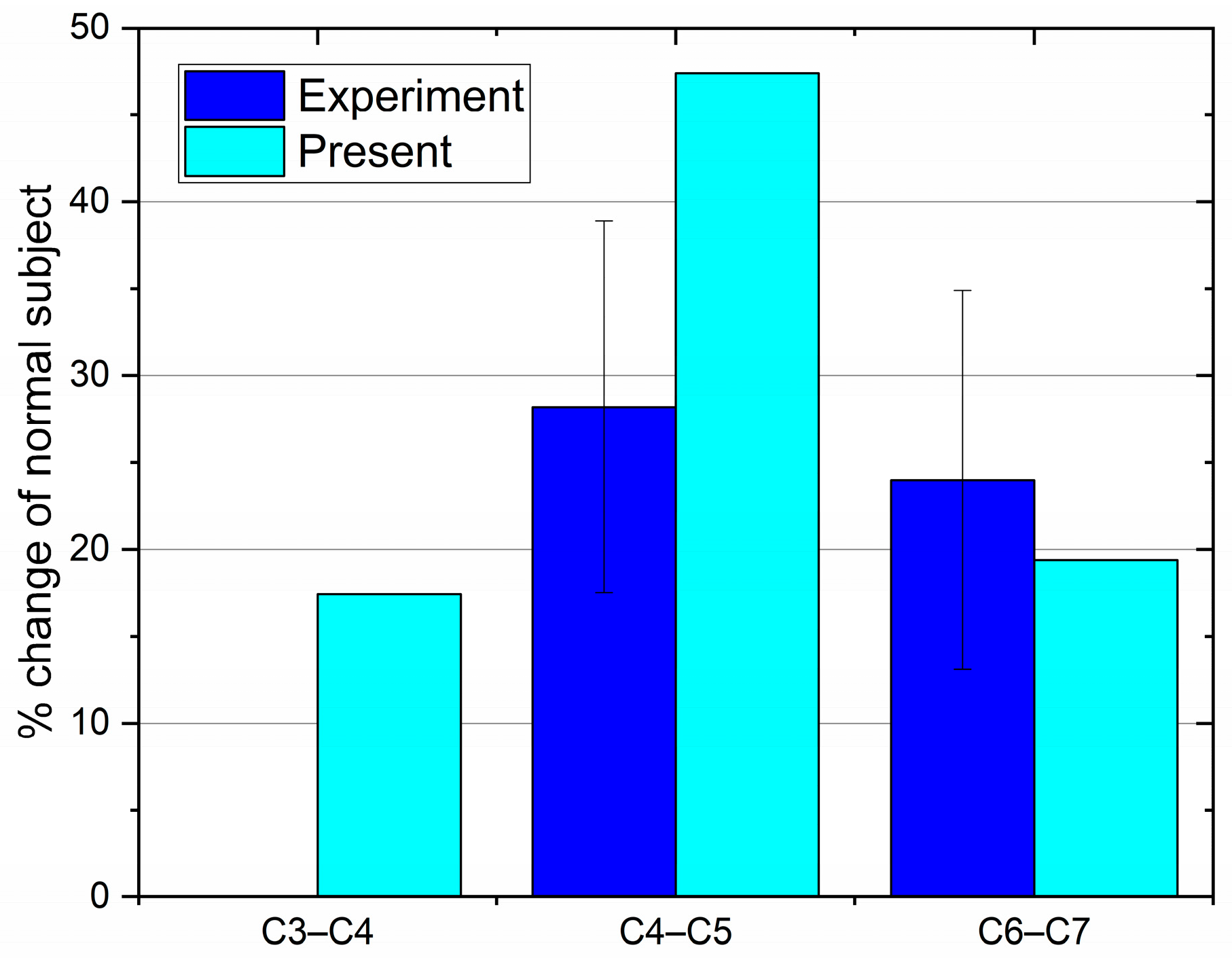
3.1. Compressive and Shear Forces
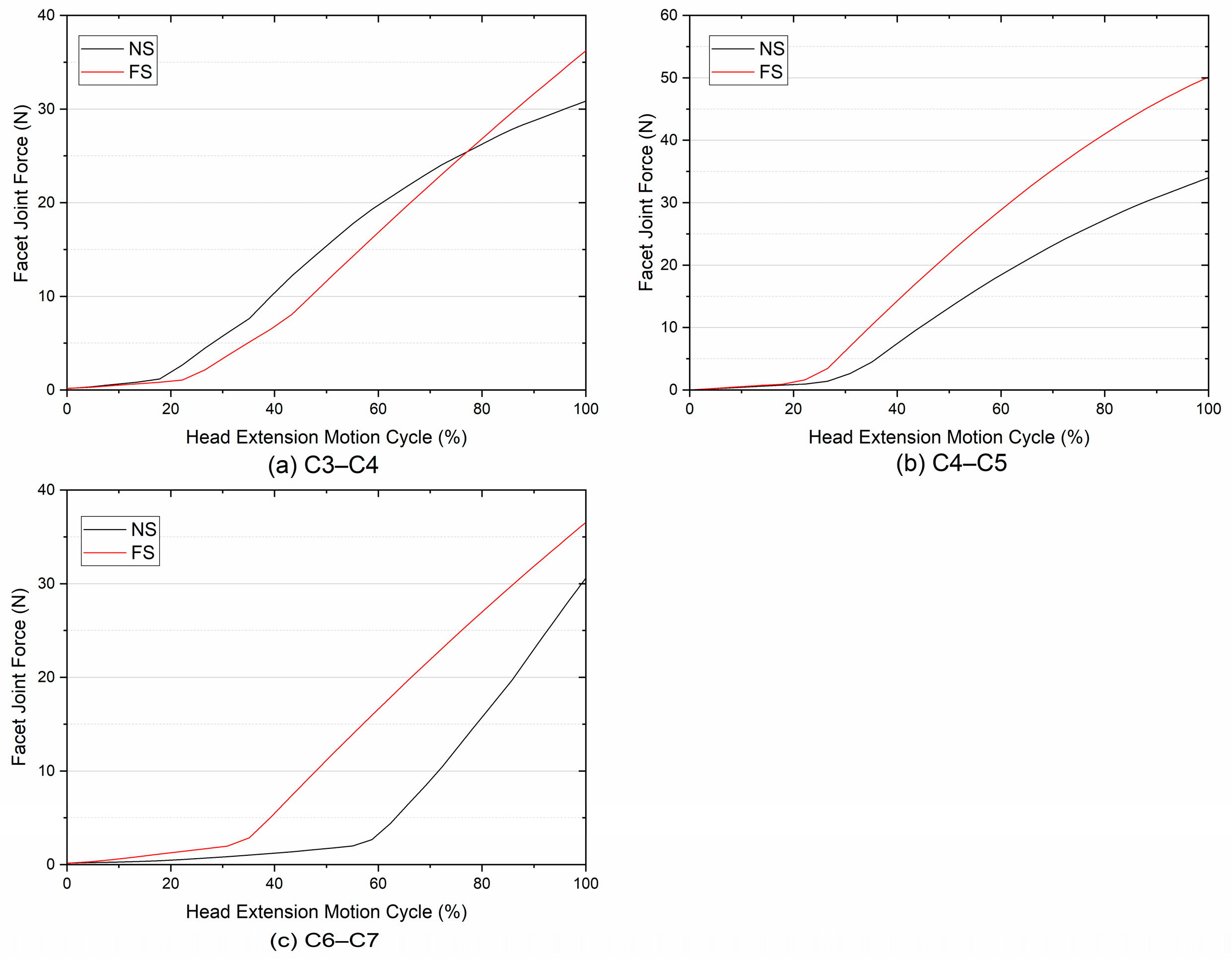
3.2. Facet Joint Force
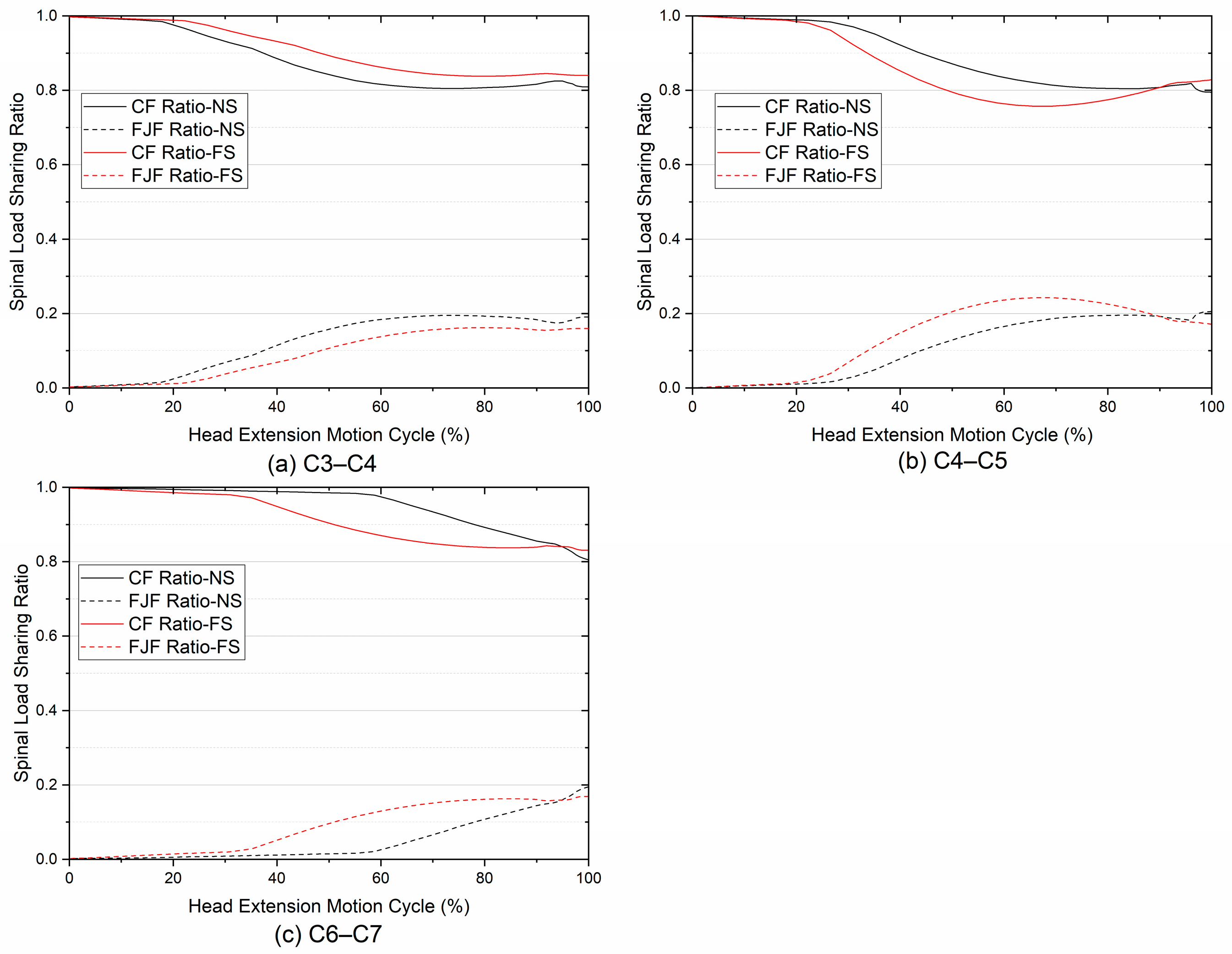
3.3. Spinal Load Sharing in Compression
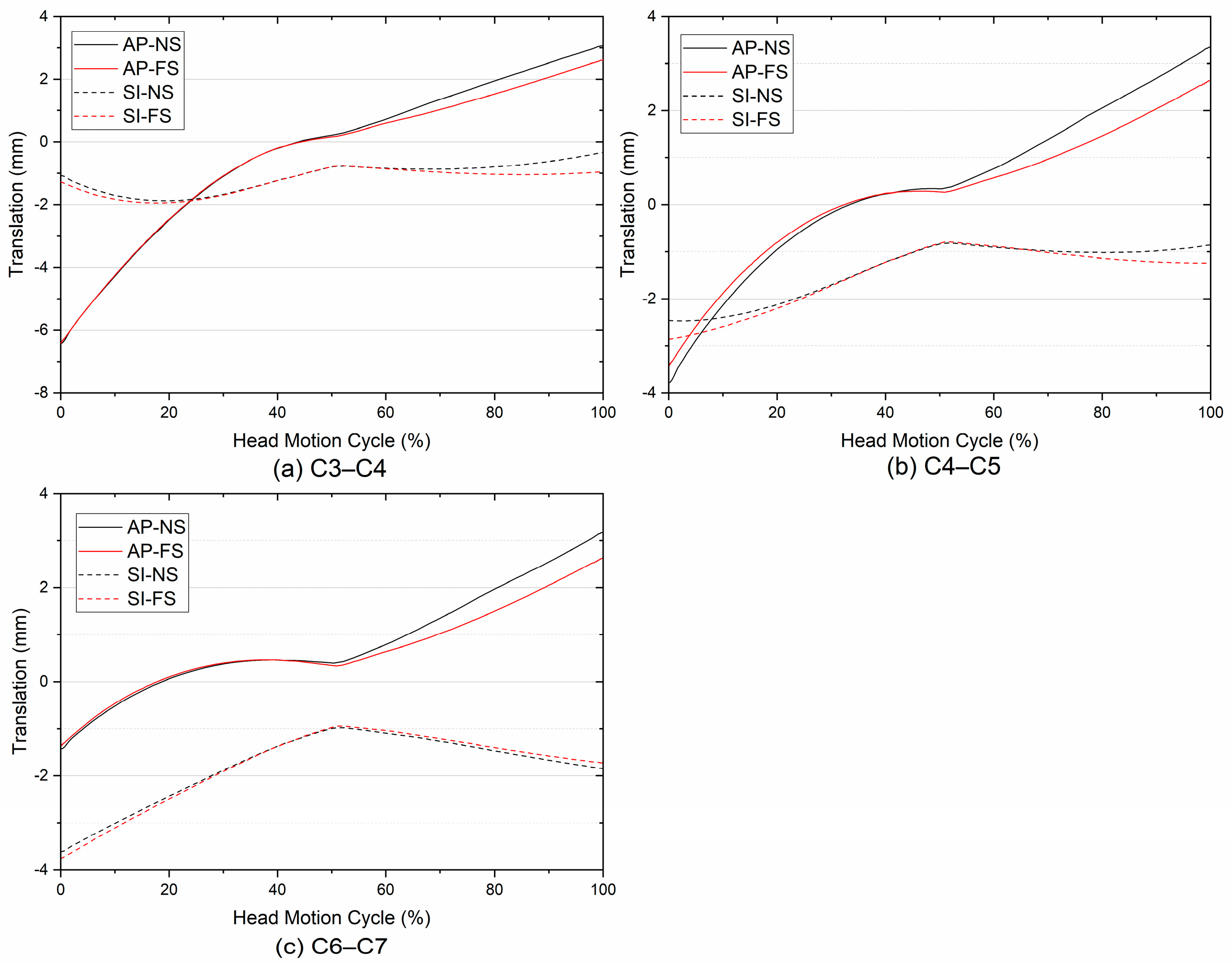
3.4. Translation
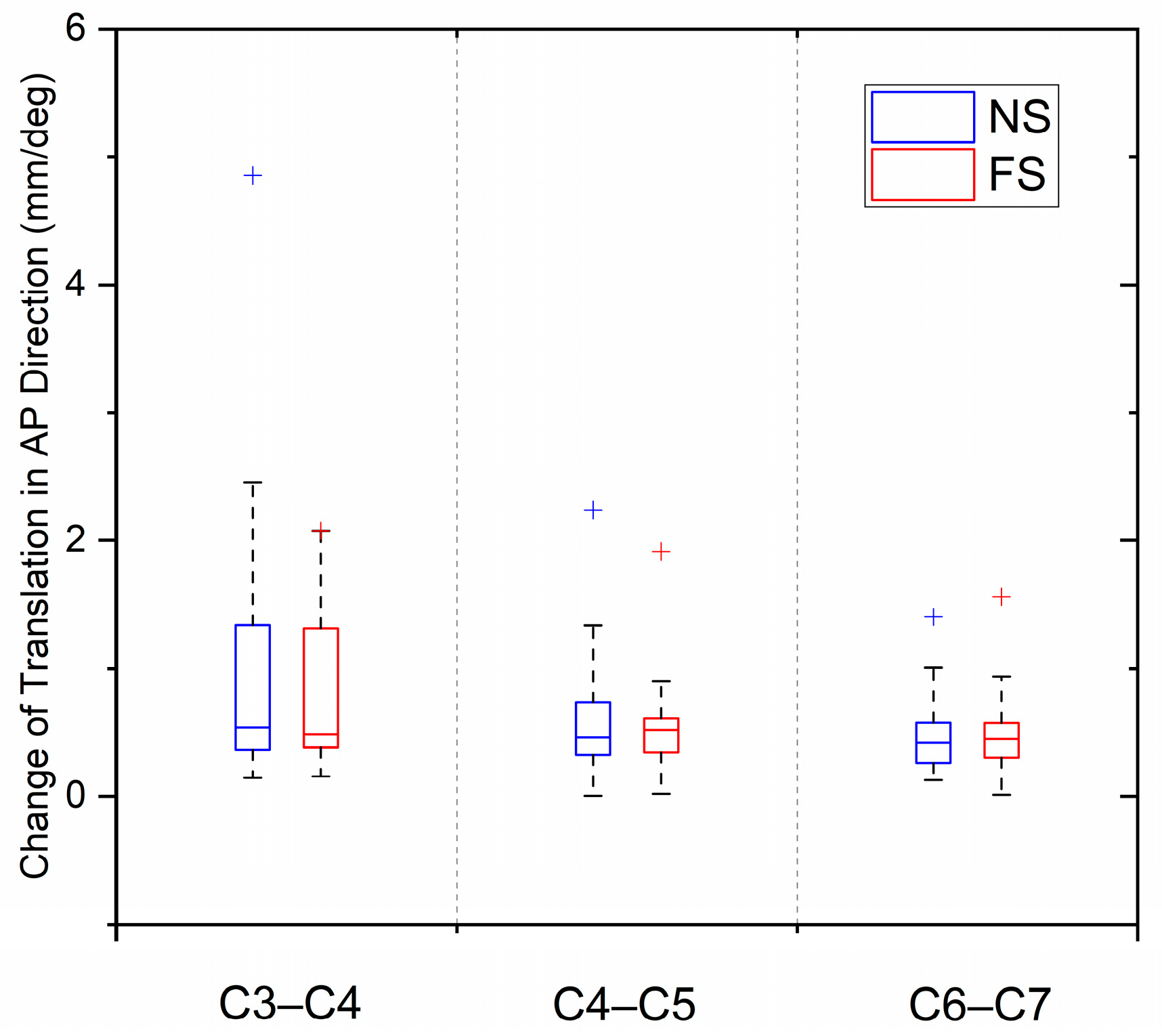
3.5. Biomechanical Comparison
4. Discussion
5. Conclusions
- (1)
- The spinal loading pattern after fusion presents a distinctly level-dependent feature, where differences in joint loading between the FS and the NS were found.
- (2)
- Based on the predicted results, the maximum CF of the FS was about 25% greater than that of the NS in the head extension phase. This phenomenon indicates that the disc would bear more loads during the head extension after spinal fusion.
- (3)
- In the head flexion phase, the same magnitudes and trends of the CFs were found, while the increments of the average CFs of the FS were mostly greater than those of the NS at the superior adjacent segments.
- (4)
- At the superior adjacent segments, there were minor variations in the A-P SFs between the FS and the NS, in addition to an obvious increase at C4–C5. At the inferior adjacent segment, the spinal fusion altered the direction of the A-P SFs.
- (5)
- Larger increases in FJFs at adjacent segments were found after fusion. The results indicated that the facet joint after fusion would share more spinal loading and even lead to facet joint degeneration.
- (6)
- Regarding the translations of ICRs, the predicted results showed that the joint internal motion during the head F/E movement might not be affected by single-level cervical spinal fusion surgery.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajaee, S.S.; Bae, H.W.; Kanim, L.E.; Delamarter, R.B. Spinal fusion in the United States: Analysis of trends from 1998 to 2008. Spine 2012, 37, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Hilibrand, A.S.; Robbins, M. Adjacent segment degeneration and adjacent segment disease: The consequences of spinal fusion? Spine J. 2004, 4, 190S–194S. [Google Scholar] [CrossRef] [PubMed]
- Alhashash, M.; Shousha, M.; Boehm, H. Adjacent Segment Disease After Cervical Spine Fusion: Evaluation of a 70 Patient Long-Term Follow-Up. Spine 2018, 43, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Song, K.J.; Choi, B.W.; Jeon, T.S.; Lee, K.B.; Chang, H. Adjacent segment degenerative disease: Is it due to disease progression or a fusion-associated phenomenon? Comparison between segments adjacent to the fused and non-fused segments. Eur. Spine J. 2011, 20, 1940–1945. [Google Scholar] [CrossRef] [PubMed]
- Saavedra-Pozo, F.M.; Deusdara, R.A.; Benzel, E.C. Adjacent segment disease perspective and review of the literature. Ochsner J. 2014, 14, 78–83. [Google Scholar] [PubMed]
- Shin, J.J. Comparison of Adjacent Segment Degeneration, Cervical Alignment, and Clinical Outcomes after One- and Multilevel Anterior Cervical Discectomy and Fusion. Neurospine 2019, 16, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Anderst, W.J.; West, T.; Donaldson, W.F., 3rd; Lee, J.Y.; Kang, J.D. Longitudinal Study of the Six Degrees of Freedom Cervical Spine Range of Motion During Dynamic Flexion, Extension, and Rotation After Single-level Anterior Arthrodesis. Spine 2016, 41, E1319–E1327. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.A.; Roughley, P.J. What is Intervertebral Disc Degeneration, and What Causes It? Spine 2006, 31, 2151–2161. [Google Scholar] [CrossRef]
- Adams, M.A.; Lama, P.; Zehra, U.; Dolan, P. Why do some intervertebral discs degenerate, when others (in the same spine) do not? Clin. Anat. 2015, 28, 195–204. [Google Scholar] [CrossRef]
- Hsieh, Y.-Y.; Tsuang, F.-Y.; Kuo, Y.-J.; Chen, C.-H.; Chiang, C.-J.; Lin, C.-L. Biomechanical analysis of single-level interbody fusion with different internal fixation rod materials: A finite element analysis. BMC Musculoskelet. Disord. 2020, 21, 100. [Google Scholar] [CrossRef]
- Schwab, J.S.; DiAngelo, D.J.; Foley, K.T. Motion Compensation Associated with Single-Level Cervical Fusion: Where Does the Lost Motion Go? Spine 2006, 31, 2439–2448. [Google Scholar] [CrossRef]
- Chang, U.K.; Kim, D.H.; Lee, M.C.; Willenberg, R.; Kim, S.H.; Lim, J. Changes in adjacent-level disc pressure and facet joint force after cervical arthroplasty compared with cervical discectomy and fusion. J. Neurosurg. Spine 2007, 7, 33–39. [Google Scholar] [CrossRef]
- Eck, J.C.; Humphreys, S.C.; Lim, T.H.; Jeong, S.T.; Kim, J.G.; Hodges, S.D.; An, H.S. Biomechanical study on the effect of cervical spine fusion on adjacent-level intradiscal pressure and segmental motion. Spine 2002, 27, 2431–2434. [Google Scholar] [CrossRef]
- Patel, V.V.; Wuthrich, Z.R.; McGilvray, K.C.; Lafleur, M.C.; Lindley, E.M.; Sun, D.; Puttlitz, C.M. Cervical facet force analysis after disc replacement versus fusion. Clin. Biomech. 2017, 44, 52–58. [Google Scholar] [CrossRef]
- Gandhi, A.A.; Grosland, N.M.; Kallemeyn, N.A.; Kode, S.; Fredericks, D.C.; Smucker, J.D. Biomechanical Analysis of the Cervical Spine Following Disc Degeneration, Disc Fusion, and Disc Replacement: A Finite Element Study. Int. J. Spine Surg. 2019, 13, 491–500. [Google Scholar] [CrossRef]
- Volkheimer, D.; Malakoutian, M.; Oxland, T.R.; Wilke, H.-J. Limitations of current in vitro test protocols for investigation of instrumented adjacent segment biomechanics critical analysis of the literature. Eur. Spine J. 2015, 24, 1882–1892. [Google Scholar] [CrossRef]
- Diao, H.; Xin, H.; Jin, Z. Prediction of in vivo lower cervical spinal loading using musculoskeletal multi-body dynamics model during the head flexion/extension, lateral bending and axial rotation. Proc. Inst. Mech. Eng. H 2018, 232, 1071–1082. [Google Scholar] [CrossRef]
- Andersen, M.S.; Damsgaard, M.; Rasmussen, J. Force-dependent kinematics: A new analysis method for non-conforming joints. In Proceedings of the 13th International Symposium on Computer Simulation in Biomechanics, Leuven, Belgium, 30 June–2 July 2011. [Google Scholar]
- Andersen, M.S.; de Zee, M.; Damsgaard, M.; Nolte, D.; Rasmussen, J. Introduction to Force-Dependent Kinematics: Theory and Application to Mandible Modeling. J. Biomech. Eng. 2017, 139, 091001. [Google Scholar] [CrossRef]
- Anderst, W.J.; Lee, J.Y.; Donaldson, W.F., 3rd; Kang, J.D. Six-degrees-of-freedom cervical spine range of motion during dynamic flexion-extension after single-level anterior arthrodesis: Comparison with asymptomatic control subjects. J. Bone Jt. Surg. Am. 2013, 95, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, J.; Damsgaard, M.; Voigt, M. Muscle recruitment by the min/max criterion-a comparative numerical study. J. Biomech. 2001, 34, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Aizawa, T.; Kanno, H.; Itoi, E. Adjacent segment degeneration after fusion spinal surgery-a systematic review. Int. Orthop. 2019, 43, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Hutton, W.C.; Toribatake, Y.; Elmer, W.A.; Ganey, T.M.; Tomita, K.; Whitesides, T.E. The effect of compressive force applied to the intervertebral disc in vivo. A study of proteoglycans and collagen. Spine 1998, 23, 2524–2537. [Google Scholar] [CrossRef] [PubMed]
- Goel, V.K.; Monroe, B.T.; Gilbertson, L.G.; Brinckmann, P. Interlaminar Shear Stresses and Laminae Separation in a Disc. Spine 1995, 20, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Benditz, A.; Auer, S.; Sporrer, J.F.; Wolkerstorfer, S.; Grifka, J.; Suess, F.; Dendorfer, S. Regarding loads after spinal fusion, every level should be seen separately: A musculoskeletal analysis. Eur. Spine J. 2018, 27, 1905–1910. [Google Scholar] [CrossRef]
- Kumaresan, S.; Yoganandan, N.; Pintar, F.A. Posterior complex contribution to the axial compressive and distraction behavior of the cervical spine. J. Musculoskelet. Res. 1998, 2, 257–265. [Google Scholar] [CrossRef]
- Anderst, W.; Baillargeon, E.; Donaldson, W.; Lee, J.; Kang, J. Motion path of the instant center of rotation in the cervical spine during in vivo dynamic flexion-extension: Implications for artificial disc design and evaluation of motion quality after arthrodesis. Spine 2013, 38, E594–E601. [Google Scholar] [CrossRef]

| Disc Level | Force (N) | −6° to −5° | −5° to −4° | −4° to −3° | −3° to −2° | −2° to −1° | −1° to 0° | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NS | FS | NS | FS | NS | FS | NS | FS | NS | FS | NS | FS | ||
| C3–C4 | CF | 134.3 | 151.0 | 107.6 | 117.3 | 83.9 | 110.1 | 79.2 | 90.6 | 76.0 | 82.7 | 68.7 | 70.7 |
| SF | 23.8 | 31.8 | 16.6 | 19.8 | 8.2 | 16.7 | 3.5 | 6.9 | 1.2 | 3.3 | −0.1 | 0.5 | |
| FJF | 30.0 | 33.4 | 25.3 | 24.4 | 16.6 | 21.0 | 8.1 | 8.4 | 2.3 | 2.6 | 0.5 | 0.5 | |
| C4–C5 | CF | 124.4 | 82.5 | 98.8 | 77.6 | 89.7 | 78.5 | 87.4 | 81.9 | 80.8 | 79.2 | 71.2 | 71.3 |
| SF | 18.1 | 20.5 | 11.2 | 12.7 | 5.4 | 5.8 | 0.8 | 1.4 | −0.6 | 0.1 | −0.6 | −0.4 | |
| FJF | 29.4 | 29.1 | 21.8 | 21.3 | 13.8 | 12.0 | 5.0 | 4.0 | 0.9 | 1.1 | 0.3 | 0.4 | |
| C6–C7 | CF | — a | 161.0 | 133.5 | 141.4 | 133.9 | 111.1 | 127.0 | 99.3 | 114.7 | 90.9 | 86.1 | 76.4 |
| SF | — | −26.1 | −10.4 | −20.9 | −11.9 | −14.2 | −11.2 | −13.4 | −8.2 | −12.8 | −2.6 | −10.0 | |
| FJF | — | 35.3 | 28.2 | 29.5 | 19.6 | 19.5 | 9.4 | 9.6 | 2.1 | 2.4 | 0.6 | 0.6 | |
| Disc Level | Force (N) | 0° to 1° a | 1° to 2° | 2° to 3° | 3° to 4° | 4° to 5° | 5° to 6° | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NS | FS | NS | FS | NS | FS | NS | FS | NS | FS | NS | FS | ||
| C3–C4 | CF | 60.2 | 58.7 | 52.9 | 52.5 | 51.9 | 57.9 | 55.2 | 64.5 | 59.3 | 71.1 | 63.6 | 78.5 |
| SF | −1.3 | −1.6 | −2.5 | −3.9 | −4.1 | −7.3 | −5.9 | −10.5 | −8.3 | −13.3 | −10.8 | −16.1 | |
| C4–C5 | CF | 62.5 | 58.8 | 55.2 | 56.8 | 54.8 | 64.7 | 58.8 | 73.2 | 62.4 | 82.8 | 66.1 | 91.1 |
| SF | −0.8 | −1.5 | −1.1 | −4.1 | −2.0 | −6.7 | −3.0 | −8.7 | −3.8 | −10.2 | −4.7 | −10.7 | |
| C6–C7 | CF | 63.2 | 63.5 | 72.8 | 58.9 | 80.3 | 64.4 | 85.3 | 70.3 | 89.5 | 76.7 | 93.2 | 84.5 |
| SF | 0.2 | −8.2 | 2.0 | −8.0 | 4.2 | −8.8 | 6.0 | −9.1 | 7.9 | −8.8 | 9.8 | −7.8 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diao, H.; Xin, H.; Jin, Z. Estimation of Cervical Spinal Loading and Internal Motion at Adjacent Segments after C5–C6 Fusion Using a Musculoskeletal Multi-Body Dynamics Model during the Head Flexion–Extension Movement. Appl. Sci. 2024, 14, 261. https://doi.org/10.3390/app14010261
Diao H, Xin H, Jin Z. Estimation of Cervical Spinal Loading and Internal Motion at Adjacent Segments after C5–C6 Fusion Using a Musculoskeletal Multi-Body Dynamics Model during the Head Flexion–Extension Movement. Applied Sciences. 2024; 14(1):261. https://doi.org/10.3390/app14010261
Chicago/Turabian StyleDiao, Hao, Hua Xin, and Zhongmin Jin. 2024. "Estimation of Cervical Spinal Loading and Internal Motion at Adjacent Segments after C5–C6 Fusion Using a Musculoskeletal Multi-Body Dynamics Model during the Head Flexion–Extension Movement" Applied Sciences 14, no. 1: 261. https://doi.org/10.3390/app14010261
APA StyleDiao, H., Xin, H., & Jin, Z. (2024). Estimation of Cervical Spinal Loading and Internal Motion at Adjacent Segments after C5–C6 Fusion Using a Musculoskeletal Multi-Body Dynamics Model during the Head Flexion–Extension Movement. Applied Sciences, 14(1), 261. https://doi.org/10.3390/app14010261






