A Modified Protocol for Staining of Undecalcified Bone Samples Using Toluidine Blue—A Histological Study in Rabbit Models
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- An, Y.H.; Moreira, P.L.; Kang, Q.K.; Gruber, H.E. Principles of Embedding and Common Protocols. In Handbook of Histology Methods for Bone and Cartilage; An, Y., Martin, K., Eds.; Humana Press Inc.: Clifton, NJ, USA, 2003; pp. 185–197. ISBN 1-59259-417-4. [Google Scholar]
- Suvarna, S.K.; Layton, C.; Bancroft, J.D. (Eds.) Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Churchill Livingstone, Elsevier: London, UK, 2019; pp. 280–305. ISBN 978-0-7020-6864-5. [Google Scholar]
- Singhrao, S.; Nicholson, K.; Crean, S. Informed choices for challenging specimens when choosing methacrylate resin systems for histology. Microsc. Res. Tech. 2012, 75, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Gayoso, J.; Garrosa, M.; Gayoso, S.; Rodríguez-Arias, C.; Martin-Ferrero, M.; Gayoso, M. Three-sectioning method: A procedure for studying hard tissues and large pieces under light and electron microscopy. Micron 2020, 132, 102841. [Google Scholar] [CrossRef] [PubMed]
- Dey, P. Decalcification of bony and hard tissue for histopathology processing. In Basic and Advanced Laboratory Techniques in Histopathology and Cytology; Springer: Singapore, 2018; pp. 35–39. [Google Scholar]
- Skiner, R. Decalcification of Bone Tissue. In Handbook of Histology Methods for Bone and Cartilage; An, Y., Martin, K., Eds.; Humana Press Inc.: Clifton, NJ, USA, 2003; pp. 167–184. ISBN 1-59259-417-4. [Google Scholar]
- Lindner, C.; PrÖhl, A.; Abels, M.; Löffler, T.; Batinic, M.; Jung, O.; Barbeck, M. Specialized Histological and Histomorphometrical Analytical Methods for Biocompatibility Testing of Biomaterials for Maxillofacial Surgery in (Pre-) Clinical Studies. In Vivo 2020, 34, 137–3152. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.R.; Burr, D.B. Techniques in Histomorphometry. In Basic and Applied Bone Biology, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 141–158. ISBN 978-0-12-813259-3. [Google Scholar]
- Gül, M.; Bayat, N.; Gül, S.; Hüz, M.; Yıldız, A.; Otlu, A. A Comparison of Three Different Agents of Decalcification for a Histological Examination of Bone Tissues. J. Turgut Ozal Med. Cent. 2014, 21, 274–279. [Google Scholar]
- Licini, C.; Farinelli, L.; Cerqueni, G.; Hosein, A.; Marchi, S.; Gigante, A.; Mattioli-Belmonte, M. Heterotopic ossification in a patient with diffuse idiopathic skeletal hyperostosis: Input from histological findings. Eur. J. Histochem. 2020, 64, 3176. [Google Scholar] [CrossRef]
- Licini, C.; Notarstefano, V.; Marchi, S.; Ciapetti, G.; Vitale-Brovarone, C.; Cerqueni, E.; Mattioli-Belmonte, M. Altered type I collagen networking in osteoporotic human femoral head revealed by histomorphometric and Fourier transform infrared imaging correlated analyses. BioFactors 2022, 48, 1089–1110. [Google Scholar] [CrossRef]
- Ren, J.; Paxton, N.; Hammond, J.; Saifzadeh, S.; Steck, R.; Lawrence, F.; Woodruff, M. Novel resin tissue array system reduces sample preparation time, labour and reagent costs in bone tissue histology. Bone 2021, 153, 116155. [Google Scholar] [CrossRef]
- Yang, R.; Davies, C.; Archer, C.; Richards, R. Immunohistochemistry of bone matrix markers in Technovit 9100 new embedded undecalcified bone sections. Eur. Cells Mater. 2003, 31, 57–71. [Google Scholar] [CrossRef]
- García, M.; Martin, A.; Fushimi, S.; Feldman, S.; Pastorino, N.; Juárez, J.; Jammal, M.; Missan, L. Optimization for Bone Samples Embedded in Methyl Methacrylate. J. Hard Tissue Biol. 2022, 31, 181–186. [Google Scholar] [CrossRef]
- Erben, R. Embedding of bone samples in methyl methacrylate: An improved method suitable for bone histomorphometry, histochemistry, and immunohistochemistry. J. Histochem. Cytochem. 1997, 45, 307–313. [Google Scholar] [CrossRef]
- Goldschlager, T.; Abdelkader, A.; Kerr, J.; Boundy, I.; Jenki, G. Undecalcified Bone Preparation for Histology, Histomorphometry and Fluorochrome Analysis. JoVE 2010, 35, e1707. [Google Scholar]
- Willbold, E.; Witte, F. Histology and research at the hard tissue–implant interface using Technovit 9100 New embedding technique. Acta Biomater. 2010, 6, 4447–4455. [Google Scholar] [CrossRef]
- Ramelt, S.; Corbeil, D.; Manthey, S.; Zwipp, H.; Hanisch, U. Immunohistochemical in situ characterization of orthopedic implants on polymethyl metacrylate embedded cutting and grinding sections. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2007, 83, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Maglio, M.; Salamanna, F.; Brogini, S.; Borsari, V.; Pagani, S.; Nicoli Aldini, N.; Giavaresi, G.; Fini, M. Histological, Histomorphometrical, and Biomechanical Studies of Bone-Implanted Medical Devices: Hard Resin Embedding. BioMed Res. Int. 2020, 2020, 1804630. [Google Scholar] [CrossRef] [PubMed]
- Vincic, L.; Weston, S.; Riddell, R. Bone core biopsies. Plastic or paraffin? Am. J. Surgeical Pathol. 1989, 13, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.; Vogel, M.; Delling, G. Undecalcified preparation of bone tissue: Report of technical experience and development of new methods. Virchows Arch. A Pathol. Anat. 1991, 418, 1–7. [Google Scholar] [CrossRef]
- Moreno-Jiménez, I.; Garske, D.; Lahr, C.; Hutmacher, D.; Cipitria, A. Targeted 2D histology and ultrastructural bone analysis based on 3D microCT anatomical locations. MethodsX 2021, 8, 101480. [Google Scholar] [CrossRef]
- Donath, K.; Breuner, G. A method for the study of undeealeified bones and teeth with attaehed soft tissues. The Sage-Schliff (sawing and grinding) Technicjue. J. Oral Pathol. 1982, 11, 318–326. [Google Scholar] [CrossRef]
- Rohrer, M.; Schubert, C. The cutting-grinding technique for histologic preparation of undecalcified bone and bone-anchored implants. Improvements in instrumentation and procedures. Oral Surg. Oral Med. Oral Pathol. 1992, 74, 73–78. [Google Scholar] [CrossRef]
- Mukhamadiyarov, R.; Sevostyanova, V.; Shishkova, D.; Nokhrin, A.; Sidorova, O.; Kutikhin, A. Grinding and polishing instead of sectioning for the tissue sampleswith a graft: Implications for light and electron microscopy. Micron 2016, 85, 1–7. [Google Scholar] [CrossRef]
- Bottagisio, M.; Coman, C.; Lovati, A. Animal models of orthopaedic infections. A review of rabbit models used to induce long bone bacterial infections. J. Med. Microbiol. 2019, 68, 506–537. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, C.; Johansson, C. Osseointegration of Implants. In Osteology Guidelines for Oral and Maxillofacial Regeneration: Preclinical Models for Translational Research; Quintessence Pub Co.: Batavia, IL, USA, 2011; pp. 103–121. ISBN 978-1-85097-211-2. [Google Scholar]
- Gruber, H.; Ingram, J. Basic Staining and Histochemical Techniques and Immunohistochemical Localizations Using Bone Sections. In Handbook of Histology Methods for Bone and Cartilage; An, Y., Martin, K., Eds.; Humana Press Inc.: Clifton, NJ, USA, 2003; pp. 281–286. ISBN 1-59259-417-4. [Google Scholar]
- Schenk, R.K.; Olah, A.J.; Herrmann, W. Preparation of calcified tissues for light microscopy. In Methods of Calcified Tissue Preparation; Dickson, G., Ed.; Elsevier: Amsterdam, The Netherlands, 1984; pp. 1–56. ISBN 9780444805805. [Google Scholar]
- Osborne, D.; Curtis, J. A Protocol for the Staining of Cement Lines in Adult Human Bone Using Toluidine Blue. J. Histotechnol. 2005, 28, 73–79. [Google Scholar] [CrossRef]
- Burr, D. Estimated intracortical bone turnover in the femur of growing macaques: Implications for their use as models in skeletal pathology. Anat. Rec. 1992, 2, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Reim, N.; Breig, B.; Stahr, K.; Eberle, J.; Hoeflich, A.; Wolf, E.; Erben, R. Cortical bone loss in androgen-deficient aged male rats is mainly caused by increased endocortical bone remodelling. J. Bone Miner. Res. 2008, 5, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Eurell, J.; Sterchi, D. Microwaveable Toluidine Blue Stain for Surface Staining of Undecalcified Bone Sections. J. Histotechnol. 1994, 17, 357–359. [Google Scholar] [CrossRef]
- Villanueva, A.; Kujawa, M.; Mathews, C.; Parfitt, A. Identification of the mineralization front: Comparisonof a modified toluidine blue stain with tetracycline fluorescence. Metab. Bone Dis. Relat. Res. 1983, 5, 41–45. [Google Scholar] [CrossRef]
- Bain, S.; Impeduglia, T.; Rubin, C. Cement line in undecalcified thin sections of cortical bone. Stain. Technol. 1990, 65, 159–163. [Google Scholar] [CrossRef]
- Metzler, P.; Wilmowsky, C.; Stadlinger, B.; Zemann, W.; Schlegel, K.; Rosiwal, S.; Rupprecht, S. Nano-crystalline diamond-coated titanium dental implants—A histomorphometric study in adult domestic pigs. J. Cranio-Maxillo-Facial Surg. 2013, 41, 532–538. [Google Scholar] [CrossRef]
- Beutel, B.; Danna, N.; Granato, R.; Bonfante, E.; Marin, C.; Tovar, N.; Suzuki, M.; Coelho, P. Implant design and its effects on osseointegration over time within cortical and trabecular bone. J. Biomed. Mater. Res. Part B 2016, 104, 1091–1097. [Google Scholar] [CrossRef]
- Cohen, O.; Ormianer, Z.; Tal, H.; Rothamel, D.; Miron Weinreb, M.; Moses, O. Differences in crestal bone-to-implant contact following an under-drilling compared to an over-drilling protocol. A study in the rabbit tibia. Clin. Oral Investig. 2016, 20, 2475–2480. [Google Scholar] [CrossRef]
- Chen, H.; Lai, W.; Chee, T.; Chan, Y.; Feng, S. Monitoring the Changes of Material Properties at Bone-Implant Interface during the Healing Process In Vivo: A Viscoelastic Investigation. BioMed Res. Int. 2017, 2017, 1945607. [Google Scholar] [CrossRef] [PubMed]
- Faeda, R.; Nascimento, S.; Santos, P.; Neto, R.; Rafael Sartori, R.; Rogério Margonar, R.; Elcio Marcantonio, E., Jr. Human non-decalcified histology of three dental implants 45 months under function—A case report. Int. J. Implant. Dent. 2019, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Guglielmotti, M.; Olmedo, D.; Cabrini, R. Research on implants and osseointegration. Periodontol. 2000 2019, 79, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Auciello, O.; Renou, S.; Kang, K.; Tasat, D.; Olmedo, D. A Biocompatible Ultrananocrystalline Diamond (UNCD) Coating for a New Generation of Dental Implants. Nanomaterials 2022, 12, 782. [Google Scholar] [CrossRef]
- Carter, D.H.; Barnes, J.M.; Aaron, J.E. Histomorphometry of fresh-frozen iliac crest bone biopsies. Calcif. Tissue Int. 1989, 44, 387–392. [Google Scholar] [CrossRef]


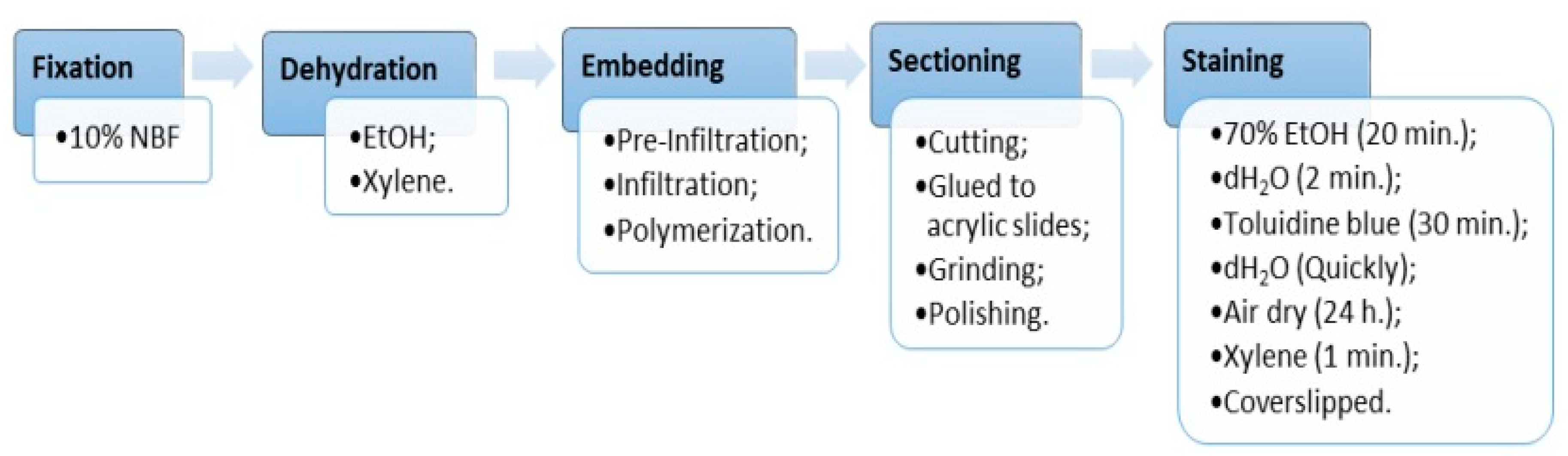
| Stages | Solution | Time [h] | Condition |
|---|---|---|---|
| Fixation | 10% NBF | 72 | RT |
| Dehydration | 70% EtOH | 4 | RT—vacuum |
| 80% EtOH | 4 | RT—vacuum | |
| 90% EtOH | 16 | RT | |
| 96% EtOH | 4 | RT | |
| 96% EtOH | 4 | RT | |
| 99.8% EtOH | 16 | RT | |
| 99.8% EtOH | 4 | RT | |
| 99.8% EtOH | 4 | RT | |
| Xylene | 16 | RT | |
| Xylene | 8 | RT | |
| Pre-infiltration 1 | Xylene + stabilized MMA (1:1) | 24 | RT—with agitation |
| Pre-infiltration 2 | 200 mL stabilized MMA + 1 g Hardener 1 | 24 | RT—with agitation |
| Pre-infiltration 3 | 200 mL destabilized MMA + 1 g Hardener 1 | 24 | 4 °C—with agitation |
| Infiltration | 250 mL destabilized MMA + 20 g PMMA + 1 g Hardener 1 | 72 | 4 °C—with agitation |
| Polymerization | Solution A + Solution B (9:1) | 120 | −20 °C; |
| Solution | Immersion Time |
|---|---|
| 0.1% formic acid | 5 min |
| dH2O | Quick rinse |
| 70% EtOH | 15 min |
| Toluidine blue | 5 min |
| dH2O | Quick rinse |
| 70% EtOH (differentiation) | 30 s |
| 95% EtOH | 30 s |
| 100% EtOH | 30 s |
| 100% EtOH | 30 s |
| Experimental Group № | Immersion Time (min) | Differentiation and Dehydration | |||||
|---|---|---|---|---|---|---|---|
| 0.1% Formic Acid | dH2O | 70% EtOH | dH2O | Toluidine Blue (5 Subgroups According to the Immersion Time) | dH2O | ||
| 1 | 5 | Quickly | 15 | 2 | 5; 10; 20; 30; 40 | Quickly | Yes |
| 2 | 5 | Quickly | - | - | 5; 10; 20; 30; 40 | Quickly | Yes |
| 3 | - | - | 15 | 2 | 5; 10; 20; 30; 40 | Quickly | Yes |
| 4 | - | - | 20 | 2 | 5; 10; 20; 30; 40 | Quickly | Yes |
| 5 | 5 | Quickly | 15 | 2 | 5; 10; 20; 30; 40 | Quickly | No |
| 6 | 5 | Quickly | - | - | 5; 10; 20; 30; 40 | Quickly | No |
| 7 | - | - | 15 | 2 | 5; 10; 20; 30; 40 | Quickly | No |
| 8 | - | - | 20 | 2 | 5; 10; 20; 30; 40 | Quickly | No |
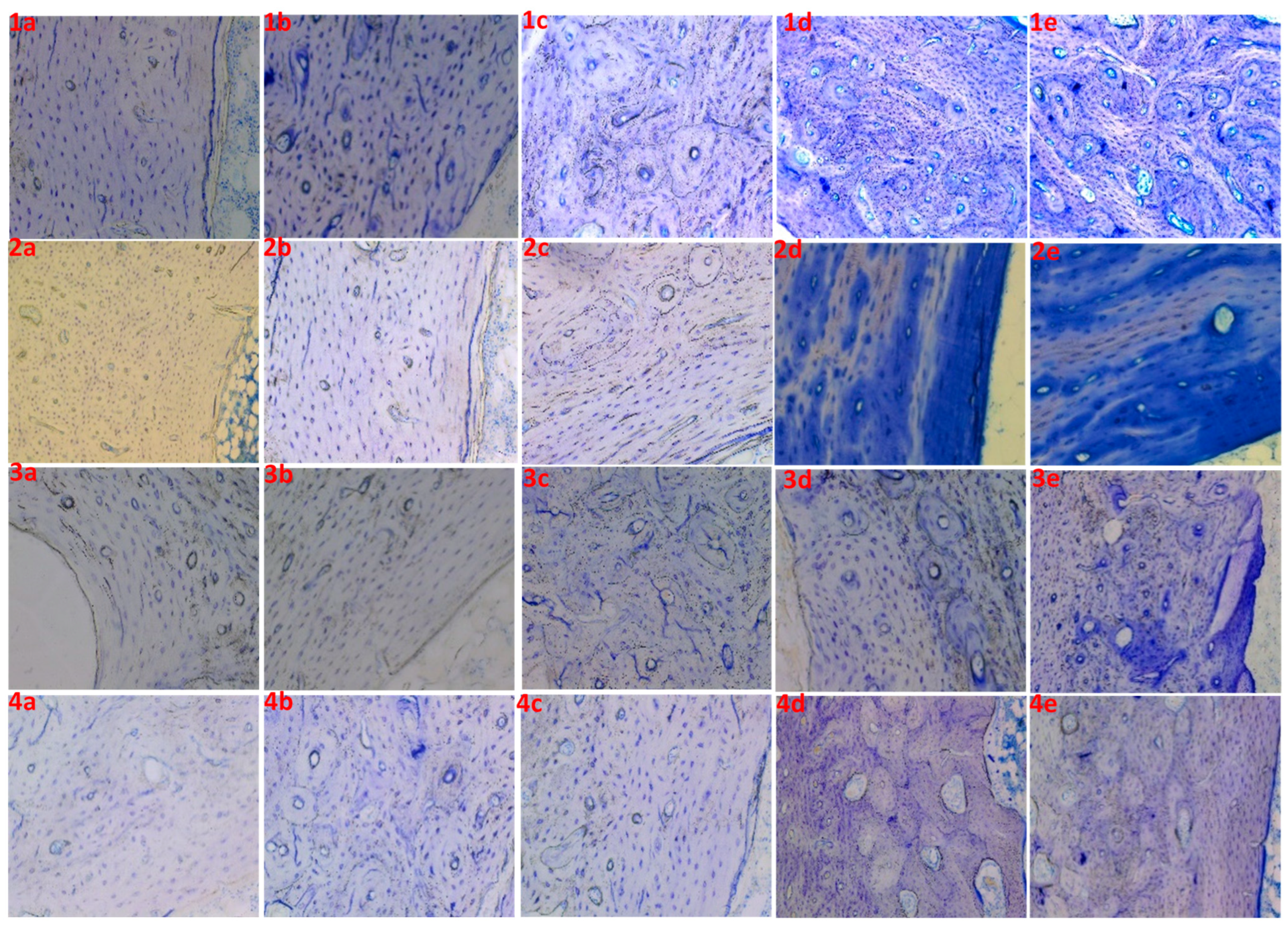
| Experimental Group № | Samples | Time in Formic Acid (min) | Time in EtOH (min) | Time in Toluidine Blue (min) | Staining Result |
|---|---|---|---|---|---|
| 1 | 1a | 5 | 15 | 5 | Poor |
| 1b | 5 | 15 | 10 | Poor | |
| 1c | 5 | 15 | 20 | Good | |
| 1d | 5 | 15 | 30 | Good | |
| 1e | 5 | 15 | 40 | Good | |
| 2 | 2a | 5 | 0 | 5 | Unstained |
| 2b | 5 | 0 | 10 | Poor | |
| 2c | 5 | 0 | 20 | Poor | |
| 2d | 5 | 0 | 30 | Unclear | |
| 2e | 5 | 0 | 40 | Unclear | |
| 3 | 3a | 0 | 15 | 5 | Unstained |
| 3b | 0 | 15 | 10 | Unstained | |
| 3c | 0 | 15 | 20 | Poor | |
| 3d | 0 | 15 | 30 | Poor | |
| 3e | 0 | 15 | 40 | Unclear | |
| 4 | 4a | 0 | 20 | 5 | Unstained |
| 4b | 0 | 20 | 10 | Poor | |
| 4c | 0 | 20 | 20 | Poor | |
| 4d | 0 | 20 | 30 | Good | |
| 4e | 0 | 20 | 40 | Good |
| Experimental Group № | Samples | Time in Formic Acid (min) | Time in EtOH (min) | Time in Toluidine Blue (min) | Staining Result |
|---|---|---|---|---|---|
| 5 | 5a | 5 | 15 | 5 | Poor |
| 5b | 5 | 15 | 10 | Poor | |
| 5c | 5 | 15 | 20 | Good | |
| 5d | 5 | 15 | 30 | Good | |
| 5e | 5 | 15 | 40 | Overstained | |
| 6 | 6a | 5 | 0 | 5 | Unstained |
| 6b | 5 | 0 | 10 | Poor | |
| 6c | 5 | 0 | 20 | Poor | |
| 6d | 5 | 0 | 30 | Unclear | |
| 6e | 5 | 0 | 40 | Unclear | |
| 7 | 7a | 0 | 15 | 5 | Unstained |
| 7b | 0 | 15 | 10 | Unstained | |
| 7c | 0 | 15 | 20 | Poor | |
| 7d | 0 | 15 | 30 | Poor | |
| 7e | 0 | 15 | 40 | Good | |
| 8 | 8a | 0 | 20 | 5 | Poor |
| 8b | 0 | 20 | 10 | Good | |
| 8c | 0 | 20 | 20 | Good | |
| 8d | 0 | 20 | 30 | Best | |
| 8e | 0 | 20 | 40 | Overstained |
| Solution | Immersion Time |
|---|---|
| 70% EtOH | 20 min |
| dH2O | 2 min |
| Toluidine blue | 30 min |
| dH2O | Quick rinse |
| Air dry | 24 h |
| Xylene | 1 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peev, S.; Parushev, I.; Yotsova, R. A Modified Protocol for Staining of Undecalcified Bone Samples Using Toluidine Blue—A Histological Study in Rabbit Models. Appl. Sci. 2024, 14, 461. https://doi.org/10.3390/app14010461
Peev S, Parushev I, Yotsova R. A Modified Protocol for Staining of Undecalcified Bone Samples Using Toluidine Blue—A Histological Study in Rabbit Models. Applied Sciences. 2024; 14(1):461. https://doi.org/10.3390/app14010461
Chicago/Turabian StylePeev, Stefan, Ivaylo Parushev, and Ralitsa Yotsova. 2024. "A Modified Protocol for Staining of Undecalcified Bone Samples Using Toluidine Blue—A Histological Study in Rabbit Models" Applied Sciences 14, no. 1: 461. https://doi.org/10.3390/app14010461
APA StylePeev, S., Parushev, I., & Yotsova, R. (2024). A Modified Protocol for Staining of Undecalcified Bone Samples Using Toluidine Blue—A Histological Study in Rabbit Models. Applied Sciences, 14(1), 461. https://doi.org/10.3390/app14010461








