Effect of Sodium Laureth Sulfate on Contact Angles of High-Impact Polystyrene and Acrylonitrile–Butadiene–Styrene from Recycled Refrigeration Equipment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Method of Selection of the Representative Sample
2.2. Pre-Identification of HIPS and ABS with Handheld NIR Spectrometer
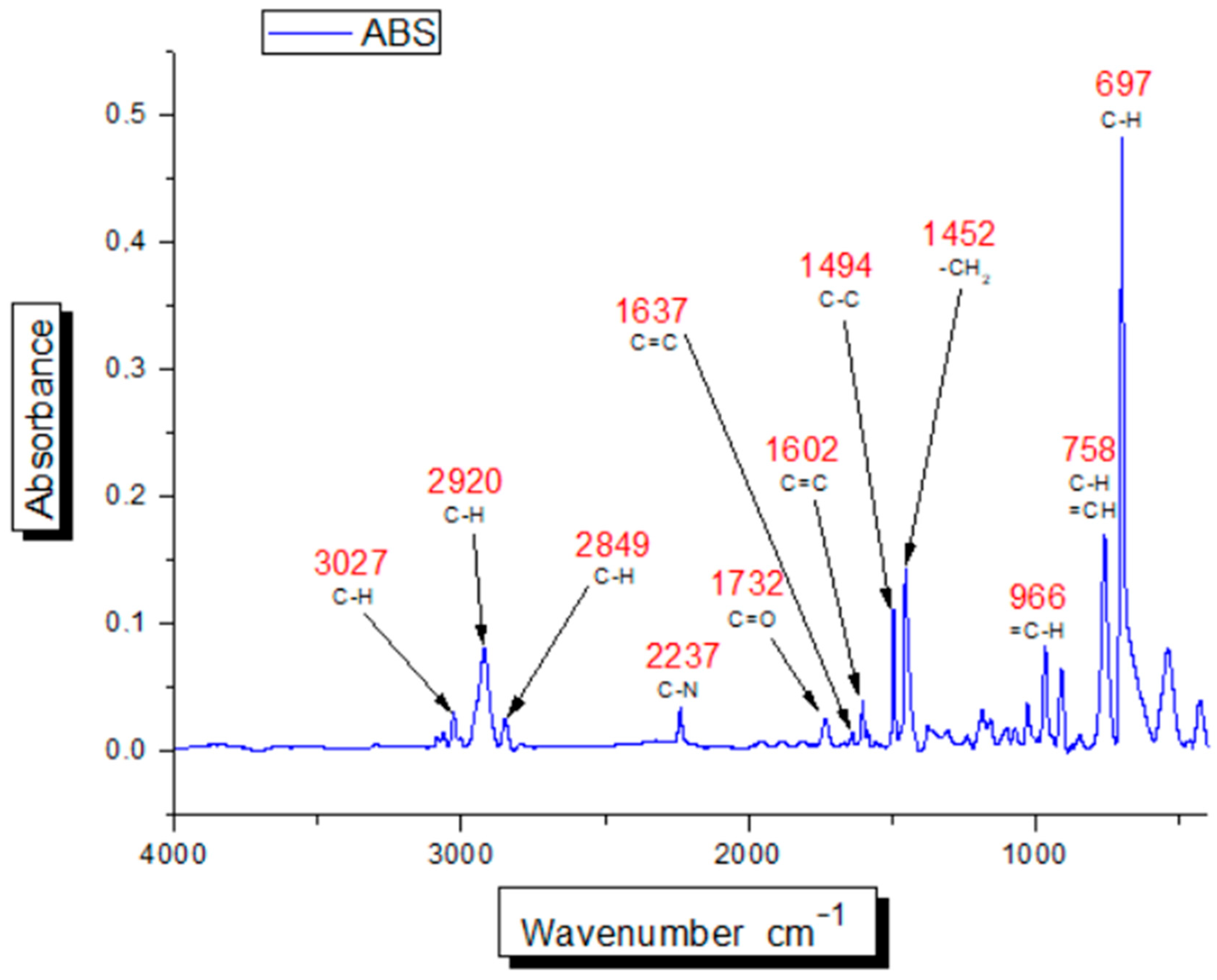
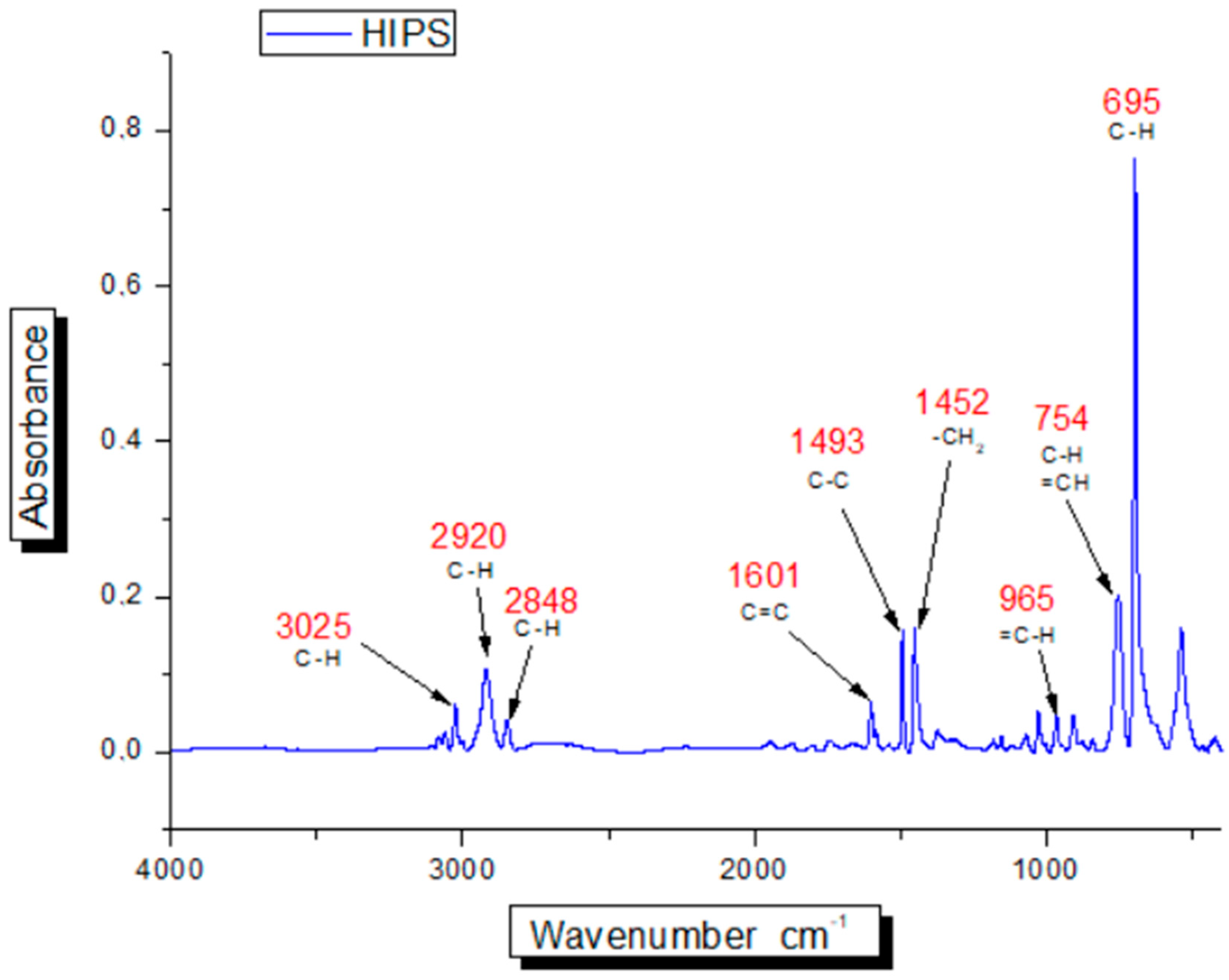
2.3. Identification of HIPS and ABS Using Stationary FTIR Spectrometer
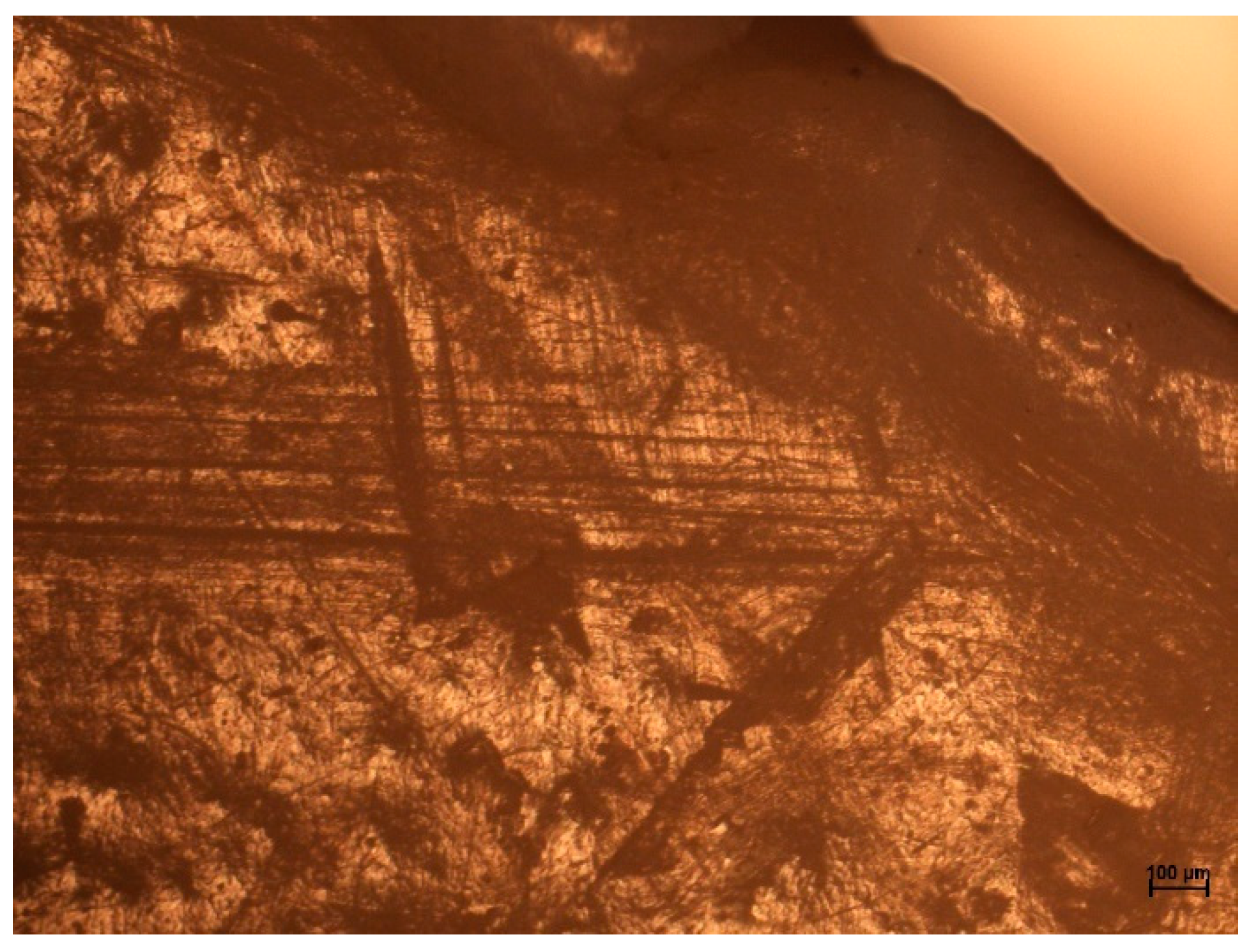
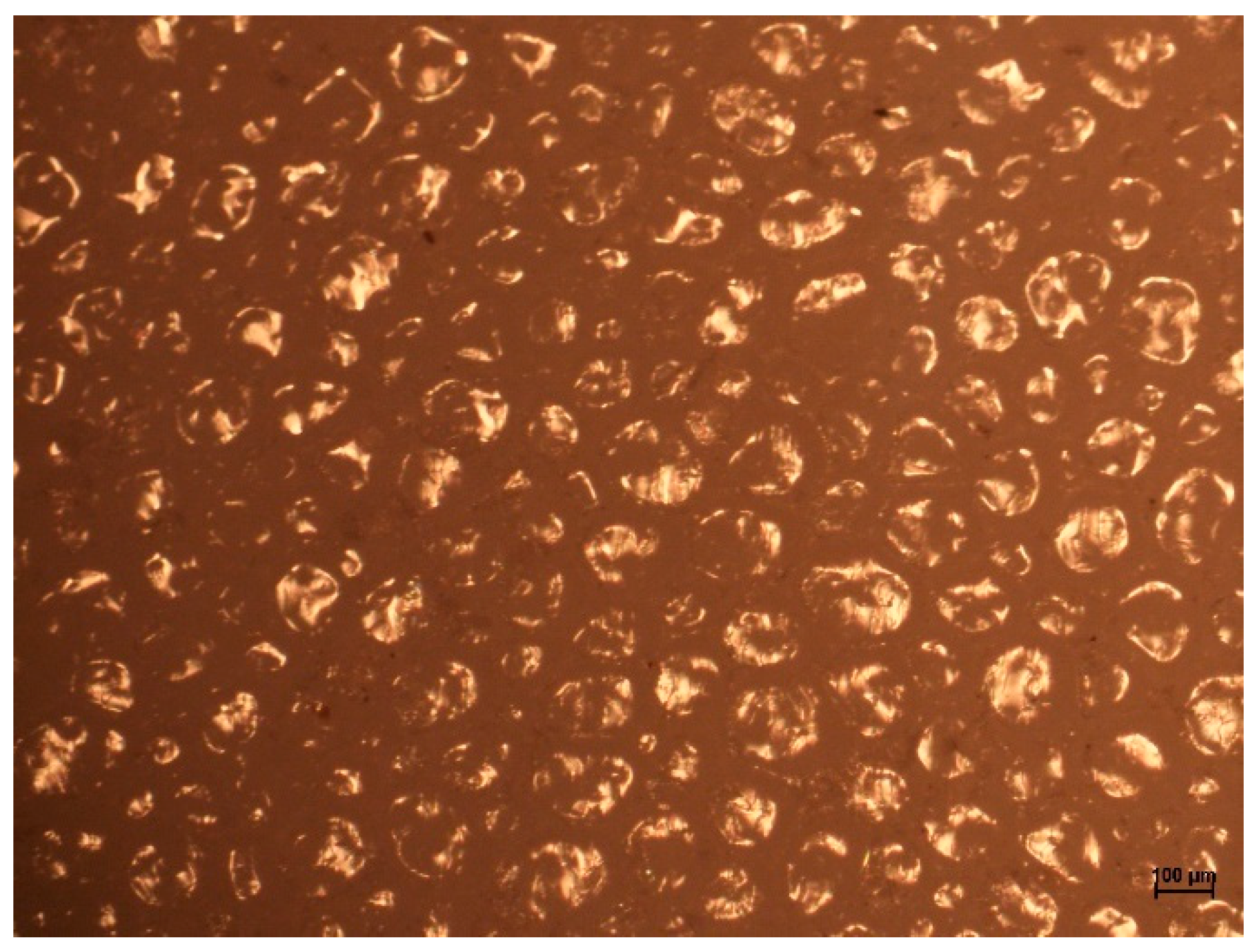
2.4. Optical Microscopy Examinations of HIPS and ABS Flakes
2.5. Testing the Moisture Content of HIPS and ABS Flakes
2.6. Rheological Examination of MFR and MVR Parameters
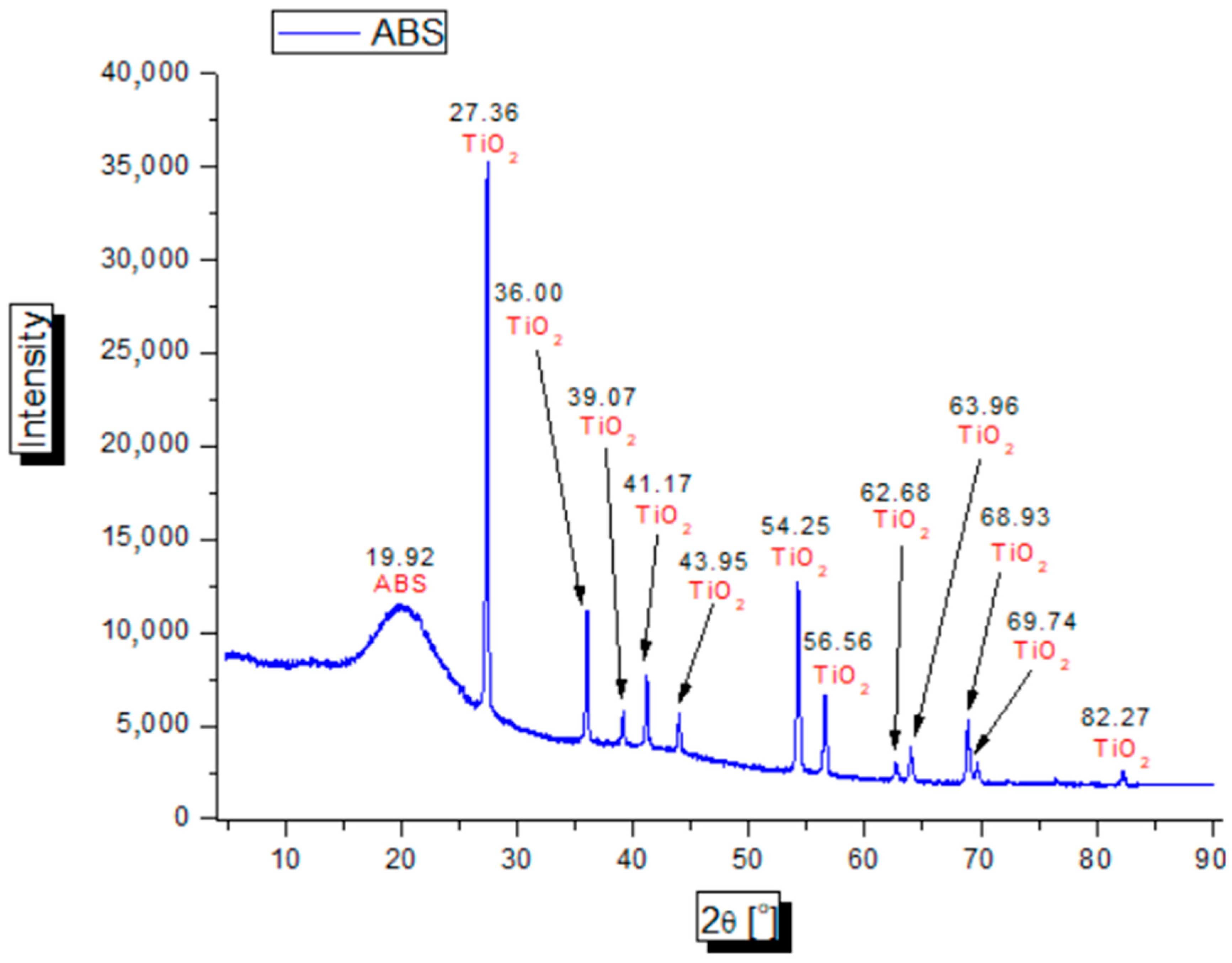
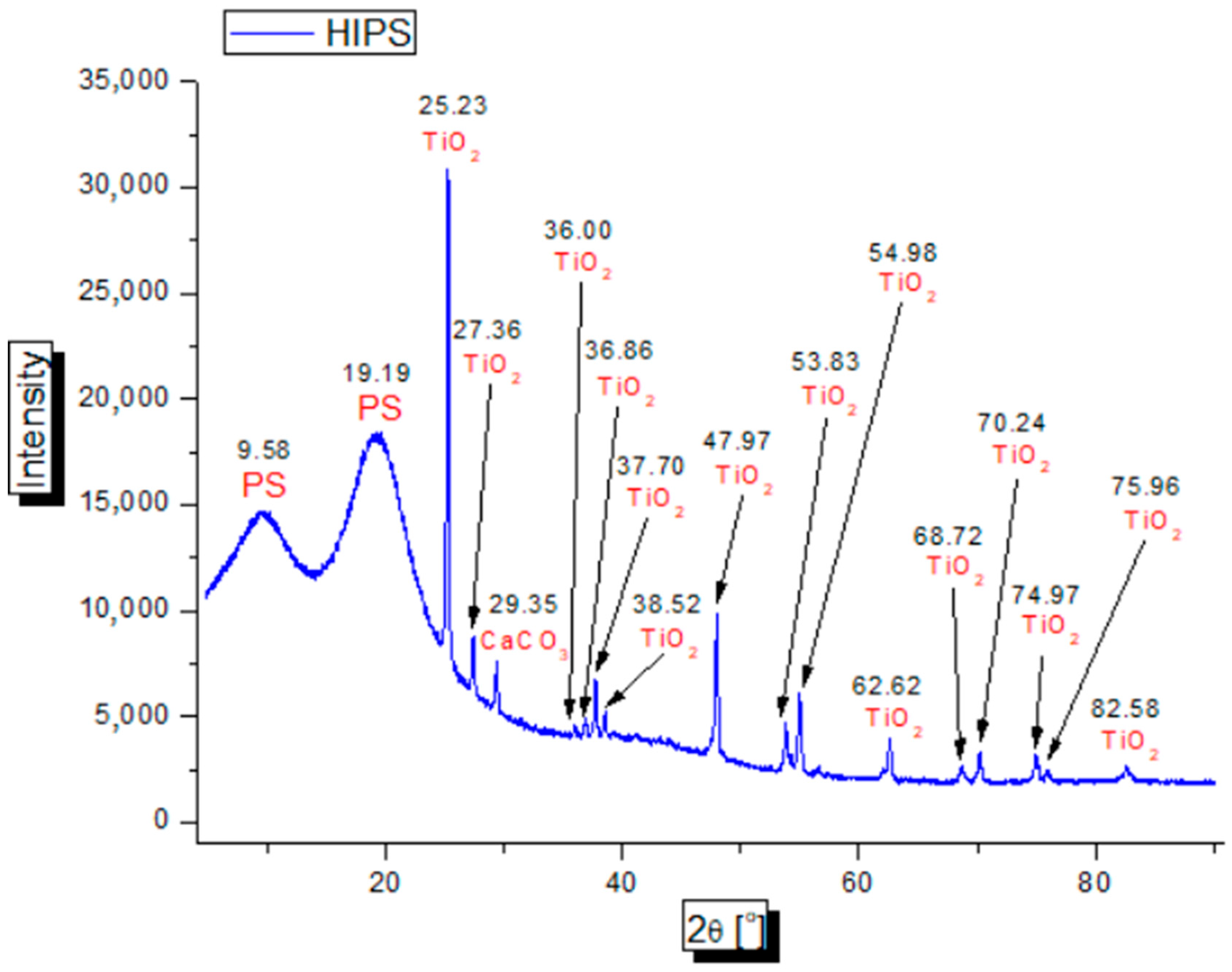
2.7. X-ray Diffraction (XRD) Testing
2.8. The Theoretical Number of the Bubbles That Should Attach to the Surface of HIPS and ABS Flakes to Have a Density Lower than That of Tap Water
2.9. Scanning Electron Microscope (SEM) Surface Analysis
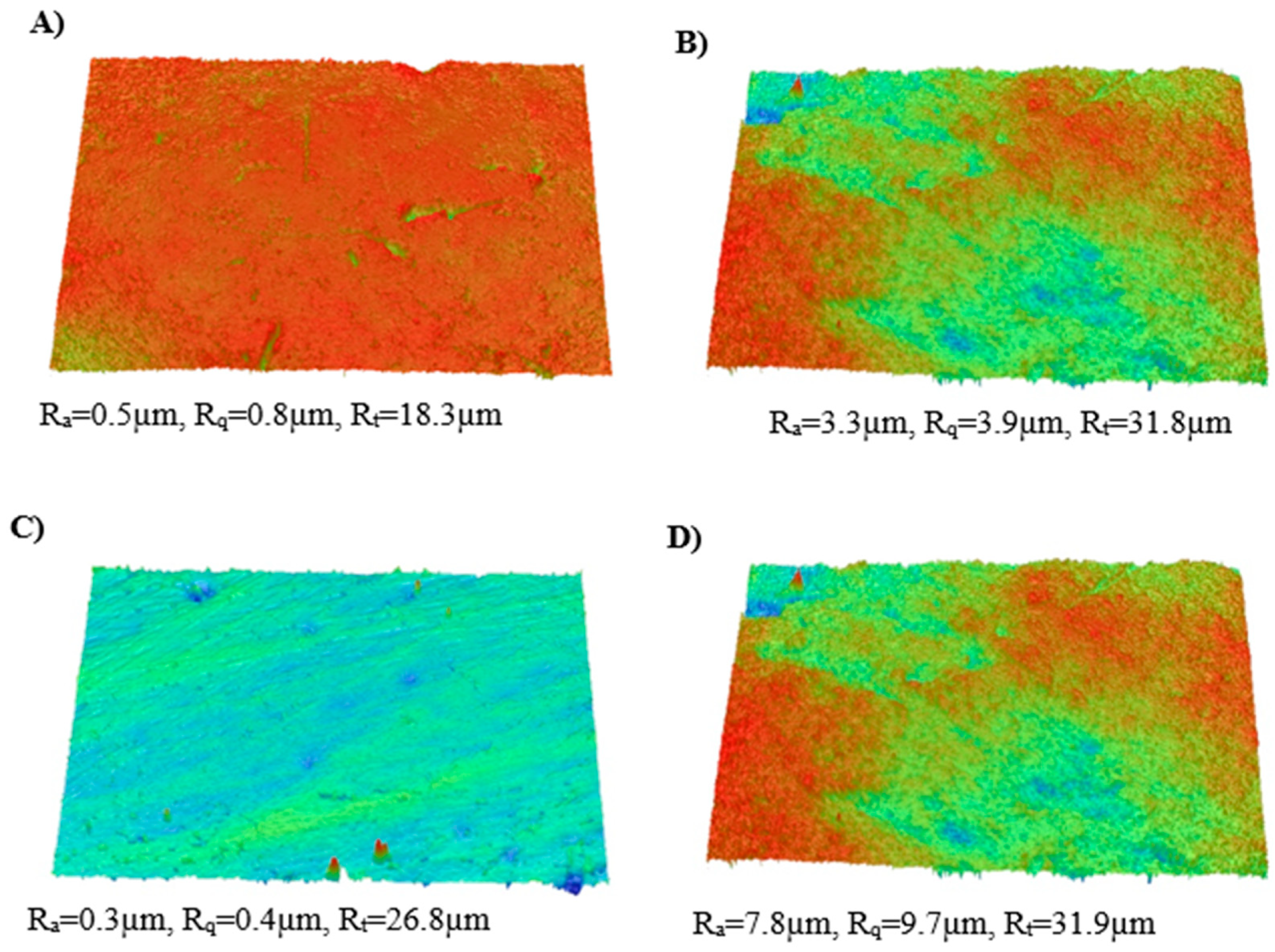
2.10. Optical Profilometry
2.11. Examination of HIPS and ABS Contact Angles by the Sessile Drop Method
3. Results
3.1. Pre-Identification of HIPS and ABS with the Handheld NIR Spectrometer
3.2. Identification of HIPS and ABS Using Stationary FTIR Spectrometer
3.3. Optical Microscopy Examinations of HIPS and ABS Flakes
3.4. Testing the Moisture Content of HIPS and ABS Flakes
3.5. Rheological Examination of MFR and MVR Parameters
3.6. Study of X-ray Diffraction (XRD)
3.7. The Theoretical Number of the Bubbles That Should Attach to the Surface of HIPS and ABS Flakes to Have a Density Lower than That of Tap Water
3.8. Scanning Electron Microscopy (SEM) Surface Analysis
3.9. Optical Profilometry
3.10. Examination of HIPS and ABS Contact Angles by the Sitting Drop Method
4. Applications
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Afroz, R.; Masud, M.M.; Akhtar, R.; Duasa, J.B. Survey and analysis of public knowledge. awareness and willingness to pay in Kuala Lumpur. Malaysia—A case study on household WEEE management. J. Clean. Prod. 2013, 52, 185–193. [Google Scholar] [CrossRef]
- Cucchiella, F.; D’Adamo, I.; Koh, S.C.L.; Rosa, P. Recycling of WEEEs: An economic assessment of present and future e-waste streams. Renew. Sust. Energy Rev. 2015, 51, 263–272. [Google Scholar] [CrossRef]
- Dodbiba, G.; Shibayama, A.; Miyazaki, T.; Fujita, T. Triboelectrostatic separation of ABS. PS and PP plastic mixture. Mater. Trans. 2003, 44, 161–166. [Google Scholar] [CrossRef]
- Fraunholcz, N. Separation of waste plastics by froth flotation—A review. Part I. Miner. Eng. 2004, 17, 261–268. [Google Scholar] [CrossRef]
- Bakker, E.J.; Rem, P.C.; Fraunholcz, N. Upgrading mixed polyolefin waste with magnetic density separation. Waste Manag. 2009, 29, 1712–1717. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, G.; Xu, Z. Tribo-charging properties of waste plastic granules in process of tribo-electrostatic separation. Waste Manag. 2015, 35, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Fang, Y.; Yuan, H.; Tan, W.; Dong, Y. Effect of the medium’s density on the hydrocyclonic separation of waste plastics with different densities. Waste Manag. 2017, 67, 27–31. [Google Scholar] [CrossRef]
- Censori, M.; La Marca, F.; Carvalho, M.T. Separation of plastics: The importance of kinetics knowledge in the evaluation of froth flotation. Waste Manag. 2016, 54, 39–43. [Google Scholar] [CrossRef]
- Truc, N.T.T.; Lee, B.K. Combining ZnO/microwave treatment for changing wettability of WEEE styrene plastics (ABS and HIPS) and their selective separation by froth flotation. Appl. Surf. Sci. 2017, 420, 746–752. [Google Scholar] [CrossRef]
- Mallampati, S.R.; Lee, C.; Park, M.H.; Lee, B. Processing plastics from ASR/ESR waste: Separation of poly vinyl chloride (PVC) by froth flotation after microwave- assisted surface modification. J. Mater. Cycles Waste Manag. 2018, 20, 91–99. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H. Fenton treatment for flotation separation of polyvinyl chloride from plastic mixtures. Sep. Purif. Technol. 2017, 187, 415–425. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Fu, J. Floatability of polymer materials modulated by frothers. Waste Manag. 2013, 33, 2623–2631. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, R.D. The use of selective depressants for the separation of ABS and HIPS by froth flotation. Miner. Eng. 2005, 18, 233–237. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Wu, B.; Liu, Q. Boiling treatment of ABS and PS plastics for flotation separation. Waste Manag. 2014, 34, 1206–1210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, H.; Wang, H.; Wang, C. Flotation separation of acrylonitrile-butadiene-styrene and polystyrene in WEEE based on oxidation of active sites. Miner. Eng. 2020, 146, 106131. [Google Scholar] [CrossRef]
- Guo, C.; Zou, Q.; Wang, J.; Wang, H.; Chen, S.; Zhong, Y. Application of surface modification using sodium hypochlorite for helping flotation separation of acrylonitrile-butadiene-styrene and polystyrene plastics of WEEE. Waste Manag. 2018, 82, 167–176. [Google Scholar] [CrossRef]
- Cui, Y.; Li, Y.; Wang, W.; Wang, X.; Lin, J.; Mai, X.; Song, G.; Naik, N.; Guo, Z. Flotation separation of acrylonitrile-butadienestyrene (ABS) and high impact polystyrene (HIPS) from waste electrical and electronic equipment (WEEE) by potassium permanganate surface modification. Sep. Purif. Technol. 2021, 269, 118767. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.; Wang, H.; Wang, C.; Huang, W. Separation of acrylonitrile-butadiene-styrene and polystyrene waste plastics after surface modification using potassium ferrate by froth flotation. Waste Manag. 2018, 78, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Baigabelov, A. Investigation of Surface Treatment and Density Separation for Selective Recycling of Plastics; Montana Tech Library: Butte, MT, USA, 2019. [Google Scholar]
- Campos, M.; Campos, R. Applications of quartering method in soils and foods. Int. J. Eng. Res. Appl. 2017, 7 Pt 2, 35–39. [Google Scholar] [CrossRef]
- Petersen, L.; Minkkinen, P.; Esbensen, K.H. Representative sampling for reliable data analysis: Theory of Sampling. Chemom. Intell. Lab. Syst. 2005, 77, 261–277. [Google Scholar] [CrossRef]
- ISO 1133-1:2022; Plastics—Determination of the Melt Mass-Flow Rate (MFR) and Melt Volume-Flow Rate (MVR) of Thermoplastics. ISO: Geneva, Switzerland, 2022.
- Shen, H.; Forssberg, E.; Pugh, R.J. Selective flotation separation of plastics by particle control. Resour. Conserv. Recycl. 2001, 33, 37–50. [Google Scholar] [CrossRef]
- Martinho, G.; Pires, A.; Saraiva, L.; Ribeiro, R. Composition of plastics from waste electrical and electronic equipment (WEEE) by direct sampling. Waste Manag. 2012, 32, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Y.; Wang, C. Surface modification and selective flotation of waste plastics for effective recycling—A review. Sep. Purif. Technol. 2019, 226, 75–94. [Google Scholar] [CrossRef]
- Turku, I.; Kasala, S.; Kärki, T. Characterization of Polystyrene Wastes as Potential Extruded Feedstock Filament for 3D Printing Lappeenranta University of Technology. Recycling 2018, 3, 57. [Google Scholar] [CrossRef]
- Notomi, M.; Kishimoto, K.; Shibuya, T.; Koizumi, T. Effects of moisture absorption on fracture behaviors of acrylonitrile-butadiene-styrene resin. J. Appl. Polym. Sci. 1999, 72, 435–442. [Google Scholar] [CrossRef]
- Turku, I.; Karki, T. Recyclability of plastic waste extracted from electrical devices. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; p. 012001. [Google Scholar]
- Bandeira, L.C.; Campos, B.M.; Ciuffi, K.J.; Nassar, E.J.; Silva, J.V.; Oliveira, M.F.; Maia, I.A. Calcium Phosphate Coatings by Sol-Gel on Acrylonitrile-butadiene-styrene Substrate. J. Braz. Chem. Soc. 2017, 28, 943–949. [Google Scholar] [CrossRef]
- Saja, S.; Al-Taweel, S.S. New route for synthesis of pure anatase TiO2 nanoparticles via utrasoundassisted sol-gel method. Chem. Pharm. Res. 2016, 8, 620–626. [Google Scholar]
- Prabhu, T.V.; Chandrasekaran, J.; Thangaraju, D.; Vivek, P.; Gopi, S. Fabrication and performance analysis of set standard natural dye-sensitized solar cell (N-DSSC) using extracted Terminalia kattapa (Red), Azadirachia indica (Green), and Clitoria ternatea (Blue) dyes with virgin Degussa p25 photo-anode. J. Mater. Sci. Mater. Electron. 2022, 33, 17331–17342. [Google Scholar] [CrossRef]
- Thamaphat, K.; Limsuwan, P. Phase Characterization of TiO2 Powder by XRD and TEM. Agric. Nat. Resour. 2008, 42, 357–361. [Google Scholar]
- Hurley, N.; Li, L.; Koenigsmann, C.; Wong, S. Surfactant-Free Synthesis of Three-Dimensional Perovskite Titania-Based Micron-Scale Motifs Used as Catalytic Supports for the Methanol Oxidation Reaction. Molecules 2021, 26, 909. [Google Scholar] [CrossRef]
- Ankasi, D.W.; Dikio, E.D. Comparative Study of Polystyrene and Polymethylmethacrylate Wastes as Adsorbents for Sorption of Pb2+ from Aqueous Solution. Asian J. Chem. 2014, 26, 8295–8302. [Google Scholar] [CrossRef]
- Sahebian, S.; Zebarjad, S.M.; Sajjadi, S.A.; Sherafat, Z.; Lazzeri, A. Effect of both uncoated and coated calcium carbonate on fracture toughness of HDPE/CaCO3 nanocomposites. J. Appl. Polym. Sci. 2007, 104, 3688–3694. [Google Scholar] [CrossRef]
- Uddin, M.J.; Cesano, F.; Chowdhury, A.R.; Trad, T.; Cravanzola, S.; Martra, G.; Mino, L.; Zecchina, A.; Scarano, D. Surface Structure and Phase Composition of TiO2 P25 Particles After Thermal Treatments and HF Etching. Front. Mater. 2020, 7, 192. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Maniadi, A.; Koudoumas, E.; Liebscher, M.; Tzounis, L. Mechanical Properties of 3D-printed acrylonitrile–butadiene–styrene, TiO2 and ATO Nanocomposites. Polymers 2020, 12, 1589. [Google Scholar] [CrossRef]
- Che Ismail, N.H.; Akil, H.M. Improving the Flexural Properties of Abs/Muscovite Composites by Introducing Modified Muscovite, The Effect of Filler Type on the Mechanical, Thermal and Rheological Properties of Cellulose-filled Thermoplastic. J. Phys. Conf. Ser. 2018, 1082, 01201. [Google Scholar] [CrossRef]
- Vilaplana, F.; Ribes-Greus, A.; Karlsson, S. Analytical strategies for the quality assessment of recycled high-impact polystyrene: A combination of thermal analysis, vibrational spectroscopy, and chromatography. Anal. Chim. Acta 2007, 604, 18–28. [Google Scholar] [CrossRef]
- Fricker, A.L.; McPhail, D.S.; Keneghan, B.; Pretzel, B. Investigating the impact of cleaning treatments on polystyrene using SEM, AFM and ToF–SIMS. Herit. Sci. 2017, 5, 28. [Google Scholar] [CrossRef]
- Martinez, M.A.; Abenojar, J.; Lopez de Armentia, S. Environmentally Friendly Plasma Activation of Acrylonitrile–Butadiene–Styrene and Polydimethylsiloxane Surfaces to Improve Paint Adhesion. Coatings 2018, 8, 428. [Google Scholar] [CrossRef]
- Kubiak, K.J.; Wilson, M.C.T.; Mathia, T.G.; Carval, P. Wettability versus roughness of engineering surfaces. Wear 2011, 271, 523–528. [Google Scholar] [CrossRef]
- Nakae, H.; Inui, R.; Hirata, Y.; Saito, H. Effect of surface roughness on wettability. Pergamon 1998, 46, 2313–2318. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Mallampati, S.R.; Lee, B.H.; Mitoma, Y.; Simion, C. Selective sequential separation of ABS/HIPS and PVC from automobile and electronic waste shredder residue by hybrid nano-Fe/Ca/CaO assisted ozonisation process. Waste Manag. 2017, 60, 428–438. [Google Scholar] [CrossRef] [PubMed]







| Material | Mass [g] | % Mass [%] |
|---|---|---|
| PS | 100.40 | 57.44 |
| ABS | 68.60 | 39.24 |
| PP | 4.80 | 2.75 |
| PA | 1.00 | 0.57 |
| TOTAL | 174.80 | 100.00 |
| Number of Sample | Mass before Test [g] | Moisture Content [%] |
|---|---|---|
| 1 | 14.123 | 0.064 |
| 2 | 14.057 | 0.078 |
| 3 | 14.079 | 0.092 |
| 4 | 14.051 | 0.071 |
| 5 | 14.010 | 0.100 |
| STANDARD DEVIATION | 0.015 | |
| AVERAGE | 0.081 | |
| Number of Sample | Mass before Test [g] | Moisture Content [%] |
|---|---|---|
| 1 | 12.997 | 0.208 |
| 2 | 14.165 | 0.163 |
| 3 | 14.076 | 0.142 |
| 4 | 14.090 | 0.163 |
| 5 | 13.701 | 0.161 |
| STANDARD DEVIATION | 0.024 | |
| AVERAGE | 0.167 | |
| Test Number | MFR [g/10 min] | MVR [cm3/10 min] | Density [g/cm3] |
|---|---|---|---|
| 1 | 3.41 | 3.52 | 0.97 |
| 2 | 3.43 | 3.47 | 0.99 |
| 3 | 4.12 | 4.20 | 0.98 |
| 4 | 3.78 | 3.78 | 1.00 |
| 5 | 4.42 | 4.44 | 1.00 |
| STANDARD DEVIATION | 0.44 | 0.43 | 0.01 |
| AVERAGE | 3.83 | 3.88 | 0.99 |
| Test Number | MFR [g/10 min] | MVR [cm3/10 min] | Density [g/cm3] |
|---|---|---|---|
| 1 | 32.52 | 32.45 | 1.00 |
| 2 | 30.48 | 29.89 | 1.02 |
| 3 | 33.12 | 33.30 | 0.99 |
| 4 | 32.04 | 31.59 | 1.01 |
| 5 | 30.48 | 29.03 | 1.05 |
| STANDARD DEVIATION | 1.20 | 1.77 | 0.02 |
| AVERAGE | 31.73 | 31.25 | 1.02 |
| No. | Mass of ABS > 8 mm [g] | Mass of HIPS > 8 mm [g] | Mass of ABS 4–8 mm [g] | Mass of HIPS 4–8 mm [g] |
|---|---|---|---|---|
| 1 | 0.979 | 0.231 | 0.051 | 0.248 |
| 2 | 0.301 | 0.244 | 0.140 | 0.202 |
| 3 | 0.422 | 0.387 | 0.096 | 0.053 |
| 4 | 0.429 | 0.432 | 0.088 | 0.181 |
| 5 | 0.231 | 0.448 | 0.065 | 0.268 |
| STANDARD DEVIATION | 0.295 | 0.104 | 0.034 | 0.084 |
| AVERAGE | 0.350 | 0.369 | 0.116 | 0.146 |
| No. | ABS Density [g/cm3] at T = 25 °C | HIPS Density [g/cm3] at T = 25 °C |
|---|---|---|
| 1 | 1.106 | 1.036 |
| 2 | 1.028 | 1.018 |
| 3 | 1.060 | 1.047 |
| 4 | 1.051 | 1.037 |
| 5 | 1.054 | 1.024 |
| 6 | 1.067 | 1.019 |
| 7 | 1.028 | 1.024 |
| 8 | 1.027 | 1.005 |
| 9 | 1.085 | 1.011 |
| 10 | 1.023 | 1.008 |
| STANDARD DEVIATION | 0.028 | 0.014 |
| AVERAGE | 1.053 | 1.023 |
| Material and Its Dimensions | Minimum Number of Air Bubbles [pcs.] |
|---|---|
| ABS > 8 mm | 19 |
| HIPS > 8 mm | 10 |
| ABS 4–8 mm | 4 |
| HIPS 4–8 mm | 4 |
| ABS 2.5–4 mm | 1 |
| HIPS 2.5–4 mm | 1 |
| ABS < 2.5 mm | 1 |
| HIPS < 2.5 mm | 1 |
| SLES Concentration [g/L] | HIPS Contact Angle | Standard Deviation | ABS Contact Angle | Standard Deviation |
|---|---|---|---|---|
| 0.00 | 72.30 | 4.36 | 73.00 | 4.36 |
| 0.10 | 61.02 | 5.16 | 50.26 | 3.93 |
| 0.15 | 54.71 | 2.90 | 61.57 | 4.52 |
| 0.20 | 66.80 | 5.23 | 56.70 | 4.90 |
| 0.25 | 52.64 | 3.38 | 55.67 | 3.50 |
| 0.30 | 49.01 | 5.48 | 56.48 | 3.45 |
| 0.40 | 53.38 | 4.42 | 53.16 | 4.16 |
| 0.50 | 41.46 | 4.00 | 37.57 | 2.70 |
| 0.60 | 41.45 | 3.64 | 38.32 | 3.11 |
| 0.70 | 38.33 | 4.21 | 37.35 | 4.81 |
| 0.80 | 52.37 | 3.19 | 56.88 | 5.58 |
| 0.90 | 49.10 | 3.02 | 51.39 | 3.93 |
| 1.00 | 57.42 | 3.33 | 54.07 | 4.23 |
| 1.10 | 44.74 | 4.09 | 47.59 | 3.90 |
| 1.20 | 36.20 | 4.31 | 39.19 | 3.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malinowska, B.; Munoz Esteban, J.L.; Pakhlov, E.; Terpiłowski, K. Effect of Sodium Laureth Sulfate on Contact Angles of High-Impact Polystyrene and Acrylonitrile–Butadiene–Styrene from Recycled Refrigeration Equipment. Appl. Sci. 2024, 14, 4407. https://doi.org/10.3390/app14114407
Malinowska B, Munoz Esteban JL, Pakhlov E, Terpiłowski K. Effect of Sodium Laureth Sulfate on Contact Angles of High-Impact Polystyrene and Acrylonitrile–Butadiene–Styrene from Recycled Refrigeration Equipment. Applied Sciences. 2024; 14(11):4407. https://doi.org/10.3390/app14114407
Chicago/Turabian StyleMalinowska, Benita, Julio León Munoz Esteban, Evgeniy Pakhlov, and Konrad Terpiłowski. 2024. "Effect of Sodium Laureth Sulfate on Contact Angles of High-Impact Polystyrene and Acrylonitrile–Butadiene–Styrene from Recycled Refrigeration Equipment" Applied Sciences 14, no. 11: 4407. https://doi.org/10.3390/app14114407
APA StyleMalinowska, B., Munoz Esteban, J. L., Pakhlov, E., & Terpiłowski, K. (2024). Effect of Sodium Laureth Sulfate on Contact Angles of High-Impact Polystyrene and Acrylonitrile–Butadiene–Styrene from Recycled Refrigeration Equipment. Applied Sciences, 14(11), 4407. https://doi.org/10.3390/app14114407







