An Innovative Method of Leaching of Battery Masses Produced in the Processing of Li-Ion Battery Scrap
Abstract
:1. Introduction
- -
- -
- the use of waste separators from used cells as ecological reducers of cathode metals for the roasting process [9];
- -
- the recovery of by-products of LIB hydrometallurgical processes, including graphite (C/graphite), sodium sulphate (Na2SO4) and lithium carbonate (Li2CO3) [10];
- -
- the selective recovery of lithium by an acid-free mechanical–chemical method [11];
- -
- the selective leaching of nickel, cobalt, manganese, lithium and iron [12];
- -
- or the innovative co-fusion of used Li-ion materials with copper slag [13].
| Author | Title | Patent Application or Patent Number |
|---|---|---|
| Akkuuser Ltd. [27] | Battery recycling method | US 8 979 006 B2 |
| Duesenfeld GmBH [28] | Recycling method for treating used batteries, particularly rechargeable batteries, and battery processing installation | US 2019/0260101 A1 |
| Recupyl [29] | Method for the recycling of mixed batteries and cells comprising lithium-based anodes | EP 1733451 B1 |
| Retriev Technology [30,31] Taxco [32] | (1) Recovery of lithium-ion batteries (2) Process for recovering and regenerating lithium cathode material from lithium-ion batteries (3) Li reclamation process | (1) 8 616 475 (2) 8 882 007 (3) 5 888 463 |
| Umicore NV SA [33,34] | (1) Process for recycling li-ion batteries (2) Process for the recovery of lithium | (1) WO 2011/035915 A1 (2) WO 2018/184876 A1 |
| Worcester Polytechnic Institute [35] | Method and apparatus for recycling lithium-ion batteries | US10522884B2 |
| Commissariat a l’Energie Atomique et aux Energies Alternatives [36] | Method for recycling the electrolyte of a Li-ion battery and method for recycling Li-ion batteries | US10497993B2 |
| Li-Cycle Corp. [37] | Process, apparatus and system for recovering materials from batteries | 11077452 |
| Lithium Inc. [38] | Lithium-ion battery recycling process | CA3076688C |
| SOLVAY SA [39] | A process for manufacturing nickel sulphate | WO 2021/105365 A1 |
| Urban Mining PYT LTD [40] | Process for the recovery of cobalt, lithium and other metals from spent lithium-based batteries and other feeds | US2021079495A1 |
| Smith W.N., Swoffer S.D. [41] | Process for recycling cobalt and nickel from lithium-ion batteries | 113116208 |
| Laucournet R., Barthelemy S., Diaferia N. [42] | Method for recycling lithium batteries and/or electrodes of such batteries | 9312581 |
| Marsh W., Rice University [43] | Recycling Li-ion batteries using green chemicals and processes | 20200399737 |
| Yi-Lung J., Jiun-Ren U.L., Je-Yuan S. [44] | Metal recovery method of wasted lithium-ion battery using sulfuric acid | TW501294B |
| Or PRESS FRIMET [45] | A process for recovering metals from recycled rechargeable batteries | CA3109084A1 |
| Gagne-Bourque D.M.C, Nadeau E., Couture B. [46] | Lithium-ion battery recycling process | WO2019060996A1 |
| Ho K.P., Wang R., Shen P. [47] | Method for recycling lithium-ion battery | US20180013181A1 |
| JX Nippon Mining and Metals Corp. [48] | Method for recovering metals from recycled lithium-ion battery materials | JP6334450B2 |
| Li-metal [49] | Granted patent for producing refined lithium metal from lithium carbonate | CA3179470 |
2. Materials and Methods
2.1. Materials
2.2. Single-Stage Acid Leaching Method with the Addition of Hydrogen Peroxide—Selection of the Leaching Agent
2.3. Single-Stage Acid Leaching Method with the Addition of Hydrogen Peroxide—Selection of the Phase Ratio
2.4. Two-Stage Counter-Current Acid Leaching Method with the Addition of Hydrogen Peroxide
3. Results and Discussion
3.1. Results of the Single-Stage Acid Leaching Method with the Addition of Hydrogen Peroxide—Selection of the Leaching Agent
3.1.1. Results of the Single-Stage Acid Leaching with the Addition of 1 dm3 of Hydrogen Peroxide—Selection of the Leaching Agent
3.1.2. Results of the Single-Stage Acid Leaching with the Addition of 2 dm3 of Hydrogen Peroxide—Selection of the Leaching Agent
3.2. Results of the Single-Stage Acid Leaching Method with the Addition of Hydrogen Peroxide—Selection of the Leaching Agent
3.3. Results of the Two-Stage Counter-Current Acid Leaching with the Addition of Hydrogen Peroxide
3.4. Results of the Analysis of the Composition of Sludge Left after Single-Stage and Two-Stage Counter-Current Acid Leaching with the Addition of Hydrogen Peroxide
4. Conclusions
- The leaching efficiency of cobalt and other valuable components from the battery masses increases with increasing concentrations of sulfuric acid in the range from 15% to 20%, with the addition of 1 dm3 or 2 dm3 of hydrogen peroxide;
- The leaching of a 1 kg portion of the battery mass (regardless of the material composition) in the 15% or 20% sulfuric acid solution with the addition of 1 dm3 or 2 dm3 of H2O2, for 2 h, with a solid-to-liquid-phase ratio of 1:5 (or even 1:4) at 60 °C ensures a leaching efficiency of cobalt, nickel, copper and lithium above 95%;
- The use of the two-stage counter-current leaching of the battery mass in the selected conditions ensures the leaching efficiency of cobalt, nickel, copper and lithium at a level significantly above 95%, while obtaining the concentration of cobalt in the after-leaching solution at a level of nearly 50 g/dm3, reducing the duration of leaching in a single stage up to 1 h and, most importantly, reducing the amount of waste solutions and the consumption of H2O2 and sulfuric acid.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neumann, J.; Petranikova, M.; Meeus, M.; Gamarra, J.D.; Younesi, R.; Winter, M.; Nowak, S. Recycling of Lithium-Ion Batteries—Current State of the Art, Circular Economy, and Next Generation Recycling. Adv. Energy Mater. 2022, 12, 2102917. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, M.; Liu, T.; Wang, Y.; Liu, Y.; Nai, J.; Zhang, L.; Zhang, S.; Tao, X. Direct recovery: A sustainable recycling technology for spent lithium-ion battery. Energy Storage Mater. 2023, 54, 120–134. [Google Scholar] [CrossRef]
- Ali, H.; Khan, H.A.; Pecht, M. Preprocessing of spent lithium-ion batteries for recycling: Need, methods, and trends. Renew. Sustain. Energy Rev. 2022, 168, 112809. [Google Scholar] [CrossRef]
- Pražanová, A.; Knap, V.; Stroe, D.-I. Literature Review, Recycling of Lithium-Ion Batteries from Electric Vehicles, Part I: Recycling Technology. Energies 2022, 15, 1086. [Google Scholar] [CrossRef]
- Pražanová, A.; Knap, V.; Stroe, D.-I. Literature Review, Recycling of Lithium-Ion Batteries from Electric Vehicles, Part II: Environmental and Economic Perspective. Energies 2022, 15, 7356. [Google Scholar] [CrossRef]
- Islam, M.T.; Iyer-Raniga, U. Lithium-Ion Battery Recycling in the Circular Economy: A Review. Recycling 2022, 7, 33. [Google Scholar] [CrossRef]
- Vanderburgt, S.; Santos, R.M.; Chiang, Y.W. Is it worthwhile to recover lithium-ion battery electrolyte during lithium-ion battery recycling? Resour. Conserv. Recycl. 2023, 189, 106733. [Google Scholar] [CrossRef]
- Sobianowska-Turek, A.; Łoś, P.; Fornalczyk, A.; Zygmunt, M. Potential market value of electrolyte condensate recovered from LIBs mechanical treatment. Gaz Woda I Tech. Sanit. 2023, 6, 19–20. [Google Scholar] [CrossRef]
- Hou, W.; Xuanrui, H.; Tang, R.; Min, Y.; Qunjie, X.; Hu, Z.; Shi, P. Repurposing of spent lithium-ion battery separator as a green reductant for efficiently refining the cathode metals. Waste Manag. 2023, 155, 129–136. [Google Scholar] [CrossRef]
- Cao, Y.; Li, L.; Liu, Z.; Wang, L.; Wu, F.; You, J. Co-products recovery does not necessarily mitigate environmental and economic tradeoffs in lithium-ion battery recycling. Resour. Conserv. Recycl. 2023, 188, 106689. [Google Scholar] [CrossRef]
- Zhang, Q.; Fan, E.; Lin, J.; Sun, S.; Zhang, X.; Chen, R.; Wu, F.; Li, L. Acid-free mechanochemical process to enhance the selective recycling of spent LiFePO4 batteries. J. Hazard. Mater. 2023, 443, 130160. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, W.; Liu, X.; Tang, J.; Su, F.; Li, Z.; Yang, J.; Me, Y. One-step selective separation and efficient recovery of valuable metals from mixed spent lithium batteries in the phosphoric acid system. Waste Manag. 2023, 155, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Qu, G.; Li, B.; Wei, Y. A novel approach for the recovery and cyclic utilization of valuable metals by co-smelting spent lithium-ion batteries with copper slag. Chem. Eng. J. 2023, 451, 138897. [Google Scholar] [CrossRef]
- Patent Office of the Republic of Poland. Available online: https://uprp.gov.pl/pl (accessed on 3 August 2022).
- European Patent Office. Available online: https://worldwide.espacenet.com/ (accessed on 3 August 2022).
- World Intellectual Property Organization. Available online: https://www.wipo.int/patentscope/en/ (accessed on 3 August 2022).
- Google Patents. Available online: https://patents.google.com (accessed on 3 August 2022).
- Justia Patents. Available online: https://patents.justia.com/ (accessed on 3 August 2022).
- Jung, J.C.-Y.; Sui, P.-C.; Jiujun, Z. A review of recycling spent lithium-ion battery cathode materials using hydrometallurgical treatments. J. Energy Storage 2021, 35, 102217. [Google Scholar] [CrossRef]
- Yu, W.; Guo, Y.; Xu, S.; Yang, Y.; Zhao, Y.; Zhang, J. Comprehensive recycling of lithium-ion batteries: Fundamentals, pretreatment, and perspectives. Energy Storage Mater. 2023, 54, 172–220. [Google Scholar] [CrossRef]
- Urbańska, W. Recovery of Co, Li, and Ni from spent Li-ion batteries by the inorganic and/or organic reducer assisted leaching method. Minerals 2020, 10, 555. [Google Scholar] [CrossRef]
- Yang, J.; Lai, Y.; Liu, F.; Jia, M.; Jiang, L. Countercurrent leaching of Ni, Co, Mn, and Li from spent lithium-ion batteries. Waste Manag. Res. 2020, 38, 1358–1366. [Google Scholar] [CrossRef]
- Meshram, P.; Abhilash, S.V.; Sainio, T. Solvent Extraction for Separation of 99.9% Pure Cobalt and Recovery of Li, Ni, Fe, Cu, Al from Spent LIBs. Metals 2022, 12, 1056. [Google Scholar] [CrossRef]
- Chabhadiya, K.; Srivastava, R.R.; Pathak, P. Two-step leaching process and kinetics for an eco-friendly recycling of critical metals from spent Li-ion batteries. J. Environ. Chem. Eng. 2021, 9, 105232. [Google Scholar] [CrossRef]
- Nayl, A.A.; Elkhashab, R.A.; Badawy, S.M.; El-Khateeb, M.A. Acid leaching of mixed spent Li-ion batteries. Arab. J. Chem. 2017, 10, S3632–S33639. [Google Scholar] [CrossRef]
- Chen, L.; Chao, Y.; Li, X.; Li, X.; Zhou, G.; Lu, Q.; Hua, M.; Li, H.; Ni, X.; Wu, P.; et al. Engineering a tandem leaching system for the highly selective recycling of valuable metals from spent Li-ion batteries. Green Chem. 2021, 23, 2177. [Google Scholar] [CrossRef]
- Akkuser Ltd. Battery Recycling Method. U.S. Patent US8979006B2, 3 August 2022.
- Duesenfeld GmBH. Recycling Method for Treating Used Batteries, in Particular Rechargeable Batteries, and Battery Processing Installation. Google Patents US 2019/0260101 A1, 3 August 2022.
- Recupyl. Method for the Recycling of Mixed Batteries and Cells Comprising Lithium-Based Anodes. Google Patents EP 1733451 B1, 3 August 2022.
- Retriev Technology. Recovery of Lithium-Ion Batteries. Justia Patents 8 616 475, 3 August 2022.
- Retriev Technology. Process for Recovering and Regenerating Lithium Cathode Material from Lithium Ion Batteries. Justia Patents 888 2007, 3 August 2022.
- Toxco. Li Reclamation Process. Justia Patents 5 888 463, 3 August 2022.
- Umicore. Process for Recycling Li-Ion Batteries. Google Patents EP3087208A1, 3 August 2022.
- Umicore. Process for the Recovery of Lithium. WO2018184876A1, 4 October 2018. Available online: https://worldwide.espacenet.com/patent/search/family/058501374/publication/WO2018184876A1?q=wo2018184876a1 (accessed on 3 August 2022).
- Worcester Polytechnic Institute. Method and Apparatus for Recycling Lithium-Ion Batteries. 2022. Available online: https://patents.google.com/patent/US10522884B2/en?q=batteries+recycling+Li-ion&oq=batteries+recycling+Li-ion (accessed on 3 August 2022).
- Commissariat a l'Energie Atomique et aux Energies Alternatives, Method for Recycling the Electrolyte of a Li-Ion Battery and Method for Recycling Li-Ion Batteries. US10497993B2, 20 May 2021. Available online: https://worldwide.espacenet.com/patent/search/family/051987239/publication/US10497993B2?q=pn%3DUS10497993B2 (accessed on 3 August 2022).
- Li-Cycle Corp. Process, Apparatus, and System for Recovering Materials from Batteries. Justia Patents 11077452, 3 August 2022.
- Lithium Inc. Lithium-Ion Batteries Recycling Process. Google Patents CA3076688C, 3 August 2022.
- SOLVAY SA. A Process for Manufacturing Nickel Sulphate. Google Patents WO 2021/105365 A1, 12 August 2022. [Google Scholar]
- Urban Mining PYT LTD. Process for the Recovery of Cobalt, Lithium and other Metals from Spent Lithium-Based Batteries and Other Feeds. US2021079495A1, 18 March 2021. Available online: https://worldwide.espacenet.com/patent/search/family/064565602/publication/US2021079495A1?q=pn%3DUS2021079495A1 (accessed on 3 August 2022).
- Smith, W.N.; Swoffer, S.D. Process for Recycling Cobalt and Nickel from Lithium-Ion Batteries. Justia Patents 113116208, 3 August 2022. [Google Scholar]
- Laucournet, R.; Barthelemy, S.; Diaferia, N. Method for Recycling Lithium Batteries and/or Electrodes of such Batteries. Justia Patents 9312581US, 3 August 2022. [Google Scholar]
- Marsh, W. Rice University, Recycling Li-Ion Batteries Using Green Chemicals and Processes. Justia Patents 20200399737, 3 August 2022. [Google Scholar]
- Yi-Lung, J.; Jiun-Ren, U.L.; Je-Yuan, S. Metal Recovery Method of Wasted Lithium-Ion Battery Using Sulfuric Acid. TW501294B; Espacenet—Search Results, 3 August 2022. [Google Scholar]
- Or PRESS FRIMET. A Process for Recovering Metals from Recycled Rechargeable Batteries. Google Patents CA3109084A1, 3 August 2022.
- Gagne-Bourque, D.M.C.; Nadeau, E.; Couture, B. Lithium-Ion Batteries Recycling Process. Google Patents WO2019060996A1, 3 August 2022. [Google Scholar]
- Ho, K.P.; Wang, R.; Shen, P. Method for Recycling Lithium-Ion Battery. Google Patents US20180013181A1, 3 August 2022. [Google Scholar]
- JX Nippon Mining and Metals Corp. Method for Recovering Metals from Recycled Lithium-Ion Battery Materials. Google Patents JP6334450B2, 8 August 2022.
- Li-Metal Corp. Available online: https://investors.li-metal.com/news/news-details/2023/Li-Metal-Granted-Patent-for-Production-of-Refined-Lithium-Metal/default.aspx (accessed on 22 May 2022).
- Elemental Strategic Metals. Available online: https://elementalbatteries.com/ (accessed on 27 May 2023).
- Instytut Łukasiewicz—Centre of Analytical Chemistry in the Łukasiewicz Research Network—Institute of Non-Ferrous Metals. Available online: http://www.imn.gliwice.pl/content/506/centrum_chemii_analitycznej (accessed on 22 May 2022).
- Diekmann, J.; Hanisch, C.; Froböse, L.; Schälicke, G.; Loellhoeffel, T.; Fölster, A.-S.; Kwade, A. Ecological Recycling of Lithium-Ion Batteries from Electric Vehicles with Focus on Mechanical Processes. J. Electrochem. Soc. 2017, 164, A6184–A6191. [Google Scholar] [CrossRef]
- Abramowicz, M.; Osiał, M.; Urbańska, W.; Walicki, M.; Wilczewski, S.; Pregowska, A.; Skórczewska, K.; Jenczyk, P.; Warczak, M.; Pisarek, M.; et al. Upcycling of Acid-Leaching Solutions from Li-Ion Battery Waste Treatment through the Facile Synthesis of Magnetorheological Fluid. Molecules 2023, 28, 2558. [Google Scholar] [CrossRef] [PubMed]
- Guimarăes, L.F.; Botelho Junior, A.B.; Espinose, D.C.R. Sulfuric acid leaching of metals from waste Li-ion batteries without using reducing agent. Miner. Eng. 2022, 185, 107597. [Google Scholar] [CrossRef]
- Peng, C.; Hamuyumi, J.; Wilson, P.; Lundström, M. Selective reductive leaching of cobalt and lithium from industrially crushed waste Li-ion batteries in sulfuric acid system. Waste Manag. 2018, 76, 582–590. [Google Scholar] [CrossRef]
- Chen, W.-S.; Ho, H.-J. Recovery of Valuable Metals from Lithium-Ion Batteries NMC Cathode Waste Materials by Hydrometallurgical Methods. Metals 2018, 8, 321. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, T.; Zou, Q.; Yang, F.; Wang, Y.; Wang, S.; Wang, C. Solvent extraction of Ni and Co from Ni-laterite leach solutions using a new synergistic system consisting of Versatic 10 acid, Mextral 6103H and Aliquat 336 with elemental mass balance for leaching, precipitation, solvent extraction, scrubbing and stripping. Hydrometallurgy 2022, 208, 105822. [Google Scholar] [CrossRef]
- Kazem-Ghamsari, A.; Abdollahi, H. Electrowinning of Nickel and Cobalt from Non-circulated Sulfate Electrolyte. Trans. Indian Inst. Met. 2022, 75, 1141–1151. [Google Scholar] [CrossRef]
- Elemental Strategic Metals. Method of Carrying out the Process of Two-Stage Leaching of Battery Masses. Patent Application WIPO ST 10/C PL440688, 18 March 2022.
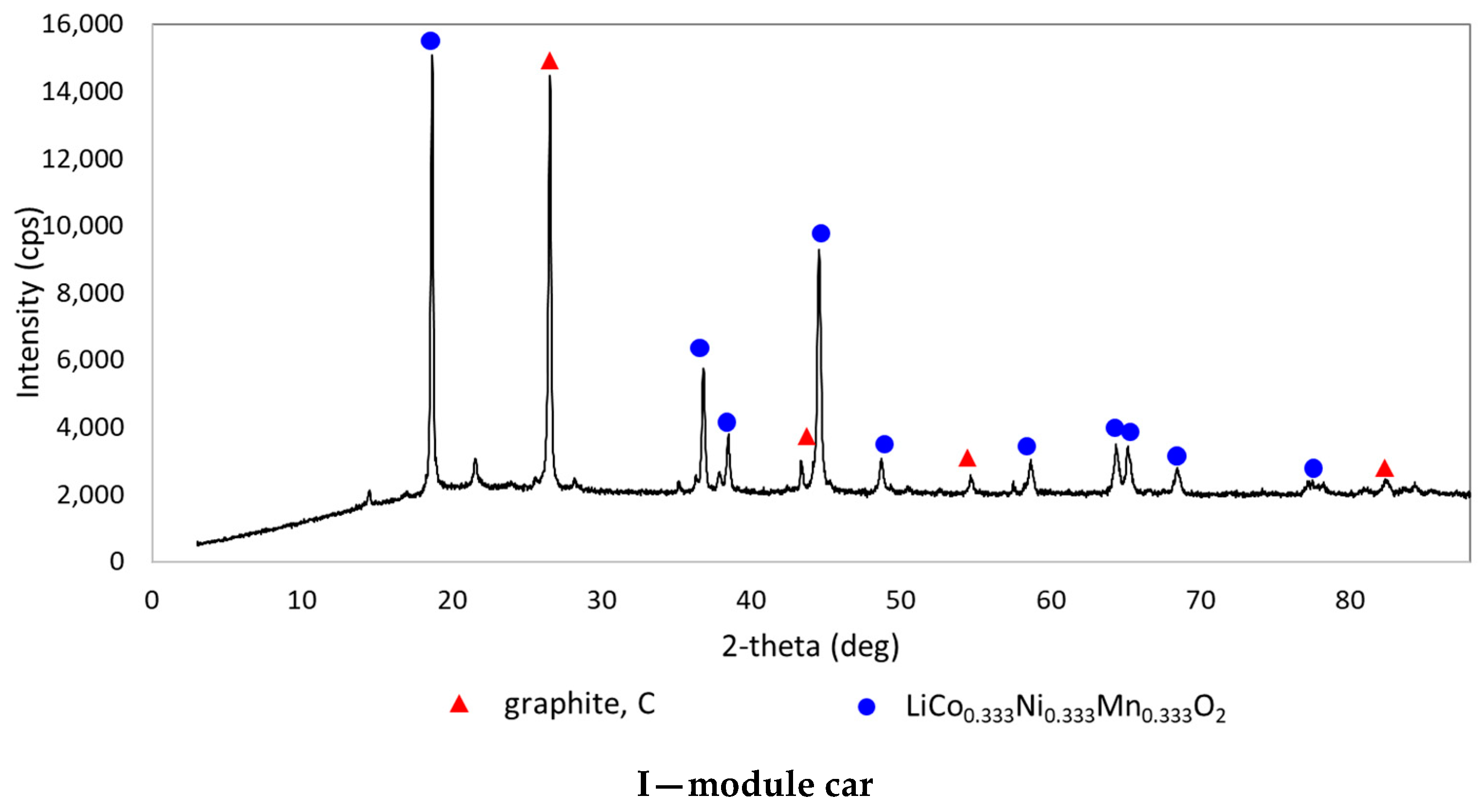
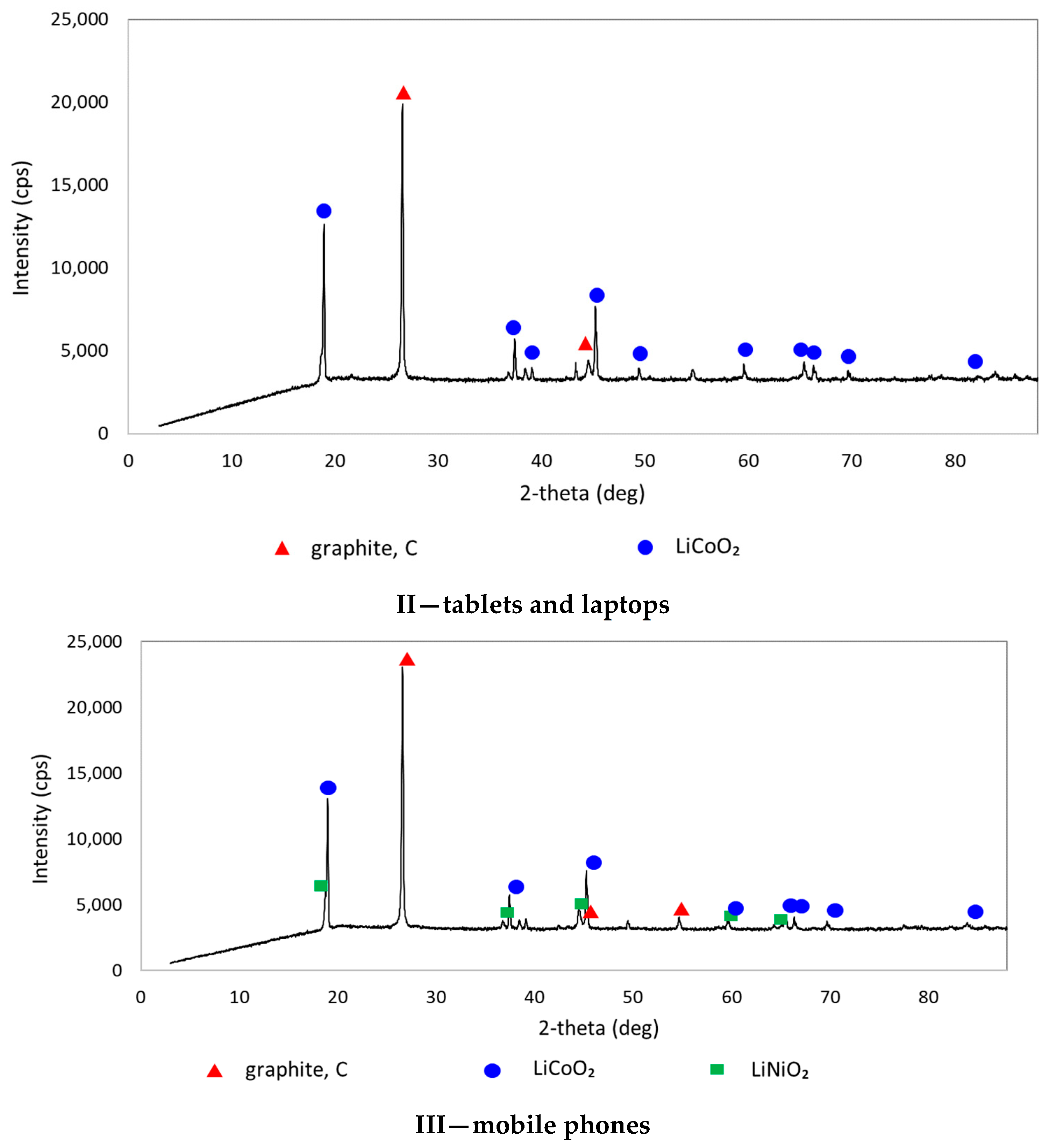

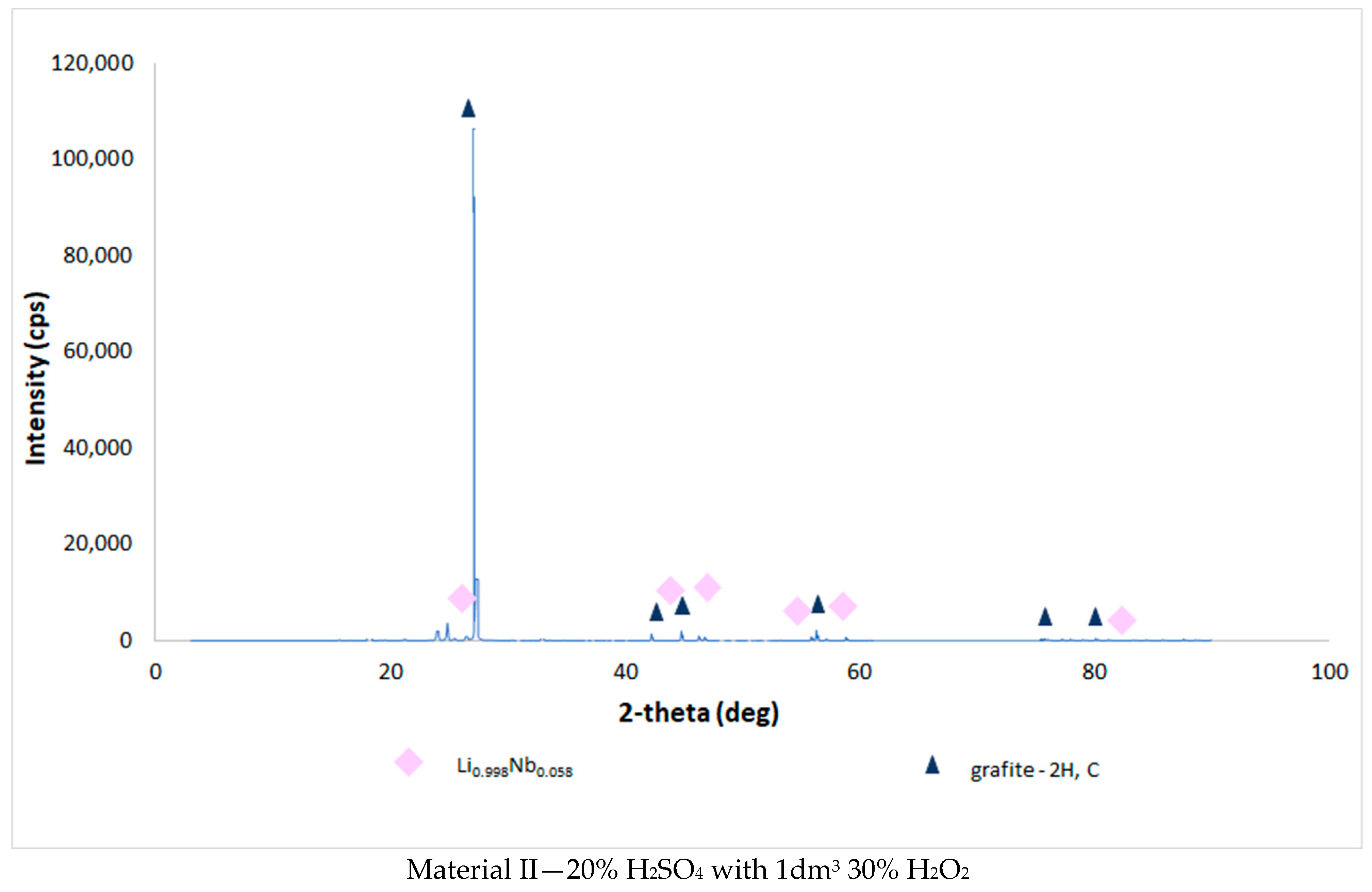

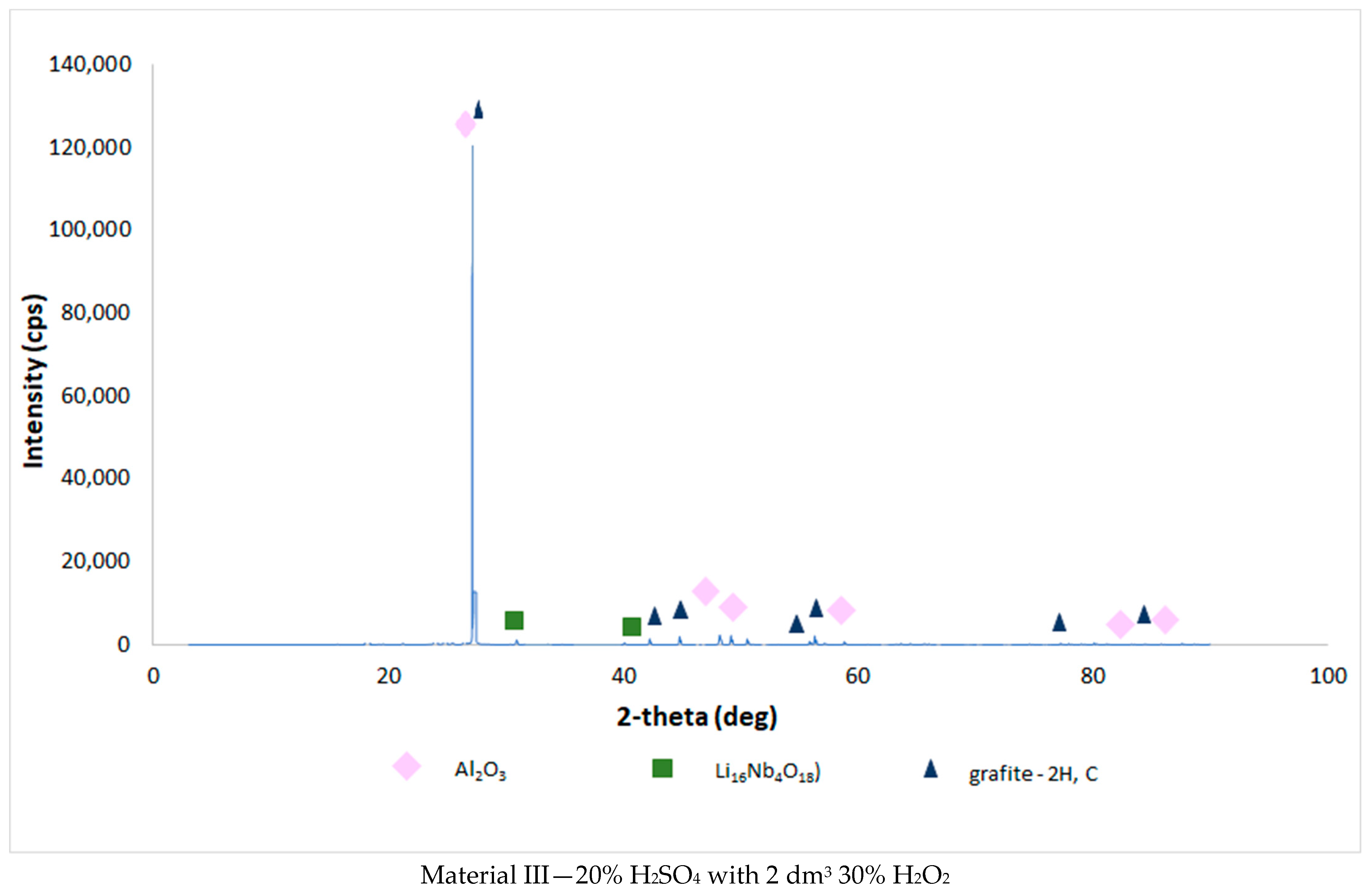
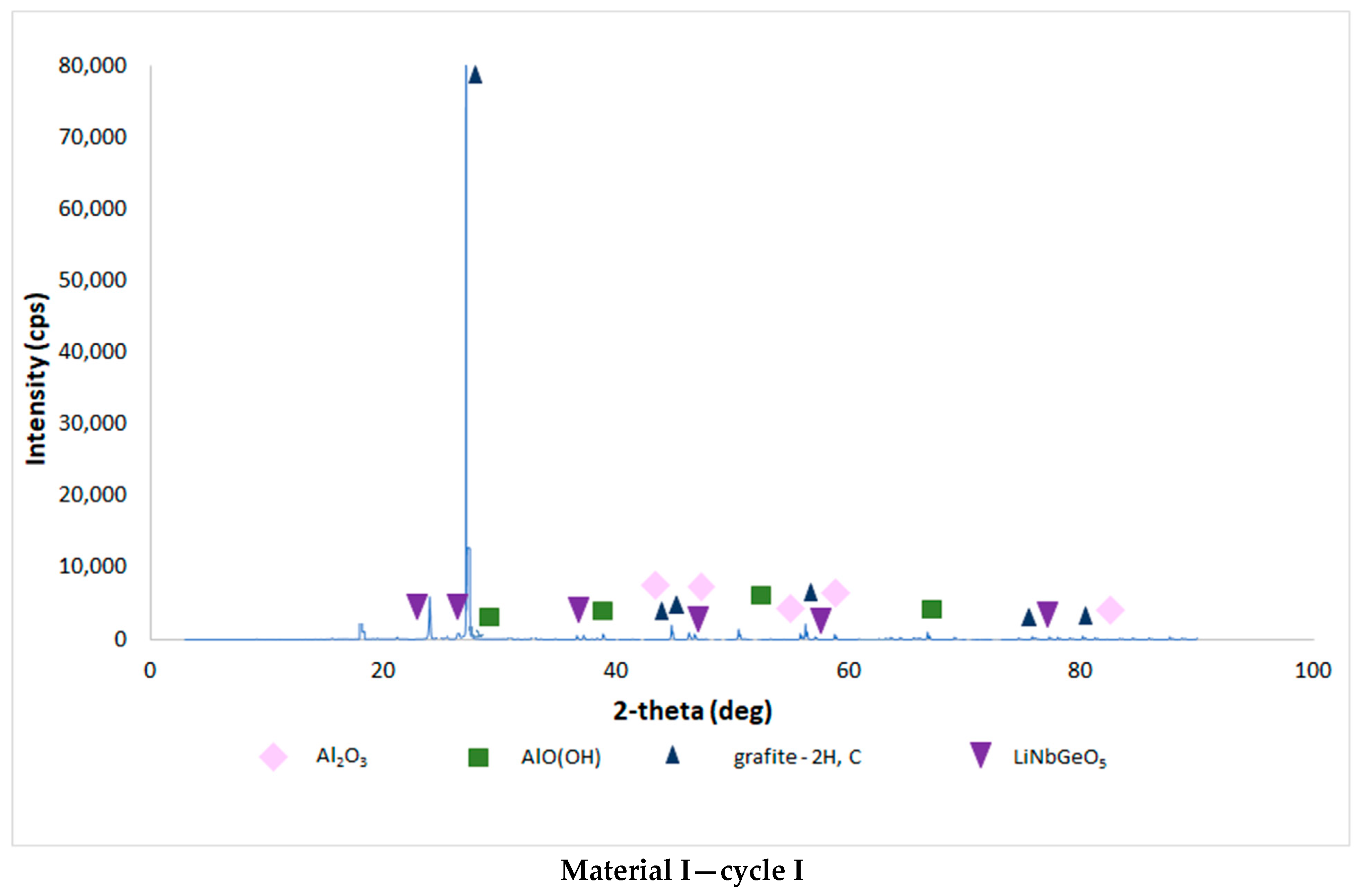
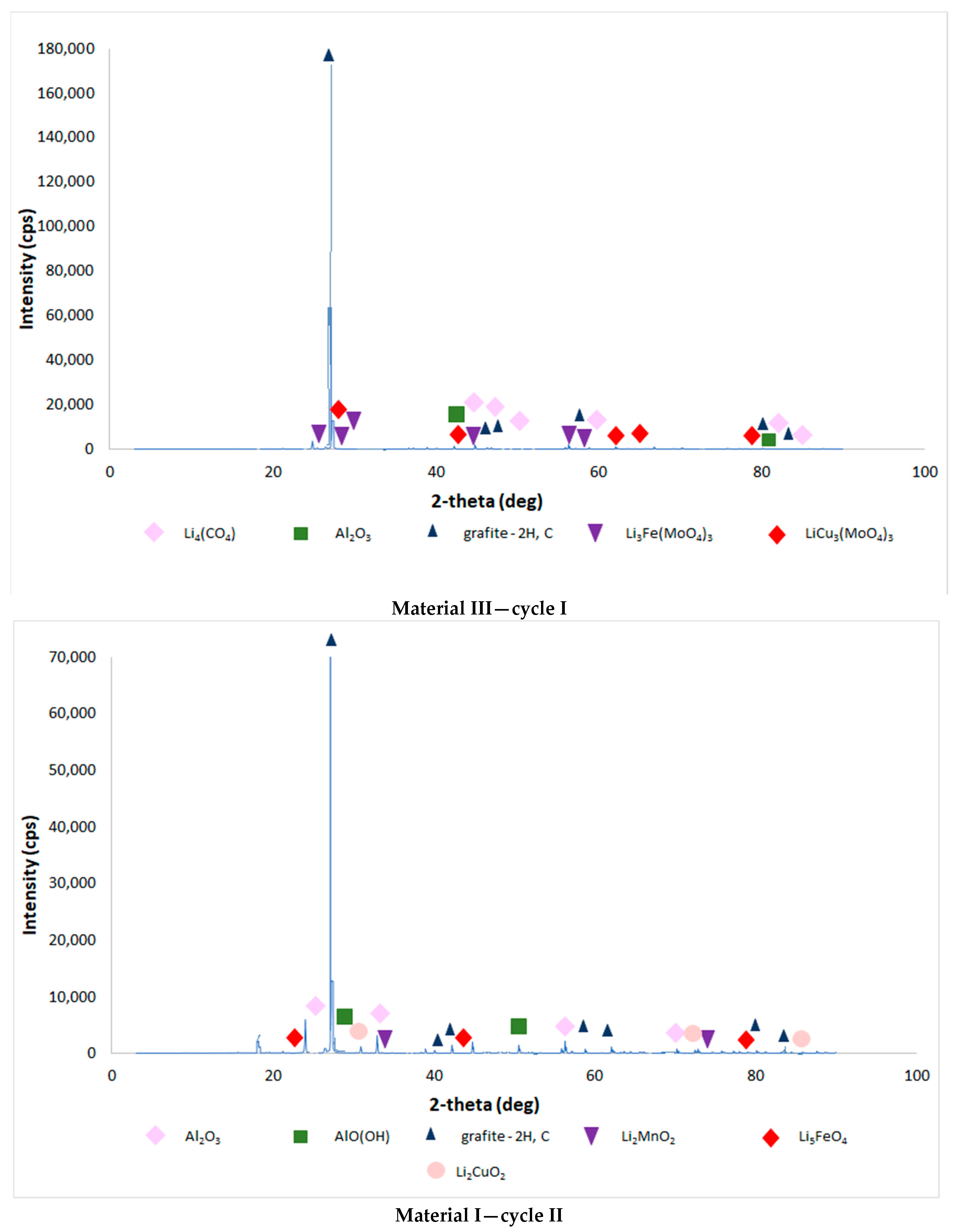
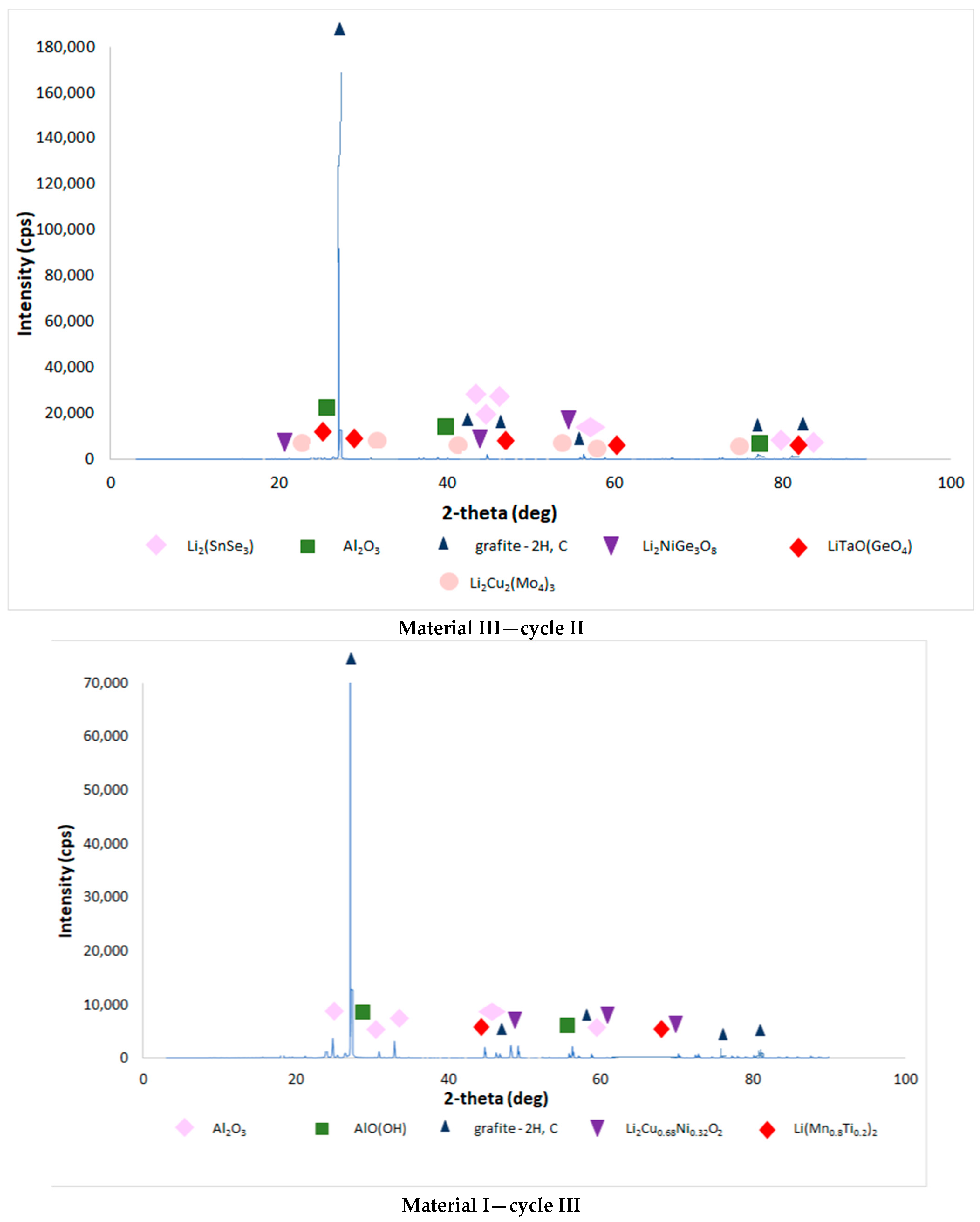

| Chemical Composition (wt%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Material | C | Co | Ni | Mn | Li | Al | Cu | P | F | Cl | Fe |
| I—module car | 34.30 | 9.33 | 9.83 | 8.70 | 3.64 | 4.28 | 2.73 | 0.84 | 3.22 | 0.04 | 0.31 |
| II—tablets and laptops | 38.20 | 29.70 | 1.96 | 0.81 | 3.79 | 1.57 | 1.53 | 0.49 | 1.84 | <0.01 | 0.39 |
| III—mobile phones | 39.20 | 27.00 | 2.66 | 1.51 | 3.79 | 1.35 | 1.37 | 0.47 | 2.02 | <0.01 | 0.34 |
| Material | H2SO4 Concentration (%) | Volume of the Solution after Leaching (cm3) | Volume of 30% H2O2 (dm3) | pH | Free Acid Concentration (g/dm3) | Concentration of the Element in the Solution after Leaching (g/dm3) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Co | Ni | Li | Cu | Mn | Fe | P | F | ||||||
| I | 15 | 4840 | 1.0 | 1.7 | 5.9 | 14.70 | 15.60 | 5.59 | 3.45 | 12.90 | 0.57 | 1.13 | 4.80 |
| 20 | 5700 | 0.5 | 48.5 | 14.90 | 15.70 | 5.67 | 3.98 | 13.20 | 0.50 | 1.06 | 4.30 | ||
| II | 15 | 4920 | 0.7 | 15.2 | 45.90 | 3.03 | 5.29 | 2.35 | 1.31 | 0.35 | 0.55 | 3.05 | |
| 20 | 5960 | 0.4 | 62.7 | 43.90 | 3.28 | 6.04 | 2.56 | 1.43 | 0.42 | 0.33 | 2.60 | ||
| III | 15 | 5300 | 0.7 | 31.0 | 31.10 | 3.80 | 5.02 | 1.91 | 2.19 | 0.51 | 0.65 | 1.65 | |
| 20 | 5520 | 0.3 | 86.0 | 36.60 | 4.21 | 5.50 | 2.00 | 2.26 | 0.61 | 0.69 | 2.00 | ||
| I | 15 | 5900 | 2.0 | 0.8 | 13.7 | 14.40 | 15.40 | 5.83 | 3.88 | 12.50 | 0.37 | 1.31 | 4.20 |
| 20 | 6660 | 0.4 | 59.3 | 12.80 | 13.60 | 5.10 | 3.41 | 11.0 | 0.43 | 2.86 | 3.55 | ||
| II | 15 | 5620 | 0.7 | 7.4 | 34.40 | 2.46 | 5.82 | 1.93 | 1.25 | 0.11 | 0.47 | 2.55 | |
| 20 | 6040 | 0.4 | 44.6 | 38.20 | 1.81 | 4.90 | 1.37 | 1.24 | 0.43 | 0.65 | 2.45 | ||
| III | 15 | 5920 | 0.8 | 25.0 | 25.10 | 2.75 | 4.10 | 1.30 | 1.51 | 0.42 | 0.61 | 1.40 | |
| 20 | 5960 | 0.4 | 66.0 | 38.50 | 3.75 | 5.74 | 1.90 | 2.10 | 0.51 | 0.99 | 1.95 | ||
| Material | Solid to Liquid Phase Ratio | Volume of the Solution after Leaching (cm3) | pH | Free Acid Concentration (g/dm3) | Concentration of the Element in the Solution after Leaching (g/dm3) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Co | Ni | Li | Cu | Mn | Fe | P | F | |||||
| I | 1:5 | 5700 | 0.45 | 48.5 | 14.90 | 15.70 | 5.67 | 3.98 | 13.20 | 0.50 | 1.06 | 4.30 |
| 1:4 | 1885 | 0.60 | 40.2 | 24.30 | 26.00 | 9.58 | 5.54 | 19.00 | 0.70 | 1.51 | 5.85 | |
| II | 1:5 | 5960 | 0.60 | 62.7 | 43.90 | 3.28 | 6.04 | 2.56 | 1.43 | 0.42 | 0.33 | 2.60 |
| 1:4 | 1980 | 0.60 | 65.1 | 63.90 | 4.63 | 8.89 | 3.62 | 1.85 | 0.57 | 0.82 | 3.55 | |
| III | 1:5 | 5520 | 0.39 | 85.8 | 36.40 | 4.21 | 5.50 | 2.00 | 2.26 | 0.64 | 0.69 | 2.00 |
| 1:4 | 2150 | 0.50 | 49.0 | 51.50 | 5.24 | 7.92 | 2.75 | 3.07 | 0.67 | 1.03 | 2.00 | |
| Material | Cycle | Volume of the Solution after Leaching (cm3) | pH | Concentration of the Element in the Solution after Leaching (g/dm3) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I Stage | II Stage | Co | Ni | Li | Cu | Mn | Fe | P | F | |||
| I | 1 | 4900 | - | 0.2 | 11.10 | 13.20 | 6.51 | 4.74 | 8.89 | 0.42 | 1.05 | 4.80 |
| - | 6800 | 0.2 | 5.73 | 5.09 | 0.84 | 0.55 | 5.23 | 0.09 | 0.18 | 0.33 | ||
| 2 | 6100 | - | 0.4 | 14.70 | 15.50 | 5.68 | 3.65 | 12.60 | 0.41 | 0.99 | 4.40 | |
| - | 6300 | 0.1 | 6.44 | 5.69 | 0.98 | 0.99 | 5.63 | 0.13 | 0.17 | 0.33 | ||
| 3 | 5840 | - | 0.3 | 16.60 | 17.40 | 6.39 | 4.50 | 13.70 | 0.63 | 1.01 | 4.40 | |
| - | 6380 | 0.1 | 6.61 | 5.52 | 1.15 | 1.04 | 5.12 | 0.29 | 0.24 | 0.43 | ||
| II | 1 | 4700 | - | 0.1 | 30.00 | 2.73 | 6.32 | 3.00 | 1.26 | 0.50 | 0.50 | 3.14 |
| - | 6000 | 0.1 | 24.00 | 0.55 | 1.32 | 0.23 | 0.41 | 0.12 | 0.15 | 0.20 | ||
| 2 | 5510 | - | 0.4 | 47.90 | 2.52 | 5.63 | 1.84 | 1.33 | 0.49 | 0.58 | 2.98 | |
| - | 6240 | 0.1 | 25.80 | 0.97 | 1.49 | 0.52 | 0.57 | 0.16 | 0.20 | 0.22 | ||
| 3 | 5700 | - | 0.5 | 51.60 | 3.34 | 6.67 | 2.41 | 1.46 | 0.52 | 0.55 | 2.76 | |
| - | 5900 | 0.1 | 21.90 | 1.17 | 1.67 | 0.69 | 0.70 | 0.19 | 0.21 | 0.22 | ||
| III | 1 | 4900 | - | 0.1 | 26.60 | 4.03 | 5.83 | 2.10 | 1.92 | 0.71 | 0.68 | 2.20 |
| - | 6600 | 0.1 | 19.40 | 0.70 | 1.10 | 0.18 | 0.72 | 0.06 | 0.08 | 0.18 | ||
| 2 | 6000 | - | 0.4 | 38.80 | 3.18 | 5.14 | 1.51 | 1.64 | 0.54 | 0.64 | 1.60 | |
| - | 6500 | 0.1 | 19.80 | 1.10 | 1.28 | 0.24 | 0.86 | 0.07 | 0.07 | 0.16 | ||
| 3 | 5860 | - | 0.4 | 38.10 | 3.57 | 5.35 | 1.60 | 1.81 | 0.56 | 0.62 | 1.59 | |
| - | 6420 | 0.1 | 21.80 | 1.15 | 1.37 | 0.28 | 0.97 | 0.09 | 0.06 | 0.16 | ||
| Material | The Initial Weight of the Battery Mass or after-Leaching Sludge (g) | Volume of 30% H2O2 (dm3) | Composition of the Battery Mass or after-Leaching Sludge (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Co | Ni | Li | Cu | Mn | Fe | P | F | |||
| I | 1000 | - | 9.33 | 9.83 | 3.64 | 2.73 | 8.70 | 0.31 | 0.84 | 3.22 |
| 431 | 1 | 0.15 | 0.16 | 0.09 | 0.09 | 0.13 | 0.15 | 0.31 | 2.15 | |
| 413 | 2 | 0.08 | 0.09 | 0.06 | 0.06 | 0.06 | 0.09 | 0.17 | 2.16 | |
| II | 1000 | - | 29.70 | 1.96 | 3.79 | 1.53 | 0.81 | 0.39 | 0.49 | 1.84 |
| 457 | 1 | 7.23 | 0.08 | 0.34 | 0.08 | 0.08 | 0.33 | 0.20 | 1.18 | |
| 391 | 2 | 1.42 | 0.05 | 0.12 | 0.11 | 0.01 | 0.21 | 0.20 | 1.33 | |
| III | 1000 | - | 27.00 | 2.66 | 3.79 | 1.37 | 1.51 | 0.34 | 0.47 | 2.02 |
| 448 | 1 | 7.06 | 0.14 | 0.42 | 0.08 | 0.07 | 0.08 | 0.09 | 1.36 | |
| 386 | 2 | 0.83 | 0.03 | 0.07 | 0.02 | <0.01 | 0.08 | 0.09 | 1.50 | |
| Material | The Initial Weight of the Battery Mass or after-Leaching Sludge (g) | Cycle | Composition of the Battery Mass or after-Leaching Sludge (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Co | Ni | Li | Cu | Mn | Fe | P | F | |||
| I | 1000 | 0 | 9.33 | 9.83 | 3.64 | 2.73 | 8.70 | 0.31 | 0.84 | 3.22 |
| 422 | 1 | 0.05 | 0.06 | 0.05 | 0.05 | 0.04 | 0.10 | 0.11 | 2.07 | |
| 440 | 2 | 0.22 | 0.20 | 0.08 | 0.14 | 0.17 | 0.16 | 0.16 | 1.92 | |
| 418 | 3 | 0.22 | 0.21 | 0.08 | 0.10 | 0.16 | 0.14 | 0.14 | 2.01 | |
| II | 1000 | 0 | 29.70 | 1.96 | 3.79 | 1.53 | 0.81 | 0.39 | 0.49 | 1.84 |
| 394 | 1 | 0.37 | 0.02 | 0.05 | 0.05 | 0.01 | 0.11 | 0.17 | 1.33 | |
| 404 | 2 | 0.71 | 0.04 | 0.06 | 0.06 | 0.01 | 0.10 | 0.19 | 1.38 | |
| 405 | 3 | 0.75 | 0.07 | 0.06 | 0.07 | 0.01 | 0.19 | 0.19 | 1.35 | |
| III | 1000 | 0 | 27.00 | 2.66 | 3.79 | 1.37 | 1.51 | 0.34 | 0.47 | 2.02 |
| 381 | 1 | 0.30 | 0.03 | 0.03 | 0.01 | 0.01 | 0.14 | 0.07 | 1.52 | |
| 373 | 2 | 0.48 | 0.04 | 0.04 | 0.02 | 0.02 | 0.13 | 0.09 | 1.47 | |
| 369 | 3 | 0.33 | 0.03 | 0.03 | 0.01 | 0.01 | 0.08 | 0.08 | 1.49 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leszczyńska-Sejda, K.; Chmielarz, A.; Kopyto, D.; Ochmański, M.; Benke, G.; Palmowski, A.; Sobianowska-Turek, A.; Łoś, P.; Fornalczyk, A.; Zygmunt, M.; et al. An Innovative Method of Leaching of Battery Masses Produced in the Processing of Li-Ion Battery Scrap. Appl. Sci. 2024, 14, 397. https://doi.org/10.3390/app14010397
Leszczyńska-Sejda K, Chmielarz A, Kopyto D, Ochmański M, Benke G, Palmowski A, Sobianowska-Turek A, Łoś P, Fornalczyk A, Zygmunt M, et al. An Innovative Method of Leaching of Battery Masses Produced in the Processing of Li-Ion Battery Scrap. Applied Sciences. 2024; 14(1):397. https://doi.org/10.3390/app14010397
Chicago/Turabian StyleLeszczyńska-Sejda, Katarzyna, Andrzej Chmielarz, Dorota Kopyto, Michał Ochmański, Grzegorz Benke, Arkadiusz Palmowski, Agnieszka Sobianowska-Turek, Przemysław Łoś, Agnieszka Fornalczyk, Michał Zygmunt, and et al. 2024. "An Innovative Method of Leaching of Battery Masses Produced in the Processing of Li-Ion Battery Scrap" Applied Sciences 14, no. 1: 397. https://doi.org/10.3390/app14010397
APA StyleLeszczyńska-Sejda, K., Chmielarz, A., Kopyto, D., Ochmański, M., Benke, G., Palmowski, A., Sobianowska-Turek, A., Łoś, P., Fornalczyk, A., Zygmunt, M., & Goc, K. (2024). An Innovative Method of Leaching of Battery Masses Produced in the Processing of Li-Ion Battery Scrap. Applied Sciences, 14(1), 397. https://doi.org/10.3390/app14010397







