Study on the Alkali–Sulfur Co-Activation and Mechanical Properties of Low-Carbon Cementitious Composite Materials Based on Electrolytic Manganese Residue, Carbide Slag, and Granulated Blast-Furnace Slag
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Analysis
2.2. Experimental Methods
2.2.1. Preparation and Curing Procedures
2.2.2. Test Methods
- (1)
- (2)
- Particle size distribution test method: The particle size distribution of the prepared powder was assessed using the Morphologi 4-ID particle size and shape analyzer (Malvern Panalytical, Malvern, UK).
- (3)
- Mechanical property test: The bending and compressive strength of the mortar at 3 d, 7 d, and 28 d were tested using a mortar press in accordance with the GB/T 17671-1999 [27] “Cement Mortar Strength Inspection Method (ISO Method)” standard.
- (4)
- Hydration heat test: The hydration heat release process of the slurry after 72 h was examined at 25 °C using an isothermal calorimeter (I-CAL4000/8000) manufactured by Calmetrix, Boston, MA, USA.
- (5)
- X-ray diffraction test: The mineral composition of the sample powder was analyzed using an X-ray diffractometer (X’PERT PRO) (Malvern Panalytical, Malvern, UK). The diffraction angle ranged from 5° to 80°, and the rate was set at 4°/min.
- (6)
- Scanning electron microscopy test: A scanning electron microscope (JSM-6380LV, JEOL Ltd., Tokyo, Japan) was employed to observe the micro-morphology of the sample under the following conditions: voltage 5 kV, current 5 μA, magnification 5000× g, scale 10 μm, and glided sample surface.
- (7)
- Thermogravimetric differential thermogravimetry test: Approximately 3 mg of the sample was weighed and placed into the crucible. The thermogravimetric analyzer (Q500 IR) (Newcastle, DE, USA) was utilized to conduct tests in a nitrogen atmosphere, with a heating rate of 10 °C/min.
3. Results and Discussion
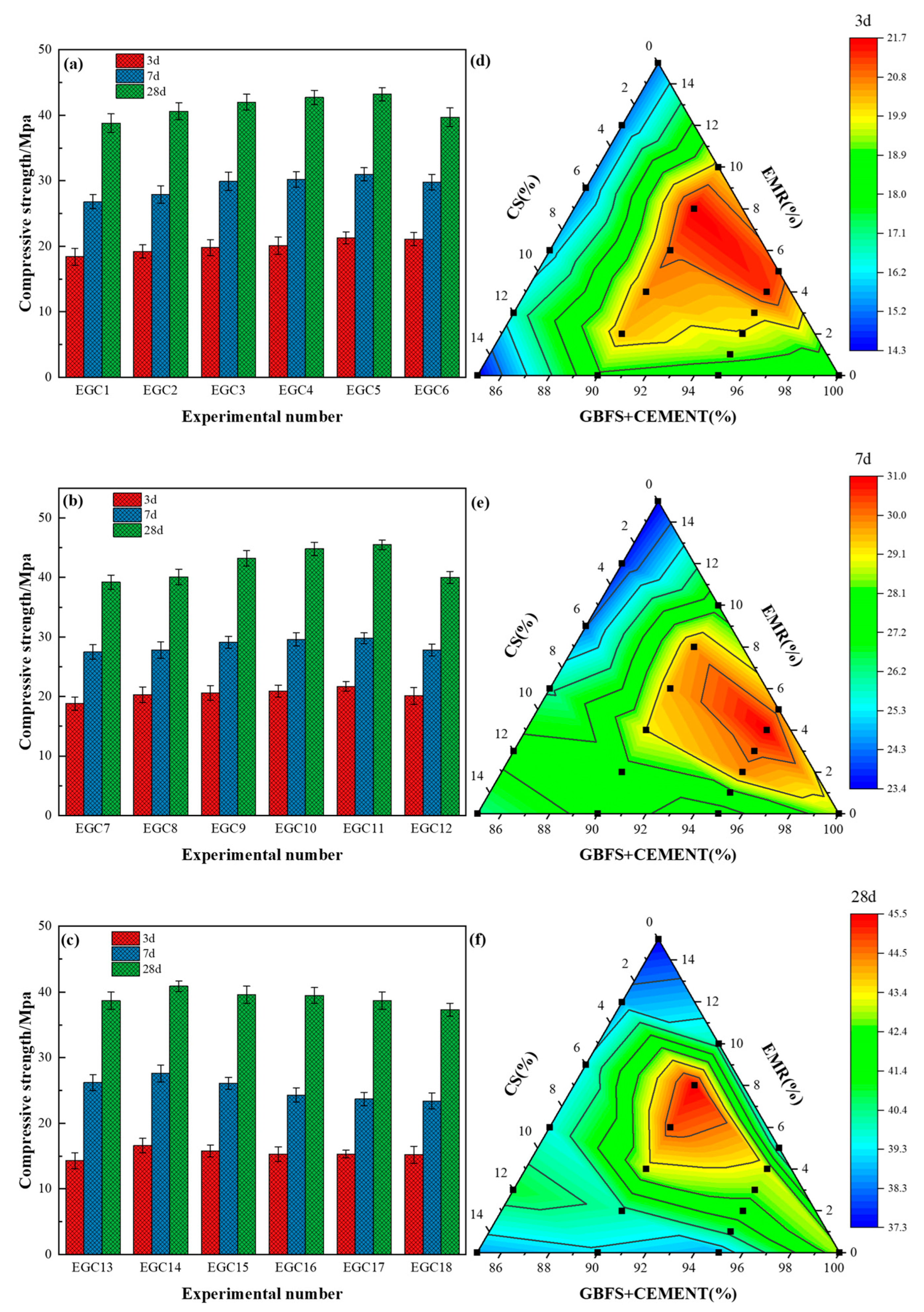
3.1. Compressive Strength
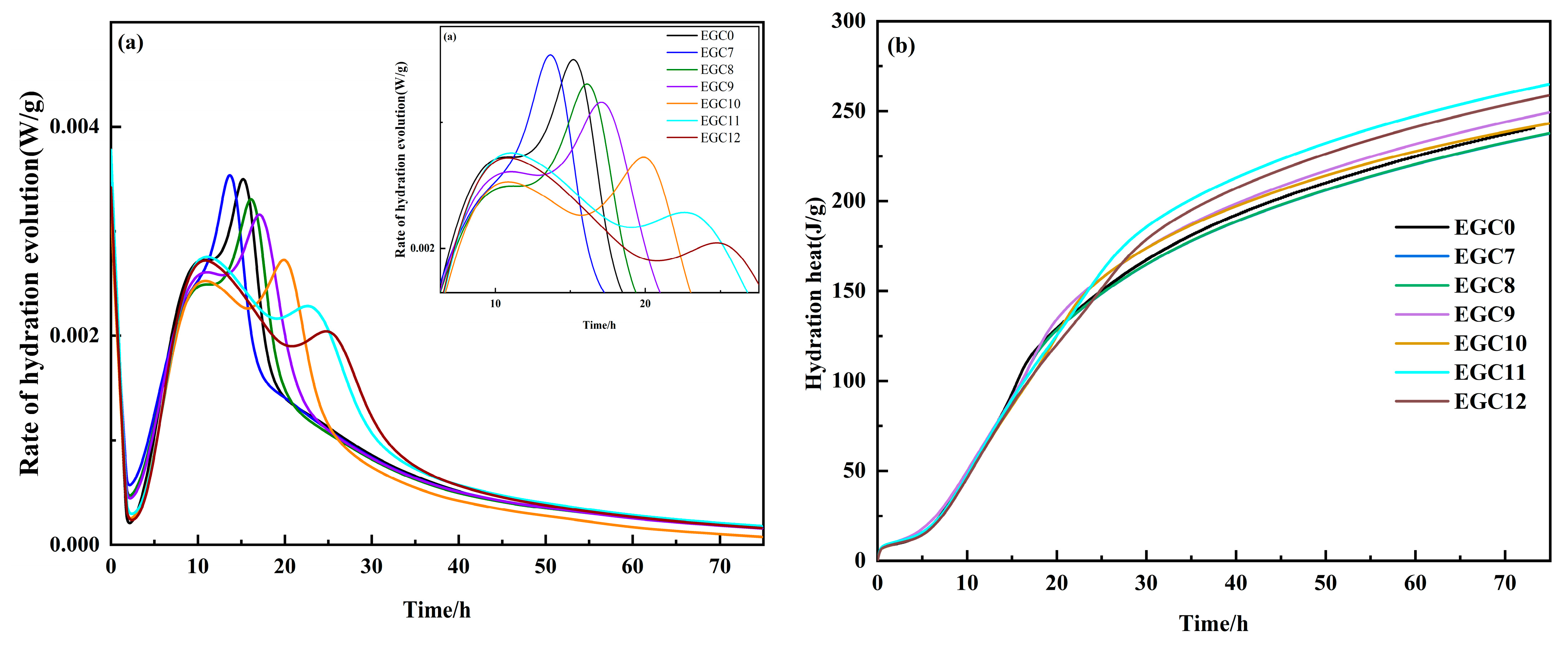
3.2. Thermal Analysis of Hydration
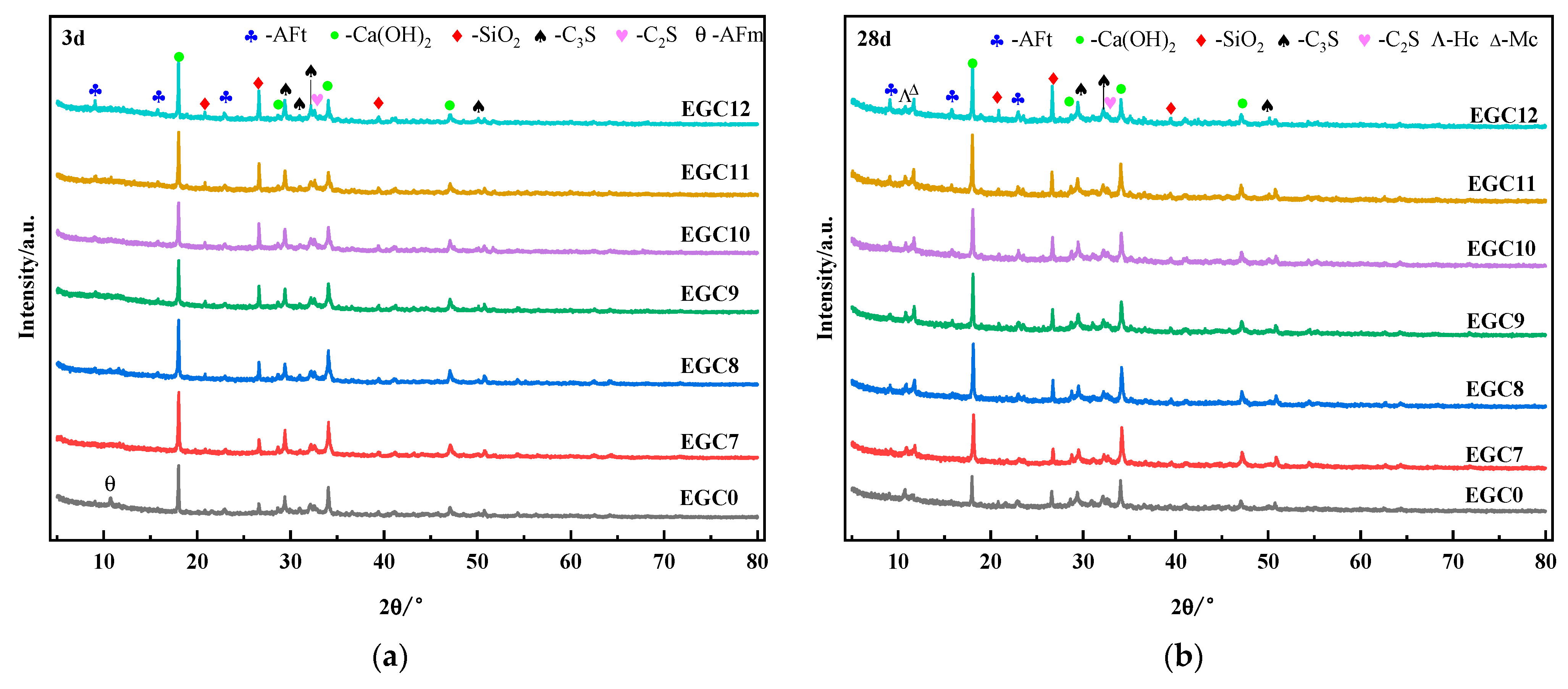
3.3. X-ray Diffraction Analysis
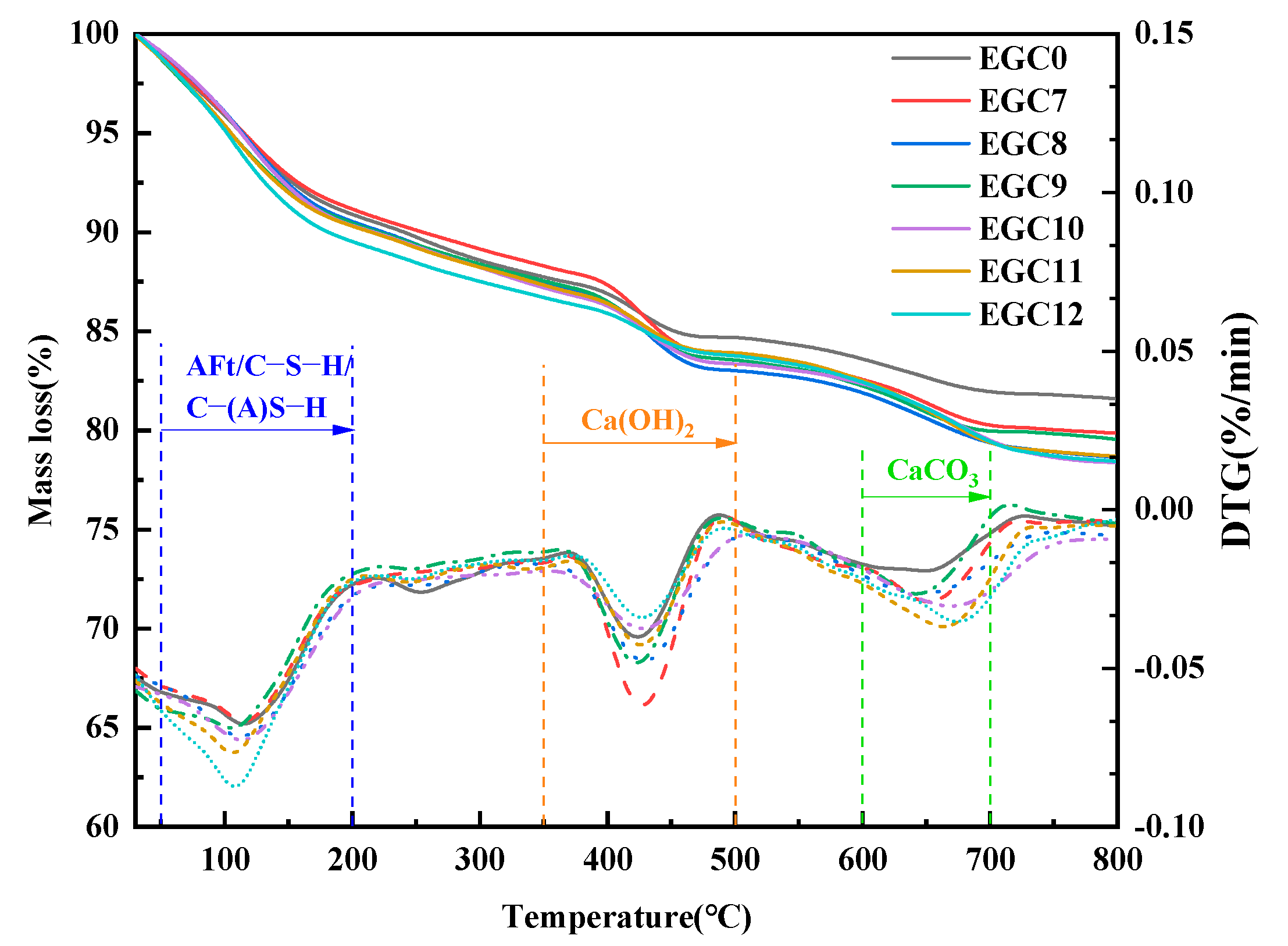
3.4. Thermogravimetric Differential Thermogravimetry Analysis
3.5. Scanning Electron Microscopy Analysis
3.6. Discussion
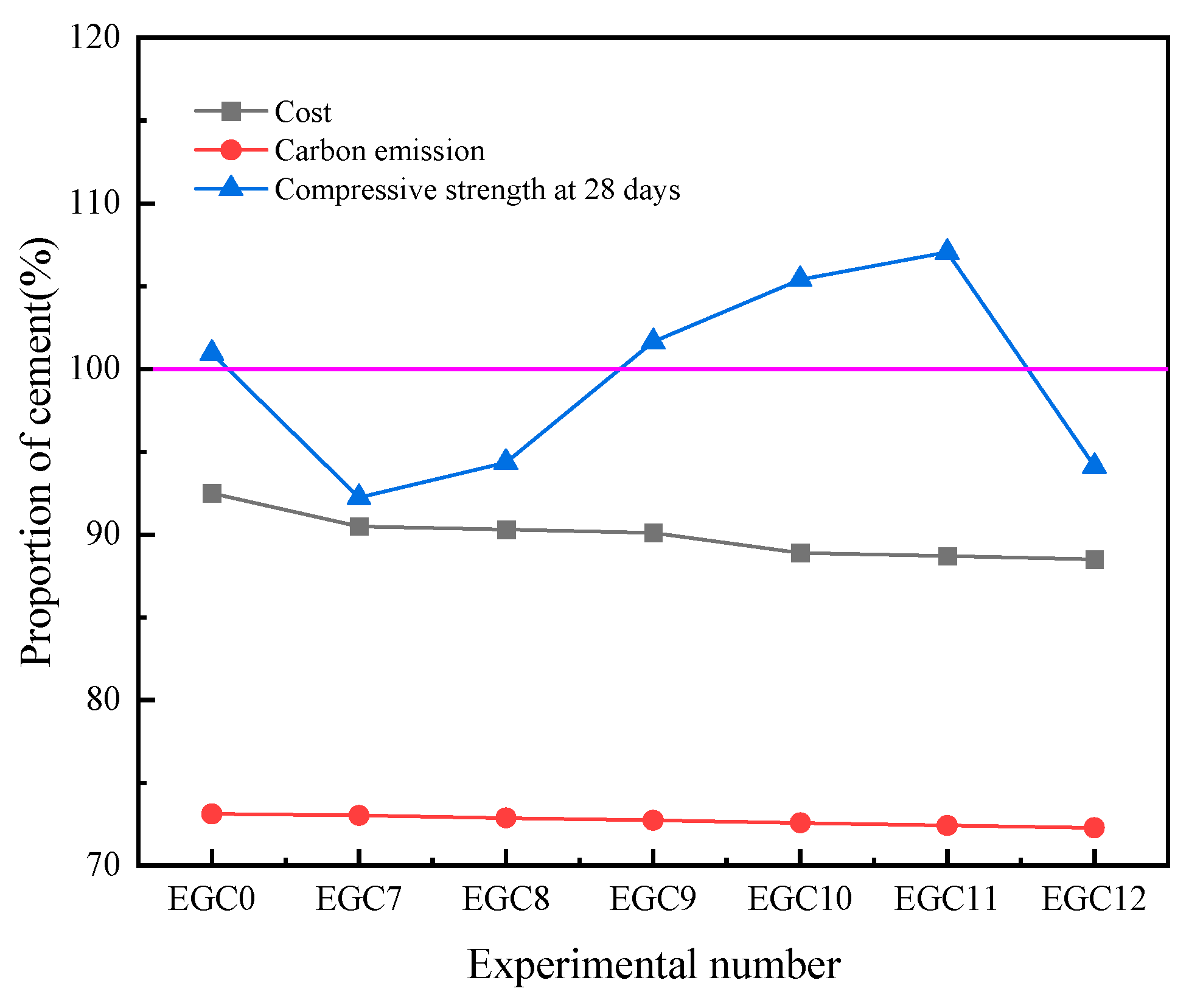
3.7. Prediction of Economic and Environmental Benefits
4. Conclusions
- EMR and CS effectively enhanced the cementitious activity of GBFS and improved the mechanical properties of the multicomponent cementitious materials, with the most significant improvement observed in early strength. Notably, the EGC11 group, featuring 20% GBFS content, 8% EMR content, and 2% CS content, exhibited the optimal performance, achieving a 28 d compressive strength of 45.5 MPa, with lower costs and carbon emissions than conventional cement.
- The addition of 30% GBFS alone reduced the hydration reaction rate and the total cumulative heat release. However, the incorporation of CS and EMR effectively increased the hydration reaction rate and the total cumulative heat release of the slurry. This suggests that alkali–sulfur co-activation effectively enhanced the cementitious activity of GBFS and the degree of hydration reaction.
- The main hydration products of the CS–EMR–GBFS solid waste cementitious material were AFt and C–S–H gels. With the increase in the EMR content and the decrease in the CS content, the AFt and C–S–H gel contacts continued to increase, and a large amount of AFt was interspersed and grew in the C–S–H gels.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Type | CS-GBFS | EMR-GBFS | CS-EMR-GBFS |
|---|---|---|---|
| Principle | Alkali-activated | Sulfate-activated | Alkali–sulfur co-activation |
| Compressive strength | 3 d > 20 MPa, 28 d > 30 MPa | 3 d > 17 MPa, 28 d > 44 MPa | 3 d > 20 MPa, 28 d > 45 MPa |
| Hydration heat | 24 h heat release > 100 J/g | 72 h heat release > 250 J/g | 72 h heat release > 250 J/g |
| Type of hydration products | C-(A)-S-H | AFt | C-(A)-S-H, AFt |
| Advantage | High early strength, fast setting time. | High EMR content, and the later strength data are considerable. | Excellent data for each age. |
| Deficiency | The cost is high, and the later strength is not considerably improved. | EMR requires high temperature treatment and high energy consumption. | - |
References
- Zhang, J.; Tan, H.; He, X.; Yang, W.; Deng, X. Utilization of carbide slag-granulated blast furnace slag system by wet grinding as low carbon cementitious materials. Constr. Build. Mater. 2020, 249, 118763. [Google Scholar] [CrossRef]
- Nie, S.; Zhou, J.; Yang, F.; Lan, M.; Li, J.; Zhang, Z.; Chen, Z.; Xu, M.; Li, H.; Sanjayan, J.G. Analysis of theoretical carbon dioxide emissions from cement production: Methodology and application. J. Clean. Prod. 2022, 334, 130270. [Google Scholar] [CrossRef]
- Sousa, V.; Bogas, J.A.; Real, S.; Meireles, I. Industrial production of recycled cement: Energy consumption and carbon dioxide emission estimation. Environ. Sci. Pollut. Res. 2023, 30, 8778–8789. [Google Scholar] [CrossRef]
- Gou, M.; Zhao, M.; Zhou, L.; Zhao, J.; Hou, W.; Ma, W.; Hou, Z. Hydration and mechanical properties of FGD gypsum-cement-mineral powder composites. J. Build. Eng. 2023, 69, 106288. [Google Scholar] [CrossRef]
- Dai, J.; Gong, C.; Wang, Y.; Huo, L.; Lu, L. Effect of nano-silica on structure and properties of high sulfate resistant Portland cement mixed with mineral powder or fly ash. J. Build. Eng. 2023, 66, 105843. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, R.; Liu, P.; Liu, C.; Sun, L.; Zhang, H. Effects of silica fume on the abrasion resistance of low-heat Portland cement concrete. Constr. Build. Mater. 2022, 329, 127165. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, J.; Yan, C.; Yin, L.; Wang, X.; Liu, S. Hydration characteristics of low carbon cementitious materials with multiple solid wastes. Constr. Build. Mater. 2022, 322, 126366. [Google Scholar] [CrossRef]
- Ren, C.; Li, K.; Wang, Y.; Li, Y.; Tong, J.; Cai, J. Preparation and Hydration Mechanisms of Low Carbon Ferrochrome Slag-Granulated Blast Furnace Slag Composite Cementitious Materials. Materials 2023, 16, 2385. [Google Scholar] [CrossRef]
- Yu, X.; Shi, J.; He, Z.; Yalçınkaya, Ç.; Revilla-Cuesta, V.; Gencel, O. Review of the materials composition and performance evolution of green alkali-activated cementitious materials. Clean Technol. Environ. Policy 2023, 25, 1439–1459. [Google Scholar] [CrossRef]
- Zhou, J.; Qian, S.; Sierra Beltran, M.G.; Ye, G.; van Breugel, K.; Li, V.C. Development of engineered cementitious composites with limestone powder and blast furnace slag. Mater. Struct. 2010, 43, 803–814. [Google Scholar] [CrossRef]
- Ogirigbo, O.R.; Black, L. Influence of slag composition and temperature on the hydration and microstructure of slag blended cements. Constr. Build. Mater. 2016, 126, 496–507. [Google Scholar] [CrossRef]
- Cai, L.; Li, X.; Ma, B.; Lv, Y. Effect of binding materials on carbide slag based high utilization solid-wastes autoclaved aerated concrete (HUS-AAC): Slurry, physic-mechanical property and hydration products. Constr. Build. Mater. 2018, 188, 221–236. [Google Scholar] [CrossRef]
- Yang, J.; Ma, L.; Liu, H.; Wei, Y.; Keomounlath, B.; Dai, Q. Thermodynamics and kinetics analysis of Ca-looping for CO2 capture: Application of carbide slag. Fuel 2019, 242, 1–11. [Google Scholar] [CrossRef]
- Liu, Y.; Chang, C.W.; Namdar, A.; She, Y.; Lin, C.H.; Yuan, X.; Yang, Q. Stabilization of expansive soil using cementing material from rice husk ash and calcium carbide residue. Constr. Build. Mater. 2019, 221, 1–11. [Google Scholar] [CrossRef]
- Yi, Y.; Zheng, X.; Liu, S.; Al-Tabbaa, A. Comparison of reactive magnesia-and carbide slag-activated ground granulated blastfurnace slag and Portland cement for stabilisation of a natural soil. Appl. Clay Sci. 2015, 111, 21–26. [Google Scholar] [CrossRef]
- Zhao, Y.; Gu, X.; Xu, X.; Zhang, Z. Using calcium carbide residue to prepare ecological alkali activated slag composites: Effect of anion type. Ceram. Int. 2023, 49, 25092–25104. [Google Scholar] [CrossRef]
- Hanjitsuwan, S.; Phoo-ngernkham, T.; Li, L.; Damrongwiriyanupap, N.; Chindaprasirt, P. Strength development and durability of alkali-activated fly ash mortar with calcium carbide residue as additive. Constr. Build. Mater. 2018, 162, 714–723. [Google Scholar] [CrossRef]
- Li, M.; Tan, H.; Zhang, J.; Deng, X.; Kong, X.; Chen, P.; Jian, S.; He, X.; Yang, J. Enhancement in compressive strength of carbide slag activated ground granulated blast furnace slag by introducing CaCl2 and NaCl. Constr. Build. Mater. 2023, 385, 131071. [Google Scholar] [CrossRef]
- He, D.; Shu, J.; Wang, R.; Chen, M.; Wang, R.; Gao, Y.; Liu, R.; Liu, Z.; Xu, Z.; Tan, D.; et al. A critical review on approaches for electrolytic manganese residue treatment and disposal technology: Reduction, pretreatment, and reuse. J. Hazard. Mater. 2021, 418, 126235. [Google Scholar] [CrossRef]
- Wang, D.; Fang, J.; Wang, Q.; Liu, Y. Utilizing desulphurized electrolytic-manganese residue as a mineral admixture: A feasibility study. Cem. Concr. Compos. 2022, 134, 104822. [Google Scholar] [CrossRef]
- Shu, J.; Liu, R.; Liu, Z.; Chen, H.; Tao, C. Simultaneous removal of ammonia and manganese from electrolytic metal manganese residue leachate using phosphate salt. J. Clean. Prod. 2016, 135, 468–475. [Google Scholar] [CrossRef]
- Tang, B.; Gao, S.; Wang, Y.; Liu, X.; Zhang, N. Pore structure analysis of electrolytic manganese residue based permeable brick by using industrial CT. Constr. Build. Mater. 2019, 208, 697–709. [Google Scholar] [CrossRef]
- Wang, J.; Peng, B.; Chai, L.; Zhang, Q.; Liu, Q. Preparation of electrolytic manganese residue–ground granulated blastfurnace slag cement. Powder Technol. 2013, 241, 12–18. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, X.; Zhang, Y.; Tang, B.; Mukiza, E. Investigation on sulfate activation of electrolytic manganese residue on early activity of blast furnace slag in cement-based cementitious material. Constr. Build. Mater. 2019, 229, 116831. [Google Scholar] [CrossRef]
- GB/T 208-2014; Cement Density Determination Method. General Administration of Quality Supervision, Inspection and Quarantine: Beijing, China, 2014; pp. 1–3.
- GB/T 8074-2008; Cement-Specific Surface Area Determination Method: Burr’s Method. General Administration of Quality Supervision, Inspection and Quarantine: Beijing, China, 2008; pp. 1–4.
- GB/T 17671-1999; Test Method for Cementitious Sand Strength (ISO Method). The State Bureau of Quality and Technical Supervision: Beijing, China, 1999; p. 9.
- Zhou, L.; Chen, P.; Hu, C.; Xia, H.; Liang, Z. Study on the Mechanical Properties and Hydration Behavior of Steel Slag–Red Mud–Electrolytic Manganese Residue Based Composite Mortar. Appl. Sci. 2023, 13, 5913. [Google Scholar] [CrossRef]
- He, D.; Chen, M.; Liu, H.; Wang, J. Preparation of activated electrolytic manganese residue-slag-cement ternary blended cementitious material: Hydration characteristics and carbon reduction potential. Constr. Build. Mater. 2024, 425, 135990. [Google Scholar] [CrossRef]
- Ji, X.; Wang, Z.; Zhang, H.; Wang, X.; Huo, J.; Zhang, T. Optimization design and characterization of slag cementitious composites containing carbide slag and desulfurized gypsum based on response surface methodology. J. Build. Eng. 2023, 77, 107441. [Google Scholar] [CrossRef]
- Zhang, S.; Niu, D. Hydration and mechanical properties of cement-steel slag system incorporating different activators. Constr. Build. Mater. 2023, 363, 129981. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, J.; He, X.; Zhang, Y.; Su, Y.; Tan, H. Sustainable clinker-free solid waste binder produced from wet-ground granulated blast-furnace slag, phosphogypsum and carbide slag. Constr. Build. Mater. 2022, 330, 127218. [Google Scholar] [CrossRef]
- Haha, M.B.; Le Saout, G.; Winnefeld, F.; Lothenbach, B. Influence of activator type on hydration kinetics, hydrate assemblage and microstructural development of alkali activated blast-furnace slags. Cem. Concr. Res. 2011, 41, 301–310. [Google Scholar] [CrossRef]
- Li, N.; Farzadnia, N.; Shi, C. Microstructural changes in alkali-activated slag mortars induced by accelerated carbonation. Cem. Concr. Res. 2017, 100, 214–226. [Google Scholar] [CrossRef]
- Wang, J.; Ma, B.; Tan, H.; Du, C.; Chu, Z.; Luo, Z.; Wang, P. Hydration and mechanical properties of cement-marble powder system incorporating triisopropanolamine. Constr. Build. Mater. 2021, 266, 121068. [Google Scholar] [CrossRef]
- Masoudi, R.D.H.R.; Hooton, R.D. Examining the hydration mechanism of supersulfated cements made with high and low-alumina slags. Cem. Concr. Comp. 2019, 103, 193–203. [Google Scholar] [CrossRef]
- Jin, Z.; Ma, B.; Su, Y.; Lu, W.; Qi, H.; Hu, P. Effect of calcium sulphoaluminate cement on mechanical strength and waterproof properties of beta-hemihydrate phosphogypsum. Constr. Build. Mater. 2020, 242, 118198. [Google Scholar] [CrossRef]
- Wang, F.; Long, G.; Bai, M.; Shi, Y.; Zhou, J.L. Feasibility of low-carbon electrolytic manganese residue-based supplementary cementitious materials. Sci. Total Environ. 2023, 883, 163672. [Google Scholar] [CrossRef]
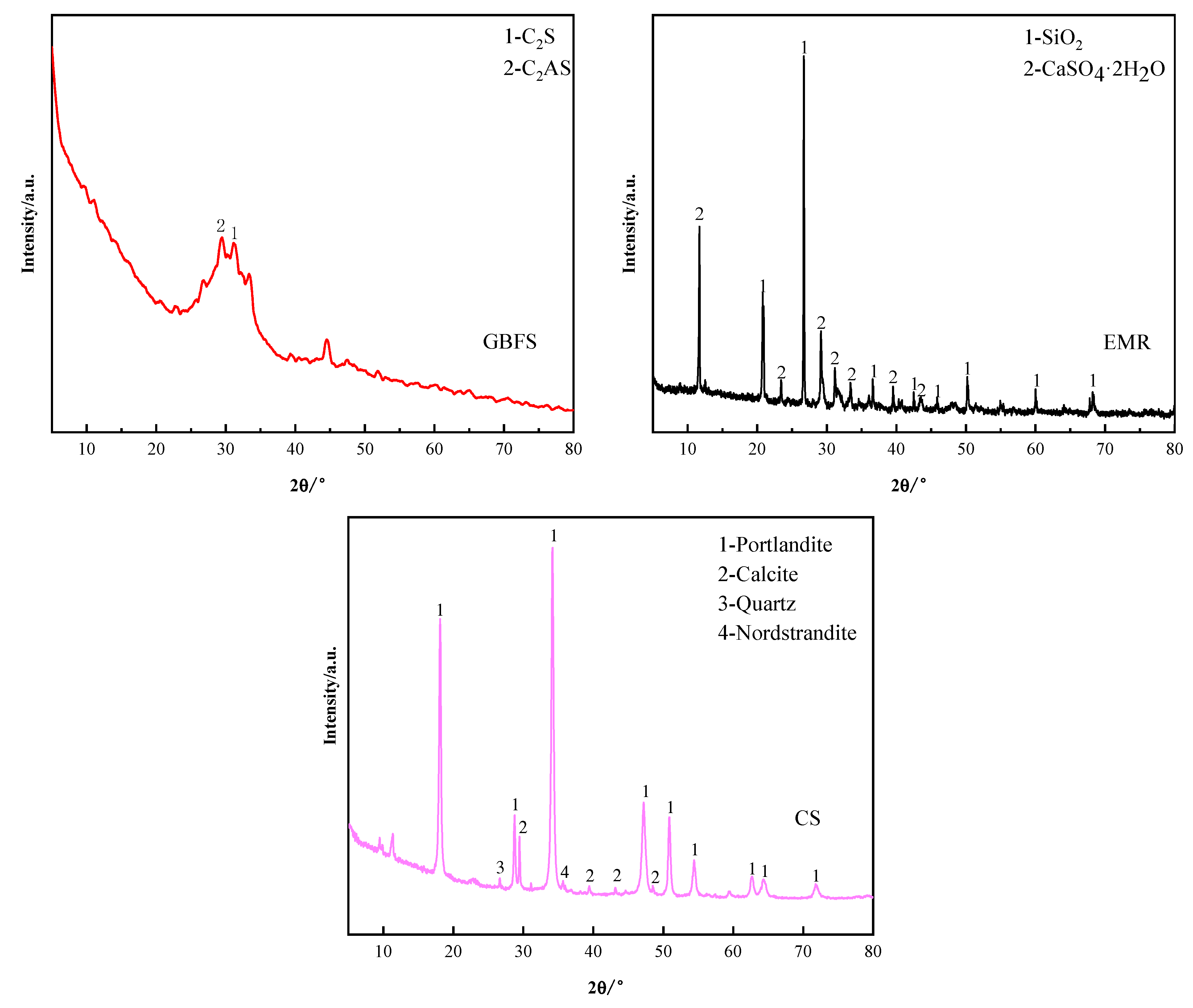
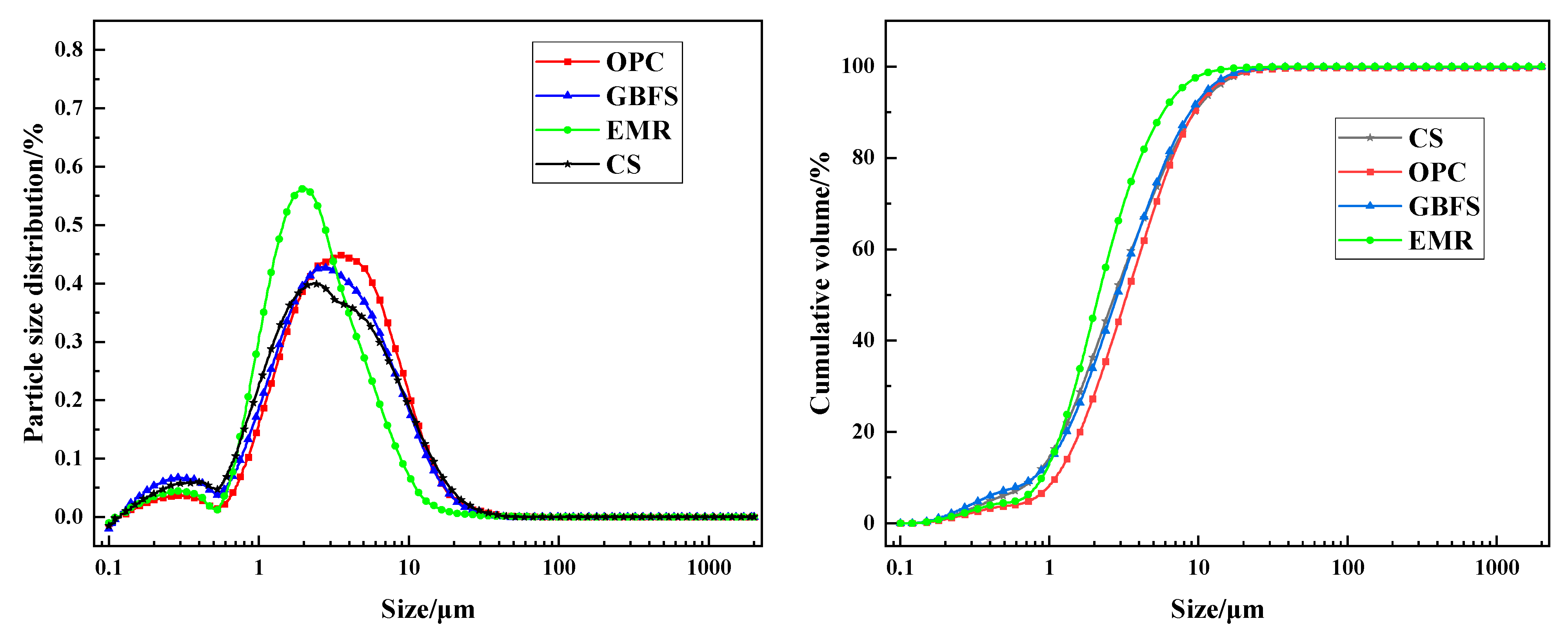



| Density (kg/m3) | Specific Surface Area (m2/kg) | Flexural Strength (MPa) | Compressive Strength (MPa) | |||
|---|---|---|---|---|---|---|
| 3 d | 28 d | 3 d | 28 d | |||
| OPC | 3.20 | 360.80 | 5.2 | 7.5 | 24.5 | 46.4 |
| Fe2O3 | Al2O3 | TiO2 | SiO2 | CaO | MgO | Na2O | K2O | MnO | SO3 | Others | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| OPC | 3.71 | 5.64 | 0.3 | 24.76 | 60.25 | 0.88 | 0.16 | 0.7 | 0.32 | - | 3.28 |
| GBFS | 0.81 | 11.79 | 1.94 | 25.26 | 57.54 | - | - | 0.7 | 0.54 | 1.16 | 0.26 |
| EMR | 11.03 | 5.09 | 0.26 | 29.98 | 20.14 | 2.55 | 2.24 | 0.71 | 9.50 | 17.06 | 1.44 |
| CS | 0.63 | 1.58 | 0.04 | 4.80 | 92.07 | 0.14 | 0.03 | 0.02 | 0.04 | 0.62 | 0.05 |
| Experiment Number | Cement/% | EMR/% | GBFS/% | CS/% | W/C | Sand/g |
|---|---|---|---|---|---|---|
| PO425 | 100 | - | - | - | 0.5 | 1350 |
| EGC0 | 70 | - | 30 | - | ||
| EGC1 | 70 | 0 | 25 | 5 | ||
| EGC2 | 70 | 1 | 25 | 4 | ||
| EGC3 | 70 | 2 | 25 | 3 | ||
| EGC4 | 70 | 3 | 25 | 2 | ||
| EGC5 | 70 | 4 | 25 | 1 | ||
| EGC6 | 70 | 5 | 25 | 0 | ||
| EGC7 | 70 | 0 | 20 | 10 | ||
| EGC8 | 70 | 2 | 20 | 8 | ||
| EGC9 | 70 | 4 | 20 | 6 | ||
| EGC10 | 70 | 6 | 20 | 4 | ||
| EGC11 | 70 | 8 | 20 | 2 | ||
| EGC12 | 70 | 10 | 20 | 0 | ||
| EGC13 | 70 | 0 | 15 | 15 | ||
| EGC14 | 70 | 3 | 15 | 12 | ||
| EGC15 | 70 | 6 | 15 | 9 | ||
| EGC16 | 70 | 9 | 15 | 6 | ||
| EGC17 | 70 | 12 | 15 | 3 | ||
| EGC18 | 70 | 15 | 15 | 0 |
| Samples | Mass Loss Ratio (%) | |
|---|---|---|
| 50–200 °C | 350–500 °C | |
| EGC0 | 7.89 | 3.05 |
| EGC7 | 7.73 | 5.26 |
| EGC8 | 8.37 | 5.21 |
| EGC9 | 8.42 | 4.83 |
| EGC10 | 8.47 | 3.84 |
| EGC11 | 8.78 | 3.40 |
| EGC12 | 9.27 | 2.94 |
| Cost (RMB/t) | Carbon Emission (kg/kg) | Compressive Strength at 28 Days (MPa) | |
|---|---|---|---|
| EMR | 20 | 0.007 | - |
| CS | 60 | 0.067 | - |
| GBFS | 300 | 0.083 | - |
| Cement | 400 | 0.8 | 42.5 |
| EGC0 | 370 | 0.5849 | 42.9 |
| EGC7 | 362 | 0.5843 | 39.2 |
| EGC8 | 361.2 | 0.5831 | 40.1 |
| EGC9 | 360.4 | 0.5819 | 43.2 |
| EGC10 | 359.6 | 0.5807 | 44.8 |
| EGC11 | 358.8 | 0.5795 | 45.5 |
| EGC12 | 358.0 | 0.5783 | 40.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.; Liu, R.; Jing, D.; Lu, F.; Zhao, Y.; Xie, Z.; Huang, W.; Chen, T. Study on the Alkali–Sulfur Co-Activation and Mechanical Properties of Low-Carbon Cementitious Composite Materials Based on Electrolytic Manganese Residue, Carbide Slag, and Granulated Blast-Furnace Slag. Appl. Sci. 2024, 14, 4355. https://doi.org/10.3390/app14114355
Liang J, Liu R, Jing D, Lu F, Zhao Y, Xie Z, Huang W, Chen T. Study on the Alkali–Sulfur Co-Activation and Mechanical Properties of Low-Carbon Cementitious Composite Materials Based on Electrolytic Manganese Residue, Carbide Slag, and Granulated Blast-Furnace Slag. Applied Sciences. 2024; 14(11):4355. https://doi.org/10.3390/app14114355
Chicago/Turabian StyleLiang, Jianbo, Rongjin Liu, Daiyan Jing, Fuhua Lu, Yanrong Zhao, Zhihan Xie, Wanyu Huang, and Tingchao Chen. 2024. "Study on the Alkali–Sulfur Co-Activation and Mechanical Properties of Low-Carbon Cementitious Composite Materials Based on Electrolytic Manganese Residue, Carbide Slag, and Granulated Blast-Furnace Slag" Applied Sciences 14, no. 11: 4355. https://doi.org/10.3390/app14114355





