A Narrative Review of Metabolomic Insights into Olive Oil’s Nutritional Value
Abstract
:1. Introduction
1.1. Importance of Olive Oil in Health and Nutrition
1.2. Introduction to Metabolomics
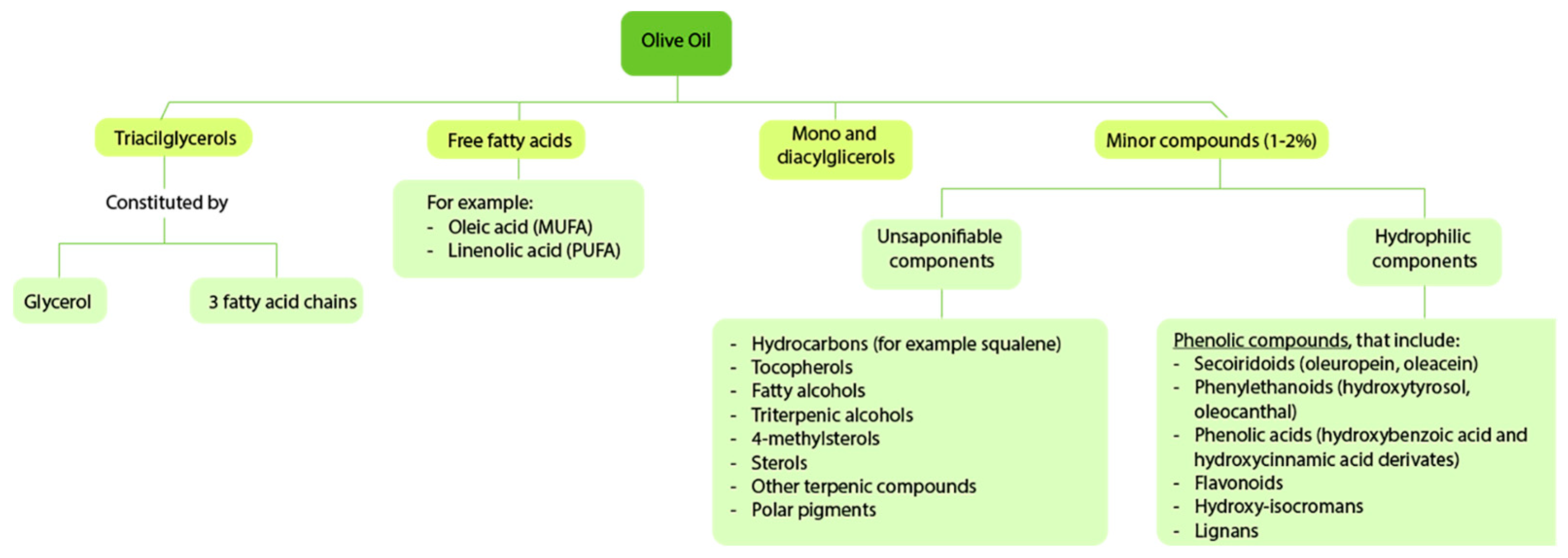
2. Olive Oil Metabolomics
2.1. Oleic Acid
2.2. Mono- and Diacylglycerols
2.3. Minor Compounds
2.3.1. Phenolic Compounds
Oleuropein
Hydroxytyrosol
Oleocanthal and Hydroxy-Isocromanans
Flavonoids and Lignans
2.4. Tocopherols
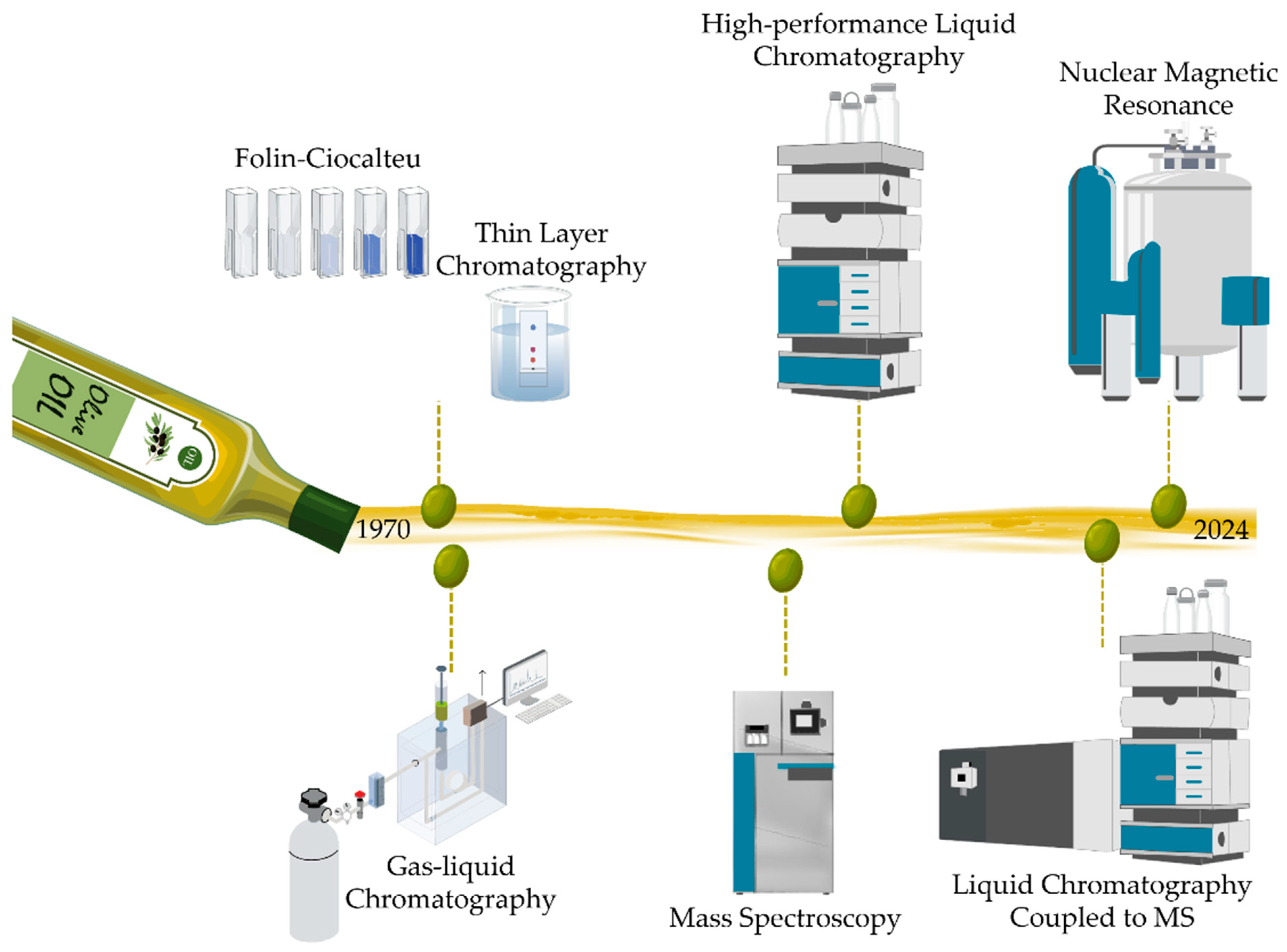
3. Techniques Used for Metabolite Profiling and Evolution
4. Case Studies and Applications
5. Challenges and Future Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guzmán Álvarez, J.; Hernández Rodríguez, P.; Gómez Calero, J.A.; Lora González, A. Olivares de España: Recorrido por la Biografía del Olivar, su Memoria y sus Paisajes; Almuzara: Cordoba, Spain, 2020. [Google Scholar]
- García-Calzón, S.; Martínez-González, M.A.; Razquin, C.; Corella, D.; Salas-Salvadó, J.; Martínez, J.A.; Zalba, G.; Marti, A. Pro12Ala polymorphism of the PPARγ2 gene interacts with a mediterranean diet to prevent telomere shortening in the PREDIMED-NAVARRA randomized trial. Circ. Cardiovasc. Genet. 2015, 8, 91–99. [Google Scholar] [CrossRef]
- Martínez-Lapiscina, E.H.; Clavero, P.; Toledo, E.; Estruch, R.; Salas-Salvadó, J.; San Julián, B.; Sanchez-Tainta, A.; Ros, E.; Valls-Pedret, C.; Martinez-Gonzalez, M. Mediterranean diet improves cognition: The PREDIMED-NAVARRA randomised trial. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1318–1325. [Google Scholar] [CrossRef]
- Xia, M.; Zhong, Y.; Peng, Y.; Qian, C. Olive oil consumption and risk of cardiovascular disease and all-cause mortality: A meta-analysis of prospective cohort studies. Front. Nutr. 2022, 9, 1041203. [Google Scholar] [CrossRef]
- Delgado-Lista, J.; Alcala-Diaz, J.F.; Torres-Peña, J.D.; Quintana-Navarro, G.M.; Fuentes, F.; Garcia-Rios, A.; Ortiz-Morales, A.M.; Gonzalez-Requero, A.I.; Perez-Caballero, A.I.; Yubero-Serrano, E.M.; et al. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): A randomised controlled trial. Lancet 2022, 399, 1876–1885. [Google Scholar] [CrossRef]
- Tsolaki, M.; Lazarou, E.; Kozori, M.; Petridou, N.; Tabakis, I.; Lazarou, I.; Karakota, M.; Saoulidis, I.; Melliou, E.; Magiatis, P. A Randomized Clinical Trial of Greek High Phenolic Early Harvest Extra Virgin Olive Oil in Mild Cognitive Impairment: The MICOIL Pilot Study. J. Alzheimers Dis. 2020, 78, 801–817. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Lindon, J.C.; Holmes, E. ‘Metabonomics’: Understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 1999, 29, 1181–1189. [Google Scholar] [CrossRef]
- Ulaszewska, M.M.; Weinert, C.H.; Trimigno, A.; Portmann, R.; Andres Lacueva, C.; Badertscher, R.; Brennan, L.; Brunius, C.; Bub, A.; Capozzi, F.; et al. Nutrimetabolomics: An Integrative Action for Metabolomic Analyses in Human Nutritional Studies. Mol. Nutr. Food Res. 2019, 63, e1800384. [Google Scholar] [CrossRef]
- Amin, A.M.; Mostafa, H.; Khojah, H.M.J. Insulin resistance in Alzheimer’s disease: The genetics and metabolomics links. Clin. Chim. Acta 2023, 539, 215–236. [Google Scholar] [CrossRef]
- Shibutami, E.; Takebayashi, T. A Scoping Review of the Application of Metabolomics in Nutrition Research: The Literature Survey 2000–2019. Nutrients 2021, 13, 3760. [Google Scholar] [CrossRef]
- García-Cañas, V.; Simó, C. Food Metabolomics—An Overview; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Gao, Q.; Praticò, G.; Scalbert, A.; Vergères, G.; Kolehmainen, M.; Manach, C.; Brennan, L.; Afman, L.A.; Wishart, D.S.; Andres-Lacueva, C.; et al. A scheme for a flexible classification of dietary and health biomarkers. Genes Amp. Nutr. 2017, 12, 34. [Google Scholar] [CrossRef]
- Termentzi, A.; Halabalaki, M.; Skaltsounis, A.L. 6—From Drupes to Olive Oil: An Exploration of Olive Key Metabolites. In Olive and Olive Oil Bioactive Constituents; Boskou, D., Ed.; AOCS Press: Urbana, IL, USA, 2015; pp. 147–177. [Google Scholar] [CrossRef]
- Covas, M.I.; Konstantinidou, V.; Fitó, M. Olive oil and cardiovascular health. J. Cardiovasc. Pharmacol. 2009, 54, 477–482. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef]
- Simopoulos, A.P.; Visioli, F. More on Mediterranean Diets; Karger: Basel, Switzerland, 2007. [Google Scholar]
- Sales-Campos, H.; Souza, P.R.; Peghini, B.C.; da Silva, J.S.; Cardoso, C.R. An overview of the modulatory effects of oleic acid in health and disease. Mini. Rev. Med. Chem. 2013, 13, 201–210. [Google Scholar]
- Terés, S.; Barceló-Coblijn, G.; Benet, M.; Alvarez, R.; Bressani, R.; Halver, J.E.; Escribá, P.V. Oleic acid content is responsible for the reduction in blood pressure induced by olive oil. Proc. Natl. Acad. Sci. USA 2008, 105, 13811–13816. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, R.; Chang, M.; Zhang, H.; Jin, Q.; Wang, X. Dietary oleic acid supplementation and blood inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2022, 62, 2508–2525. [Google Scholar] [CrossRef]
- Rehman, K.; Haider, K.; Jabeen, K.; Akash, M.S.H. Current perspectives of oleic acid: Regulation of molecular pathways in mitochondrial and endothelial functioning against insulin resistance and diabetes. Rev. Endocr. Metab. Disord. 2020, 21, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Medeiros-de-Moraes, I.M.; Gonçalves-de-Albuquerque, C.F.; Kurz, A.R.M.; Oliveira, F.M.J.; de Abreu, V.H.P.; Torres, R.C.; Carvalho, V.F.; Estato, V.; Bozza, P.T.; Sperandio, M.; et al. Omega-9 Oleic Acid, the Main Compound of Olive Oil, Mitigates Inflammation during Experimental Sepsis. Oxid. Med. Cell Longev. 2018, 2018, 6053492. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.K.; Albrecht, F.; Rohrer, C.; Koeberle, A.; Werz, O.; Schlörmann, W.; Glei, M.; Lorkowski, S.; Wallert, M. Olive Oil Extracts and Oleic Acid Attenuate the LPS-Induced Inflammatory Response in Murine RAW264.7 Macrophages but Induce the Release of Prostaglandin E2. Nutrients 2021, 13, 4437. [Google Scholar] [CrossRef] [PubMed]
- Santa-María, C.; López-Enríquez, S.; Montserrat-de la Paz, S.; Geniz, I.; Reyes-Quiroz, M.E.; Moreno, M.; Palomares, F.; Sobrino, F.; Alba, G. Update on Anti-Inflammatory Molecular Mechanisms Induced by Oleic Acid. Nutrients 2023, 15, 224. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D. Fatty Acid Oxidation and Its Relation with Insulin Resistance and Associated Disorders. Ann. Nutr. Metab. 2016, 68 (Suppl. S3), 15–20. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Wei, Y.; Sowers, J.R. Role of mitochondrial dysfunction in insulin resistance. Circ. Res. 2008, 102, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Farrokhi, F.R.; Butler, A.E.; Sahebkar, A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell. Physiol. 2019, 234, 8152–8161. [Google Scholar] [CrossRef] [PubMed]
- Debbabi, M.; Zarrouk, A.; Bezine, M.; Meddeb, W.; Nury, T.; Badreddine, A.; Karym, E.M.; Sghaier, R.; Bretillon, L.; Guyot, S.; et al. Comparison of the effects of major fatty acids present in the Mediterranean diet (oleic acid, docosahexaenoic acid) and in hydrogenated oils (elaidic acid) on 7-ketocholesterol-induced oxiapoptophagy in microglial BV-2 cells. Chem. Phys. Lipids 2017, 207, 151–170. [Google Scholar] [CrossRef]
- Amtul, Z.; Westaway, D.; Cechetto, D.F.; Rozmahel, R.F. Oleic acid ameliorates amyloidosis in cellular and mouse models of Alzheimer’s disease. Brain Pathol. 2011, 21, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Ubaid, S.; Rumman, M.; Singh, B.; Akhtar, M.S.; Mahdi, A.A.; Pandey, S. Elucidating the Neuroprotective Role of Formulated Camel α-Lactalbumin–Oleic Acid Complex by Curating the SIRT1 Pathway in Parkinson’s Disease Model. ACS Chem. Neurosci. 2020, 11, 4416–4425. [Google Scholar] [CrossRef]
- Maruyama, T.; Tanabe, S.; Uyeda, A.; Suzuki, T.; Muramatsu, R. Free fatty acids support oligodendrocyte survival in a mouse model of amyotrophic lateral sclerosis. Front. Cell. Neurosci. 2023, 17, 1081190. [Google Scholar] [CrossRef]
- Trostchansky, A.; Mastrogiovanni, M.; Miquel, E.; Rodríguez-Bottero, S.; Martínez-Palma, L.; Cassina, P.; Rubbo, H. Profile of Arachidonic Acid-Derived Inflammatory Markers and Its Modulation by Nitro-Oleic Acid in an Inherited Model of Amyotrophic Lateral Sclerosis. Front. Mol. Neurosci. 2018, 11, 131. [Google Scholar] [CrossRef]
- Carrillo, C.; Cavia Mdel, M.; Alonso-Torre, S.R. Antitumor effect of oleic acid; mechanisms of action: A review. Nutr. Hosp. 2012, 27, 1860–1865. [Google Scholar] [CrossRef]
- Feltes, M.M.C.; de Oliveira, D.; Block, J.M.; Ninow, J.L. The Production, Benefits, and Applications of Monoacylglycerols and Diacylglycerols of Nutritional Interest. Food Bioprocess Technol. 2013, 6, 17–35. [Google Scholar] [CrossRef]
- Lee, W.J.; Zhang, Z.; Lai, O.M.; Tan, C.P.; Wang, Y. Diacylglycerol in food industry: Synthesis methods, functionalities, health benefits, potential risks and drawbacks. Trends Food Sci. Technol. 2020, 97, 114–125. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. Biological Relevance of Extra Virgin Olive Oil Polyphenols Metabolites. Antioxidants 2018, 7, 170. [Google Scholar] [CrossRef]
- Bulotta, S.; Celano, M.; Lepore, S.M.; Montalcini, T.; Pujia, A.; Russo, D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: Focus on protection against cardiovascular and metabolic diseases. J. Transl. Med. 2014, 12, 219. [Google Scholar] [CrossRef] [PubMed]
- Hassen, I.; Casabianca, H.; Hosni, K. Biological activities of the natural antioxidant oleuropein: Exceeding the expectation—A mini-review. J. Funct. Foods 2015, 18, 926–940. [Google Scholar] [CrossRef]
- Khalil, A.A.; Rahman, M.M.; Rauf, A.; Islam, M.R.; Manna, S.J.; Khan, A.A.; Ullah, S.; Akhtar, M.N.; Aljohani, A.S.M.; Abdulmonem, W.A.; et al. Oleuropein: Chemistry, extraction techniques and nutraceutical perspectives-An update. Crit. Rev. Food Sci. Nutr. 2023, 2023, 2218495. [Google Scholar] [CrossRef]
- Janahmadi, Z.; Nekooeian, A.A.; Moaref, A.R.; Emamghoreishi, M. Oleuropein Offers Cardioprotection in Rats with Acute Myocardial Infarction. Cardiovasc. Toxicol. 2015, 15, 61–68. [Google Scholar] [CrossRef]
- Fabiani, R.; Rosignoli, P.; De Bartolomeo, A.; Fuccelli, R.; Servili, M.; Montedoro, G.F.; Morozzi, G. Oxidative DNA damage is prevented by extracts of olive oil, hydroxytyrosol, and other olive phenolic compounds in human blood mononuclear cells and HL60 cells. J. Nutr. 2008, 138, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.H. Cardioprotective and neuroprotective roles of oleuropein in olive. Saudi. Pharm. J. 2010, 18, 111–121. [Google Scholar] [CrossRef]
- Singh, I.; Mok, M.; Christensen, A.M.; Turner, A.H.; Hawley, J.A. The effects of polyphenols in olive leaves on platelet function. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 127–132. [Google Scholar] [CrossRef]
- Andreadou, I.; Papaefthimiou, M.; Zira, A.; Constantinou, M.; Sigala, F.; Skaltsounis, A.L.; Tsantili-Kakoulidou, A.; Iliodromitis, E.K.; Kremastinos, D.T.; Mikros, E. Metabonomic identification of novel biomarkers in doxorubicin cardiotoxicity and protective effect of the natural antioxidant oleuropein. NMR Biomed. 2009, 22, 585–592. [Google Scholar] [CrossRef]
- Marcelino, G.; Hiane, P.A.; Freitas, K.C.; Santana, L.F.; Pott, A.; Donadon, J.R.; Guimarães, R.C.A. Effects of Olive Oil and Its Minor Components on Cardiovascular Diseases, Inflammation, and Gut Microbiota. Nutrients 2019, 11, 1826. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, G.; Maisto, M.; Schisano, C.; Barrea, L.; Ciampaglia, R.; Novellinoet, E. Oleuropein as a novel anti-diabetic nutraceutical. An overview. Arch. Diabetes Obes. 2018, 1, 54–58. [Google Scholar] [CrossRef]
- Butt, M.S.; Tariq, U.; Iahtisham Ul, H.; Naz, A.; Rizwan, M. Neuroprotective effects of oleuropein: Recent developments and contemporary research. J. Food Biochem. 2021, 45, e13967. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Xu, J.; Zou, X.; Li, Y.; Chen, C.; Zheng, A.; Li, H.; Li, H.; Szeto, I.M.-Y.; Shi, Y. Hydroxytyrosol prevents diet-induced metabolic syndrome and attenuates mitochondrial abnormalities in obese mice. Free. Radic. Biol. Med. 2014, 67, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, M.; Sarriá, B.; Largo, C.; Martínez-López, S.; Madrona, A.; Espartero, J.L.; Bravo, L.; Mateos, R. Comparative evaluation of the metabolic effects of hydroxytyrosol and its lipophilic derivatives (hydroxytyrosyl acetate and ethyl hydroxytyrosyl ether) in hypercholesterolemic rats. Food Funct. 2014, 5, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ai, Q.-D.; Wei, Y.-H. Potential role of hydroxytyrosol in neuroprotection. J. Funct. Foods 2021, 82, 104506. [Google Scholar] [CrossRef]
- Imran, M.; Nadeem, M.; Gilani, S.A.; Khan, S.; Sajid, M.W.; Amir, R.M. Antitumor Perspectives of Oleuropein and Its Metabolite Hydroxytyrosol: Recent Updates. J. Food. Sci. 2018, 83, 1781–1791. [Google Scholar] [CrossRef] [PubMed]
- Rafehi, H.; Ververis, K.; Karagiannis, T.C. Mechanisms of action of phenolic compounds in olive. J. Diet. Suppl. 2012, 9, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Pojero, F.; Aiello, A.; Gervasi, F.; Caruso, C.; Ligotti, M.E.; Calabrò, A.; Procopio, A.; Candore, G.; Accardi, G.; Allegra, M. Effects of Oleuropein and Hydroxytyrosol on Inflammatory Mediators: Consequences on Inflammaging. Int. J. Mol. Sci. 2022, 24, 380. [Google Scholar] [CrossRef]
- Gong, D.; Geng, C.; Jiang, L.; Cao, J.; Yoshimura, H.; Zhong, L. Effects of hydroxytyrosol-20 on carrageenan-induced acute inflammation and hyperalgesia in rats. Phytother. Res. 2009, 23, 646–650. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, J.; Zhong, L. Hydroxytyrosol inhibits pro-inflammatory cytokines, iNOS, and COX-2 expression in human monocytic cells. Naunyn Schmiedebergs Arch. Pharmacol. 2009, 379, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, M.C.; De Stefano, D.; Di Meglio, P.; Irace, C.; Savarese, M.; Sacchi, R.; Cinelli, M.P.; Carnuccio, R. Hydroxytyrosol, a phenolic compound from virgin olive oil, prevents macrophage activation. Naunyn Schmiedebergs Arch. Pharmacol. 2005, 371, 457–465. [Google Scholar] [CrossRef]
- Rosignoli, P.; Fuccelli, R.; Fabiani, R.; Servili, M.; Morozzi, G. Effect of olive oil phenols on the production of inflammatory mediators in freshly isolated human monocytes. J. Nutr. Biochem. 2013, 24, 1513–1519. [Google Scholar] [CrossRef]
- Serra, G.; Deiana, M.; Spencer, J.P.E.; Corona, G. Olive Oil Phenolics Prevent Oxysterol-Induced Proinflammatory Cytokine Secretion and Reactive Oxygen Species Production in Human Peripheral Blood Mononuclear Cells, Through Modulation of p38 and JNK Pathways. Mol. Nutr. Food Res. 2017, 61, 1700283. [Google Scholar] [CrossRef]
- Souza, P.A.; Marcadenti, A.; Portal, V.L. Effects of Olive Oil Phenolic Compounds on Inflammation in the Prevention and Treatment of Coronary Artery Disease. Nutrients 2017, 9, 1087. [Google Scholar] [CrossRef] [PubMed]
- Lucas, L.; Russell, A.; Keast, R. Molecular mechanisms of inflammation. Anti-inflammatory benefits of virgin olive oil and the phenolic compound oleocanthal. Curr. Pharm. Des. 2011, 17, 754–768. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Giusti, L.; Hrelia, S. New neuroprotective perspectives in fighting oxidative stress and improving cellular energy metabolism by oleocanthal. Neural Regen. Res. 2019, 14, 1217–1218. [Google Scholar] [CrossRef]
- Pang, K.-L.; Chin, K.-Y. The Biological Activities of Oleocanthal from a Molecular Perspective. Nutrients 2018, 10, 570. [Google Scholar] [CrossRef]
- Ballard, C.R.; Maróstica, M.R. Chapter 10—Health Benefits of Flavonoids. In Bioactive Compounds; Campos, M.R.S., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 185–201. [Google Scholar] [CrossRef]
- López-Biedma, A.; Sánchez-Quesada, C.; Delgado-Rodríguez, M.; Gaforio, J.J. The biological activities of natural lignans from olives and virgin olive oils: A review. J. Funct. Foods 2016, 26, 36–47. [Google Scholar] [CrossRef]
- Harwood, J.L.; Yaqoob, P. Nutritional and health aspects of olive oil. Eur. J. Lipid Sci. Technol. 2002, 104, 685–697. [Google Scholar] [CrossRef]
- Lanza, B.; Ninfali, P. Antioxidants in Extra Virgin Olive Oil and Table Olives: Connections between Agriculture and Processing for Health Choices. Antioxidants 2020, 9, 41. [Google Scholar] [CrossRef]
- Tucker, J.M.; Townsend, D.M. Alpha-tocopherol: Roles in prevention and therapy of human disease. Biomed. Pharmacother. 2005, 59, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Mathur, P.; Ding, Z.; Saldeen, T.; Mehta, J.L. Tocopherols in the Prevention and Treatment of Atherosclerosis and Related Cardiovascular Disease. Clin. Cardiol. 2015, 38, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Sozen, E.; Demirel, T.; Ozer, N.K. Vitamin E: Regulatory role in the cardiovascular system. IUBMB Life 2019, 71, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Icer, M.A.; Arslan, N.; Gezmen-Karadag, M. Effects of vitamin E on neurodegenerative diseases: An update. Acta. Neurobiol. Exp. 2021, 81, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.B.; Ellis, D.I. Metabolomics: Current analytical platforms and methodologies. TrAC Trends Anal. Chem. 2005, 24, 285–294. [Google Scholar] [CrossRef]
- Bajoub, A.; Carrasco-Pancorbo, A.; Ouazzani, N.; Fernández-Gutiérrez, A. UHPLC–MS in Virgin Olive Oil Analysis. In Ultra Performance Liquid Chromatography Mass Spectrometry; CRC Press: Boca Raton, FL, USA, 2014; pp. 213–242. [Google Scholar] [CrossRef]
- Angerosa, F.; d’Alessandro, N.; Konstantinou, P.; Di Giacinto, L. GC-MS evaluation of phenolic compounds in virgin olive oil. J. Agric. Food Chem. 1995, 43, 1802–1807. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; De Mello, J.C. Application and Analysis of the Folin Ciocalteu Method for the Determination of the Total Phenolic Content from Limonium Brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef]
- Lercker, G.; Rodriguez-Estrada, M.T. Chromatographic analysis of unsaponifiable compounds of olive oils and fat-containing foods. J. Chromatogr. A 2000, 881, 105–129. [Google Scholar] [CrossRef] [PubMed]
- Lioupi, A.; Nenadis, N.; Theodoridis, G. Virgin olive oil metabolomics: A review. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2020, 1150, 122161. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, X.; Bai, C.; Zhao, C.; Lu, G.; Xu, G. LC-MS-based metabonomics analysis. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008, 866, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Kopka, J. Current challenges and developments in GC–MS based metabolite profiling technology. J. Biotechnol. 2006, 124, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Janson, J.C.; Jönsson, J.Å. Introduction to chromatography. In Protein Purification: Principles, High Resolution Methods, and Applications; Wiley: Hoboken, NJ, USA, 2011; pp. 23–50. [Google Scholar]
- Kazakevich, Y.; LoBrutto, R. Introduction. In HPLC for Pharmaceutical Scientists; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 1–24. [Google Scholar] [CrossRef]
- Hatzakis, E. Nuclear Magnetic Resonance (NMR) Spectroscopy in Food Science: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 189–220. [Google Scholar] [CrossRef] [PubMed]
- Dais, P.; Spyros, A. 31P NMR spectroscopy in the quality control and authentication of extra-virgin olive oil: A review of recent progress. Magn. Reson. Chem. 2007, 45, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Rubert, J.; Zachariasova, M.; Hajslova, J. Advances in high-resolution mass spectrometry based on metabolomics studies for food—A review. Food Addit. Contam. Part A 2015, 32, 1685–1708. [Google Scholar] [CrossRef] [PubMed]
- Rubió, L.; Farràs, M.; de La Torre, R.; Macià, A.; Romero, M.-P.; Valls, R.M.; Solà, R.; Farré, M.; Fitó, M.; Motilva, M.-J. Metabolite profiling of olive oil and thyme phenols after a sustained intake of two phenol-enriched olive oils by humans: Identification of compliance markers. Food Res. Int. 2014, 65, 59–68. [Google Scholar] [CrossRef]
- Domínguez-Perles, R.; Auñón, D.; Ferreres, F.; Gil-Izquierdo, A. Gender differences in plasma and urine metabolites from Sprague-Dawley rats after oral administration of normal and high doses of hydroxytyrosol, hydroxytyrosol acetate, and DOPAC. Eur. J. Nutr. 2017, 56, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Alemán-Jiménez, C.; Domínguez-Perles, R.; Medina, S.; Prgomet, I.; López-González, I.; Simonelli-Muñoz, A.; Campillo-Cano, M.; Auñón, D.; Ferreres, F.; Gil-Izquierdo, Á. Pharmacokinetics and bioavailability of hydroxytyrosol are dependent on the food matrix in humans. Eur. J. Nutr. 2021, 60, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Sakavitsi, M.E.; Breynaert, A.; Nikou, T.; Lauwers, S.; Pieters, L.; Hermans, N.; Halabalaki, M. Availability and Metabolic Fate of Olive Phenolic Alcohols Hydroxytyrosol and Tyrosol in the Human GI Tract Simulated by the In Vitro GIDM-Colon Model. Metabolites 2022, 12, 391. [Google Scholar] [CrossRef] [PubMed]
- Luque-Córdoba, D.; Ledesma-Escobar, C.A.; Priego-Capote, F. Qualitative and quantitative determination of phenols and their metabolites in urine by in-syringe solid-phase extraction and LC-MS/MS analysis for evaluation of virgin olive oil metabolism. Talanta 2024, 266, 125029. [Google Scholar] [CrossRef]
- Khymenets, O.; Fitó, M.; Touriño, S.; Muñoz-Aguayo, D.; Pujadas, M.; Torres, J.L.; Joglar, J.; Farré, M.; Covas, M.-I.; Torre, R.d.l. Antioxidant Activities of Hydroxytyrosol Main Metabolites Do Not Contribute to Beneficial Health Effects after Olive Oil Ingestion. Drug Metab. Dispos. 2010, 38, 1417–1421. [Google Scholar] [CrossRef]
- Qusa, M.H.; Abdelwahed, K.S.; Hill, R.A.; El Sayed, K.A. S-(−)-Oleocanthal Ex Vivo Modulatory Effects on Gut Microbiota. Nutrients 2023, 15, 618. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Fresno, R.; Llorach, R.; Urpi-Sarda, M.; Lupianez-Barbero, A.; Estruch, R.; Corella, D.; Fitó, M.; Arós, F.; Ruiz-Canela, M.; Salas-Salvadó, J.; et al. Metabolomic pattern analysis after mediterranean diet intervention in a nondiabetic population: A 1- and 3-year follow-up in the PREDIMED study. J. Proteome. Res. 2015, 14, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A.; Vazquez-Martin, A.; Garcia-Villalba, R.; Carrasco-Pancorbo, A.; Oliveras-Ferraros, C.; Fernandez-Gutierrez, A.; Segura-Carretero, A. tabAnti-HER2 (erbB-2) oncogene effects of phenolic compounds directly isolated from commercial Extra-Virgin Olive Oil (EVOO). BMC Cancer 2008, 8, 377. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Arroyo, S.; Gómez-Martínez, A.; Rocamora-Reverte, L.; Quirantes-Piné, R.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Ferragut, J.A. Application of nanoLC-ESI-TOF-MS for the metabolomic analysis of phenolic compounds from extra-virgin olive oil in treated colon-cancer cells. J. Pharm. Biomed. Anal. 2012, 63, 128–134. [Google Scholar] [CrossRef]
- Nikou, T.; Liaki, V.; Stathopoulos, P.; Sklirou, A.D.; Tsakiri, E.N.; Jakschitz, T.; Bonn, G.; Trougakos, I.P.; Halabalaki, M.; Skaltsounis, L.A. Comparison survey of EVOO polyphenols and exploration of healthy aging-promoting properties of oleocanthal and oleacein. Food Chem. Toxicol. 2019, 125, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the extraction method on the recovery of bioactive phenolic compounds from food industry by-products. Food Chem. 2022, 378, 131918. [Google Scholar] [CrossRef] [PubMed]
- Olmo-García, L.; Carrasco-Pancorbo, A. Chromatography-MS based metabolomics applied to the study of virgin olive oil bioactive compounds: Characterization studies, agro-technological investigations and assessment of healthy properties. TrAC Trends Anal. Chem. 2021, 135, 116153. [Google Scholar] [CrossRef]
- Vazquez-Aguilar, A.; Sanchez-Rodriguez, E.; Rodriguez-Perez, C.; Rangel-Huerta, O.D.; Mesa, M.D. Metabolomic-Based Studies of the Intake of Virgin Olive Oil: A Comprehensive Review. Metabolites 2023, 13, 472. [Google Scholar] [CrossRef]
- Niklas, J.; Heinzle, E. Metabolic flux analysis in systems biology of mammalian cells. Adv. Biochem. Eng. Biotechnol. 2012, 127, 109–132. [Google Scholar] [CrossRef]
- Caruana, E.J.; Roman, M.; Hernández-Sánchez, J.; Solli, P. Longitudinal studies. J. Thorac. Dis. 2015, 7, E537–E540. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Salas-Salvadó, J.; Estruch, R.; Corella, D.; Fitó, M.; Ros, E. Benefits of the Mediterranean Diet: Insights From the PREDIMED Study. Prog. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef] [PubMed]
| Technique for the Analysis of Olive Oil | Strengths | Drawbacks |
|---|---|---|
| LC-MS | Does not require derivation prior to analysis. Ionization methods (such as ESI) can be applied to allow simultaneous analysis of several classes of metabolites. Sample preparation is relatively simple. The possibility of using different analytical platforms, such as time of flight (TOF) analyzers or orbital ion traps (Orbitraps), guarantees high resolution. | Ionization methods can lead to the formation of dimers or adducts, which within a single compound amplify the spectrum of molecular characteristics. Matrix effects and ion suppression arising from co-eluting compounds are common challenges associated with this methodology. Systems using TOF have decreased resolution for lower masses. Systems with Orbitraps exhibit variations in the MS/MS spectra, depending on concentration. |
| GC-MS | Chemical derivation is able to increase the thermal stability, sensitivity, volatility, and detector response. The application of ionization methods can occur. Capable of overcoming the challenges associated with LC-MS, such as matrix effects and ion suppression arising from co-eluting compounds. Provides superior chromatographic resolution. It is possible to acquire high-resolution MS/MS spectra (TOF). | Requires compounds in volatile form, and thus chemical derivation can be performed before the analysis. The fragmentation caused by ionization can be too extensive. Primarily applicable for the separation and analysis of low-molecular-weight compounds. To ensure sufficient numbers of points per peak, especially in the case of GCxGC separation, fast acquisition speeds may be required. |
| CE-MS | Small samples and decreased reagent volumes can be used. Minimal or no organic solvent consumption. It uses simple fused silica capillaries instead of expensive LC columns. Ionization methods can be used as well (ESI or MALDI). | Salt and lipids can affect reproducibility and the possibility of comparing data, and hence sample cleanness is required. Capillary coating is needed to prevent protein adhesion and, consequently, the alteration of retention times for metabolites. The usage of MALDI is not only time-consuming but also requires fractioning on a target plate. It can be associated with loss of resolution. |
| NMR spectroscopy | This allows easy quantification of metabolites. No sample pretreatment is required. There is no need to pre-select the conditions to perform the analysis, guaranteeing the generation of spectra with a high information content. The chemical shifts are relatively stable, which allows the results to be reproducible under consistent experimental conditions. | The effectiveness is not consistently high, particularly when attempting to quantify certain minor compounds such as mono- or diacylglycerols. |
| Technique | Aim | Compounds Analyzed | Results | Reference |
|---|---|---|---|---|
| HPLC/MS/MS | Identification of biomarkers of exposure to phenolic compounds | HT sulfate, homovanillic alcohol sulfate, HT acetate sulfate, homovanilic acid sulfate, HT glucuronide, and homovanilic alcohol glucuronide | HT sulfate and HT acetate sulfate could be used as biomarkers for the intake of bioactive compounds | Rubió et al. [84] |
| UPLC-MS/MS | ||||
| UHPLC-ESI-QqQ-MS/MS | Evaluate the bioavailability of phenolic compounds | HT, HT acetate, DOPAC, homovanilic alcohol, and tyrosol | Despite having similar levels of absorption and metabolism, HT, HT acetate, and DOPAC levels did not increase proportionally with dosage, possibly due to metabolic saturation and transporter limitations | Domínguez-Perles et al. [85] |
| UHPLC-ESI-QqQ-MS/MS | Evaluate whether different food matrices influenced the pharmacokinetics and bioavailability of HT and its metabolites | HT, HT acetate, and DOPAC | The food matrix and nature of the oil influenced both factors being studied, with the best option being EVOO | Alemán-Jiménez et al. [86] |
| UPLC-ESI-HRMS | Assess the metabolism and bioavailability of HT and tyrosol using a gastrointestinal dialysis-colon model | HT, tyrosol, and their derivates | The spectral analysis provided insights into the derivates of HT and tyrosol, elucidating potential mechanisms for their chemical transformation in the distinct conditions along the gastrointestinal tract | Sakavitsi et al. [87] |
| LC-ESI-QqQ-MS/MS | Evaluate the bioavailability of phenolic compounds | HT and tyrosol | This approach allowed a quantitative and qualitative characterization of the metabolism of phenolic compounds in VOO | Luque-Córdoba et al. [88] |
| UPLC-MS | Evaluate the bioavailability of phenolic compounds and assess whether metabolites derived from polyphenols that are present in biological samples are related to the health benefits of polyphenols | 3′-O-HT-glucuronide, 4′-O-HT-glucuronide, and 4′-O-glucuronides of tyrosol and homovanillyl alcohol | Polyphenols have low bioavailability, and conjugated metabolites derived from HT have non-significant antioxidant activities, and hence they are not responsible for the health benefits associated with olive oil | Khymenets et al. [89] |
| H NMR | Study the implications of a phenolic compound in the gut microbiota, potentially aiding in the prediction of bacterial distribution in the human body | S-(-)-oleocanthal | Polyphenols have effects on the levels of numerous bacteria of known importance in human health | Qusa et al. [90] |
| HPLC | ||||
| H NMR | Assess the effects of MD on urine metabolites in the context of the PRIMED trial | Several metabolites from the MD, including carbohydrates, creatine, creatinine, amino acids, lipids, and microbial cometabolites | There were clear differences in the metabolites present in the urine between the three groups (MD + EVOO, MD + nuts, and low-fat diet) | Vázquez-Fresno et al. [91] |
| HPLC | Evaluate the protective effect of olive oil against tumor cells | HT, tyrosol, elenolic acid, (+)-pinoresinol, 1-(+)-acetoxypinoresinol, deacetoxy OLE aglycone, ligstroside aglycone, and OLE aglycone | Polyphenols promote proteasomal degradation of the protein HER2 | Menendez et al. [92] |
| CE-MS | ||||
| nanoLC-ESI-TOF-MS | Evaluate the effects of olive oil components on colon cancer cells | HT, elenolic acid, luteolin, vanillin, OLE aglycone, HT acetate, 4-OH-benzoic acid, vanillin acid, 10-H-OLE aglycone, syringarenisol, acetoxy-pinoresinol, pinoresinol, apigenin, methyl-DOA, and DOA | Phenolic compounds have antiproliferative and pro-apoptotic effects on colon cancer cells | Fernández-Arroyo et al. [93] |
| HPLC with diode array detection (DAD) | Assess the beneficial health effects of isolated polyphenols | HT, tyrosol, oleocanthal, and oleacein | Oleocanthal and oleacein have not only antioxidant properties but also healthy aging-promoting effects | Nikou et al. [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, M.; Rodríguez-Pérez, M.; Calabrò, A.; Burgos-Ramos, E.; Accardi, G.; Silva, P. A Narrative Review of Metabolomic Insights into Olive Oil’s Nutritional Value. Appl. Sci. 2024, 14, 4203. https://doi.org/10.3390/app14104203
Gonçalves M, Rodríguez-Pérez M, Calabrò A, Burgos-Ramos E, Accardi G, Silva P. A Narrative Review of Metabolomic Insights into Olive Oil’s Nutritional Value. Applied Sciences. 2024; 14(10):4203. https://doi.org/10.3390/app14104203
Chicago/Turabian StyleGonçalves, Marta, María Rodríguez-Pérez, Anna Calabrò, Emma Burgos-Ramos, Giulia Accardi, and Paula Silva. 2024. "A Narrative Review of Metabolomic Insights into Olive Oil’s Nutritional Value" Applied Sciences 14, no. 10: 4203. https://doi.org/10.3390/app14104203
APA StyleGonçalves, M., Rodríguez-Pérez, M., Calabrò, A., Burgos-Ramos, E., Accardi, G., & Silva, P. (2024). A Narrative Review of Metabolomic Insights into Olive Oil’s Nutritional Value. Applied Sciences, 14(10), 4203. https://doi.org/10.3390/app14104203








