Effect of Sodium Phosphate and Cellulose Ethers on MgO/SiO2 Cements for the 3D Printing of Forsterite Bioceramics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Cement Formulations
2.3. Characterization of MgO/SiO2 Cements
2.3.1. Fluidity and Hardening
2.3.2. Gillmore Test
2.3.3. Printability of the Cement Pastes
2.3.4. X-ray Diffraction
2.4. Preparation of the Bioceramics
2.5. Characterization of the Bioceramics
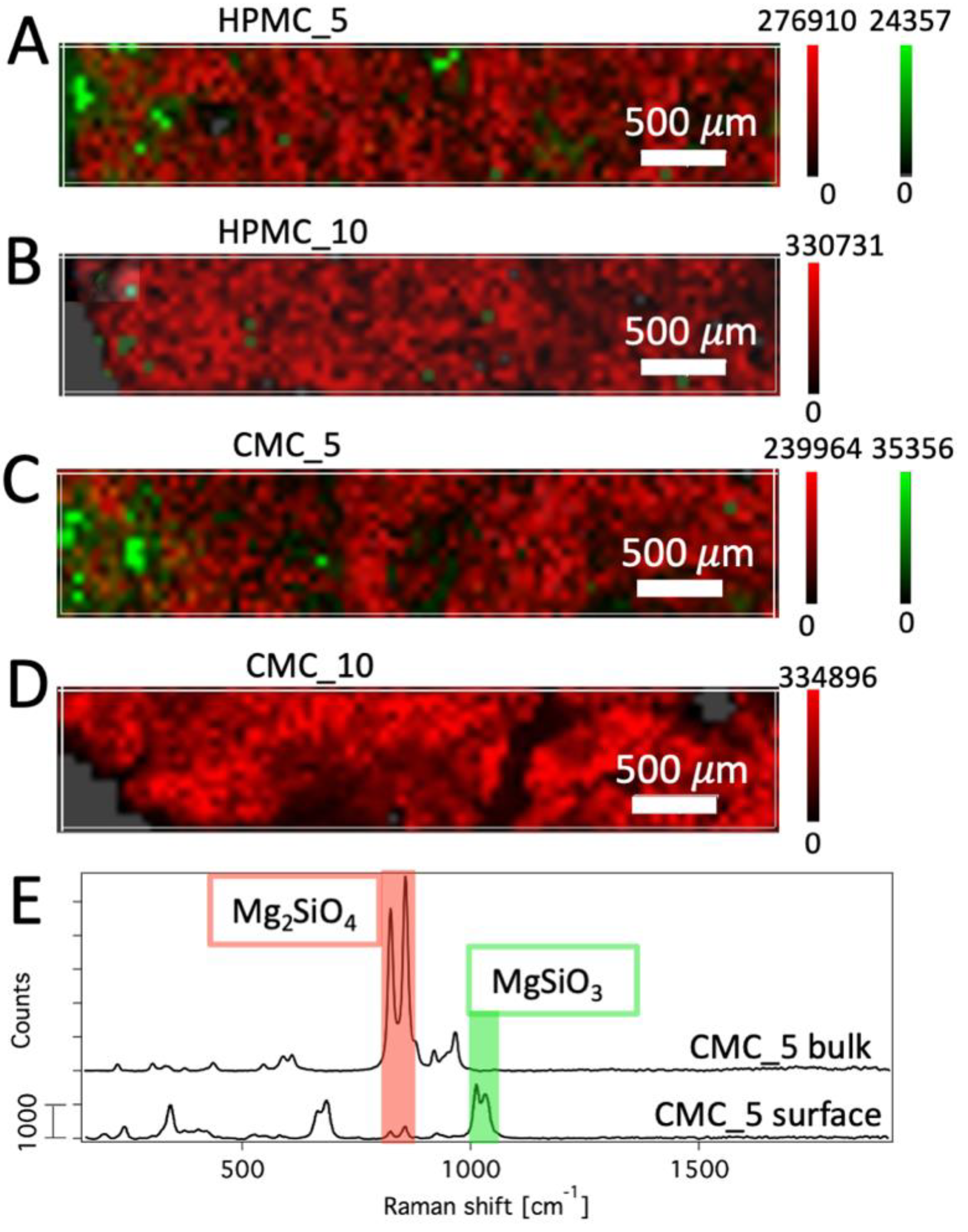
2.5.1. Confocal Raman Microscopy
2.5.2. Field Emission-Scanning Electron Microscopy (FE-SEM)
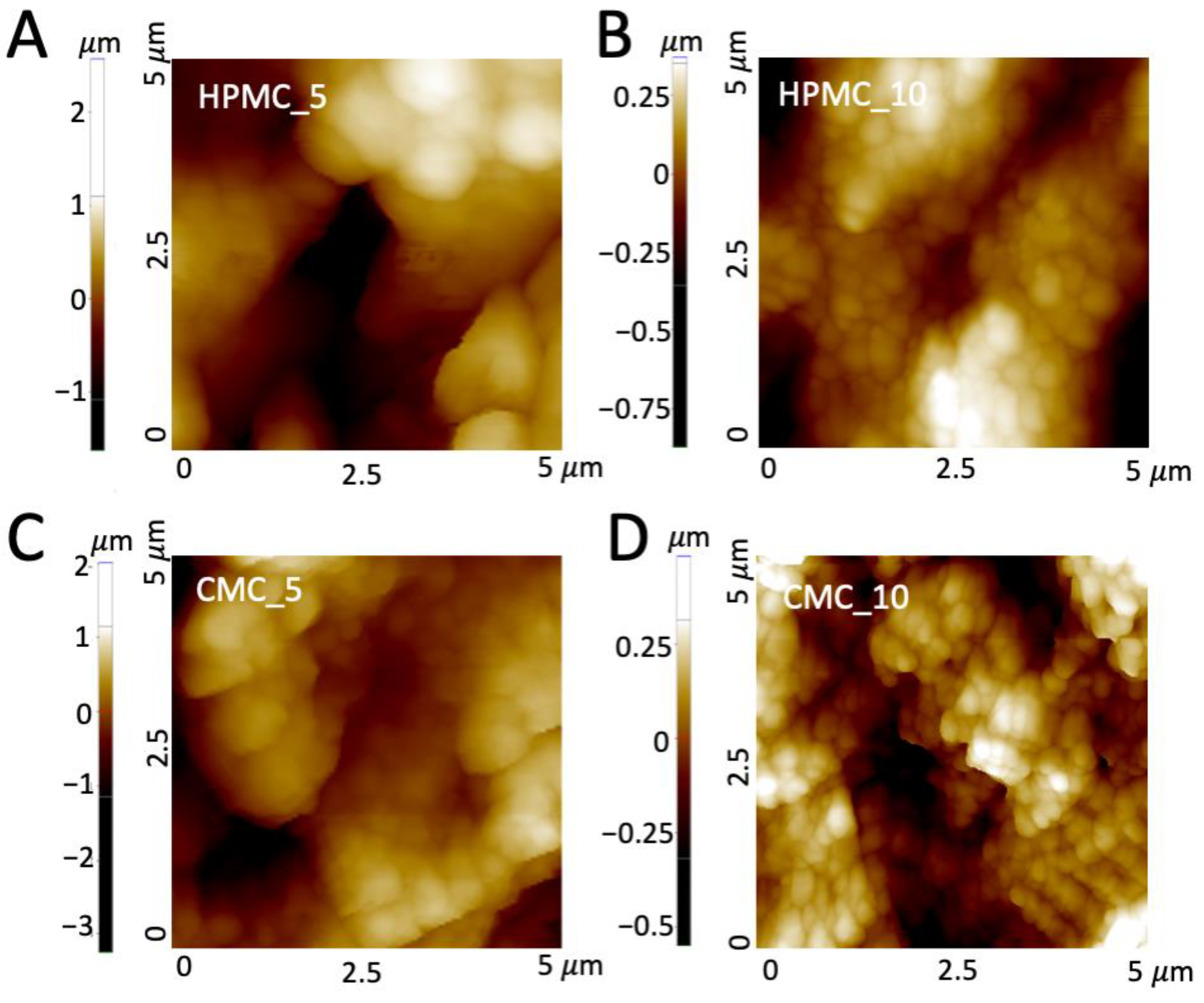
2.5.3. Atomic Force Microscopy (AFM)
2.5.4. Gas Porosimetry
2.5.5. Compressive Strength Test
3. Results and Discussion
3.1. Characterization of the Cement Pastes
3.1.1. Preliminary Experiments: Selection of the Formulations
3.1.2. Printability and Setting Properties
3.2. Characterization of the 3D-Printed Bioceramics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tonelli, M.; Faralli, A.; Ridi, F.; Bonini, M. 3D Printable Magnesium-Based Cements towards the Preparation of Bioceramics. J. Colloid Interface Sci. 2021, 598, 24–35. [Google Scholar] [CrossRef]
- Nabiyouni, M.; Brückner, T.; Zhou, H.; Gbureck, U.; Bhaduri, S.B. Magnesium-Based Bioceramics in Orthopedic Applications. Acta Biomater. 2018, 66, 23–43. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Ma, L.; Zhang, B.; Sun, J.; Sun, Y.; Fan, Y.; Gou, Z.; Zhou, C.; Zhang, X. Creating Hierarchical Porosity Hydroxyapatite Scaffolds with Osteoinduction by Three-Dimensional Printing and Microwave Sintering. Biofabrication 2017, 9, 45008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Sun, H.; Wu, L.; Ma, L.; Xing, F.; Kong, Q.; Fan, Y.; Zhou, C.; Zhang, X. 3D Printing of Calcium Phosphate Bioceramic with Tailored Biodegradation Rate for Skull Bone Tissue Reconstruction. Bio-Des. Manuf. 2019, 2, 161–171. [Google Scholar] [CrossRef]
- Leng, B.; Jin, X.; Lin, Q.; Chen, L.; Wang, Y.; Du, Z.; Lin, K.; Chang, J.; Gu, X.; Wang, C. A Comparative Study of Proliferation and Osteogenic Differentiation of Rat Adipose-Derived Stem Cells in β-Tricalcium Phosphate (β-TCP), Forsterite (Mg2SiO4) and Clinoenstatite (MgSiO3). Chin. Sci. Bull. 2013, 58, 3033–3042. [Google Scholar] [CrossRef]
- Feng, X.; Wu, Y.; Bao, F.; Chen, X.; Gong, J. Comparison of 3D-Printed Mesoporous Calcium Silicate/Polycaprolactone and Mesoporous Bioacive Glass/Polycaprolactone Scaffolds for Bone Regeneration. Microporous Mesoporous Mater. 2019, 278, 348–353. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Ma, B.; Zhu, H.; Huan, Z.; Ma, N.; Wu, C.; Chang, J. 3D-Printed Bioactive Ca 3 SiO 5 Bone Cement Scaffolds with Nano Surface Structure for Bone Regeneration. ACS Appl. Mater. Interfaces 2017, 9, 5757–5767. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chang, J. A Review of Bioactive Silicate Ceramics. Biomed. Mater. 2013, 8, 032001. [Google Scholar] [CrossRef]
- Apelt, D.; Theiss, F.; El-Warrak, A.O.; Zlinszky, K.; Bettschart-Wolfisberger, R.; Bohner, M.; Matter, S.; Auer, J.A.; von Rechenberg, B. In Vivo Behavior of Three Different Injectable Hydraulic Calcium Phosphate Cements. Biomaterials 2004, 25, 1439–1451. [Google Scholar] [CrossRef]
- Venkatraman, S.K.; Swamiappan, S. Review on Calcium- and Magnesium-Based Silicates for Bone Tissue Engineering Applications. J. Biomed. Mater. Res. Part A 2020, 108, 1546–1562. [Google Scholar] [CrossRef]
- Devi, K.B.; Tripathy, B.; Kumta, P.N.; Nandi, S.K.; Roy, M. In Vivo Biocompatibility of Zinc-Doped Magnesium Silicate Bio-Ceramics. ACS Biomater. Sci. Eng. 2018, 4, 2126–2133. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, Z.; Wu, Q.; Yi, D.; Friis, T.; Zheng, X.; Chang, J.; Jiang, X.; Xiao, Y. Clinoenstatite Coatings Have High Bonding Strength, Bioactive Ion Release, and Osteoimmunomodulatory Effects That Enhance in Vivo Osseointegration. Biomaterials 2015, 71, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Bakhsheshi-Rad, H.R.; Najafinezhad, A.; Hadisi, Z.; Iqbal, N.; Daroonparvar, M.; Sharif, S.; Ismail, A.F.; Akbari, M.; RamaKrishna, S.; Berto, F. Characterization and Biological Properties of Nanostructured Clinoenstatite Scaffolds for Bone Tissue Engineering Applications. Mater. Chem. Phys. 2021, 259, 123969. [Google Scholar] [CrossRef]
- Choudhary, R.; Chatterjee, A.; Venkatraman, S.K.; Koppala, S.; Abraham, J.; Swamiappan, S. Antibacterial Forsterite (Mg2SiO4) Scaffold: A Promising Bioceramic for Load Bearing Applications. Bioact. Mater. 2018, 3, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Ghomi, H.; Jaberzadeh, M.; Fathi, M.H. Novel Fabrication of Forsterite Scaffold with Improved Mechanical Properties. J. Alloys Compd. 2011, 509, L63–L68. [Google Scholar] [CrossRef]
- Jin, X.; Chang, J.; Zhai, W.; Lin, K. Preparation and Characterization of Clinoenstatite Bioceramics. J. Am. Ceram. Soc. 2011, 94, 66–70. [Google Scholar] [CrossRef]
- Kharaziha, M.; Fathi, M.H. Synthesis and Characterization of Bioactive Forsterite Nanopowder. Ceram. Int. 2009, 35, 2449–2454. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, L.; Ren, Z.; Gao, J.; Chen, X.; Liu, X.; Ge, T. A Novel and Green Preparation of Porous Forsterite Ceramics with Excellent Thermal Isolation Properties. Ceram. Int. 2019, 45, 2953–2961. [Google Scholar] [CrossRef]
- Chen, L.; Ye, G.; Wang, Q.; Blanpain, B.; Malfliet, A.; Guo, M. Low Temperature Synthesis of Forsterite from Hydromagnesite and Fumed Silica Mixture. Ceram. Int. 2015, 41, 2234–2239. [Google Scholar] [CrossRef]
- Vlasopoulos, N. Process for Producing Cement Binder Compositions Containing Magnesium. International Patent WO 2012/028471 A1, 8 March 2012. [Google Scholar]
- Jin, F.; Al-Tabbaa, A. Strength and Hydration Products of Reactive MgO–Silica Pastes. Cem. Concr. Compos. 2014, 52, 27–33. [Google Scholar] [CrossRef]
- Tonelli, M.; Martini, F.; Calucci, L.; Fratini, E.; Geppi, M.; Ridi, F.; Borsacchi, S.; Baglioni, P. Structural Characterization of Magnesium Silicate Hydrate: Towards the Design of Eco-Sustainable Cements. Dalton Trans. 2016, 45, 3294–3304. [Google Scholar] [CrossRef] [PubMed]
- Martini, F.; Tonelli, M.; Geppi, M.; Ridi, F.; Borsacchi, S.; Calucci, L. Hydration of MgO/SiO2 and Portland Cement Mixtures: A Structural Investigation of the Hydrated Phases by Means of X-Ray Diffraction and Solid State NMR Spectroscopy. Cem. Concr. Res. 2017, 102, 60–67. [Google Scholar] [CrossRef]
- Martini, F.; Borsacchi, S.; Geppi, M.; Tonelli, M.; Ridi, F.; Calucci, L. Monitoring the Hydration of MgO-Based Cement and Its Mixtures with Portland Cement by 1 H NMR Relaxometry. Microporous Mesoporous Mater. 2018, 269, 26–30. [Google Scholar] [CrossRef]
- Panda, B.; Sonat, C.; Yang, E.-H.; Tan, M.J.; Unluer, C. Use of Magnesium-Silicate-Hydrate (M-S-H) Cement Mixes in 3D Printing Applications. Cem. Concr. Compos. 2021, 117, 103901. [Google Scholar] [CrossRef]
- Khalil, A.; Wang, X.; Celik, K. 3D Printable Magnesium Oxide Concrete: Towards Sustainable Modern Architecture. Addit. Manuf. 2020, 33, 101145. [Google Scholar] [CrossRef]
- Bonini, M. Physico-Chemical Challenges in 3D Printing of Polymeric Nanocomposites and Hydrogels for Biomedical Applications. J. Nanosci. Nanotechnol. 2021, 21, 2778–2792. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Ruan, S.; Li, M.; Mo, L.; Unluer, C.; Tan, M.J.; Qian, S. Feasibility Study on Sustainable Magnesium Potassium Phosphate Cement Paste for 3D Printing. Constr. Build. Mater. 2019, 221, 595–603. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, M.; Xu, J.; Li, L.; Huang, Y.; Yang, L.; Zhao, P.; Lu, L. Mix Design and Rheological Properties of Magnesium Potassium Phosphate Cement Composites Based on the 3D Printing Extrusion System. Constr. Build. Mater. 2021, 284, 122797. [Google Scholar] [CrossRef]
- Götz, L.-M.; Holeczek, K.; Groll, J.; Jüngst, T.; Gbureck, U. Extrusion-Based 3D Printing of Calcium Magnesium Phosphate Cement Pastes for Degradable Bone Implants. Materials 2021, 14, 5197. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, M.; Jin, Y.; Lu, L.; Li, L. Rheology Control towards 3D Printed Magnesium Potassium Phosphate Cement Composites. Compos. Part B Eng. 2022, 239, 109963. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, X.; Sanjayan, J.; Zou, P.X.W.; Ding, Z.-K. A Feasibility Study on HPMC-Improved Sulphoaluminate Cement for 3D Printing. Materials 2018, 11, 2415. [Google Scholar] [CrossRef]
- Buswell, R.A.; Leal de Silva, W.R.; Jones, S.Z.; Dirrenberger, J. 3D Printing Using Concrete Extrusion: A Roadmap for Research. Cem. Concr. Res. 2018, 112, 37–49. [Google Scholar] [CrossRef]
- Hambach, M.; Rutzen, M.; Volkmer, D. Chapter 5—Properties of 3D-Printed Fiber-Reinforced Portland Cement Paste. In 3D Concrete Printing Technology; Sanjayan, J.G., Nazari, A., Nematollahi, B., Eds.; Butterworth-Heinemann: Oxford, UK, 2019; pp. 73–113. ISBN 978-0-12-815481-6. [Google Scholar]
- Shakor, P.; Sanjayan, J.; Nazari, A.; Nejadi, S. Modified 3D Printed Powder to Cement-Based Material and Mechanical Properties of Cement Scaffold Used in 3D Printing. Constr. Build. Mater. 2017, 138, 398–409. [Google Scholar] [CrossRef]
- Sonebi, M.; Amziane, S.; Perrot, A. Mechanical Behavior of 3D Printed Cement Materials. In 3D Printing of Concrete; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 101–124. ISBN 978-1-119-61075-5. [Google Scholar]
- Ogur, E.; Botti, R.; Bortolotti, M.; Colombo, P.; Vakifahmetoglu, C. Synthesis and Additive Manufacturing of Calcium Silicate Hydrate Scaffolds. J. Mater. Res. Technol. 2021, 11, 1142–1151. [Google Scholar] [CrossRef]
- Pei, P.; Wei, D.; Zhu, M.; Du, X.; Zhu, Y. The Effect of Calcium Sulfate Incorporation on Physiochemical and Biological Properties of 3D-Printed Mesoporous Calcium Silicate Cement Scaffolds. Microporous Mesoporous Mater. 2017, 241, 11–20. [Google Scholar] [CrossRef]
- Van Der Putten, J.; Deprez, M.; Cnudde, V.; De Schutter, G.; Van Tittelboom, K. Microstructural Characterization of 3D Printed Cementitious Materials. Materials 2019, 12, 2993. [Google Scholar] [CrossRef] [PubMed]
- Gauss, C.; Pickering, K.L.; Muthe, L.P. The Use of Cellulose in Bio-Derived Formulations for 3D/4D Printing: A Review. Compos. Part C Open Access 2021, 4, 100113. [Google Scholar] [CrossRef]
- Dai, L.; Cheng, T.; Duan, C.; Zhao, W.; Zhang, W.; Zou, X.; Aspler, J.; Ni, Y. 3D Printing Using Plant-Derived Cellulose and Its Derivatives: A Review. Carbohydr. Polym. 2019, 203, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Jiang, Y.; Ren, H.; Deng, S.; Sun, J.; Cheng, F.; Jing, J.; Chen, Y. 3D-Printed Carbon-Based Conformal Electromagnetic Interference Shielding Module for Integrated Electronics. Nano-Micro Lett. 2024, 16, 85. [Google Scholar] [CrossRef]
- Ridi, F.; Fratini, E.; Alfani, R.; Baglioni, P. Influence of Acrylic Superplasticizer and Cellulose-Ether on the Kinetics of Tricalcium Silicate Hydration Reaction. J. Colloid Interface Sci. 2013, 395, 68–74. [Google Scholar] [CrossRef]
- Long, W.-J.; Tao, J.-L.; Lin, C.; Gu, Y.; Mei, L.; Duan, H.-B.; Xing, F. Rheology and Buildability of Sustainable Cement-Based Composites Containing Micro-Crystalline Cellulose for 3D-Printing. J. Clean. Prod. 2019, 239, 118054. [Google Scholar] [CrossRef]
- Zaid, O.; El Ouni, M.H. Advancements in 3D Printing of Cementitious Materials: A Review of Mineral Additives, Properties, and Systematic Developments. Constr. Build. Mater. 2024, 427, 136254. [Google Scholar] [CrossRef]
- Muhammad Salman, N.; Ma, G.; Ijaz, N.; Wang, L. Importance and Potential of Cellulosic Materials and Derivatives in Extrusion-Based 3D Concrete Printing (3DCP): Prospects and Challenges. Constr. Build. Mater. 2021, 291, 123281. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.; Chen, Y.; Zhang, C.; Ma, L.; Deng, Z.; Chen, C.; Zhang, Y.; Pan, J.; Banthia, N. Influence of Hydroxypropyl Methylcellulose and Silica Fume on Stability, Rheological Properties, and Printability of 3D Printing Foam Concrete. Cem. Concr. Compos. 2021, 122, 104158. [Google Scholar] [CrossRef]
- Schmitt, M.; Weiss, P.; Bourges, X.; Amador del Valle, G.; Daculsi, G. Crystallization at the Polymer/Calcium-Phosphate Interface in a Sterilized Injectable Bone Substitute IBS. Biomaterials 2002, 23, 2789–2794. [Google Scholar] [CrossRef] [PubMed]
- Plank, J.; Sakai, E.; Miao, C.W.; Yu, C.; Hong, J.X. Chemical Admixtures—Chemistry, Applications and Their Impact on Concrete Microstructure and Durability. Cem. Concr. Res. 2015, 78, 81–99. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Yang, Y.; Shu, X.; Ran, Q. Effect of Side Chains in Block Polycarboxylate Superplasticizers on Early-Age Properties of Cement Paste. J. Therm. Anal. Calorim. 2018, 133, 1439–1446. [Google Scholar] [CrossRef]
- Benard, P.; Garrault, S.; Nonat, A.; Cau-dit-Coumes, C. Influence of Orthophosphate Ions on the Dissolution of Tricalcium Silicate. Cem. Concr. Res. 2008, 38, 1137–1141. [Google Scholar] [CrossRef]
- Tonelli, M.; Martini, F.; Milanesi, A.; Calucci, L.; Geppi, M.; Borsacchi, S.; Ridi, F. Effect of Phosphate Additives on the Hydration Process of Magnesium Silicate Cements. J. Therm. Anal. Calorim. 2019, 138, 3311–3321. [Google Scholar] [CrossRef]
- ASTM C266-21; Standard Test Method for Time of Setting of Hydraulic-Cement Paste by Gillmore Needles. American Society for Testing and Materials: West Conshohocken, PA, USA, 2021.
- Fu, Z.; Angeline, V.; Sun, W. Evaluation of Printing Parameters on 3D Extrusion Printing of Pluronic Hydrogels and Machine Learning Guided Parameter Recommendation. Int. J. Bioprint. 2021, 7, 434. [Google Scholar] [CrossRef]
- Sonat, C.; Dung, N.T.; Unluer, C. Performance and Microstructural Development of MgO–SiO2 Binders under Different Curing Conditions. Constr. Build. Mater. 2017, 154, 945–955. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Walling, S.A.; Kinoshita, H.; Bernal, S.A.; Collier, N.C.; Provis, J.L. Structure and Properties of Binder Gels Formed in the System Mg(OH)2–SiO2–H2O for Immobilisation of Magnox Sludge. Dalton Trans. 2015, 44, 8126–8137. [Google Scholar] [CrossRef]
- Tay, Y.W.D.; Qian, Y.; Tan, M.J. Printability Region for 3D Concrete Printing Using Slump and Slump Flow Test. Compos. Part B Eng. 2019, 174, 106968. [Google Scholar] [CrossRef]
- Papachristoforou, M.; Mitsopoulos, V.; Stefanidou, M. Evaluation of Workability Parameters in 3D Printing Concrete. Procedia Struct. Integr. 2018, 10, 155–162. [Google Scholar] [CrossRef]
- Gillispie, G.; Prim, P.; Copus, J.; Fisher, J.; Mikos, A.G.; Yoo, J.J.; Atala, A.; Lee, S.J. Assessment Methodologies for Extrusion-Based Bioink Printability. Biofabrication 2020, 12, 022003. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Naghieh, S.; Xu, C.; Wang, C.; Sun, W.; Chen, X. Printability in Extrusion Bioprinting. Biofabrication 2021, 13, 033001. [Google Scholar] [CrossRef]
- Ni, S.; Chou, L.; Chang, J. Preparation and Characterization of Forsterite (Mg2SiO4) Bioceramics. Ceram. Int. 2007, 33, 83–88. [Google Scholar] [CrossRef]
- Kumar, S.A. Eco-Friendly Nano-Hybrid Materials for Advanced Engineering Applications; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-77188-295-8. [Google Scholar]
- Lakshmi, R. Wollastonite/Forsterite Composite Scaffolds Offer Better Surface for Hydroxyapatite Formation. Bull. Mater. Sci. 2019, 42, 1–7. [Google Scholar] [CrossRef]
- Jin, S.; Zhou, J.; Zhao, X.; Sun, L. Quantitative Relationship between Pore Size Distribution and Compressive Strength of Cementitious Materials. Constr. Build. Mater. 2021, 273, 121727. [Google Scholar] [CrossRef]
- Gerhardt, L.-C.; Boccaccini, A.R. Bioactive Glass and Glass-Ceramic Scaffolds for Bone Tissue Engineering. Materials 2010, 3, 3867–3910. [Google Scholar] [CrossRef] [PubMed]





| Sample | Printing Speed [mm/s] | Rp [mm] | Extrudability |
|---|---|---|---|
| HPMC_5 | 2.5 | 0.44 | Continuous and controllable |
| 5 | 0.35 | Continuous and controllable | |
| 7.5 | 0.43 | Continuous and controllable | |
| 10 | 0.49 | Discontinuous | |
| HPMC_10 | 2.5 | 0.37 | Continuous and controllable |
| 5 | 0.36 | Discontinuous | |
| 7.5 | 0.47 | Discontinuous | |
| 10 | 0.36 | Discontinuous | |
| CMC_5 | 2.5 | 0.19 | Discontinuous |
| 5 | 0.18 | Continuous and controllable | |
| 7.5 | 0.47 | Discontinuous | |
| 10 | 0.23 | Discontinuous | |
| CMC_10 | 2.5 | 0.18 | Continuous and controllable |
| 5 | 0.15 | Continuous and controllable | |
| 7.5 | 0.50 | Discontinuous | |
| 10 | 0.29 | Discontinuous |
| Sample | Ra (nm) | SSA (m2/g) | Total Pore Volume (mL/g) | Compressive Strength (MPa) |
|---|---|---|---|---|
| HPMC_5 | 464 ± 152 | 5.6 | 0.035 | 0.55 ± 0.16 |
| HPMC_10 | 212 ± 67 | 3.7 | 0.017 | 0.51 ± 0.11 |
| CMC_5 | 360 ± 72 | 3.5 | 0.016 | 0.40 ± 0.06 |
| CMC_10 | 180 ± 94 | 2.1 | 0.013 | 0.38 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheli, L.; Bonini, M.; Tonelli, M. Effect of Sodium Phosphate and Cellulose Ethers on MgO/SiO2 Cements for the 3D Printing of Forsterite Bioceramics. Appl. Sci. 2024, 14, 4410. https://doi.org/10.3390/app14114410
Cheli L, Bonini M, Tonelli M. Effect of Sodium Phosphate and Cellulose Ethers on MgO/SiO2 Cements for the 3D Printing of Forsterite Bioceramics. Applied Sciences. 2024; 14(11):4410. https://doi.org/10.3390/app14114410
Chicago/Turabian StyleCheli, Lorenzo, Massimo Bonini, and Monica Tonelli. 2024. "Effect of Sodium Phosphate and Cellulose Ethers on MgO/SiO2 Cements for the 3D Printing of Forsterite Bioceramics" Applied Sciences 14, no. 11: 4410. https://doi.org/10.3390/app14114410





