Abstract
The current high-salinity wastewater treatment technology is complex, costly, and carries the risk of secondary contamination. As a traditional desalination technology, the combined method using frozen technology has broad development prospects in wastewater treatment. This study investigates the desalination effects of waste leachate using three different methods: the frozen–gravity method (FGM), frozen–centrifugal method (FCM), and frozen–blowing methods (FBMs), under various experimental conditions. The results showed that the salt rejection of all three methods could reach more than 75% under the conditions of a freezing time of 12 h, freezing temperature of −15 °C, and ice production rate of 40%; the salt rejection of FGM increased at higher ambient temperatures, but it was not conducive to the removal of organic pollutants; the salt rejection of FCM was sensitive to the centrifugal time and centrifugal speed, with a significant correlation (p < 0.05), the increase in centrifugal time and centrifugal speed can help to improve the salt rejection, and the increase in centrifugal speed in the range of 1000–2000 rpm can accelerate the discharge of concentrated brine more effectively; the frozen–crushed–blowing method (FCBM) in FBM has a salt rejection as high as 93.86% at an ice production rate of 25.80%, which reduces the salinity of the effluent from 4.07% to 0.25%, speeds up the desalination process, and improves the salt rejection compared to the other methods. This study provides a new perspective and reference for the treatment of high-saline wastewater.
1. Introduction
The emission of high-salt wastewater in China is generally decreasing due to the rapid development of the country’s economy and accelerated urbanization; however, the overall volume remains substantial [1]. The rapid rise in municipal waste production has significantly strained the treatment facility. When waste is deposited in landfills or stockpiled, rainwater and groundwater seep in, resulting in the generation of waste leachate that is highly chromatic, toxic, and challenging to handle. This waste leachate contains dangerously high levels of salt and poses a severe threat to both the environment and human health [2,3]. To address the challenge of treating high-salt wastewater, it has become crucial to discover effective methods for separating and treating salt in leachate treatment processes.
As one of the emerging desalination technologies, the freezing method, is based on the theory of solid–liquid phase equilibrium; due to the brine, with the pure water freezing point being low, when the temperature drops below the freezing point, the pure water from the liquid phase converts to solid-phase ice. Moreover, owing to ice’s singular mineral structure, foreign atoms are unable to permeate the ice crystals, resulting in the continuous aggregation of water molecules and the formation of pure ice, with salt ions being excluded from the ice crystals. The freezing method has received key attention from experts and scholars at home and abroad because of its advantages, such as less fouling, simple operation, no secondary pollution, and non-selectivity for wastewater treatment [4]. In addition, the ice crystals produced in the treatment process can be stored as cold energy to meet the needs of cold storage and refrigeration [5]; the nitrogen, phosphorus, and other elements in the concentrated effluent are recycled to produce nutrient-rich salt fertilizer [6]. Theoretically, all methods have certain drawbacks, such as reverse osmosis cannot treat strongly acidic (pH < 2) or strongly alkaline (pH > 12) wastewater, and evaporation uses high quantities of energy and is costly [7]. However, the freezing method also has some insurmountable drawbacks. Generally, electrical energy is used as the main source of cold energy, and in addition to the electrical energy consumption of the ice-making system, other electrical energy consumption, such as the operation of equipment, is also included. Although it is more energy-efficient than evaporation, it uses more energy than reverse osmosis, which increases energy costs and results in additional CO2 emissions. The search for a suitable source of cold is key to the industrial application of the chilling process. In recent years, several studies have favored using liquefied natural gas (LNG) for refrigeration applications, which releases 830 kJ/kg of cooling energy when gasified [8]. Therefore, using LNG for refrigerated seawater desalination is an effective way to use resources while reducing the electrical energy consumption and providing a continuous supply of cold energy. For this reason, LNG is increasingly being used for freezing desalination [9]. Undeniably, the freezing method generates a large amount of concentrate during waste leachate treatment, leading to a waste of resources and environmental pollution problems. However, solutions to the problem of concentrate disposal have been actively sought globally for many years. In addition, during the early formation of ice crystals, the rapid freezing speed and high abundance of needle-shaped ice crystals per unit volume cause salt to bind to the surface, creating saltwater bubbles that trap salt ions within the ice structure [10,11]. As such, the primary challenge in the industrial implementation of the freezing combination technique lies in efficiently separating salt ions from the resulting ice bodies.
The desalination of waste leachate is essentially the same as desalination, in that the salt ions are effectively separated to achieve a higher quality of effluent. In recent years, many scholars have devoted themselves to the treatment of high-salt wastewater by the freezing method, focusing on the analysis of how to effectively separate salt ions. Some researchers have compared immersion, water addition, and gravity methods to separate salt ions encapsulated in ice crystals. They found that the salt rejection of the ice crystals increased from 59.06% to 91.61% after immersion treatment, but the ice production rate decreased from 66.72% to 55.02%. The gravity method had a longer processing time than the immersion method, but the water production rate was high and desalination was good, with a maximum effect of 99.8% [12]. There are also scientists researching the microwave combined gravity desalination of seawater, with salt rejections of more than 90% [13].
In this study, three combined freezing methods were selected for waste leachate treatment, including the frozen–gravity method (FGM), frozen–centrifugal method (FCM), and frozen–blowing method (FBM). The effects of each method were explored separately, and the three methods were compared. A new desalination method based on FBM is proposed, the frozen–crushed–blowing method (FCBM), which provides a new reference for the treatment of high-saline wastewater, such as waste leachate, by the freezing method and an important theoretical and scientific basis for the early realization of the industrial application of the freezing method.
2. Materials and Methods
2.1. Experimental Materials
The experimental treatment water samples were of two types: one was a 5% high-saline simulated wastewater prepared with NaCl (AR, Tianjin Dengfeng), and the other was waste leachate taken from the sewage treatment station of a local waste incineration plant. The freezing generator uses a polypropylene container, and the freezing unit was a commercial freezer model DB/BC-303KEM (Midea, Foshan, China), with a refrigeration temperature range of 40–0 °C and a standard power consumption of 0.69 (kw·h) for 24 h.
The ice crystal desalting device included funnels (wall thickness 2 mm, diameter 18 cm, cylinder height 13 cm, and cone height 4.3 cm); a 2 L stainless steel container (316 austenitic steel); a hair dryer (model KF-8894, Guangdong Huanengda Electric Appliance Co., Ltd., Jieyang, China); a filter centrifuge (TD5F, Shanghai Huazhou Filtration Equipment Co., Ltd., Shanghai, China); and an ice crusher (TH-168, Vismi, Foshan, China). Other major instruments included a portable conductivity meter (DDBJ-350, Beijing Oriental Sail Technology Co., Ltd., Beijing, China), a total organic carbon analyzer (TOC-2000, Shenghan Technology Co., Ltd., Shanghai, China), and an artificial climatic chamber (MGC-850HP, Ningbo Haishu Saifu Experimental Equipment Co., Ltd., Ningbo, China, temperature control range 4–50 °C).
2.2. Experimental Methods and Processes
To simulate the top-down freezing process in nature, the periphery of the container was wrapped with insulating cotton to prevent heat exchange between the container wall and the outside, and an opening was provided at the top to ensure that the cold air could only be applied to the solution from above. Take 1 L of the prepared high-saline solution (prepared with NaCl) and place it in a freezing container. Freeze at −10 °C for 12 h, 15 h, 18 h, 21 h, and 24 h, respectively. After freezing, remove the ice and place it on a stainless steel funnel. After gravity desalting for 100 min, the remaining ice crystals are collected. After the temperature of the melted ice water has risen to room temperature, its indicators are measured to obtain the optimum freezing time; the samples are frozen at −10 °C, −15 °C, −20 °C, −25 °C, and −30 °C for 12 h to obtain the optimum freezing temperature.
The waste leachate was treated before the experiment and stored in a cool place after filtration to prevent water evaporation from affecting the detection of water quality indicators. Firstly, FGM was used to conduct desalination experiments on waste leachate using the ice production rate as an independent variable to explore the trend of the desalination effect under different ice production rates (40%, 50%, 60%, 70%, and 80%). In addition, the changes in salt rejection under the average temperatures in winter and summer were compared (based on the temperatures in Northern China, with an average temperature of 5 °C in winter and 28 °C in summer). The FCM uses an ice crusher to crush ice into small ice cubes about 6 mm in diameter. The crushed ice crystals are placed in a filtered centrifuge at a speed of 2000 rpm, and experiments are conducted at different centrifugal times (1 min, 2 min, 3 min, 4 min, and 5 min) and 2 min with different rotational speeds (1000 rpm, 1500 rpm, 2000 rpm, 2500 rpm, and 3000 rpm) as independent variables. The FBM includes the FGBM and FCBM. The FGBM involves placing ice on a blowing device for blowing after 100 min of gravitational action, with blowing times set at 5 min, 10 min, 15 min, and 20 min. The FCBM first crushes the ice in the ice crusher into small ice cubes of about 6 mm in diameter, places them in a funnel, and then applies a blowing effect. The blowing time is set to 1 min, 2 min, 3 min, 4 min, and 5 min. Finally, the treated ice crystals are collected, and the salinity of the meltwater is measured after it has melted to room temperature.
All experiments were performed three times, with the data organized using Excel 2018 software and plotted using Origin2017 (OriginLab Corp, Northampton, MA, USA) and Visio2019 (Microsoft Corporation Corp, Redmond, WA, USA) software; SPSS Version 25.0 (IBM Corp, Armonk, NY, USA) software was used for data analysis and correlation and significance studies between variables. Polyfit and one-way ANOVA methods were used.
2.3. Methods and Equations
In this paper, the ice production rate refers to the ratio of ice obtained after desalination to the total mass of wastewater, and the salt rejection is defined as the ratio of the difference between the salinity of the initial wastewater and the salinity of the ice meltwater to the initial wastewater salinity. The conductivity removal rate also responds to the effectiveness of the treatment process in removing salt from the water and is defined as the ratio of the difference between the initial wastewater conductivity and ice meltwater conductivity to the initial wastewater conductivity. The formulas are as follows:
where m1 is the mass of ice crystals after the desalination treatment, and m0 is the mass of the initial solution. σ0 is the conductivity of the initial solution, and σ1 is the conductivity measured after the ice meltwater. S0 is the salinity of the initial solution, and S1 is the salinity measured after the ice meltwater.
3. Results and Discussion
3.1. Freezing Parameter Settings
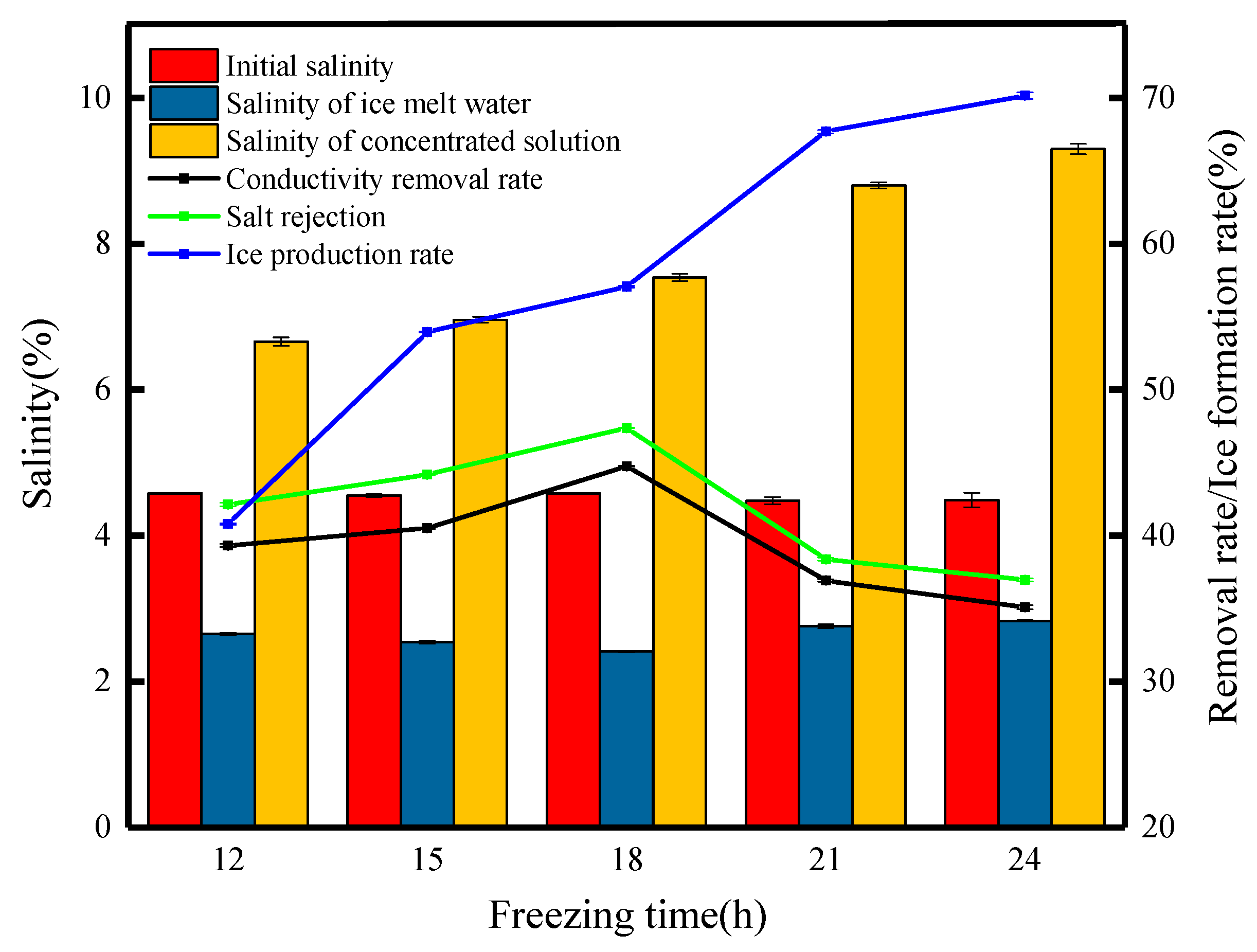
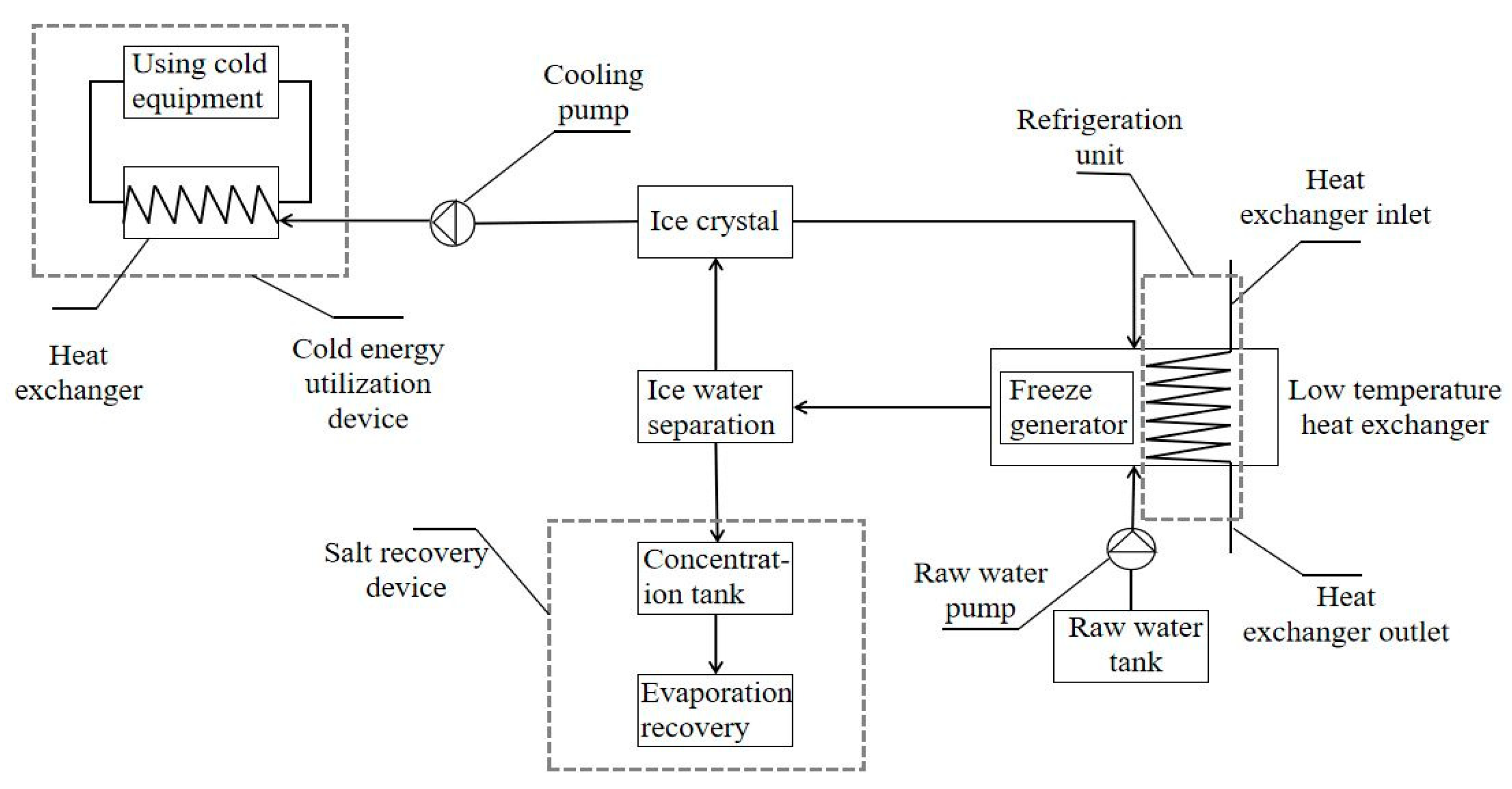
During the freezing process, the water in the solution freezes in solid form, while the solutes are more inclined to be retained in the non-ice phase of the concentrate, and with the prolongation of the freezing time, the ice crystal structure grows and gradually excludes the solutes, thus improving the salt rejection [14]. Figure 1 shows the variation in the water quality parameters at different freezing times. The experiment found that the salinity of the concentrated solution increased with the increasing freezing time, increasing from 6.66% to 9.3%. The increase in salinity of the concentrated solution is mainly due to the longer freezing time, which increases the density and hardness of the ice due to the mutual compression between the ice crystals. During the freezing process, it is difficult for heat to penetrate the interior of the ice, and the melting rate of the ice is relatively slow, which reduces the dilution of the concentrated solution in the stainless steel bucket under the funnel [15]. As the freezing time increases, the ice production rate for the same gravity time tends to increase. This is mainly because prolonged freezing compacts and hardens the ice body, making it more difficult for brine channels to form. The salinity of the ice meltwater decreased and then increased with the freezing time to 2.65%, 2.54%, 2.41%, 2.76%, and 2.83%, respectively, which were significantly lower than the initial salinity of 5%. This result indicates that the salts in the wastewater were effectively removed by the freezing treatment, and the salinity of the ice meltwater fluctuated with the increase in the freezing time. The main reason is that increasing the freezing time means that the solution absorbs more cold energy, causing more water molecules to freeze and reducing the amount of water molecules lost during separation so that the salinity of the ice meltwater decreases, but beyond a certain freezing time, the salinity of the ice meltwater increases as the concentrate becomes more saline and viscous, more likely to adhere to the ice crystals and more difficult to separate. The salt rejection and conductivity removal rates had approximately the same trend, which was increasing with the freezing time first and decreasing after the freezing time reached 18 h. The salt rejections were 42.14%, 44.18%, 47.38%, 38.39%, and 36.97%, respectively. This result shows that the salt rejection is the highest when the freezing time is 18 h. After 18 h, the ice production rate reaches a higher degree, which will inevitably entrain more salt impurities and lead to a decrease in salt rejection. To summarize, the freezing time of 18 h is the best desalination time.
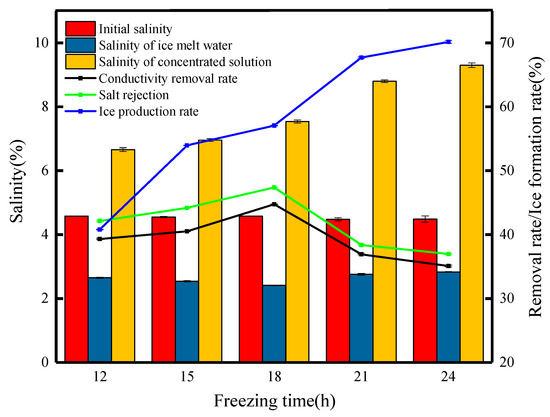
Figure 1.
Changes in the water quality parameters at different freezing times.
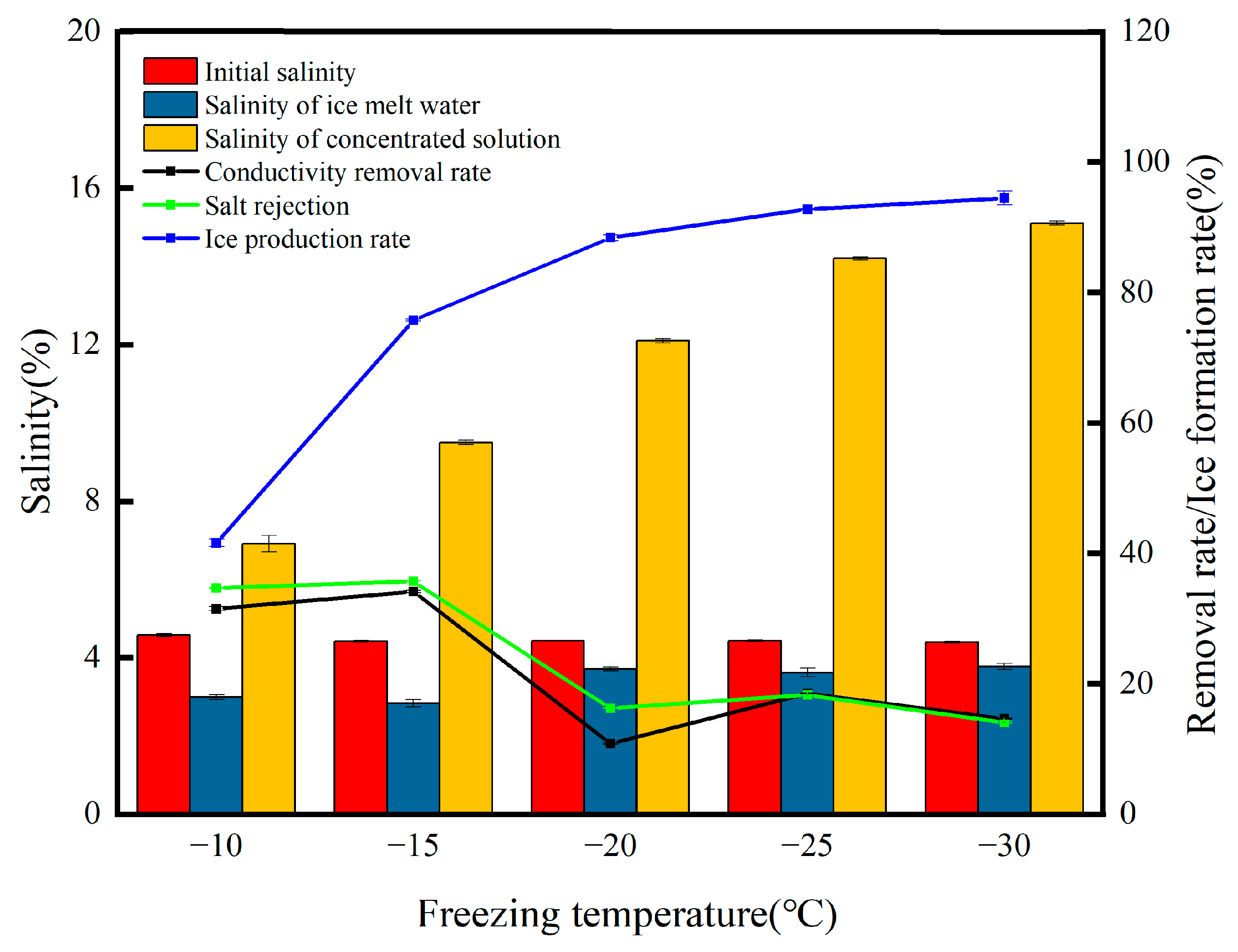
The freezing temperature is one of the important parameters in the freezing process (Figure 2), and as the freezing temperature was reduced from −10 °C to −30 °C, the lower the freezing temperature at the same freezing time, the higher the ice production rate, which increased from 41.61% at the beginning to 94.48%. There is no particular pattern in the change in salt rejection throughout the process, but overall, the salt rejection decreases from 34.71% to 14.09% with the decreasing freezing temperature, and the salinity of the ice meltwater increases from 2.99% to 3.78% initially. Such a result is not unexpected. Concentrates cannot be produced in large quantities at lower temperatures, and the high ice production rate leads to a significant reduction in the migration capacity of the salt ions, which are more transiently held in the ice phase, resulting in a reduction in the salt rejection rate. Liu et al. [16] investigated the effect of freezing temperatures on Ca2+ mobility and the removal rate in the process of PF, and the results showed that, when the freezing temperature was reduced from −5 °C to −25 °C, most of the Ca2+ migrated to the concentrate, and the Ca2+ removal rate of the ice meltwater was reduced from 98.18% to 90.35%, proving that the freezing temperature, as the main parameter, was not the lower the temperature, the better the desalination effect. The salinity of the concentrate with the reduction in temperature showed an upward trend, from −10 °C with 6.91% gradually rising to 15.1%, with a concentration multiplication rate of 3.43. This experimental phenomenon is mainly the lower temperature of the ice produced by the heat capacity, and the heat conduction speed is slow, melting is slower, the salt ions migrate to the concentrate faster than the speed of the icicle melting, and the salt ions migrate to the concentrate in larger proportions [16]. From Figure 2, it can be seen that the highest salt rejection of 35.75% was achieved at a freezing temperature of −15 °C. Therefore, −15 °C is the optimum freezing temperature.
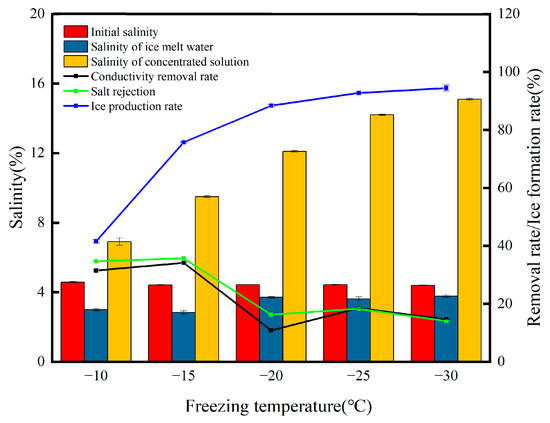
Figure 2.
Changes in the water quality parameters at different freezing temperatures.
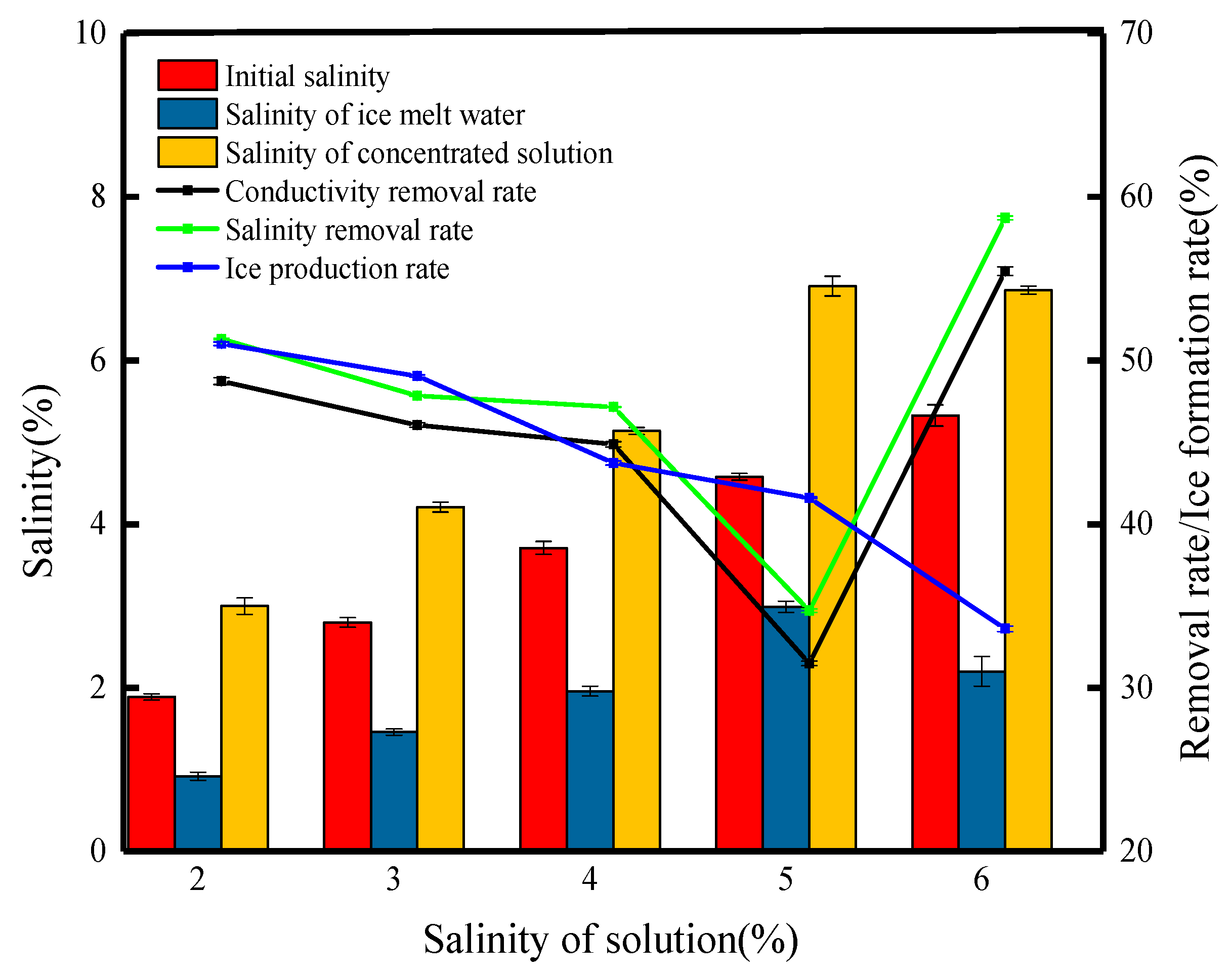
Changes in the water quality parameters at different salinities of the solution are shown in Figure 3. In the process of ice formation, the ice constantly excludes salt ions, and the salinity of the formulated high-salt solution is in the range of 2–5%; with the increase in the initial salinity, the concentration of salt ions per unit area increases and the more salt ions are encapsulated, which makes it more difficult to separate the brine, resulting in a decreasing trend in the salt rejection, which is reduced from the beginning at 51.32% to 34.72%. Moharramzadeh et al. [17], who carried out desalination studies on artificial seawater (35.6 g/L) and concentrated brine (2600 mg/L), showed that the salt removal rate was 74% and 97.7%, respectively, which also indicates that high-concentration brines are more difficult to treat. When the initial salinity was 5%, the salinity of the frozen treated meltwater was 2.99%, and all other conditions remained the same; the increase in the salinity of the initial solution caused the brine channels to become overloaded, and the salt ions in the ice body could not be removed in time to be reintroduced into the solution as the ice body melted, increasing in the salinity of the meltwater. The salt in the solution interferes with the crystallization process of water molecules, resulting in lower freezing temperatures, low hardness, and easy loosening of block ice formed from solutions with higher salinity [18]. As in the experiment, the ice body at 6% initial salinity was loose and in the form of loose flakes when placed in a funnel, and similar results were reported in the study by Teraoka et al. [19]. In this case, the short brine channel makes it very easy to separate the brine from the ice, and the desalination rate is 58.72%. Due to the faster melting rate of the ice flakes, the desalination is better compared to other initial concentrations, but the lowest ice formation rate is only 33.62%. In summary, by analyzing the change in the desalination rate and the special experimental situation, we believe that the lower the salinity of the solution, the better the desalination effect and the more difficult the treatment of high-concentration wastewater.
Figure 3.
Changes in the water quality parameters at different salinities of the solution.
3.2. The Desalination Effect of Different Desalination Methods on Waste Leachate
The complexity of the water quality will directly affect the salt rejection, so the water quality indexes of waste leachate were tested [20]. Compared with the desalination study of Yang Hui et al. [21], the composition of seawater and waste leachate is very different, mainly because most seawater is soluble inorganic salts such as NaCl and the content of soluble organic matter (DOM) is only 0.2–2.0 mg C/L, which is very small and has a small effect on the salt rejection [22,23]. During the freezing process, the concentration of the concentrated solution continues to increase, and the polarity of the solution also increases. This leads to a decrease in the solubility of less polar organic compounds, which precipitate and adhere to the surface of ice crystals. These organic compounds become trapped in the ice crystals, and when the ice melts, they dissolve in the meltwater, ultimately affecting the desalination efficiency [24,25]. The water quality indicators for waste leachate are shown in the Table 1.

Table 1.
Selected water quality indicators for waste leachate.
3.2.1. Research on the Desalination Effect of the FGM on Waste Leachate
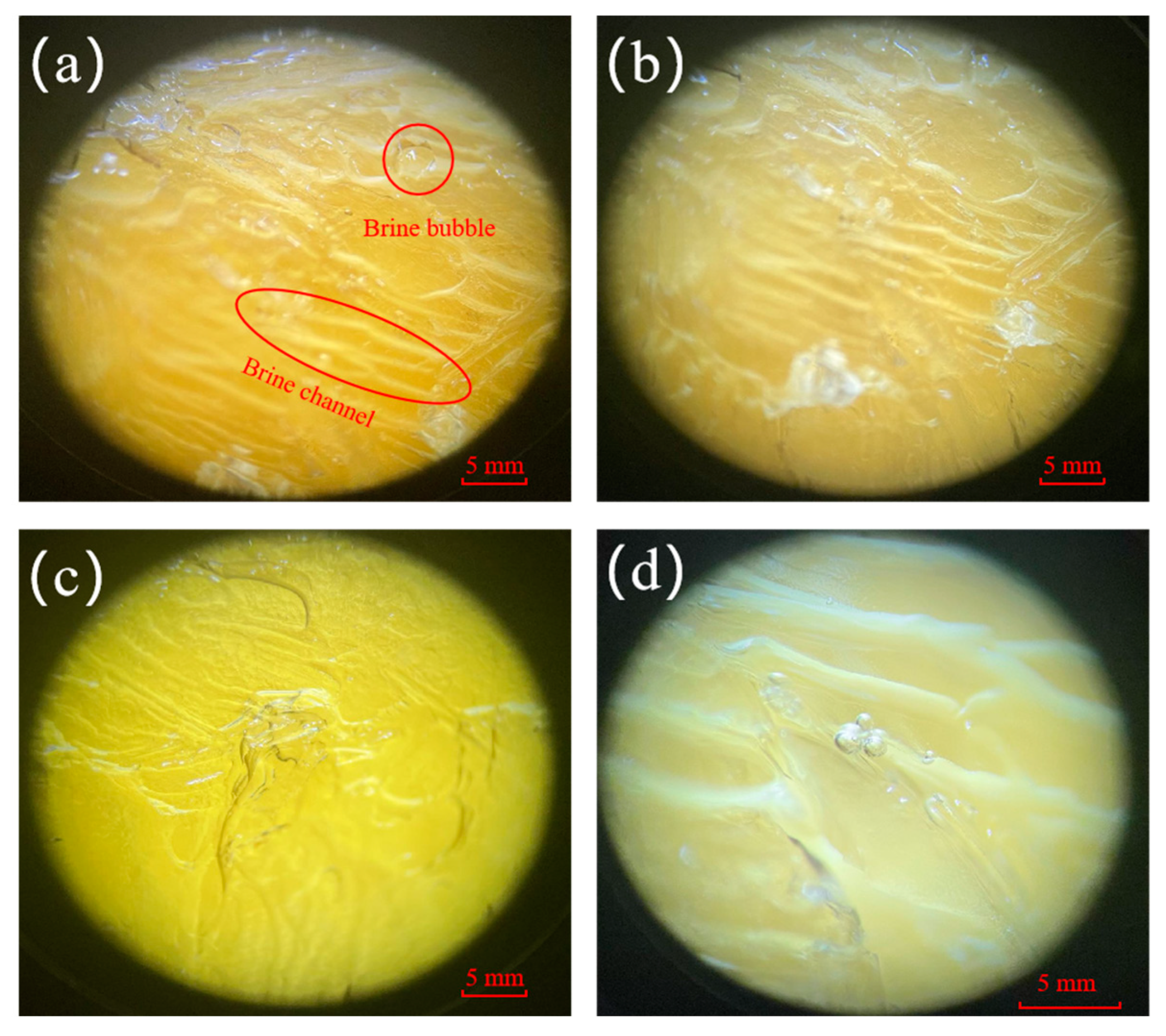
The experiments use the freezing method as the basis of the study and combine it with other methods. The ice matrix itself consists of pure ice grains (columns or flakes), and under gravity, salt ions erode the ice body and form brine channels that gradually penetrate downward until they detach from the ice body [26,27]. The freezing temperature and solution concentration affect the formation of brine channels [28], and under different experimental conditions, the brine channels produced inside the ice body show different characteristics. Figure 4 shows the microstructural observation diagram of brine bubbles and brine channels at different stages under the microscope. By observing the micrographs, it can be seen that brine bubbles of different sizes and shapes with irregular distribution were formed inside the ice body under the four experimental conditions (Figure 4a–d). The brine channels looked like a regular arrangement in the ice body, which was generally in a tree-like structure accompanied by tiny branches. With the prolongation of the sampling time, it can be seen that the brine channels become wider, and the color of the ice body gradually tends to be transparent from yellowish brown.
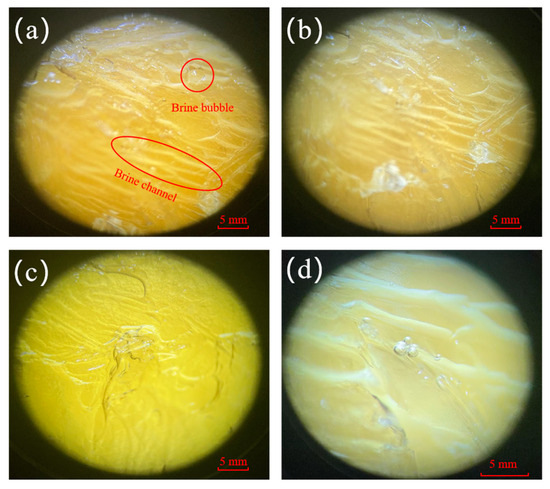
Figure 4.
Microstructural observation diagram of brine bubble vesicles and brine channels at different stages of microscopy: (a) 80% ice production stage, (b) 70% ice production stage, (c) 60% ice production stage, and (d) 50% ice production stage.
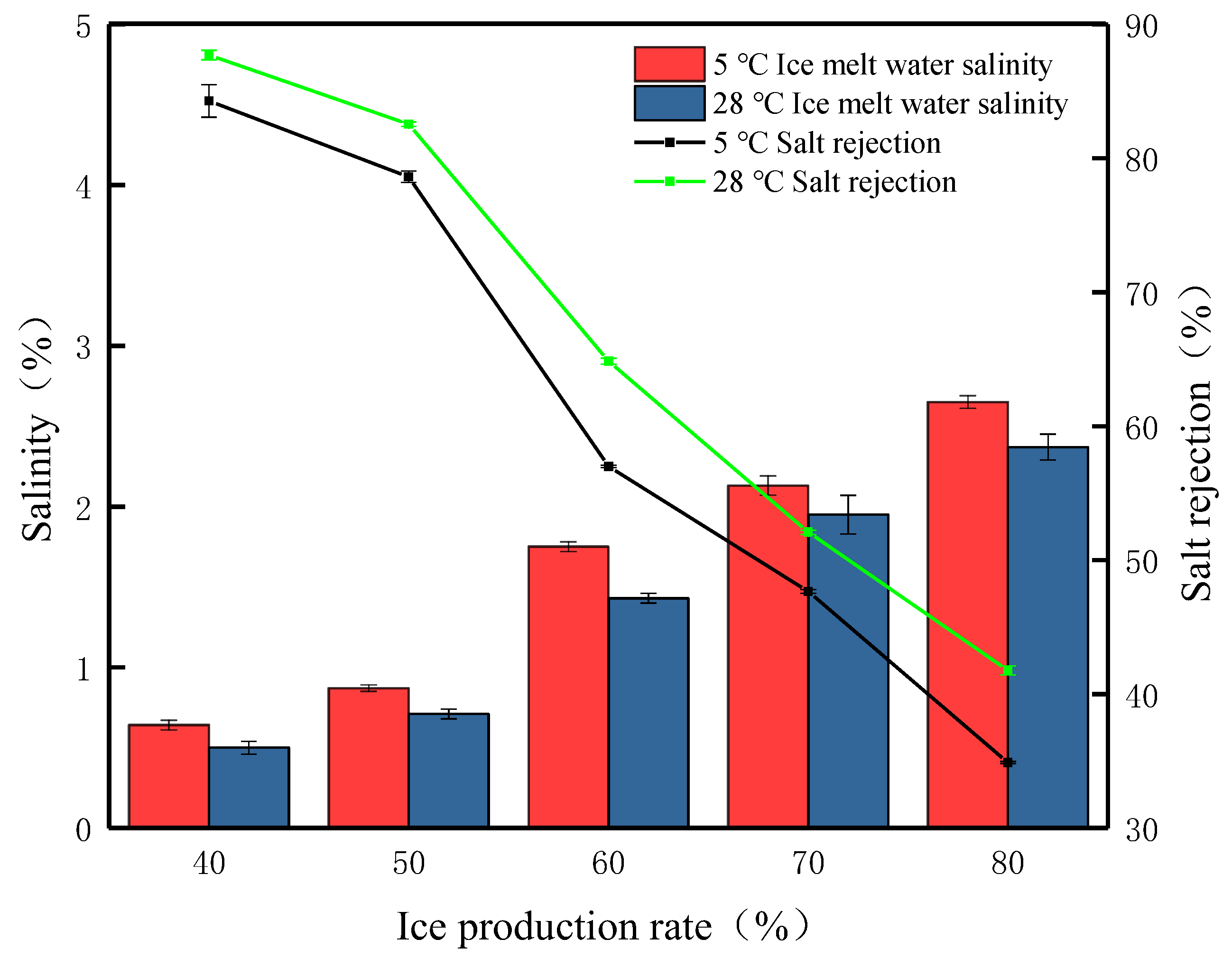
Under simulated winter average temperature conditions (5 °C), gravity desalination experiments were carried out on waste leachate at different ice production rates. The results are presented in Figure 5; the removal efficiency was 84.28% under the condition of the 40% ice production rate. At this time, it was observed that there were a large number of pores on the ice surface produced by brine erosion, and there were a small number of brown solids distributed above the ice body, which were precipitation as the organic matter adsorbed and formed, with a final detection of ice meltwater salinity of 0.64%. When the ice production rate was 50%, the salinity of the ice meltwater was 0.87%, and the salt rejection was 78.62%. When the ice production rate was 60%, the salt rejection decreased the most, the salt rejection was 57%, and the salinity of the ice meltwater was 1.75%. When the ice production rate continued to increase, reaching 70% and 80%, the salt rejection continued to decrease, falling below 50%, accompanied by the salinity of the ice meltwater increasing to 2.65%. This suggests that, at an ambient temperature of 5 °C, an increase in the ice production rate leads to an increase in the number of salt ions retained in the ice body, reducing the passage of salt channels, and fewer salt ions leaving the ice body, leading to a reduction in the salt rejection. Through the study of the salt rejection at different salt rejections under the simulated average winter temperature, it is found that longer times for brine channels to form at higher salt rejections and the formation of brine channels that do not penetrate the entire ice sheet but only form short brine channels within the ice or a small number of narrow brine channels that penetrate the ice sheet result in a slow desalination process. A low salt rejection means that the desalination process takes longer. During this time, the brine channels are fully open, and salt ions are continuously discharged from the brine channels, reducing the salt retention rate in the ice body.
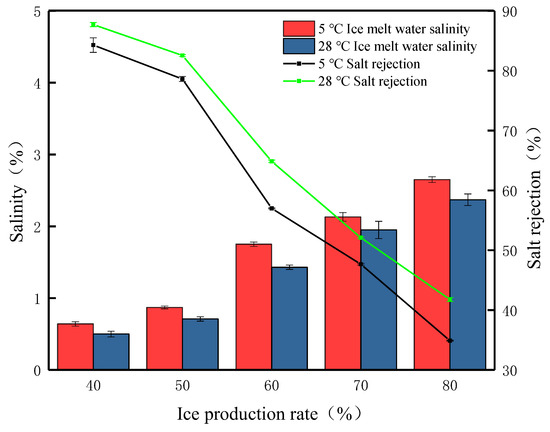
Figure 5.
The FGM desalination parameters at different ambient temperatures.
At the average summer temperature, the trend in salt rejection with different ice production rates is roughly the same as at the average winter temperature. Under the external environment of 28 °C, when the ice production rate is 40%, the salinity of the ice meltwater is 0.5%, and the highest salt rejection is 87.71%. As the ice production rate increased, the salt rejection gradually decreased to 82.55%, 64.86%, and 52.09%, respectively, until the ice production rate of 80%, when the salt rejection was 41.77%. This phenomenon was consistent with the study of Yang et al. [29]. Average summer temperatures compared to average winter temperatures results in higher salt rejection. However, it was found that the color of the ice body gradually changed from yellow-brown to transparent at the winter temperature, but the color of the ice body remained brown and did not change much during the gravitational desalination process at the average summer temperature. We carried out the analysis and detection of this phenomenon and found that the TOC content of ice meltwater at an ambient temperature of 28 °C is 1.86 times that of 5 °C. This is mainly caused by organic residues. The salt is mainly a combination of anions and cations formed into compounds, the organic molecular structure is complex, and the molecular size is often larger, with higher ambient temperatures. To achieve a predetermined rate of ice in a short period, the formation of a brine channel is difficult to pass through the macromolecules of organic matter, and the result is that the residue in the ice body cannot be discharged promptly. Therefore, higher ambient temperatures are detrimental to the removal of organic matter for the same ice production rate. Considering the ice meltwater salinity, with the same ice production rate conditions, the summer ice meltwater salinity is lower than the winter but one cannot simply think that gravity desalination at higher temperatures has a better desalination effect; when at a certain temperature, the ice body will melt a large amount of water, which will reduce the salt rejection.
3.2.2. Research on the Desalination Effect of the FCM on Waste Leachate
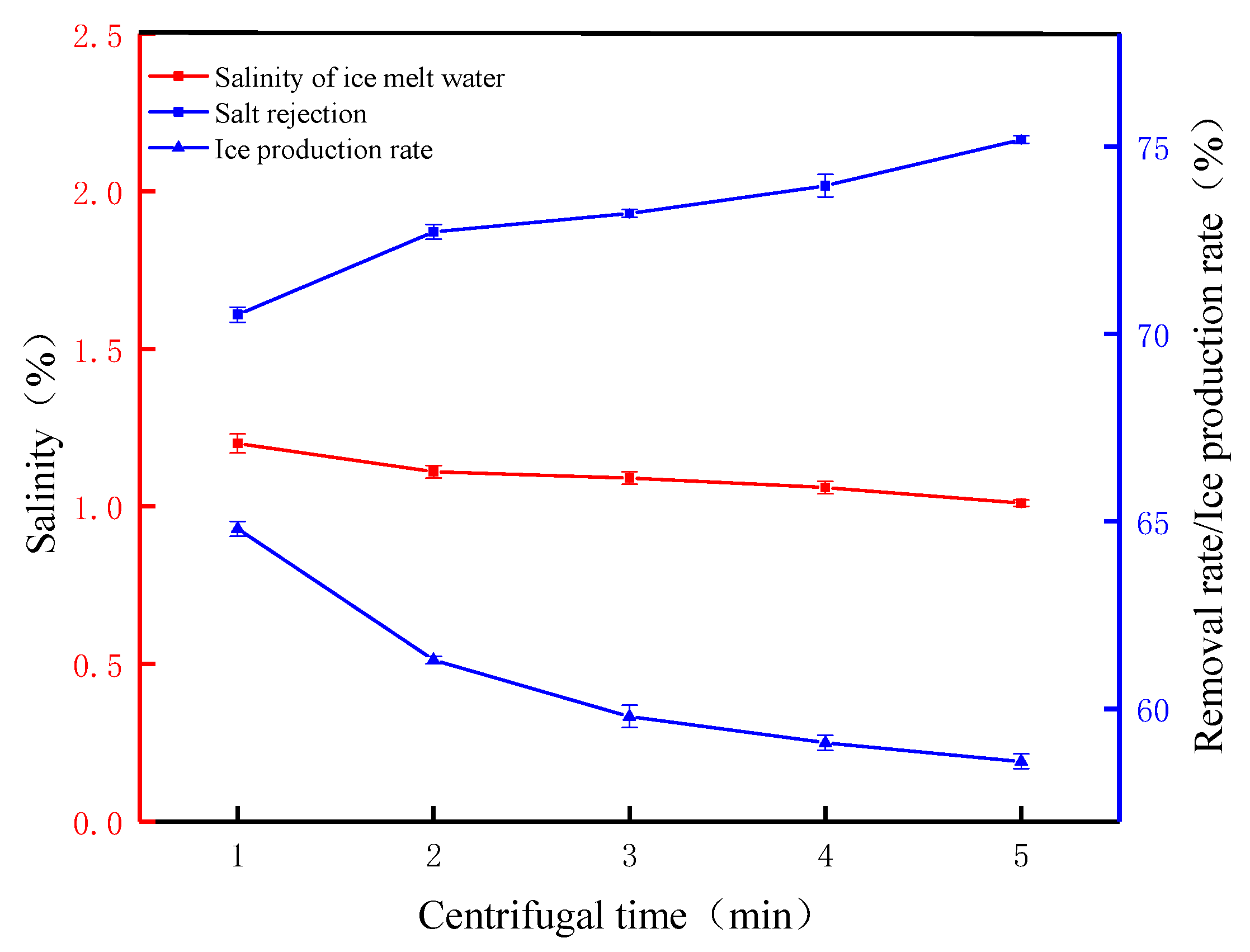
Figure 6 shows the FCM desalination parameters at different centrifugal times (speed 2000 rpm). The surface area of the crushed ice crystals was larger and more concentrated brine was attached to the surface, and most of the concentrated brine was separated by centrifugal force after it was applied. When the centrifugal time was 1 min, the salt ions attached to the surface of the ice crystals were separated, and the salt rejection was 70.51%. The results show that 1 min of centrifugal force released most of the salt ions from the ice crystals. As the centrifugal time increases, the salt rejection increases from 70.51% to 75.18%. Although the salt rejection increased, the rate of was is slow, mainly due to the low melting tendency inside the broken ice crystals. The melted water is temporarily stored inside the ice crystals. Due to the small volume of the ice crystals, the salt ions carried inside are relatively small and are carried out under the action of centrifugal force [30]. The ice production rate continues to decrease from 64.87% to 58.68%, which is consistent with the FGM’s conclusion that increasing the salt rejection reduces the ice production rate but reduces the pure water loss rate due to the relatively fast FCM process, which avoids losses due to the melting of ice crystals during operation. From the perspective of the salinity of the melted ice water, after 5 min of centrifugal force, the salinity of the melted ice water decreased from 4.07% to 1.01%, indicating that the salinity of the melted ice water decreased with the increase in the centrifugal time. These results indicate that centrifugal time, as an important parameter, has a significant effect on salt rejection, and salt rejection increases with the increase in centrifugal time. This result is consistent with the research of other scientists [31].
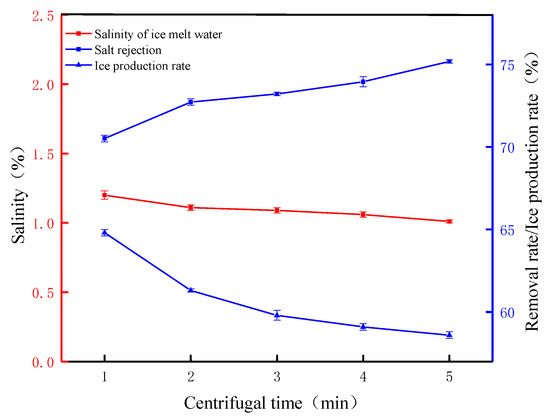
Figure 6.
The FCM desalination parameters at different centrifugal times (speed 2000 rpm).
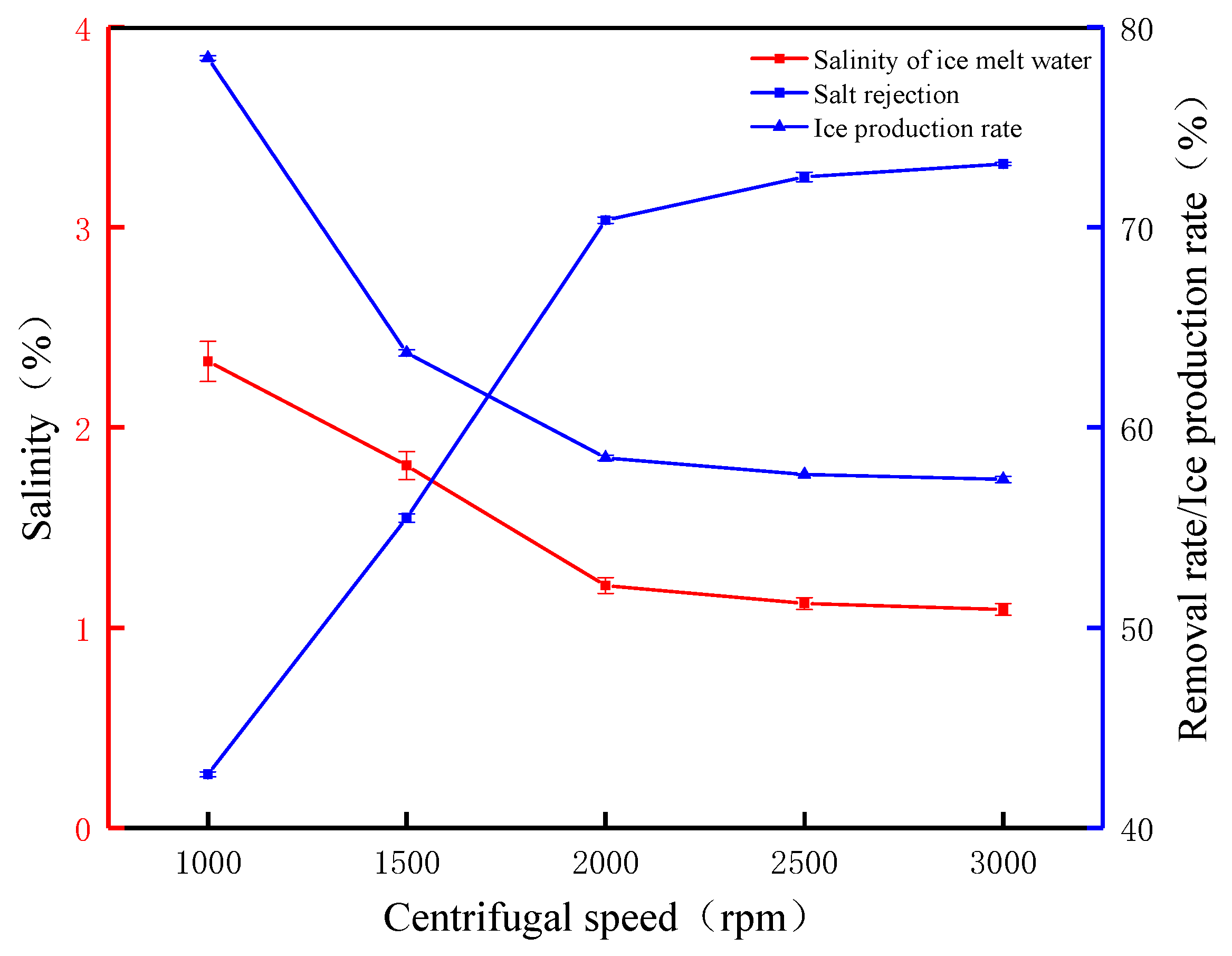
To further investigate the effect of the centrifugal speed on the salt rejection, experiments were carried out with the centrifugal speed as a variable. The effect of the centrifugal rotational speed on the salt rejection and ice production rate is shown in Figure 7. When the rotational speed was 1000 rpm, the salt rejection and ice production rates were 42.67% and 78.46%, respectively. At this time, the rotational speed was low, and the centrifugal force was not enough to overcome the friction and other hindrances, resulting in a lower salt rejection. When the rotational speed was 1500 rpm, the salt rejection was 55.48%, but the ice production rate decreased by 14.74% until the rotational speed was increased to 2000 rpm when the salt rejection was 70.36%. This indicates that, as the centrifugal rotational speed increases, the centrifugal force increases, which accelerates the settling rate of salt molecules, thus greatly improving the salt rejection. At 2000 rpm increased to 3000 rpm, the salt rejection increased from 72.52% to 73.16%, which was only a 0.64% increase in salt rejection. The centrifugal process observed that, when the rotational speed was continuously increased, a collapsed layer of gradually increasing thickness was formed at the bottom inside the centrifuge, causing ice crystals to collect on the bottom inner wall of the centrifuge. The experimental results and phenomena showed that most of the salts were discharged when the rotational speed was above 2000 rpm, the salt rejection changed slowly, and the surface of the ice crystals tended to be dry. Changes in the salinity of ice meltwater can more clearly reflect the relationship between salt rejection and rotational speed; before the centrifuge rotational speed of 2000 rpm, the trend of ice meltwater salinity is obvious, from 2.33% to 1.21%, and after that, the increase in the rotational speed of ice meltwater salinity will be 1.09% from 1.21%, and the ice production rate decreases to 57.41%. This also proves that the centrifugal speed has a significant impact on salt rejection and also proves that the increase in salt rejection requires a decrease in the ice production rate as a cost. In summary, when using the FCM to treat waste leachate, increasing the centrifugal time and speed has a positive effect on the removal rate. Increasing the centrifugal speed within a certain range can accelerate the removal of concentrated saline water.
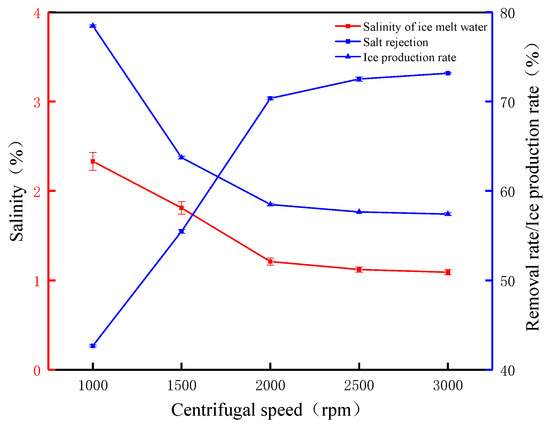
Figure 7.
The FCM desalination parameters under different centrifugal speeds (time 2 min).
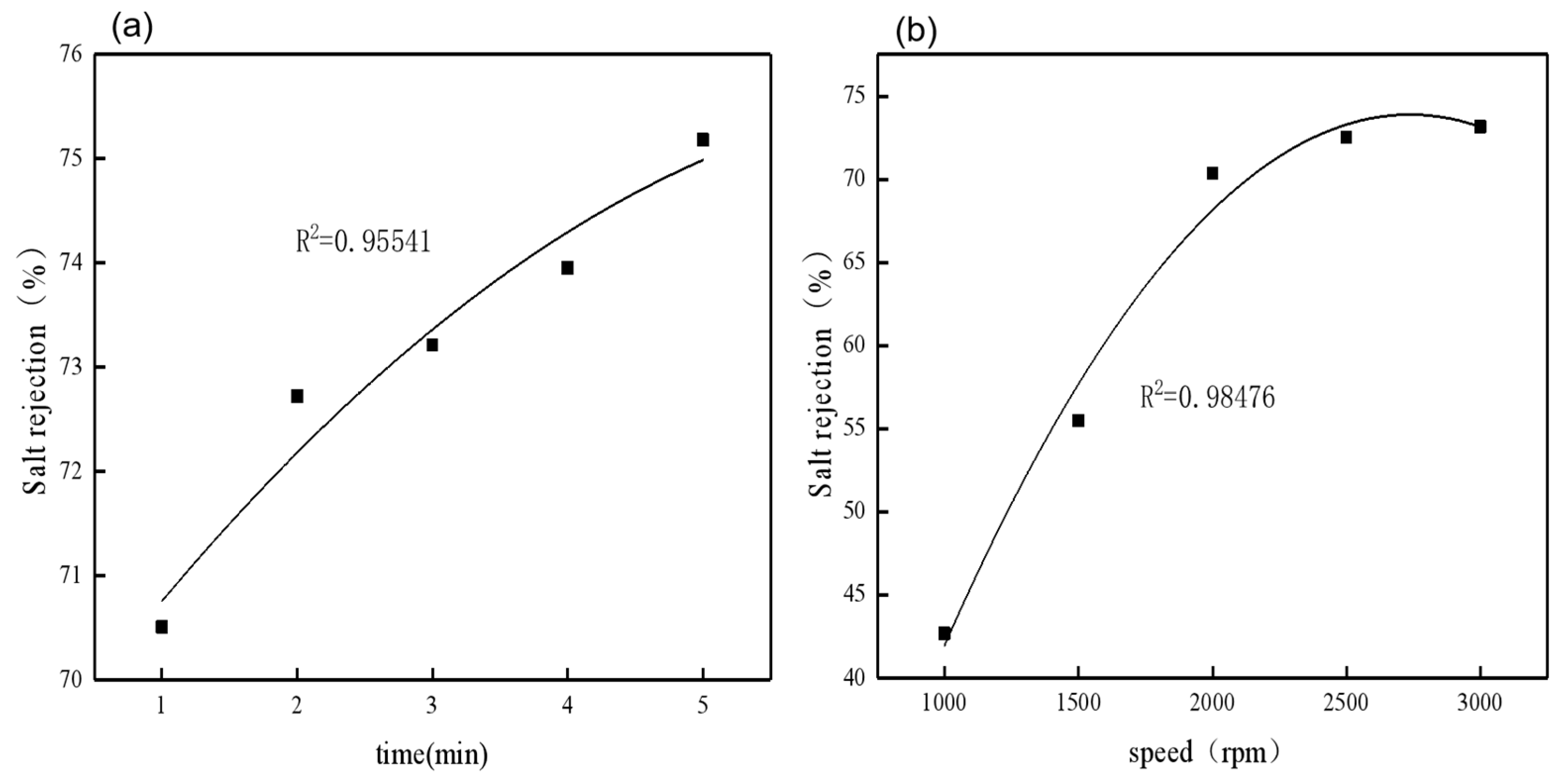
After polynomial fitting of the centrifugal time and centrifugal speed, it can be seen that the two factors have a good correlation with the salt rejection, with correlation coefficients (R2) of 0.95541 and 0.98476, respectively (Figure 8). At the same time, using analysis of variance (ANOVA), it can be concluded that the correlation between salt rejection and centrifugal time (p = 0.007, F = 45.292) and centrifugal speed (p = 0.026, F = 16.747) is high, and the p-values are all less than 0.05 (Table 2), indicating a significant correlation.
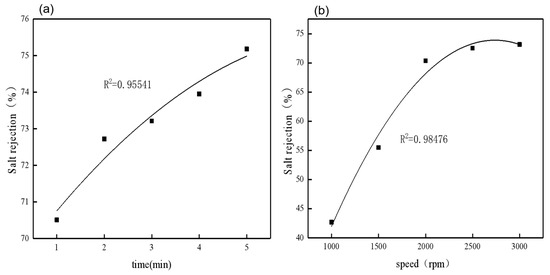
Figure 8.
The relationship between salt rejection and centrifugal time, (a) and the relationship between salt rejection and centrifugal speed (b).

Table 2.
ANOVA for the salt percent removal and process parameters.
3.2.3. Research on the Desalination Effect of the FBM on Waste Leachate
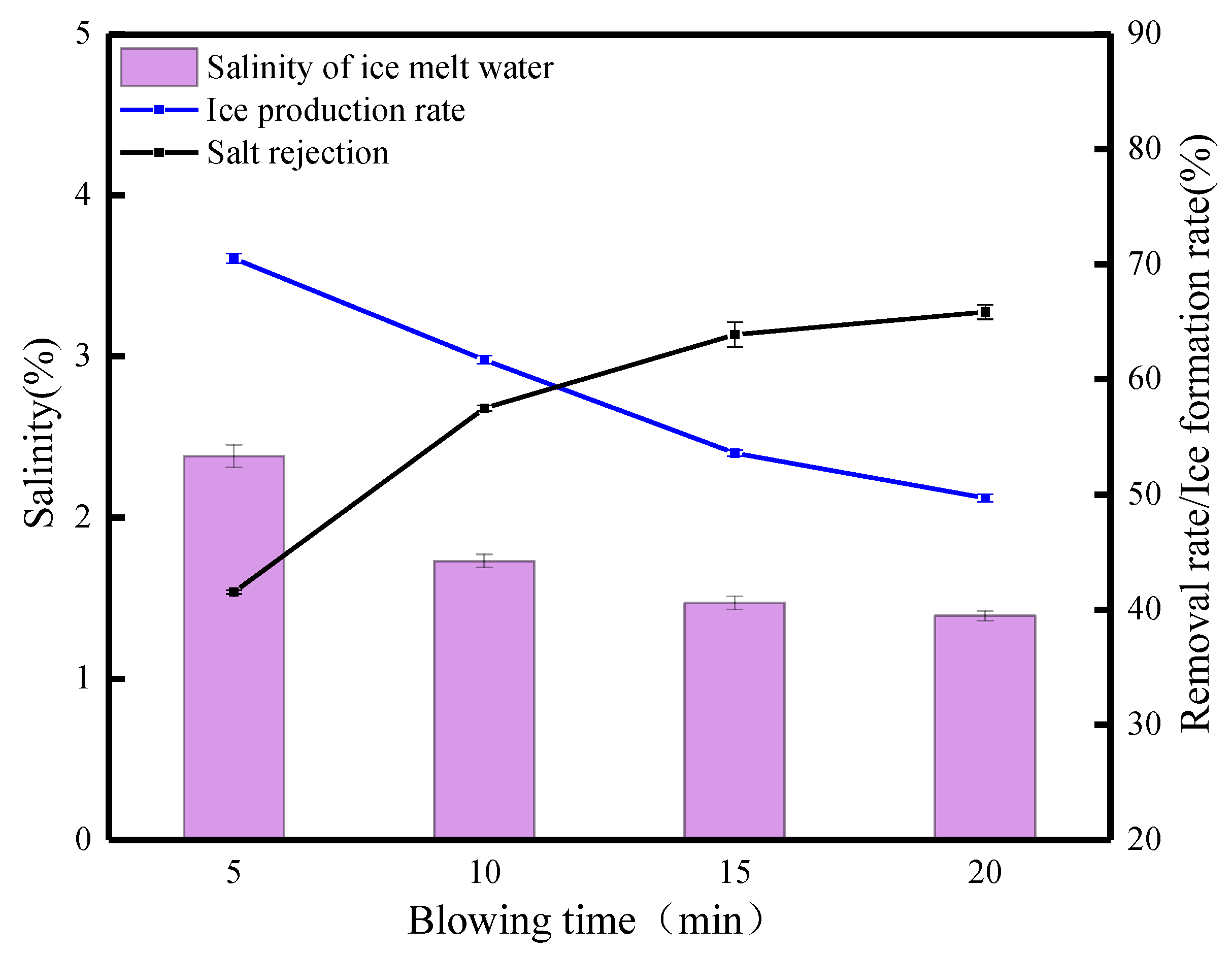
The FBM as a potential desalination method is based on the research of Rees Jones et al. [32] on desalination in saline canals. Therefore, we consider whether the FGBM can improve the desalination effect. The basis for this idea is mainly the following two factors: the formation of brine channels under gravity and the kinetic properties of the blowing wind so that the salt molecules produce displacement, vibration, or deformation and accelerate the separation from the ice. The blowing wind can accelerate the melting of the ice, and meltwater can be used as a detergent to clean the brine channels to achieve purification of the ice. Thus, a study of desalination after 100 min of gravity combined with different blowing times was initiated. Figure 9 shows the desalination parameters of waste leachate after 100 min of gravity combined with blowing. With the increase in blowing time, the salt rejection gradually increased from 41.52% at the beginning to 65.85%, and the ice production rate decreased from 70.53% to 49.72%. The salinity of the ice meltwater decreased from 3.24% to 1.39%. When the blowing time was 5 min, the salt rejection was 41.52%. When the blowing time was 10 min, the salt rejection increased to 57.49% when the salinity of the ice meltwater was 1.72%. When blowing for 15 min, the salt rejection was 63.88%, and the ice production rate was 53.61%. When the blowing action was 20 min, the salt rejection was 65.85%. Under the effects of the FGBM, the salt rejection increased continuously. Although the overall trend in salt rejection increased, the change in salt rejection from 15 to 20 min was not significant, and the salinity only decreased by 0.08%. There were two reasons for the analysis: one was that the brine channel formed at gravity 100 min was small and narrow, and the second was that the ice body was too thick, and the blowing effect could not penetrate the whole ice layer. The phenomenon observed in the experiment was that the color of the surface ice body tended to become transparent with the continuous action of the blowing wind, which indicated that, under the action of the blowing wind, the salts in the surface layer of the ice body accelerated to sink inside of the ice body under the combined actions of gravity and blowing wind, which had a good removal effect on the surface salt and organic matter. A comparative analysis showed that the FGBM was limited by two main factors, namely the large thickness of the block ice and the easy melting of the ventilated surface layer, which did not result in an advantage in the overall salt rejection of the FGBM. Therefore, the formed ice block should first be crushed before being blown.
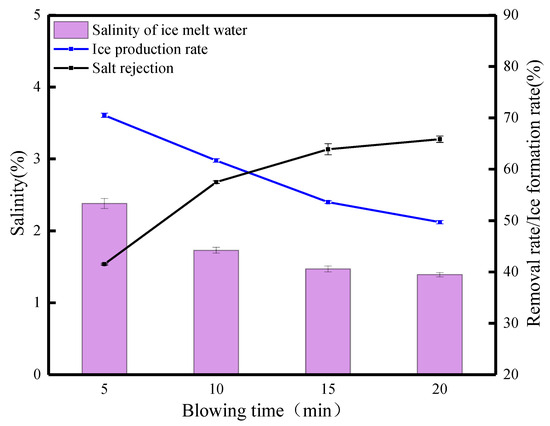
Figure 9.
Desalination parameters of the FGBM on waste leachate (gravity action for 100 min).
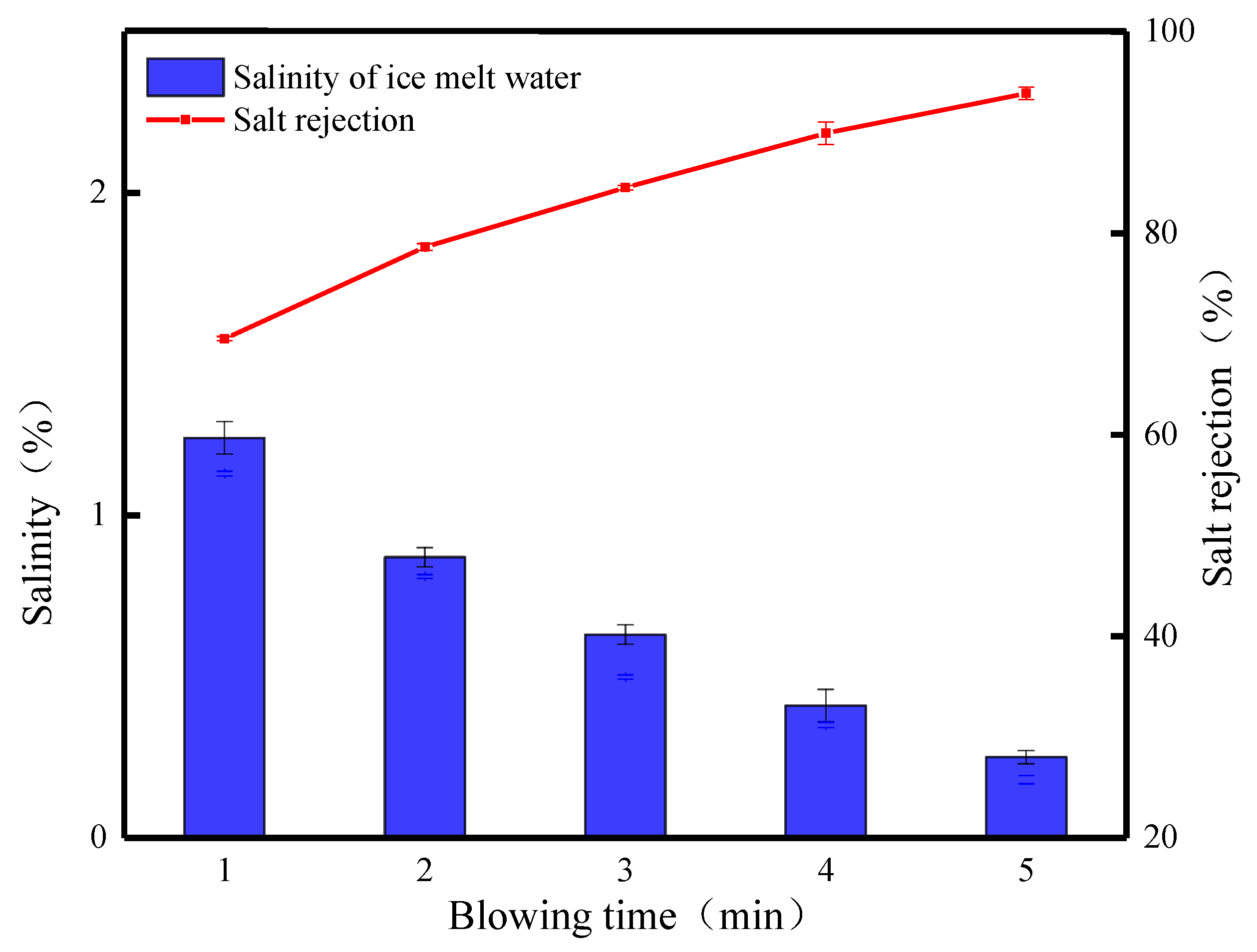
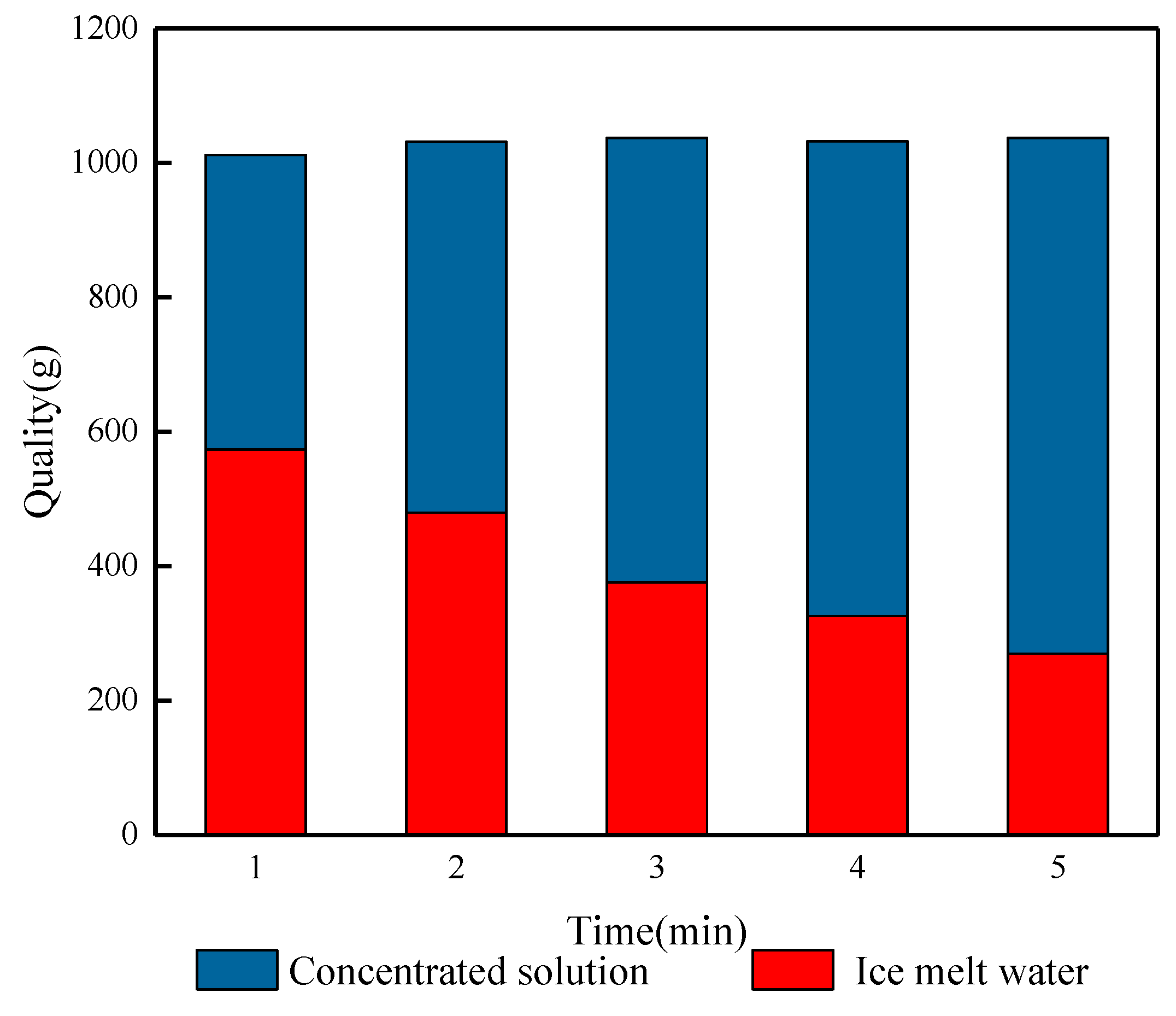
Figure 10 shows the effects of different blowing times on the salt rejection of waste leachate after crushed ice treatment. From the figure, it can be seen that the salt rejection of the FCBM changes more obviously with the increase in blowing time. When the blowing time is 1 min, the salinity of the ice meltwater is 1.24%, the salt rejection is 69.53%, and the ice production rate is 56.15%. At this time, it was observed that the broken ice in the center of the funnel was slightly yellowish brown, and the surrounding ice crystals gradually changed from yellowish brown to transparent. This phenomenon is mainly caused by the cleaning effect of meltwater on the ice crystals in the upper layer and their accumulation in the lower layer. With the prolongation of the blowing time, the salt rejection slowly increases, and when the blowing time is 2, 3, 4, and 5 min, respectively, the ice production rate decreases from 56.15% to 25.8%, and a large amount of ice melts to soak and rinse the crushed ice from the top to the bottom, which then achieves a higher rate of salt rejection, which is 24.33% higher, reaching 78.62%, 84.52%, 89.92%, and 93.86%, respectively. At low temperatures, water molecules are arranged in an orderly manner and gradually deposited on the surface of ice crystals [33]. The broken ice will be fused due to the physical properties of solidification and crystallization, and when the fine ice crystals are in contact with each other, they will interact with each other. The deposited water molecules expand to other ice crystals to form broken ice with a larger surface area and more pore space, which results in a more stable rate of salt discharge and a steady increase in salt rejection. Extending the blowing time causes the ice crystals to continuously melt and discharge the concentrated solution carrying salt ions, which increases the quality of the concentrated solution obtained, while the quality of the ice meltwater decreases, reflecting the effluent situation of the leachate wastewater treatment (Figure 11). When blowing for 5 min, the lowest salinity of the ice meltwater is 0.25%, and the salt rejection is 93.86%. The obtained concentrated solution and ice meltwater have masses of 767.46 g and 269.63 g, respectively, and the effluent is only 26%. The experimental results indicate that the desalination effect of the FCBM on waste leachate wastewater increases with the increase in the blowing time, which solves the problems of large ice thickness and low salt rejection. However, the increase in the processing time leads to a decrease in the water yield, which is also the reason why the blowing time cannot be set too long.
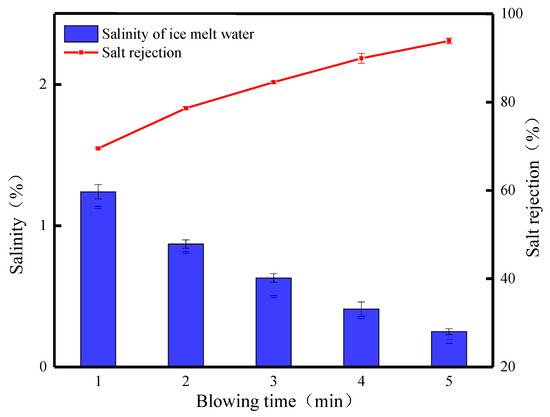
Figure 10.
Desalination parameters of the FCBM for waste leachate.
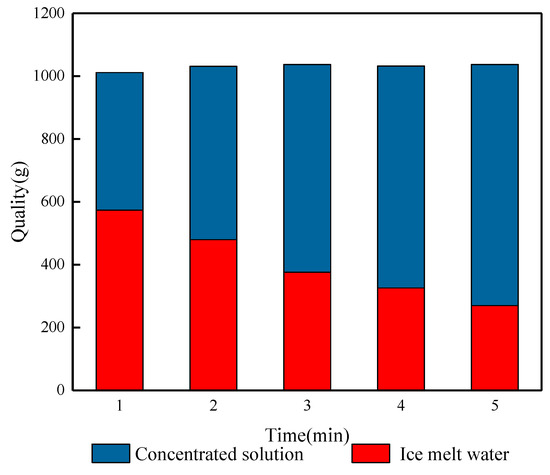
Figure 11.
Quality relationship between the ice crystal melt effluent and discharged concentrate.
3.3. Comparison of the Different Desalination Methods
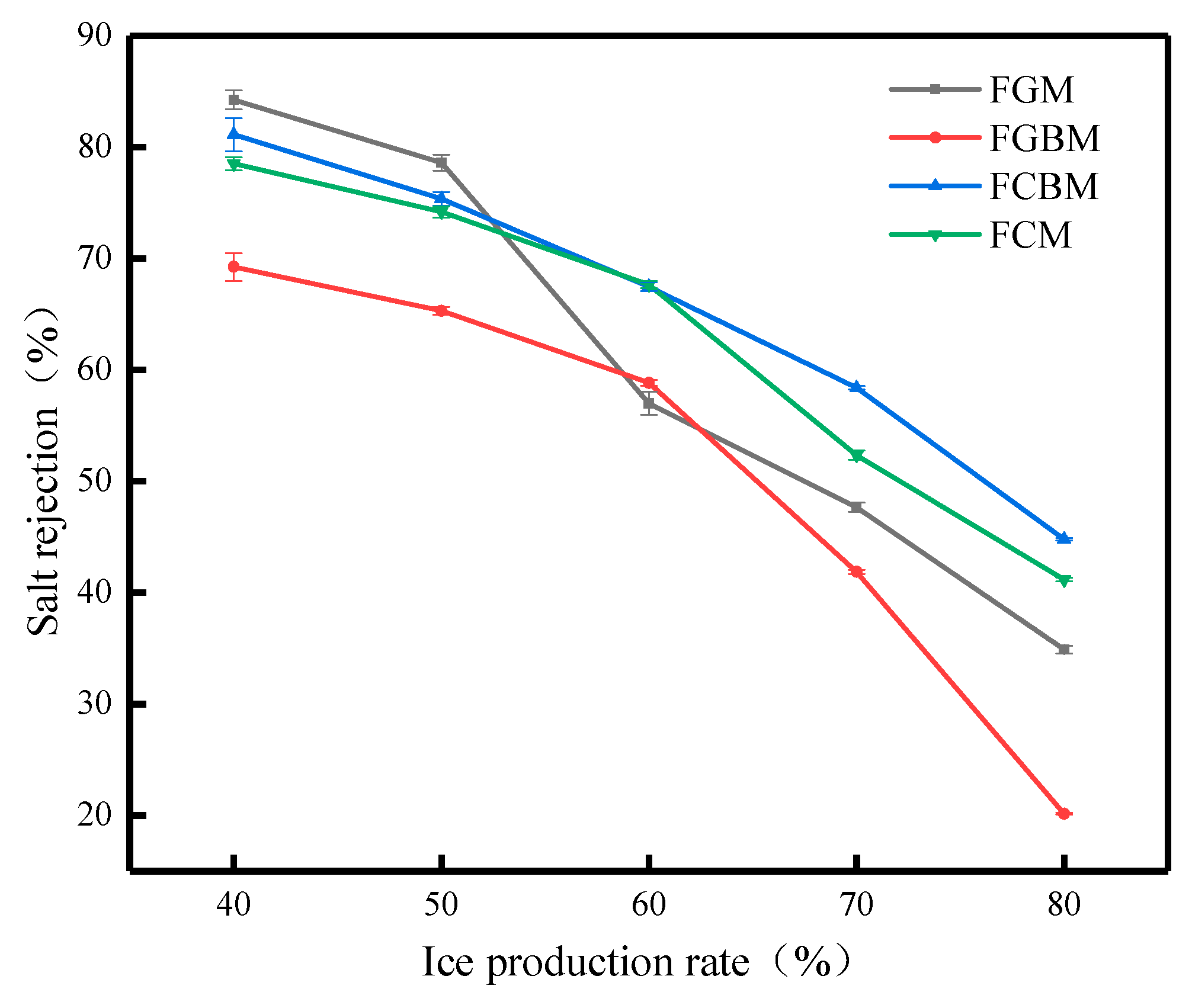
Figure 12 shows the relationship between the ice production rate and the salt rejection under different methods, and the most intuitive phenomenon in the figure is that the salt rejection decreases with the increase in the ice production rate. It is found that the above methods have some variability in the desalination effect, and this variability is based on the similarities and differences in the desalination principles of the different methods, which are mainly concentrated in the stage of high ice production rates [34]. When the ice production rate is 40%, the salt rejection in descending order is the FGM > FCBM > FCM > FGBM. Overall, however, the fluctuation of the FCBM under different ice production rates is relatively small, and its stability is higher. Compared with the FGM, the desalination process has a shorter duration. Although the FCM significantly diminishes the desalination duration, the salt rejection remains inferior to the FCBM at varying ice production rates. Therefore, the advantage of the FCBM lies in its ability to shorten the desalination process while improving the desalination efficiency.
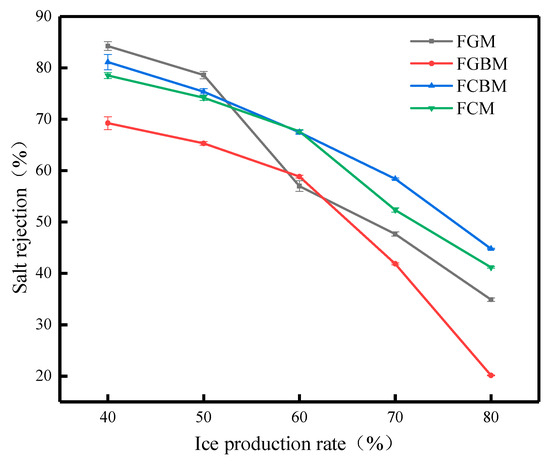
Figure 12.
Effect of each method on salt rejection at different salt rejections.
The FGM, FCM, and FBM are all methods that utilize cooling and freezing processes to achieve the icing of water molecules and separation of salt, differing only in operating principles and application scenarios, and their actual application requires a comprehensive consideration of factors such as the treatment scale, energy consumption, equipment cost, technical difficulty, and salt discharge to make a choice.
3.4. Resource Recovery Strategy
The combined freezing method is a very promising method for which a system for wastewater desalination and resource recovery and utilization has been designed (as shown in Figure 13). In the laboratory, we choose safer electricity to provide cooling energy, and in some resource-rich areas, liquefied natural gas can be used to provide cooling energy; the separated concentrated solution can be collected in a concentration tank, and valuable elements can be recycled. The collected ice crystals can be used to cool equipment in the factory area, improving the resource use efficiency.
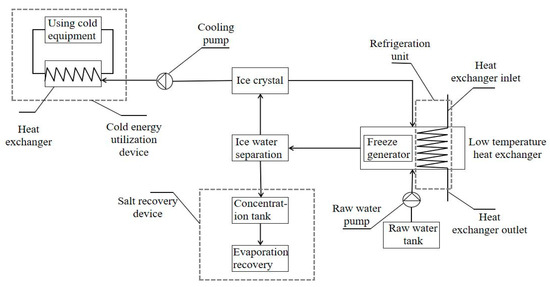
Figure 13.
A system for wastewater desalination and resource recovery and reuse.
4. Conclusions
This study focuses on evaluating and analyzing the effectiveness of three different methods of ice crystal desalination. Based on the above research, conclusions are summarized as follows:
The optimum experimental conditions for determining the freezing time and freezing temperature by the single-factor method are 18 h and −15 °C, respectively.
When the FGM is used to treat waste leachate wastewater, the rate of desalination increases as the rate of ice formation decreases. At higher ambient temperatures, the FGM has a better desalination effect, but it is not conducive to the removal of organic pollutants. When the ice production rate is 40%, the salt rejection reaches 84.28% at an average temperature of 5 °C in the winter. The salt rejection is even higher at an average temperature of 28 °C in the summer, reaching 87.71%.
The FCM is a common desalination method, and its desalination effect is influenced by the centrifugal time and rotation speed. Research has found a significant correlation between the centrifuge time, centrifuge speed, and salt rejection (p < 0.05). Increasing the centrifugal time and speed helps to improve the salt rejection, and increasing the centrifugal speed within the range of 1000–2000 rpm can accelerate the discharge of concentrated brine more effectively.
The most promising FBM is the FCBM, which has higher desalination stability compared to the FGBM. As the blowing time increases, the salt rejection continues to increase, with a salt rejection of 93.86% at 5 min of blowing. Compared to the FGM and FCM, it accelerates the desalination process and improves salt rejection.
Overall, our research results indicate that the combined freezing method for desalination is feasible and can be reasonably selected based on the water quality and effluent standards of different wastewaters. Especially when using the FCBM for wastewater treatment, shortening the desalination process and improving the desalination efficiency are undoubtedly promising. It is worth noting that the freezing method produces a large amount of concentrate in the process of waste leachate treatment, which may lead to a waste of resources and environmental pollution problems. Therefore, subsequent research will focus on improving the process parameters and recycling concentrate resources and, at the same time, pay attention to the removal of different salt ions in wastewater to gain a deeper understanding of the differences in the removal of salt ions by freezing methods.
Author Contributions
X.W.: Conceptualization, Data curation, Formal analysis, and Writing—original draft. C.Z.: Funding acquisition, Data curation, Supervision, and Writing—review and editing. B.G.: Methodology and Data curation. B.Z.: Funding acquisition and Data curation. X.L.: Investigation. Y.G.: Investigation. Y.D.: Data curation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (41877041), Qilu University of Technology (Shandong Academy of Sciences) Science Education Industry Integration Innovation Pilot Project (2020KJC-ZD13), the Natural Science Foundation of Shandong Province (ZR2022MC204), and study on the Application of Highly Substituted Cationic Starch in Textile Industry (2021CXY-09).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Santos, M.-T.; Pedro-André, L. Sludge recovery from industrial wastewater treatment. Sustain. Chem. Pharm. 2022, 29, 100803. [Google Scholar] [CrossRef]
- Carvajal-Flórez, E.; Cardona-Gallo, S.A. Technologies applicable to the removal of heavy metals from landfill leachate. Environ. Sci. Pollut. Res. 2019, 26, 15725–15753. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.P.; Wu, W.H.; Shi, P.; Guo, J.; Cheng, J. Characterization of dissolved organic matter in landfill leachate during the combined treatment process of air stripping, Fenton, SBR and coagulation. Waste Manag. 2015, 41, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Adeniyi, A.; Mbaya, R.K.K.; Onyango, M.-S.; Popoola, A.P.I.; Maree, J.P. Efficient suspension freeze desalination of mine wastewaters to separate clean water and salts. Environ. Chem. Lett. 2016, 14, 449–454. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Song, M.; Dai, B.; Sun, Z. Impacts on the solidification of water on plate surface for cold energy storage using ice slurry. Appl. Energy 2018, 227, 284–293. [Google Scholar] [CrossRef]
- John, M.; Semken, R.S.; Mikkola, A.; Häkkinen, A. Natural freeze concentration of wastewater for nutrient recovery. Cold Reg. Sci. Technol. 2023, 213, 103943. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, C.; Rong, H.; Zhao, M.; Gou, S. Treatment of electroplating wastewater using the freezing method. Sep. Purif. Technol. 2020, 234, 116043. [Google Scholar] [CrossRef]
- He, T.; Chong, Z.R.; Zheng, J.; Ju, Y.; Linga, P. LNG cold energy utilization: Prospects and challenges. Energy 2019, 170, 557–568. [Google Scholar] [CrossRef]
- Chang, J.; Zuo, J.; Lu, K.J.; Chung, T.-S. Freeze desalination of seawater using LNG cold energy. Water Res. 2016, 102, 282–293. [Google Scholar] [CrossRef]
- Zikalalaa, N.; Mareeb, J.-P.; Zvinowandac, C.; Akinwekomi, V.; Mtombeni, T.; Mpenyana-Monyatsi, L. Treatment of sulphate wastewater by freeze desalination. Desalination Water Treat. 2017, 79, 93–102. [Google Scholar] [CrossRef]
- Klotz, S.; Komatsu, K.; Pietrucci, F.; Kagi, H.; Ludl, A.-A.; Machida, S.; Hattori, T.; Sano-Furukawa, A.; Bove, L.E. Ice VII from aqueous salt solutions: From a glass to a crystal with broken H-bonds. Sci. Rep. 2016, 6, 32040. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yuanfei, J.; Rui, W.; Dong, B.; Fu, M. Effects of the soaking-related parameters in a combined freezing-based seawater desalination process. Environ. Sci. Pollut. Res. 2022, 29, 52162–52174. [Google Scholar] [CrossRef]
- Tang, W.; Tao, J.; Wang, J.; Liu, C.; Zhang, H. Sea ice desalination under gravity using microwave heating. Desalination 2018, 430, 159–164. [Google Scholar] [CrossRef]
- Shum, E.; Papangelakis, V. Water recovery from inorganic solutions via natural freezing and melting. J. Water Process Eng. 2019, 31, 100787. [Google Scholar] [CrossRef]
- Li, Z.; Yong, L.; Jianping, W.; Wu, J. Molecular dynamics simulation and experimental study into effect of temperature on hardness of ice abrasives. Front. Earth Sci. 2023, 10, 1114421. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, Y.; Tang, Y.; Xiaozhuang, W.; Chen, Z.; Nan, W.; Yucan, L. Application of progressive freeze concentration in the removal of Ca2+ from wastewater. J. Water Process Eng. 2022, 46, 102619. [Google Scholar]
- Moharramzadeh, S.; Say-Kee, O.; James, A.; Cetin, K.S. Parametric study of the progressive freeze concentration for desalination. Desalination 2021, 510, 115077. [Google Scholar] [CrossRef]
- Peng, Z.; Fuqiang, T.; Jingwei, W.; Huang, J.; Hu, H.; Darnault, C.J. A numerical model for water and heat transport in freezing soils with nonequilibrium ice-water interfaces. Water Resour. Res. 2016, 52, 7366–7381. [Google Scholar] [CrossRef]
- Teraoka, Y.; Akio, S.; Seiji, O. Formation de cristaux de glace dans une solution surrefroidie. Int. J. Refrig. 2002, 2, 218–225. [Google Scholar] [CrossRef]
- Htira, T.; Claudia, C.; Emilie, G.; Mangin, D. Experimental study of industrial wastewater treatment by freezing. J. Water Process Eng. 2018, 23, 292–298. [Google Scholar] [CrossRef]
- Yang, H.; Zhengyang, S.; Zhonglai, Z.; Zhang, H.; Yao, Y. Effects of watering parameters in a combined seawater desalination process. Desalination 2018, 42, 577–585. [Google Scholar] [CrossRef]
- Park, S.; Taewoo, N.; Jeongyeop, Y.; Kim, E.S.; Choi, I.; Park, J. Evaluating membrane fouling potentials of dissolved organic matter in brackish water. Water Res. 2019, 14, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shuang, X.; Yingzi, L.; Wang, C.; Wang, Q.; Han, Q. Effect of freezing–thawing on dissolved organic matter in water. Desalination Water Treat. 2016, 57, 17230–17240. [Google Scholar] [CrossRef]
- Yabalak, E.; Akay, S.; Kayan, B.; Gizir, A.M.; Yang, Y. Solubility and Decomposition of Organic Compounds in Subcritical Water. Molecules 2023, 28, 1000. [Google Scholar] [CrossRef] [PubMed]
- He, X.-S.; Chao, Y.; Shao-Hong, Y.; Zhang, H.; Xi, B.D.; Yu, M.D. Redox properties of compost-derived organic matter and their association with polarity and molecular weight. Sci. Total Environ. 2019, 665, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Marchenko, A.; Ben, L. The influence of closed brine pockets and permeable brine channels on the thermo-elastic properties of saline ice. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 375, 20150351. [Google Scholar] [CrossRef]
- Gu, W.; Lin, Y.-B.; Xu, Y.-J.; Chen, W.-B.; Tao, J.; Yuan, S. Gravity-induced sea ice desalination under low temperature. Cold Reg. Sci. Technol. 2013, 86, 133–141. [Google Scholar] [CrossRef]
- Yuan, H.; Kunyuan, S.; Kunwei, W.; Zhang, J.; Zhang, Z.; Zhang, L. Ice crystal growth in the freezing desalination process of binary water-NaCl system. Desalination 2020, 496, 114737. [Google Scholar] [CrossRef]
- Yang, H.; Zhonglai, Z.; Yuexin, Y.; Sun, Z. Influence of gravity-induced brine drainage on seawater ice desalination. Desalination 2017, 40, 733–740. [Google Scholar] [CrossRef]
- Petzold, G.; Jorge, M.; Paz, L.; Rojas, K.; Orellana, P. Block freeze concentration assisted by centrifugation applied to blueberry and pineapple juices. Innov. Food Sci. Emerg. Technol. 2015, 30, 192–197. [Google Scholar] [CrossRef]
- Yang, H.; Yuanfei, J.; Mengxiao, F.; Wang, R. Application of low temperature soaking liquid in combined freezing-based desalination processes in summer. Water Reuse 2021, 11, 425–438. [Google Scholar] [CrossRef]
- Jones, D.-W.R.; Grae, W. A simple dynamical model for gravity drainage of brine from growing sea ice. Geophys. Res. Lett. 2013, 40, 307–311. [Google Scholar] [CrossRef]
- Knight, C. Structural Approach to Ice Growth (and Nucleation) in Liquid Water. Cryst. Growth Des. 2019, 20, 580–589. [Google Scholar] [CrossRef]
- Yin, Y.; Yuhang, Y.; de Lourdes Mendoza, M.; Zhai, S.; Feng, W.; Wang, Y. Progressive freezing and suspension crystallization methods for tetrahydrofuran recovery from Grignard reagent wastewater. J. Clean. Prod. 2017, 144, 180–186. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).