Analyzing Temperature Distributions and Gradient Behaviors for Early-Stage Tumor Lesions in 3D Computational Model of Breast
Abstract
:1. Introduction
1.1. Theoretical Framework
1.2. Angiogenesis and Glycolysis, Processes Related to Temperature Elevations
1.3. Tissue Thermometry
2. Methodology
2.1. Heat Transfer and Bioheating Equation
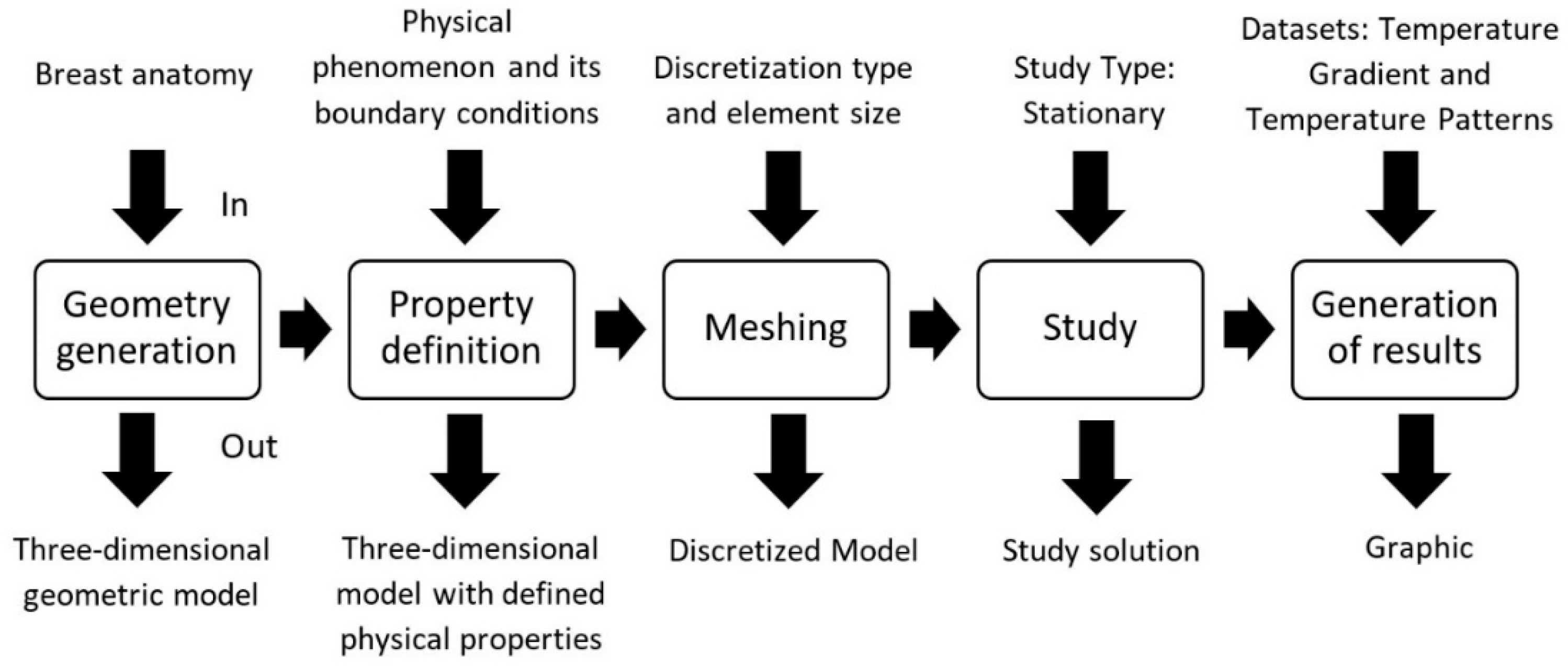
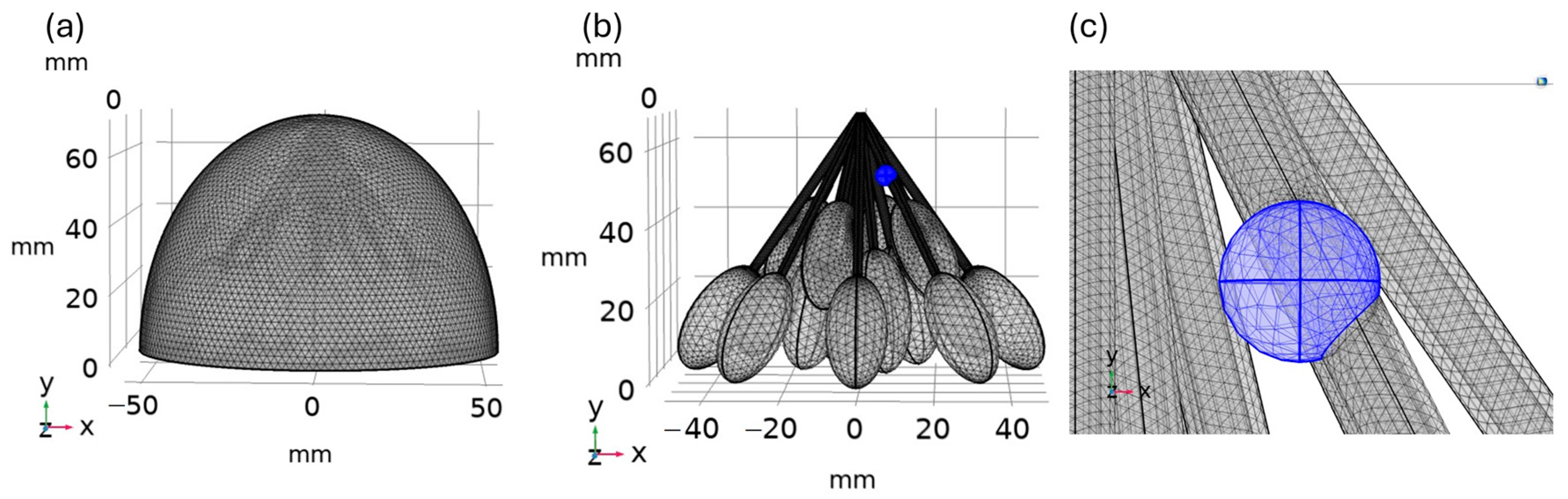
2.2. Finite Element Method and the Computational Modelling
2.3. Simulation Properties
2.4. Case Study: Stationary for 5, 6, and 8 mm Tumors and Healthy Tissue
3. Results
3.1. Results from Internal Analysis for Breast with Tumors and Healthy Tissue
3.1.1. Vertical Internal Path
3.1.2. Horizontal Internal Path
3.2. Results from Superficial Temperatures for Breast with Tumors and Healthy Tissue
4. Discussion
4.1. Modelling Constrains
- (a)
- The assumed model geometry is based on a 3D semi-ellipse shape that is commonly used in this kind of research. The actual anatomy of the breast deviates from this ideal model, which could constrain the internal and superficial thermal distributions presented here. In a real case, the subject position could be adapted in such a way as to obtain a symmetrical configuration, e.g., in a prone position with free breasts.
- (b)
- Considering the internal anatomy of the mammary gland as a cluster of lobes connected by ducts to the nipple produced a certain symmetry in terms of geometry that could idealize the results.
- (c)
- Different breast components were interfaced without considering the connective tissue between them; as a consequence, the effect of this was not considered in assessing temperature behavior at tissues boundaries.
- (d)
- All tumors were considered at the same depth and spherical geometries to evaluate thermal profiles under the same boundary conditions.
- (e)
- Environment conditions were considered stable, which means that no changes in external temperature were considered.
- (f)
- No temperature changes during the menstrual cycle were considered.
- (g)
- Natural body movements (e.g., breathing), and how they could affect the acquisition of an internal and external thermograms, were not considered.
- (h)
- No extensive clinical data were considered.
4.2. Technical and Economical Perspective
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Available online: https://www.who.int/news/item/03-02-2023-who-launches-new-roadmap-on-breast-cancer (accessed on 8 October 2023).
- Mann, R.M.; Hooley, R.; Barr, R.G.; Moy, L. Novel Approaches to Screening for Breast Cancer. Radiology 2020, 297, 266–285. [Google Scholar] [CrossRef] [PubMed]
- Hooley, R.J.; Scoutt, L.M.; Philpotts, L.E. Breast ultrasonography: State of the art. Radiology 2013, 268, 642–659. [Google Scholar] [CrossRef] [PubMed]
- Heywang-Köbrunner, S.H.; Hacker, A.; Sedlacek, S. Advantages and Disadvantages of Mammography Screening. Breast Care 2011, 6, 199–207. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dibden, A.; Offman, J.; Duffy, S.W.; Gabe, R. Worldwide Review and Meta-Analysis of Cohort Studies Measuring the Effect of Mammography Screening Programmes on Incidence-Based Breast Cancer Mortality. Cancers 2020, 12, 976. [Google Scholar] [CrossRef]
- US Preventive Services Task Force. Screening for Breast Cancer: US Preventive Services Task Force Recommendation Statement. JAMA, 2024; published online. [Google Scholar] [CrossRef]
- Marinovich, M.L.; Hunter, K.E.; Macaskill, P.; Houssami, N. Breast Cancer Screening Using Tomosynthesis or Mammography: A Meta-analysis of Cancer Detection and Recall. J. Natl. Cancer Inst. 2018, 110, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Chalabi, N.A.M.; AbuElMaati, A.A.; Elsadawy, M.E.I. Contrast-enhanced spectral mammography: Successful initial clinical institute experience. Egypt. J. Radiol. Nucl. Med. 2021, 52, 183. [Google Scholar] [CrossRef]
- Gonzalez-Hernandez, J.-L.; Recinella, A.N.; Kandlikar, S.G.; Dabydeen, D.; Medeiros, L.; Phatak, P. Technology, application and potential of dynamic breast thermography for the detection of breast cancer. Int. J. Heat. Mass. Transf. 2018, 131, 558–573. [Google Scholar] [CrossRef]
- Shrestha, S.; Kc, G.; Gurung, D.B. Transient Bioheat Equation in Breast Tissue: Effect of Tumor Size and Location. J. Adv. Appl. Math. 2020, 5, 9–19. [Google Scholar] [CrossRef]
- Tello-Mijares, S.; Woo, F.; Flores, F. Breast Cancer Identification via Thermography Image Segmentation with a Gradient Vector Flow and a Convolutional Neural Network. J. Health Eng. 2019, 2019, 9807619. [Google Scholar] [CrossRef]
- Ohashi, Y.; Uchida, I. Applying dynamic thermography in the diagnosis of breast cancer. IEEE Eng. Med. Biol. Mag. 2000, 19, 42–51. [Google Scholar] [CrossRef]
- Lozano, A., 3rd; Hayes, J.C.; Compton, L.M.; Azarnoosh, J.; Hassanipour, F. Determining the thermal characteristics of breast cancer based on high-resolution infrared imaging, 3D breast scans, and magnetic resonance imaging. Sci. Rep. 2020, 10, 10105. [Google Scholar] [CrossRef]
- Abbaci, M.; Conversano, A.; De Leeuw, F.; Laplace-Builhé, C.; Mazouni, C. Near-infrared fluorescence imaging for the prevention and management of breast cancer-related lymphedema: A systematic review. Eur. J. Surg. Oncol. 2019, 45, 1778–1786. [Google Scholar] [CrossRef] [PubMed]
- Mashekova, A.; Zhao, Y.; Ng, E.Y.; Zarikas, V.; Fok, S.C.; Mukhmetov, O. Early detection of the breast cancer using infrared technology—A comprehensive review. Therm. Sci. Eng. Prog. 2022, 27, 101142. [Google Scholar] [CrossRef]
- Ring, E.F.J.; Ammer, K. Infrared thermal imaging in medicine. Physiol. Meas. 2012, 33, R33–R46. [Google Scholar] [CrossRef]
- Chanmugam, A.; Hatwar, R.; Herman, C. Thermal analysis of cancerous breast model. Int. Mech. Eng. Congr. Expo. 2012, 2012, 134–143. [Google Scholar] [CrossRef]
- Al Husaini, M.A.S.; Habaebi, M.H.; Suliman, F.M.; Islam, M.R.; Elsheikh, E.A.A.; Muhaisen, N.A. Influence of Tissue Thermophysical Characteristics and Situ-Cooling on the Detection of Breast Cancer. Appl. Sci. 2023, 13, 8752. [Google Scholar] [CrossRef]
- Ng, E.Y.K.; Sudharsan, N.M. An improved three-dimensional direct numerical modelling and thermal analysis of a female breast with tumour. Proc. Inst. Mech. Eng. Part. H J. Eng. Med. 2001, 215, 25–38. [Google Scholar] [CrossRef]
- Saniei, E.; Setayeshi, S.; Akbari, M.E.; Navid, M. Parameter estimation of breast tumour using dynamic neural network from thermal pattern. J. Adv. Res. 2016, 7, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; Mohammadi, F.A. Thermogram assessment for tumor parameter estimation considering the body geometry by thermography. Comput. Biol. Med. 2016, 76, 80–93. [Google Scholar] [CrossRef]
- D’Alessandro, G.; Tavakolian, P.; Sfarra, S. A Review of Techniques and Bio-Heat Transfer Models Supporting Infrared Thermal Imaging for Diagnosis of Malignancy. Appl. Sci. 2024, 14, 1603. [Google Scholar] [CrossRef]
- Makrariya, A. Two dimensional finite element model of temperature distribution in dermal tissues of human breast under different stages of development. In Proceedings of the International Conference on Interdisciplinary Research in Engineering, Management, Pharmacy and Science, 19–22 February 2015; Available online: https://www.researchgate.net/publication/280623090_TWO_DIMENSIONAL_FINITE_ELEMENT_MODEL_OF_TEMPERATURE_DISTRIBUTION_IN_DERMAL_TISSUES_OF_HUMAN_BREAST_UNDER_DIFFERENT_STAGES_OF_DEVELOPMENT (accessed on 8 October 2023).
- Osman, M.M.; Afify, E.M. Thermal Modeling of the Normal Woman’s Breast. J. Biomech. Eng. 1984, 106, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Kwok, J.; Krzyspiak, J. Thermal Imaging and Analysis for Breast Tumor Detection. In BEE453: Computer Aided Engineering: Applications to Biomedical Processes; Cornell University: Ithaca, NY, USA, 2007; p. 18. [Google Scholar]
- Bardati, F.; Iudicello, S. Modeling the visibility of breast malignancy by a microwave radiometer. IEEE Trans. Biomed. Eng. 2008, 55, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Roa, I. Basic Concepts in Tumor Angiogenesis. Int. J. Med. Surg. Sci. 2014, 1, 129–138. [Google Scholar] [CrossRef]
- Shahi, P.K.; del Castillo Rueda, A.; Manga, G.P. Angiogénesis neoplásica. An. Med. Interna 2008, 25, 366–369. [Google Scholar]
- Socarrás, V.S. Papel de la angiogénesis en el crecimiento tumoral. Rev. Cuba. Investig. Biomed. 2001, 20, 223–230. Available online: http://web.a.ebscohost.com.dibpxy.uaa.mx/ehost/pdfviewer/pdfviewer?vid=1&sid=c8157159-95fc-4c94-a971-ac5193bc69f4%40sessionmgr4008 (accessed on 13 November 2019).
- Gautherie, M. Thermopathology of Breast Cancer: Measurement and Analysis of in Vivo Temperature and Blood Flow. Ann. N. Y. Acad. Sci. 1980, 335, 383–415. [Google Scholar] [CrossRef]
- Folkman, J. Tumor Angiogenesis: Therapeutic Implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef]
- Pinto, J.; Zaharia, M.; Gómez, H. Angiogénesis: Desde la biología hasta la terapia blanco dirigida. Angiogenes. Biol. Target. Ther. 2015, 5, 61–70. Available online: http://web.a.ebscohost.com.dibpxy.uaa.mx/ehost/pdfviewer/pdfviewer?vid=1&sid=a0f318e6-82bf-4619-9ec8-9a3c497e8ca8%40sdc-v-sessmgr01 (accessed on 12 November 2019). (In Spanish).
- Martínez-Ezquerro, J.D.; Herrera, L.A.; Montalvo, L.A.H.; México, D.T. ANGIOGÉNESIS: VEGF/VEGFRs como Blancos Terapéuticos en el Tratamiento Contra el Cáncer. Cancerología 2006, 1, 83–96. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Baronzio, G.F.; Gramaglia, A.; Baronzio, A.; Freitas, I. Influence of Tumor Microenvironment on Thermoresponse: Biologic and clinical implications. In Hyperthermia in Cancer Treatment: A Primer; Springer: Boston, MA, USA, 2006; pp. 67–91. [Google Scholar]
- Hannon, G.; Tansi, F.L.; Hilger, I.; Prina-Mello, A. The Effects of Localized Heat on the Hallmarks of Cancer. Adv. Therap. 2021, 4, 2000267. [Google Scholar] [CrossRef]
- Vesnin, S.; Turnbull, A.K.; Dixon, J.M.; Goryanin, I. Modern microwave thermometry for breast cancer. J. Mol. Imaging Dyn. 2017, 7, 1000136. [Google Scholar] [CrossRef]
- Gautherie, M.; Gros, C.M. Breast thermography and cancer risk prediction. Cancer 1980, 45, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Goryanin, I.; Karbainov, S.; Shevelev, O.; Tarakanov, A.; Redpath, K.; Vesnin, S.; Ivanov, Y. Passive microwave radiometry in biomedical studies. Drug Discov. Today 2020, 25, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Jolesz, F.A. MRI-guided focused ultrasound surgery. Annu. Rev. Med. 2009, 60, 417–430. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rieke, V.; Butts Pauly, K. MR thermometry. J. Magn. Reson. Imaging 2008, 27, 376–390. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Winter, L.; Oberacker, E.; Paul, K.; Ji, Y.; Oezerdem, C.; Ghadjar, P.; Thieme, A.; Budach, V.; Wust, P.; Niendorf, T. Magnetic resonance thermometry: Methodology, pitfalls and practical solutions. Int. J. Hyperth. 2016, 32, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Odéen, H.; Parker, D.L. Magnetic resonance thermometry and its biological applications—Physical principles and practical considerations. Prog. Nucl. Magn. Reson. Spectrosc. 2019, 110, 34–61. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pennes, H.H. Analysis of Tissue and Arterial Blood Temperatures in the Resting Human Forearm. J. Appl. Physiol. 1948, 1, 93–122. [Google Scholar] [CrossRef]
- TChandrupatla, R.; Belegundu, A.D. Introduction to Finite Elements in Engineering, 4th ed.; Pearson Educación: Hoboken, NJ, USA, 2011. [Google Scholar]
- Acero, M.R.V.; Bazán, I.; Ramirez-García, A. Computational Model of Breast Tissue with a Lesion defined by a Thermal Gradient. In Proceedings of the 2021 Global Medical Engineering Physics Exchanges/Pan American Health Care Exchanges (GMEPE/PAHCE), Virtual, 15–20 March 2021; pp. 1–6. [Google Scholar] [CrossRef]
- Gautherie, M.; Quenneville, Y.; Gros, C.M. Metabolic Heat Production, Growth Rate and Prognosis of Early Breast Carcinomas. Biomedicine 1975, 22, 328–336. [Google Scholar]
- Comsol. Comsol. Comsol Multiphysics. In User Guide; Comsol: Burlington, MA, USA, 2012. [Google Scholar]
- Said Camilleri, J.; Farrugia, L.; Curto, S.; Rodrigues, D.B.; Farina, L.; Caruana Dingli, G.; Bonello, J.; Farhat, I.; Sammut, C.V. Review of Thermal and Physiological Properties of Human Breast Tissue. Sensors 2022, 22, 3894. [Google Scholar] [CrossRef] [PubMed]



| Domain | Property | |||
|---|---|---|---|---|
| Cp [J/(kg °C)] | K [W/(m °C)] | ρ [kg/m3] | Qm [W/m3] | |
| Fat | 2348 [48] | 0.21 [11,48] | 911 [48] | 400 [19] |
| Gland | 3475 [10] | 0.48 [19] | 1080 [19] | 700 [19] |
| Tumor | 3475 [10] | 0.48 [19] | 1080 [19] | VAR [20] |
| Dermis | 3475 [10] | 0.3140 [10] | 1050 [10] | 368.1 [17] |
| Epidermis | 3475 [10] | 0.2093 [10] | 1050 [10] | 0 [17] |
| Domain | Property | |||
|---|---|---|---|---|
| Ta [°C] | Cp,b [J/(kg °C)] | Wb [1/s] | ρb [kg/m3] | |
| Fat | 37 | 4200 | 0.0002 | 1060 |
| Gland | 37 | 4200 | 0.0006 | 1060 |
| Tumor | 37 | 4200 | 0.012 | 1060 |
| Dermis | 37 | 4200 | 0.0002 | 1200 |
| Epidermis | 37 | 4200 | 0 | 1200 |
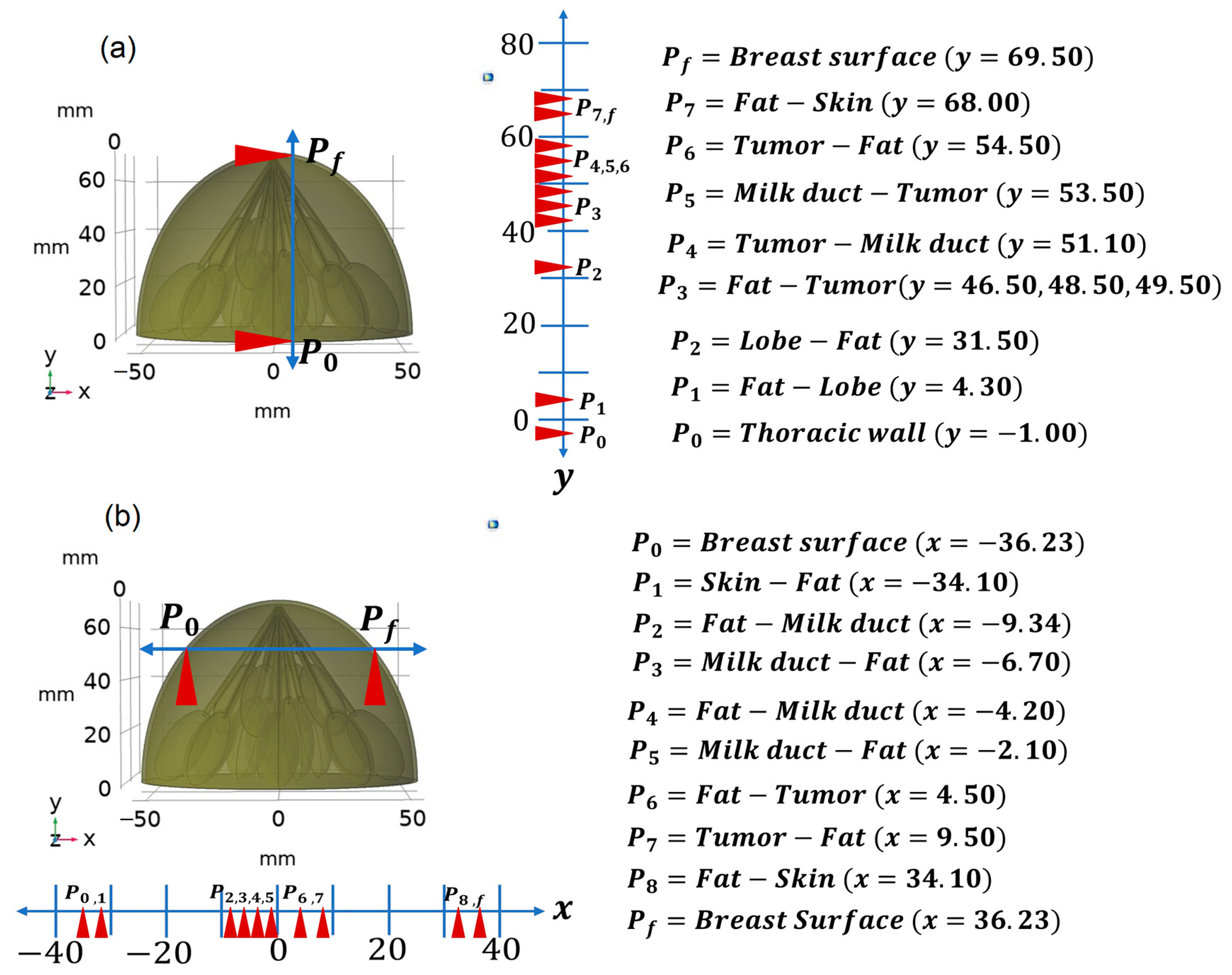
| Tumor Size (Diameter [mm]) | P0 [mm] | P1 [mm] | P2 [mm] | P3 [mm] | P4 [mm] | P5 [mm] | P6 [mm] | P7 [mm] | P8 [mm] | Pf [mm] |
|---|---|---|---|---|---|---|---|---|---|---|
| 5 | −36.23 | −34.10 | −9.34 | −6.76 | −4.2 | −2.14 | 4.5 | 9.5 | 34.1 | 36.23 |
| 6 | −36.66 | −34.54 | −9.62 | −6.88 | −4.56 | −2.14 | 4 | 10 | 34.54 | 36.66 |
| 8 | −37.47 | −35.40 | −10.05 | −7.25 | −4.76 | −2.06 | 3 | 11 | 35.40 | 37.48 |
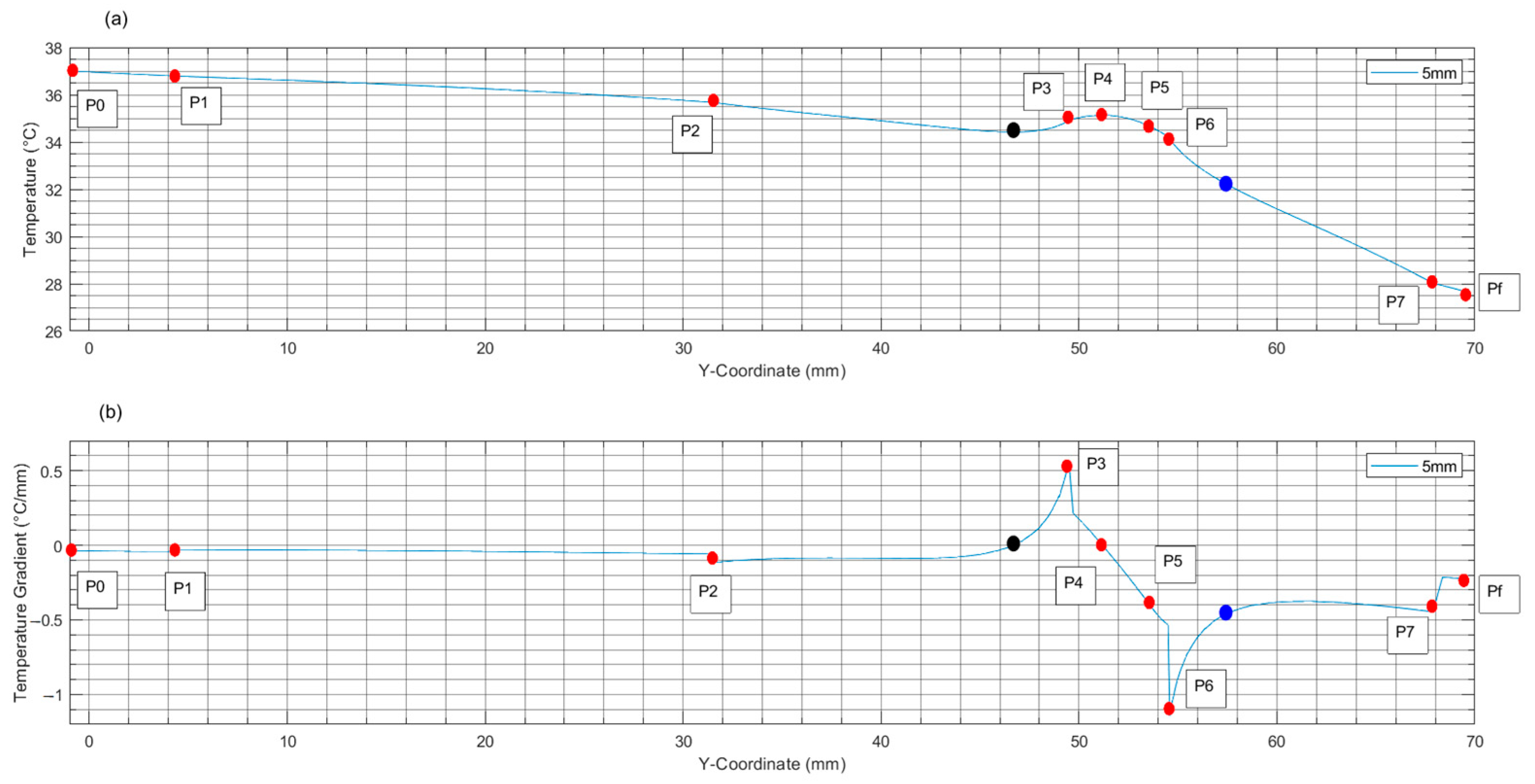
| T. D. [mm] | Healthy Tissue | Tumoral Zone | Healthy Tissue | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zone Warmed by Tumor Growth (ZWTG) | ||||||||||||
| P0 | P1 | P2 | (a) Black Point | P3 | P4 | P5 | P6 | (b) Blue Point | P7 | Pf | ||
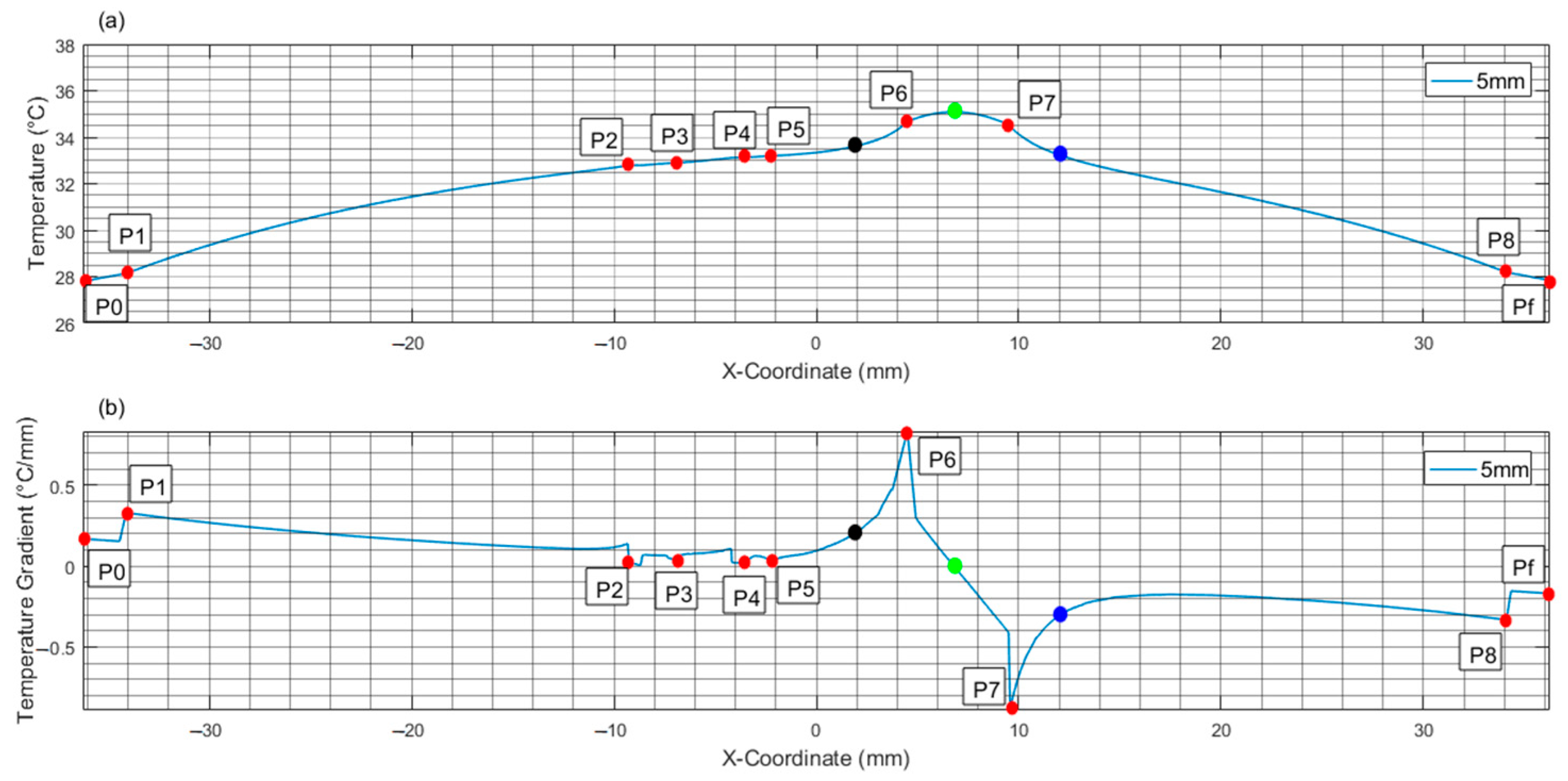
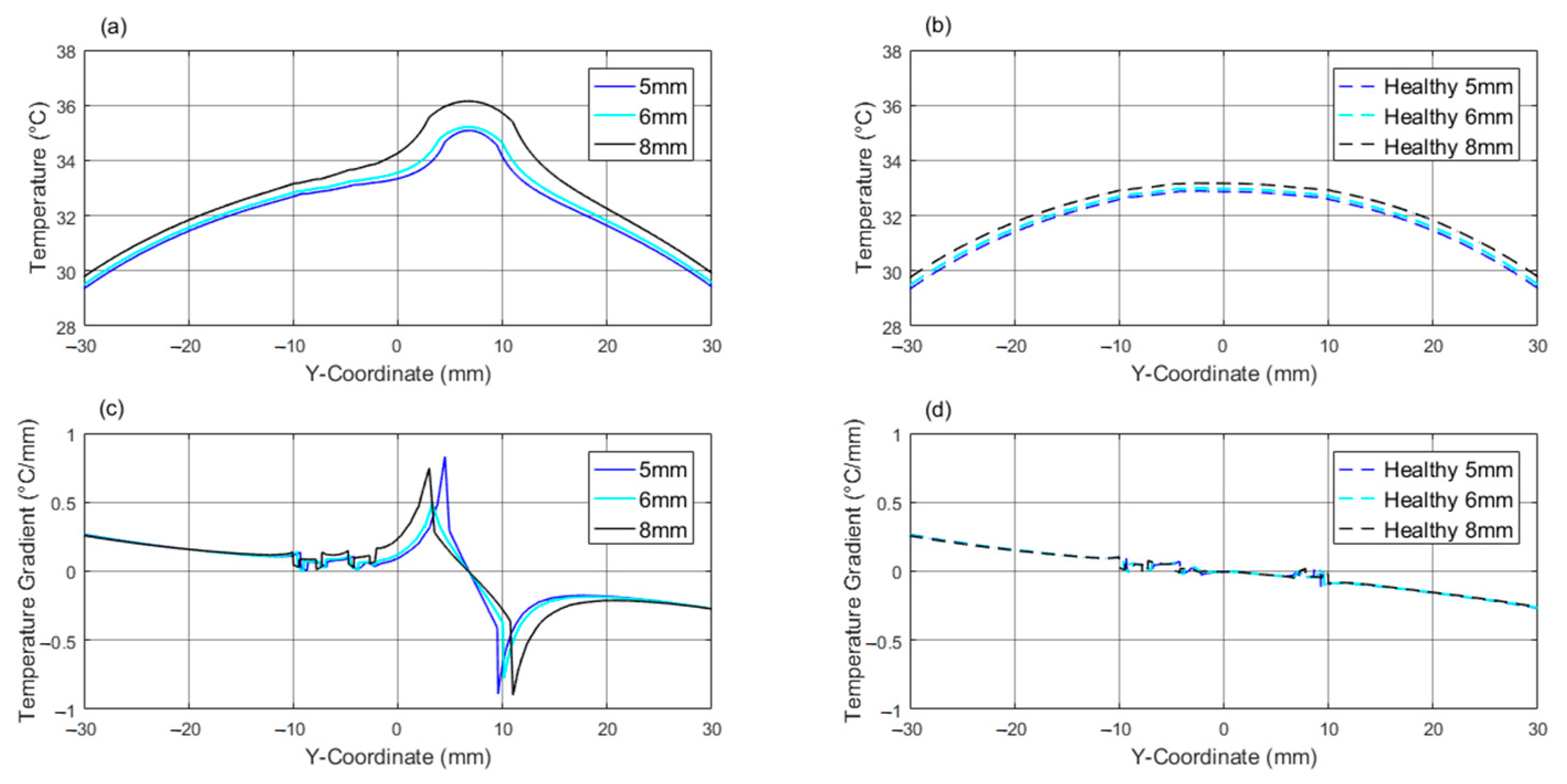
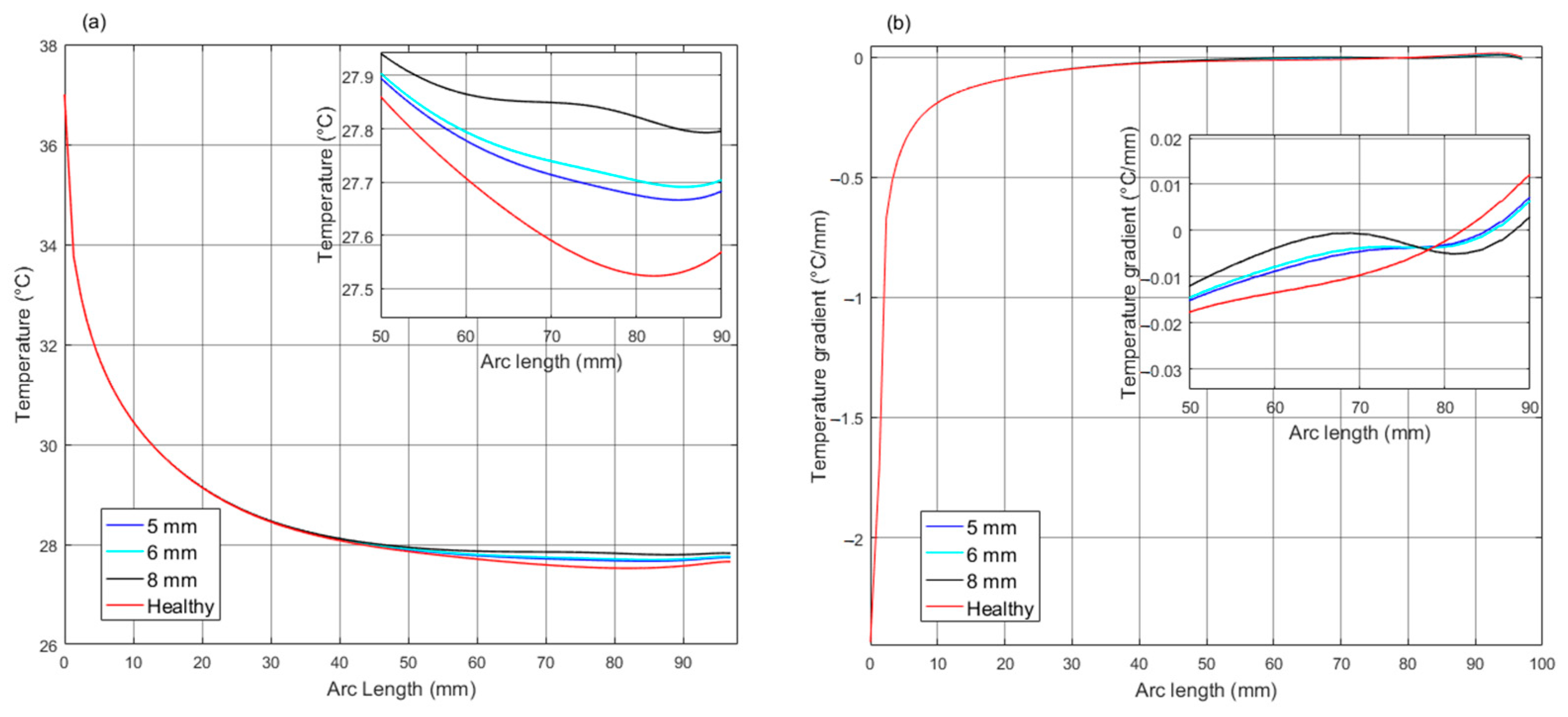
| 5 | T T | 37 −0.037 | 36.79 −0.029 | 35.68 −0.058 | 34.42 ≈0 | 34.92 0.547 | 35.13 ≈0 | 34.67 −0.399 | 34.12 −1.144 | 32.21 −0.459 | 28.03 −0.446 | 27.67 −0.224 |
| 6 | T T | 37.00 −0.029 | 36.79 −0.029 | 35.69 −0.057 | 34.64 ≈0 | 34.96 0.340 | 35.24 −0.085 | 34.69 −0.384 | 34.16 −1.035 | 32.26 −0.464 | 28.06 −0.448 | 27.69 −0.225 |
| 8 | T T | 37.00 −0.036 | 36.79 −0.028 | 35.76 −0.049 | 35.19 ≈0 | 35.73 0.375 | 36.06 −0.157 | 35.39 −0.413 | 34.84 −1.089 | 31.90 −0.457 | 28.17 −0.459 | 27.80 −0.229 |
| Healthy | T T | 37.00 −0.038 | 36.79 −0.029 | 35.63 −0.064 | -- | -- | 32.88 −0.174 | 32.45 −0.216 | -- | -- -- | 27.88 −0.431 | 27.53 −0.219 |
| T. D. [mm] | Healthy Tissue | Tumoral Zone | Healthy Tissue | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zone Warmed by Tumor Growth (ZWTG) | ||||||||||||||
| P0 | P1 | P2 | P3 | P4 | P5 | (a) Black Point | P6 | (b) Green Point | P7 | (c) Blue Point | P8 | Pf | ||
| 5 | T T | 27.79 0.167 | 28.13 0.328 | 32.79 0.016 | 32.9 0.030 | 33.13 0.025 | 33.20 0.034 | 33.62 0.199 | 34.67 0.831 | 35.08 ≈ 0 | 34.53 −0.890 | 33.22 −0.301 | 28.18 −0.333 | 27.84 −0.170 |
| H. E. C. | T T | 27.79 0.167 | 28.13 0.328 | 32.67 0.016 | 32.75 0.016 | 32.88 ≈0 | 32.9 −0.028 | -- -- | 32.8 −0.030 | 32.73 −0.011 | 32.65 −0.103 | -- -- | 28.15 −0.330 | 27.8 −0.167 |
| 6 | T T | 27.81 0.169 | 28.15 0.332 | 32.89 0.028 | 33.02 0.029 | 33.27 0.014 | 33.36 0.044 | 33.55 0.118 | 34.79 0.490 | 35.21 ≈ 0 | 34.63 −0.775 | 33.00 −0.239 | 28.20 −0.337 | 27.85 −0.172 |
| H. E. C. | T T | 27.80 0.169 | 28.14 0.331 | 32.75 0.016 | 32.84 0.011 | 32.97 −0.023 | 32.99 ≈0 | -- -- | 32.92 −0.021 | 32.83 −0.039 | 32.71 −0.092 | -- -- | 28.16 −0.333 | 27.82 −0.170 |
| 8 | T T | 27.84 0.173 | 28.18 0.340 | 33.16 0.044 | 33.33 0.034 | 33.67 0.023 | 33.86 0.070 | 34.04 0.180 | 35.56 0.749 | 36.15 ≈ 0 | 35.38 −0.898 | 33.4 −0.288 | 28.25 −0.347 | 27.90 −0.177 |
| H. E. C. | T T | 27.82 0.172 | 28.16 0.338 | 32.92 0.025 | 33.01 ≈0 | 33.15 −0.015 | 33.17 0.011 | -- -- | 33.14 −0.019 | 33.03 −0.046 | 32.84 −0.082 | -- -- | 28.18 −0.340 | 27.84 −0.172 |
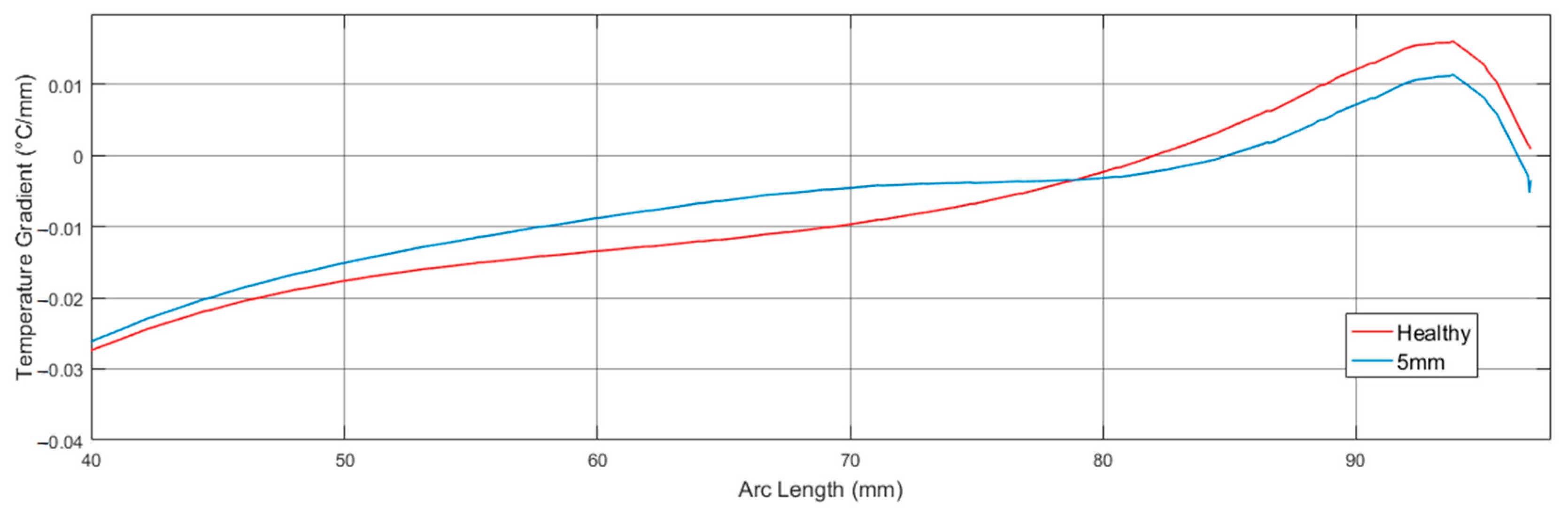
| Tumor Diameter | (A) Maximum Temperature Difference with Respect to Healthy Case | (B) Arc Length of Maximum Temperature Difference | (C) Arc Length of Zero Crossing in the Gradient Graph | (D) Arc Length of Intersection with Gradient of Healthy Case |
|---|---|---|---|---|
| (mm) | (°C) | (mm) | (mm) | (mm) |
| 5 | 0.15 | 78.88 | 84.92 | 78.88 |
| 6 | 0.18 | 78.64 | 85.55 | 78.64 |
| 8 | 0.30 | 78.03 | 88.15 | 78.03 |
| Healthy | -- | -- | 82.02 | -- |
| Tumor Diam. (mm) | Distances from Blue and Black Points to Tumor Boundaries (mm) | ZWTG (mm) |
|---|---|---|
| TS | Dv | Lv = TS + 2Dv |
| 5 | 2.78 | 10.56 |
| 6 | 3.03 | 12.06 |
| 8 | 4.53 | 17.06 |
| Tumor Diam. (mm) | Distances from Blue and Black Points to Tumor Boundaries (mm) | ZWTG (mm) |
|---|---|---|
| TS | Dh | Lh = TS + 2Dh |
| 5 | 2.52 | 10.04 |
| 6 | 3.38 | 12.76 |
| 8 | 4.10 | 16.20 |
| Tumor Diam. (mm) | Qm W/m3 | Gradient Peak Magnitude (°C/mm) | |||
|---|---|---|---|---|---|
| Vertical Path | Horizontal Path | ||||
| Posterior Boundary (P3V) | Anterior Boundary (P6V) | Right Boundary (P6H) | Left Boundary (P7H) | ||
| 5 | 120,800 | 0.547 | 1.144 | 0.831 | 0.890 |
| 6 | 60,830 | 0.340 | 1.035 | 0.490 | 0.775 |
| 8 | 68,450 | 0.375 | 1.089 | 0.749 | 0.898 |
| A. | B1. | B2. | B3. | C. | D. | E. | F. | G. | H. |
|---|---|---|---|---|---|---|---|---|---|
| Tumor Diam | Location of Tumor Projected on x Axis | Arc Length of Maximum Temp Diff | Projection of (C) on x Axis | Dist. from (D) to Center | Arc Length of Maximum Grad Diff | Projection of (F) on x Axis | Dist. from (G) to Center | ||
| Left Bound (P6H) | Right Bound (P7H) | Center | |||||||
| 5 | 4.50 | 9.5 | 7.00 | 78.88 | 3.15 | 3.85 | 67.62 | 6.40 | 0.60 |
| 6 | 4.00 | 10.00 | 7.00 | 78.64 | 3.25 | 3.75 | 66.51 | 6.80 | 0.20 |
| 8 | 3.00 | 11.00 | 7.00 | 78.03 | 3.35 | 3.65 | 64.67 | 7.50 | −0.50 |
| Tumor Diam. (mm) | Path | |||||
|---|---|---|---|---|---|---|
| Vertical | Horizontal | Superficial | ||||
| Temp Diff (°C) | Y Coord (mm) | Temp Diff (°C) | X Coord (mm) | Temp Diff (°C) | Arc Length (mm) | |
| 5 | 2.36 | 52.34 | 2.35 | 6.86 | 0.15 | 78.88 |
| 6 | 2.40 | 52.06 | 2.39 | 6.89 | 0.18 | 78.64 |
| 8 | 3.17 | 51.35 | 3.14 | 7.2 | 0.3 | 78.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acero Mendoza, R.V.; Bazán, I.; Ramírez-García, A. Analyzing Temperature Distributions and Gradient Behaviors for Early-Stage Tumor Lesions in 3D Computational Model of Breast. Appl. Sci. 2024, 14, 4538. https://doi.org/10.3390/app14114538
Acero Mendoza RV, Bazán I, Ramírez-García A. Analyzing Temperature Distributions and Gradient Behaviors for Early-Stage Tumor Lesions in 3D Computational Model of Breast. Applied Sciences. 2024; 14(11):4538. https://doi.org/10.3390/app14114538
Chicago/Turabian StyleAcero Mendoza, Ruth Valeria, Ivonne Bazán, and Alfredo Ramírez-García. 2024. "Analyzing Temperature Distributions and Gradient Behaviors for Early-Stage Tumor Lesions in 3D Computational Model of Breast" Applied Sciences 14, no. 11: 4538. https://doi.org/10.3390/app14114538






