Antifungal Effects of Fermented Sophora flavescens and Eleutherococcus sessiliflorus Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of Microbial Strains
2.2. 16S rRNA Sequence Analysis
2.3. Fermentation and Cultivation of Plant Extracts
2.4. Antifungal Activity
2.5. LC-Q-TOF/MS Analysis
2.6. Statistical Analysis
3. Results and Discussion
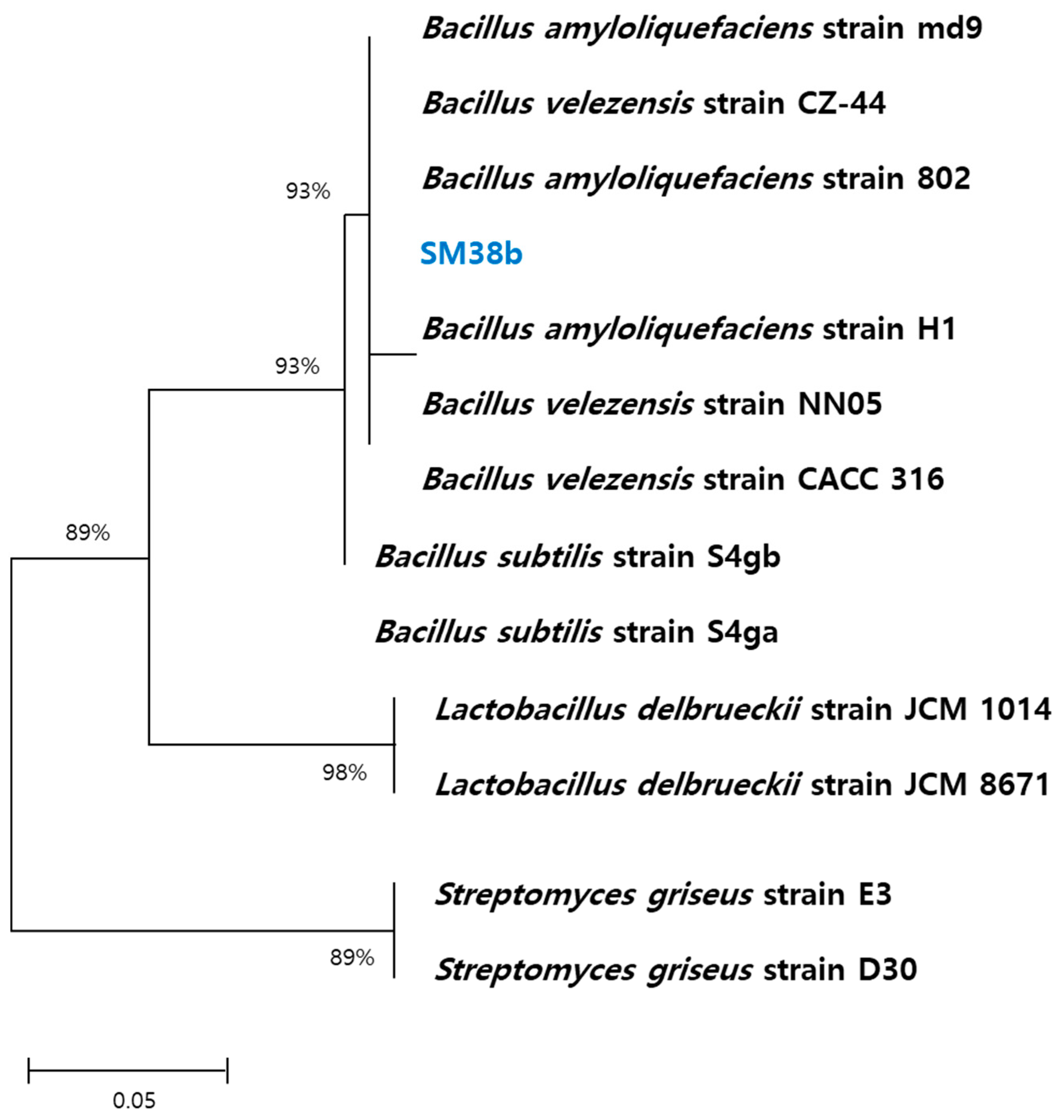
3.1. Identification of the Isolated Microbial Strains
3.2. Antifungal Effects of the Fermented and Unfermented Plant Extracts
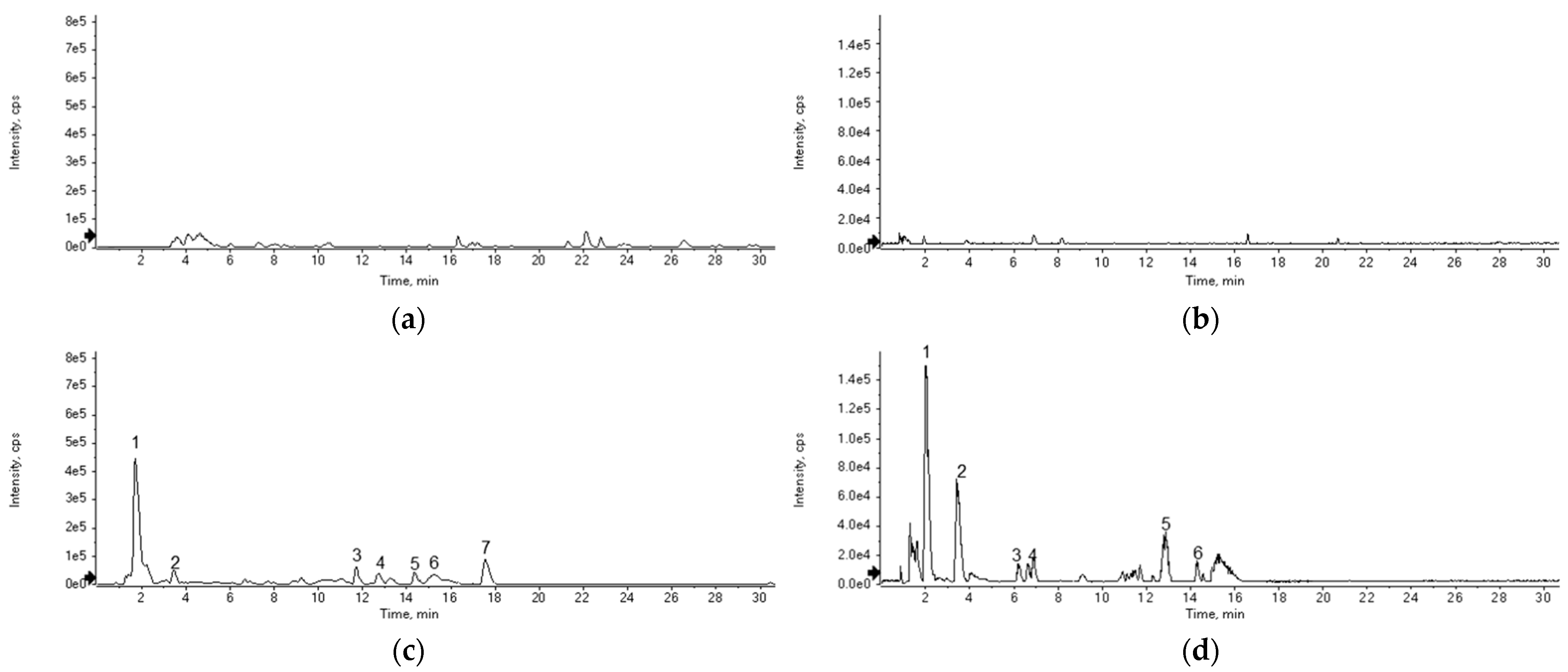
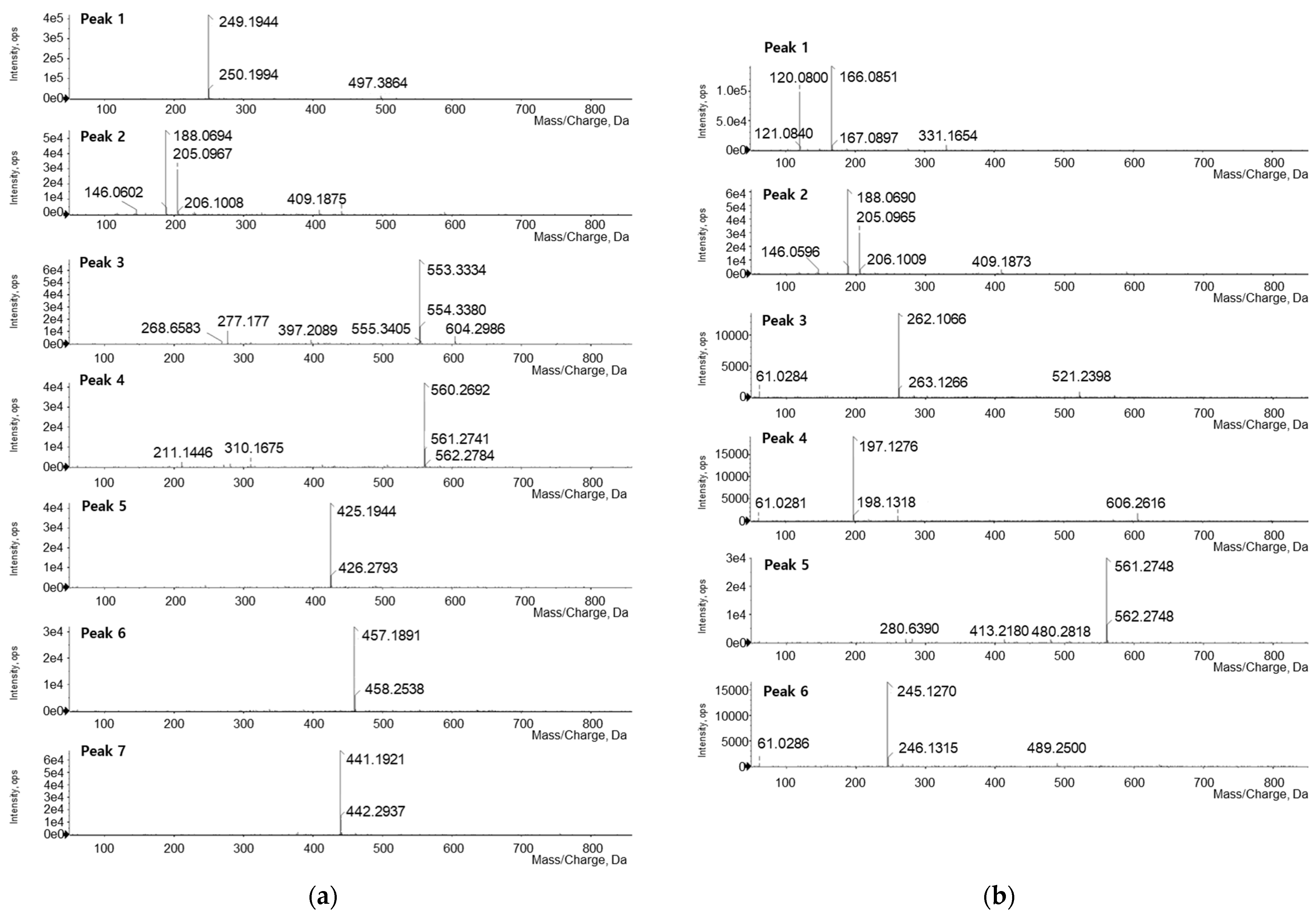
3.3. LC-Q-TOF/MS Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial Properties and Mechanism of Action of Some Plant Extracts Against Food Pathogens and Spoilage Microorganisms. Front. Microbiol. 2018, 9, 1639. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Meng, X.; Li, Y.; Zhao, C.-N.; Tang, G.-Y.; Li, H.-B. Antibacterial and Antifungal Activities of Spices. Int. J. Mol. Sci. 2017, 18, 1283. [Google Scholar] [CrossRef] [PubMed]
- Yassin, M.T.; Mostafa, A.A.-F.; Al-Askar, A.A.; Sayed, S.R. In Vitro Antimicrobial Activity of Thymus vulgaris Extracts Against Some Nosocomial and Food Poisoning Bacterial Strains. Process Biochem. 2022, 115, 152–159. [Google Scholar] [CrossRef]
- Wink, M. Modes of Action of Herbal Medicines and Plant Secondary Metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial Plant Compounds, Extracts and Essential Oils: An Updated Review on Their Effects and Putative Mechanisms of Action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef]
- Jacob, J.; Rajendran, R.U.; Priya, S.H.; Purushothaman, J.; Amma, D.K.B.N.S. Enhanced Antibacterial Metabolite Production Through the Application of Statistical Methodologies by a Streptomyces nogalater NIIST A30 Isolated from Western Ghats Forest Soil. PLoS ONE 2017, 12, e0175919. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Sinha, A.; Osborne, W.J. Microbial Secondary Metabolites: Recent Developments and Technological Challenges. In Volatiles Metabolites of Microbes; Academic Press: Cambridge, MA, USA, 2021; pp. 1–22. [Google Scholar] [CrossRef]
- Singh, B.P.; Rateb, M.E.; Rodriguez-Couto, S.; Polizeli, M.d.L.T.d.M.; Li, W.-J. Editorial: Microbial Secondary Metabolites: Recent Developments and Technological Challenges. Front. Microbiol. 2019, 10, 454593. [Google Scholar] [CrossRef]
- Moon, K.; Cha, J. Enhancement of Antioxidant and Antibacterial Activities of Salvia miltiorrhiza Roots Fermented with Aspergillus oryzae. Foods 2020, 9, 34. [Google Scholar] [CrossRef]
- Khan, A.W.; Lali, N.S.; Sabei, F.Y.; Irfan, M.I.; Naeem-Ul-Hassan, M.; Sher, M.; Safhi, A.Y.; Alsalhi, A.; Albariqi, A.H.; Kamli, F.; et al. Sunlight-Assisted Green Synthesis of Gold Nanocubes Using Horsetail Leaf Extract: A Highly Selective Colorimetric Sensor for Pb2+, Photocatalytic and Antimicrobial Agent. J. Environ. Chem. Eng. 2024, 12, 112576. [Google Scholar] [CrossRef]
- Ullah, S.; Khalid, R.; Rehman, M.F.; Irfan, M.I.; Abbas, A.; Alhoshani, A.; Anwar, F.; Amin, H.M.A. Biosynthesis of Phyto-Functionalized Silver Nanoparticles Using Olive Fruit Extract and Evaluation of Their Antibacterial and Antioxidant Properties. Front. Chem. 2023, 11, 1202252. [Google Scholar] [CrossRef]
- Hong, M.H.; Lee, J.Y.; Jung, H.; Jin, D.-H.; Go, H.Y.; Kim, J.H.; Jang, B.-H.; Shin, Y.-C.; Ko, S.-G. Sophora flavescens Aiton Inhibits the Production of Pro-Inflammatory Cytokines through Inhibition of the NF κB/IκB Signal Pathway in Human Mast Cell Line (HMC-1). Toxicol. Vitr. 2009, 23, 251–258. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.; Sa, K.; Li, H.; Chen, L. Alkaloids from the Roots of Sophora flavescens and Their Anti-Tumor Activity. Fitoterapia 2023, 171, 105685. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, X.-J.; Li, J.-J.; He, L.; Yang, Y.-R.; Zhong, F.; He, M.-H.; Shen, Y.-T.; Tu, B.; Zhang, X.; et al. A Novel Type Lavandulyl Flavonoid from Sophora flavescens as Potential Anti-Hepatic Injury Agent That Inhibit TLR2/NF-κB Signaling Pathway. J. Ethnopharmacol. 2023, 307, 116163. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Zhao, W.; Wang, Q.; Chen, L.; Sun, K.; Zhan, Z.; Wang, J. Chemical Diversity, Biological Activities and Traditional Uses of and Important Chinese Herb Sophora. Phytomedicine 2022, 100, 154054. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-J.; Zhang, X.; Shen, X.-C.; Long, Q.-D.; Xu, C.-Y.; Tan, C.-J.; Lin, Y. Phytochemistry and Biological Properties of Isoprenoid Flavonoids from Sophora flavescens Ait. Fitoterapia 2020, 143, 104556. [Google Scholar] [CrossRef] [PubMed]
- Shohael, A.M.; Chakrabarty, D.; Yu, K.W.; Hahn, E.J.; Paek, K.Y. Application of Bioreactor System for Large-Scale Production of Eleutherococcus sessiliflorus Somatic Embryos in an Air-Lift Bioreactor and Production of Eleutherosides. J. Biotechnol. 2005, 120, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Załuski, D.; Olech, M.; Verpoorte, R.; Khan, I.; Kuźniewski, R.; Nowak, R. Phytoconstituents and Nutritional Properties of the Fruits of Eleutherococcus divaricatus and Eleutherococcus sessiliflorus: A Study of Non-European Species Cultivated in Poland. Oxidative Med. Cell. Longev. 2017, 2017, 8374295. [Google Scholar] [CrossRef]
- Załuski, D.; Smolarz, H.D.; Gawlik-Dziki, U. Bioactive Compounds and Antioxidative, Antileukemic and Anti-MMPs Activity of Eleutherococcus Species Cultivated in Poland. Nat. Prod. Commun. 2012, 7, 1483–1486. [Google Scholar] [CrossRef] [PubMed]
- Rimondi, E.; Zweyer, M.; Ricci, E.; Fadda, R.; Secchiero, P. Receptor Activator of Nuclear Factor κB Ligand (RANKL) Modulates the Expression of Genes Involved in Apoptosis and Cell Cycle in Human Osteoclasts. Anat. Rec. 2007, 290, 838–845. [Google Scholar] [CrossRef]
- Muratovic, D.; Atkins, G.J.; Findlay, D.M. Is RANKL A Potential Molecular Target in Osteoarthritis? Osteoarthr. Cartil. 2024, 32, 493–500. [Google Scholar] [CrossRef]
- Sun, H.; Feng, J.; Sun, Y.; Sun, S.; Li, L.; Zhu, J.; Zang, H. Phytochemistry and Pharmacology of Eleutherococcus sessiliflorus (Rupr. & Maxim.) S.Y. Hu: A Review. Molecules 2023, 28, 6564. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.N.; Li, W.; Yan, X.T.; Yang, S.Y.; Kim, Y.H. Chemical Constituents from the Stems of Acanthopanax divaricatus var. albeofructus. Biochem. Syst. Ecol. 2014, 57, 164–168. [Google Scholar] [CrossRef]
- Jang, H.-J.; Kim, W.J.; Lee, S.U.; Kim, M.-O.; Park, M.H.; Song, S.; Kim, D.-Y.; Lee, S.M.; Yuk, H.J.; Lee, D.Y.; et al. Optimization of Chiisanoside and Chiisanogenin Isolation from Eleutherococcus sessiliflorus (Rupr. & Maxim.) Leaves for Industrial Application: A Pilot Study. Ind. Crops Prod. 2022, 185, 115099. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, M.; Zheng, J.; Gänzle, M.G. Bacillus Species in Food Fermentations: An Underappreciated Group of Organisms for Safe Use in Food Fermentations. Curr. Opin. Food Sci. 2023, 50, 101007. [Google Scholar] [CrossRef]
- Schallmey, M.; Singh, A.; Ward, O.P.; Sumi, C.D.; Yang, B.W.; Yeo, I.-C.; Hahm, Y.T.; Zhang, C.; Zhang, X.; Yao, Z.; et al. Developments in the Use of Bacillus Species for Industrial Production. Can. J. Microbiol. 2004, 50, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Ning, C.; Liu, Z.; Liang, Q.; Fu, X.; Tian, M.; Zhu, C.; Mou, H. High-Efficiency Heterologous Expression of Nattokinase Based on a Combinatorial Strategy. Process Biochem. 2023, 133, 65–74. [Google Scholar] [CrossRef]
- Sardar, R.; Asad, M.J.; Ahmad, M.S.; Ahmad, T. Optimization of Phytase Production by Bacillus sp. (HCYL03) under Solid-State Fermentation by Using Box-Behnken Design. Braz. Arch. Biol. Technol. 2022, 65, e22210307. [Google Scholar] [CrossRef]
- Toljamo, A.; Koistinen, V.; Hanhineva, K.; Kärenlampi, S.; Kokko, H. Terpenoid and Lipid Profiles Vary in Different Phytophthora cactorum–Strawberry Interactions. Phytochemistry 2021, 189, 112820. [Google Scholar] [CrossRef] [PubMed]
- Nellist, C.F.; Armitage, A.D.; Bates, H.J.; Sobczyk, M.K.; Luberti, M.; Lewis, L.A.; Harrison, R.J. Comparative Analysis of Host-Associated Variation in Phytophthora cactorum. Front. Microbiol. 2021, 12, 679936. [Google Scholar] [CrossRef]
- Lu, X.; Xu, H.; Song, W.; Yang, Z.; Yu, J.; Tian, Y.; Jiang, M.; Shen, D.; Dou, D. Rapid and Simple Detection of Phytophthora cactorum in Strawberry Using a Coupled Recombinase Polymerase Amplification–Lateral Flow Strip Assay. Phytopathol. Res. 2021, 3, 12. [Google Scholar] [CrossRef]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; Van Kan, J.A.L. Botrytis cinerea: The Cause of Grey Mould Disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Audenaert, K.; Van Labeke, M.-C.; Höfte, M. Detection of Botrytis cinerea on Strawberry Leaves upon Mycelial Infection Through Imaging Technique. Sci. Hortic. 2024, 330, 113071. [Google Scholar] [CrossRef]
- Wen, X.; Wang, Q.; Luo, H.; He, C.; An, B. A Baeyer-Villiger Monooxygenase Cgbvmo1 Is Involved in Superoxide Anion Metabolism, Cell Wall Synthesis, and Pathogenicity of Colletotrichum gloeosporioides. Postharvest Biol. Technol. 2024, 210, 112786. [Google Scholar] [CrossRef]
- Galsurker, O.; Diskin, S.; Maurer, D.; Feygenberg, O.; Alkan, N. Fruit Stem-End Rot. Horticulturae 2018, 4, 50. [Google Scholar] [CrossRef]
- Li, H.; Zhou, G.-Y.; Liu, J.-A.; Xu, J.X. Population Genetic Analyses of the Fungal Pathogen Colletotrichum fructicola on Tea-Oil Trees in China. PLoS ONE 2016, 11, e0156841. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Plant Health (PLH). Pest Categorisation of Colletotrichum fructicola. EFSA J. 2021, 19, e06803. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Li, X.; Gao, Q.-H.; Geng, C.; Duan, K. Colletotrichum Species Pathogenic to Strawberry: Discovery History, Global Diversity, Prevalence in China, and the Host Range of Top Two Species. Phytopathol. Res. 2022, 4, 42. [Google Scholar] [CrossRef]
- Li, W.; Ran, F.; Long, Y.; Mo, F.; Shu, R.; Yin, X. Evidences of Colletotrichum fructicola Causing Anthracnose on Passiflora edulis Sims in China. Pathogens 2022, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Evallo, E.; Taguiam, J.D.; Bengoa, J.; Maghirang, R.; Balendres, M.A. First Report of Colletotrichum fructicola, Causing Anthracnose of Hylocereus Plants, in the Philippines. Czech Mycol. 2021, 73, 79–90. [Google Scholar] [CrossRef]
- Lin, S.-R.; Yu, S.-Y.; Chang, T.-D.; Wen, C.-J.; Lin, Y.H.; Lin, Y.-H. First Report of Anthracnose Caused by Colletotrichum fructicola on Tea in Taiwan. Plant Dis. 2020, 105, 710. [Google Scholar] [CrossRef]
- Lim, Y.-S.; Hassan, O.; Chang, T. First Report of Anthracnose of Shine Muscat Caused by Colletotrichum fructicola in Korea. Mycobiology 2019, 48, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Rockenbach, M.F.; Velho, A.C.; Gonçalves, A.E.; Mondino, P.E.; Alaniz, S.M.; Stadnik, M.J. Genetic Structure of Colletotrichum fructicola Associated to Apple Bitter Rot and Glomerella Leaf Spot in Southern Brazil and Uruguay. Phytopathology 2016, 106, 774–781. [Google Scholar] [CrossRef]
- Velho, A.C.; Mondino, P.; Stadnik, M.J. Extracellular Enzymes of Colletotrichum fructicola Isolates Associated to Apple Bitter Rot and Glomerella Leaf Spot. Mycology 2018, 9, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, L.; Huang, X.; Huang, X.; He, C.; He, C.; Zhang, Q.-Y.; Zhang, Q.-Y.; Zou, X.; Zou, X.; et al. Novel Fungal Pathogenicity and Leaf Defense Strategies Are Revealed by Simultaneous Transcriptome Analysis of Colletotrichum fructicola and Strawberry Infected by This Fungus. Front. Plant Sci. 2018, 9, 434. [Google Scholar] [CrossRef] [PubMed]
- Grenville-Briggs, L.J.; Kushwaha, S.K.; Cleary, M.R.; Witzell, J.; Savenkov, E.I.; Whisson, S.C.; Chawade, A.; Vetukuri, R.R. Draft Genome of the Oomycete Pathogen Phytophthora cactorum Strain LV007 Isolated from European Beech (Fagus sylvatica). Genom. Data 2017, 12, 155–156. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Wu, F. Characterization of Mirnas Associated with Botrytis cinerea Infection of Tomato Leaves. BMC Plant Biol. 2015, 15, 1. [Google Scholar] [CrossRef]
- Shi, S.; Wang, J.; Liu, C.; Zheng, L. Alleviative Effects of Quercetin of Botrytis cinerea-Induced Toxicity in Zebrafish (Danio rerio) Larvae. Fish Shellfish. Immunol. 2023, 142, 109146. [Google Scholar] [CrossRef]
- Yu, L.; Lan, G.; Yang, Y.; Tang, Y.; Li, Z.; She, X.; He, Z. First Report of Anthracnose Caused by Colletotrichum fructicola on Brassica parachinensis in China. Crop Prot. 2021, 154, 105842. [Google Scholar] [CrossRef]
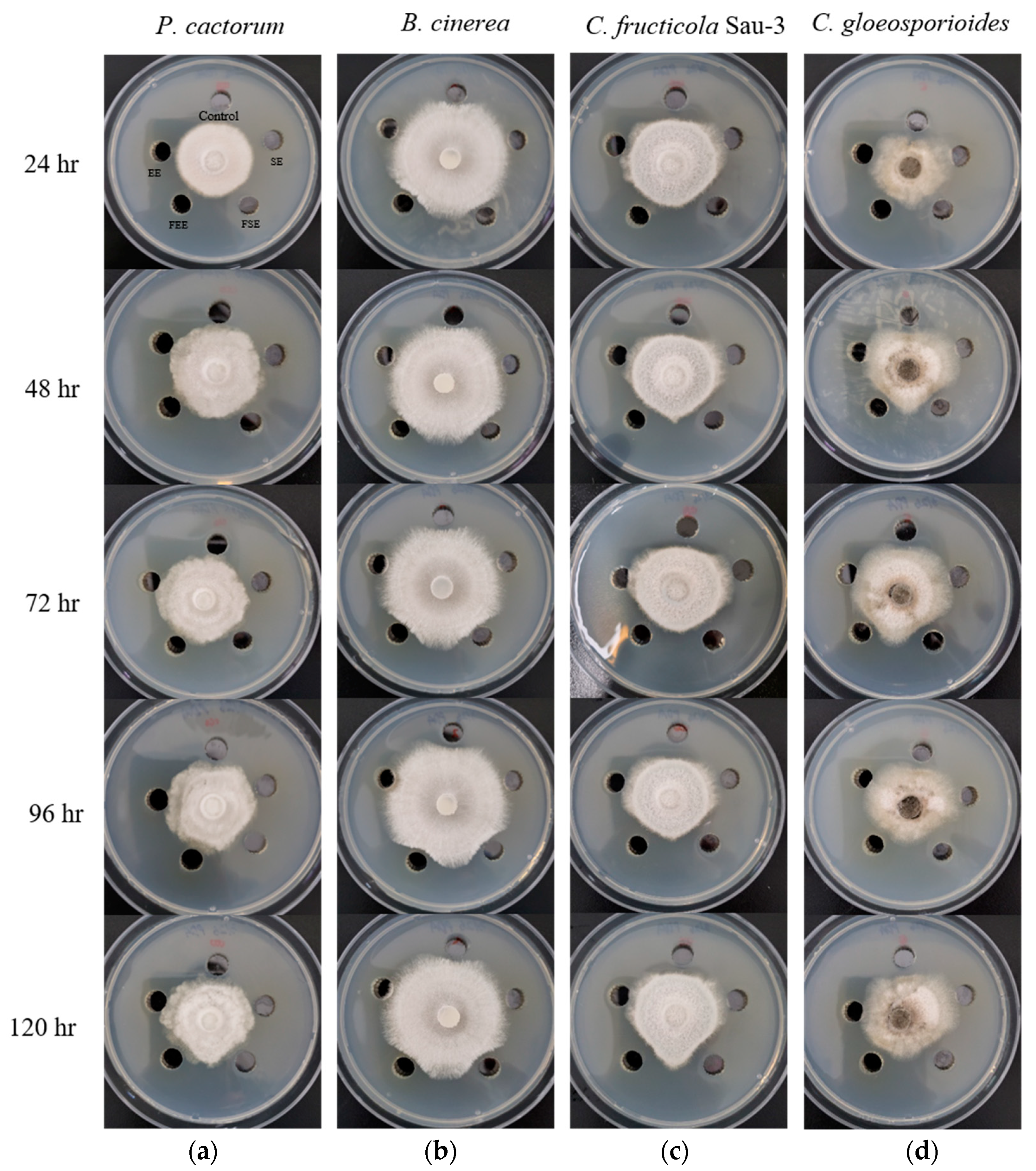
| Target Fungi | Incubation Time (h) | Antifungal Effects | |||
|---|---|---|---|---|---|
| SE a | FSE b | EE c | FEE d | ||
| P. cactorum | 24 | - e | - | - | - |
| B. cinerea | - | + | - | + | |
| C. fructicola Sau-3 | - | ++ | - | ++ | |
| C. gloeoporioides | - | ++ | - | ++ | |
| P. cactorum | 48 | + | + | - | + |
| B. cinerea | - | + | - | + | |
| C. fructicola Sau-3 | - | ++ | - | ++ | |
| C. gloeoporioides | - | ++ | - | ++ | |
| P. cactorum | 72 | + | + | - | + |
| B. cinerea | + | ++ | - | ++ | |
| C. fructicola Sau-3 | - | ++ | - | ++ | |
| C. gloeoporioides | - | ++ | - | ++ | |
| P. cactorum | 96 | + | ++ | - | ++ |
| B. cinerea | + | ++ | - | ++ | |
| C. fructicola Sau-3 | - | ++ | - | ++ | |
| C. gloeoporioides | - | +++ | - | ++ | |
| P. cactorum | 120 | + | ++ | - | ++ |
| B. cinerea | + | ++ | - | ++ | |
| C. fructicola Sau-3 | - | +++ | - | +++ | |
| C. gloeoporioides | - | +++ | - | ++ | |
| No. | tR (min) | Observed Ion (m/z) | Theoretical Ion (m/z) | Error (ppm) | Adducts | Formula | Identification |
|---|---|---|---|---|---|---|---|
| Fermented Sophora flavescens Extract | |||||||
| 1 | 1.79 | 249.1944 | 249.1967 | −9.23 | M + H | C15H24N2O | Matrine |
| 2 | 3.46 | 205.0967 | 205.0977 | −4.87 | M + H | C11H12N2O2 | Tryptophan |
| 3 | 11.72 | 553.3334 | - | - | - | - | Unknown |
| 4 | 12.72 | 560.2692 | - | - | - | - | Unknown |
| 5 | 14.39 | 425.1944 | 425.1964 | −4.70 | M + H | C25H28O6 | Kushenol F |
| 6 | 15.21 | 457.1891 | 457.1862 | +6.34 | M + H | C25H28O8 | Kushenol G |
| 7 | 17.58 | 441.1921 | 441.1913 | +1.81 | M + H | C25H28O7 | Kushenol L |
| Fermented Eleutherococcus sessiliflorus extract | |||||||
| 1 | 2.06 | 166.0851 | 166.0868 | −10.24 | M + H | C9H11NO2 | Phenylalanine |
| 2 | 3.48 | 205.0965 | 205.0977 | −5.85 | M + H | C11H12N2O2 | Tryptophan |
| 3 | 6.24 | 262.1066 | 262.1039 | +10.30 | M + H | C9H15N3O6 | Asn-Glu |
| 4 | 6.87 | 197.1276 | 197.1290 | −7.10 | M + H | C10H16N2O2 | Cyclo(-Pro-Val) |
| 5 | 12.87 | 561.2748 | - | - | - | - | Unknown |
| 6 | 14.31 | 245.1270 | 245.1290 | −8.16 | M + H | C14H16N2O2 | Cyclo(-Phe-Pro) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.Y.; Chae, M.J.; Son, Y.G.; Jo, S.M.; Kang, N.R.; Kang, S.D.; Kim, K.D.; Lee, S.W.; Kim, J.Y. Antifungal Effects of Fermented Sophora flavescens and Eleutherococcus sessiliflorus Extract. Appl. Sci. 2024, 14, 4074. https://doi.org/10.3390/app14104074
Kim JY, Chae MJ, Son YG, Jo SM, Kang NR, Kang SD, Kim KD, Lee SW, Kim JY. Antifungal Effects of Fermented Sophora flavescens and Eleutherococcus sessiliflorus Extract. Applied Sciences. 2024; 14(10):4074. https://doi.org/10.3390/app14104074
Chicago/Turabian StyleKim, Ju Yeon, Min Joo Chae, Yun Gon Son, Su Min Jo, Na Rae Kang, Seong Doo Kang, Kwang Dong Kim, Sang Won Lee, and Jeong Yoon Kim. 2024. "Antifungal Effects of Fermented Sophora flavescens and Eleutherococcus sessiliflorus Extract" Applied Sciences 14, no. 10: 4074. https://doi.org/10.3390/app14104074
APA StyleKim, J. Y., Chae, M. J., Son, Y. G., Jo, S. M., Kang, N. R., Kang, S. D., Kim, K. D., Lee, S. W., & Kim, J. Y. (2024). Antifungal Effects of Fermented Sophora flavescens and Eleutherococcus sessiliflorus Extract. Applied Sciences, 14(10), 4074. https://doi.org/10.3390/app14104074









