Volatile Organic Compounds (VOCs) in Heritage Environments and Their Analysis: A Review
Abstract
1. Introduction
- VVOCs: very volatile organic compounds, boiling point < 100 °C.
- VOCs: volatile organic compounds, boiling point ≤ 250 °C.
- SVOCs: semi-volatile organic compounds, boiling point > 250 °C, up to 380–400 °C.
2. Volatiles in Heritage Environments
2.1. Volatiles Emitted from Heritage Objects
2.2. Volatiles Emitted by Conservation Treatments
2.3. Volatiles Emitted by People
3. Olfactory Exhibitions
4. Characterization of VOC Emissions
4.1. Sampling Methods
4.1.1. Sampling Setups
4.1.2. Sampling Devices
4.2. Analysis of Volatiles
5. Museum Guidelines
6. Conclusions
- -
- The emission rates of volatiles from objects are still poorly understood, especially of potentially harmful VOCs, e.g., organic acids. Quantitatively determined emission rates are essential to enable environmental managers to establish appropriate air exchange requirements in high-density storage areas or in enclosures.
- -
- The availability of sorbents for sampling of the diverse VOCs emitted from heritage objects is still limited, especially for organic acids. Their low emission rates require either long sampling times or elevated temperatures (e.g., for model materials), which is impractical. More research and development are needed to implement more effective sorbents for sampling.
- -
- There is a significant lack of data on the thresholds for VOC concentrations in indoor heritage environments from the viewpoint of preventive conservation. More research is needed into the assessments of the relative impact of VOCs on heritage materials, as compared to temperature and relative humidity.
- -
- The recent growth in popularity of olfactory exhibitions requires the development of a practical methodology for the assessment of the effect of smells intentionally introduced into exhibition spaces on the displayed objects, as well as the development of guidelines to ensure the safety of both collections and visitors.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grzywacz, C.M. Monitoring for Gaseous Pollutants in Museum Environments; Getty Publications: Los Angeles, CA, USA, 2006. [Google Scholar]
- Alvarez-Martin, A.; Wilcop, M.; Anderson, R.; Wendt, D.; Barden, R.; Kavich, G.M. Investigation of volatile organic compounds in museum storage areas. Air Qual. Atmos. Health 2021, 14, 1797–1809. [Google Scholar] [CrossRef]
- Cincinelli, A.; Martellini, T.; Amore, A.; Dei, L.; Marrazza, G.; Carretti, E.; Belosi, F.; Ravegnani, F.; Leva, P. Measurement of volatile organic compounds (VOCs) in libraries and archives in Florence (Italy). Sci. Total Environ. 2016, 572, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Menart, E.; de Bruin, G.; Strlič, M. Effects of NO2 and acetic acid on the stability of historic paper. Cellulose 2014, 21, 3701–3713. [Google Scholar] [CrossRef]
- Lattuati-Derieux, A.; Thao, S.; Langlois, J.; Regert, M. First results on headspace-solid phase microextraction-gas chromatography/mass spectrometry of volatile organic compounds emitted by wax objects in museums. J. Chromatogr. A 2008, 1187, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Chiantore, O.; Poli, T. Indoor air quality in museum display cases: Volatile emissions, materials contributions, impacts. Atmosphere 2021, 12, 364. [Google Scholar] [CrossRef]
- Tichenor, B. Final Report: Criteria for Evaluating Programs That Assess Materials/Products to Determine Impacts on Indoor Air Quality; Contractor Report (final) submitted to USEPA; U.S. Environmental Protection Agency Office of Radiation and Indoor Air Indoor Environments Division: Washington, DC, USA, 2006.
- Lundgren, B. Materials emission of chemicals-PVC flooring materials. Indoor Air 1999, 9, 202–208. [Google Scholar] [CrossRef]
- Sawoszczuk, T.; Syguła-Cholewińska, J.; del Hoyo-Meléndez, J.M. Optimization of headspace solid phase microextraction for the analysis of microbial volatile organic compounds emitted by fungi: Application to historical objects. J. Chromatogr. A 2015, 1409, 30–45. [Google Scholar] [CrossRef] [PubMed]
- Kearney, M.; Townsend, J.H.; Parkin, I.P.; Hidalgo, M.; Curran, K. Factors affecting the practicality of solid-phase microextraction VOC analysis of artworks featuring polymeric materials in open environments. Microchem. J. 2020, 155, 104711. [Google Scholar] [CrossRef]
- Lattuati-Derieux, A.; Bonnassies-Termes, S.; Lavédrine, B. Identification of volatile organic compounds emitted by a naturally aged book using solid-phase microextraction/gas chromatography/mass spectrometry. J. Chromatogr. A 2004, 1026, 9–18. [Google Scholar] [CrossRef]
- Joblin, Y.; Moularat, S.; Anton, R.; Bousta, F.; Orial, G.; Robine, E.; Picon, O.; Bourouina, T. Detection of moulds by volatile organic compounds: Application to heritage conservation. Int. Biodeterior. Biodegrad. 2010, 64, 210–217. [Google Scholar] [CrossRef]
- Korpi, A.; Pasanen, A.L.; Pasanen, P. Volatile Compounds Originating from Mixed Microbial cultures on building materials under various humidity conditions. Appl. Environ. Microbiol. 1998, 64, 2914–2919. [Google Scholar] [CrossRef] [PubMed]
- Strlič, M.; Thomas, J.; Trafela, T.; Cséfalvayová, L.; Cigić, I.K.; Kolar, J.; Cassar, M. Material degradomics: On the smell of old books. Anal. Chem. 2009, 81, 8617–8622. [Google Scholar] [CrossRef] [PubMed]
- Tétreault, J. Airborne Pollutants in Museums, Galleries and Archives: Risk Assessment, Control Strategies and Preservation Management; Canadian Conservation Institute: Ottawa, ON, Canada, 2003. [Google Scholar]
- Kumar, A.; Víden, I. Volatile organic compounds: Sampling methods and their worldwide profile in ambient air. Environ. Monit. Assess. 2007, 131, 301–321. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Misztal, P.K.; Nazaroff, W.W.; Goldstein, A.H. Siloxanes are the most abundant volatile organic compound emitted from engineering students in a classroom. Environ. Sci. Technol. Lett. 2015, 2, 303–307. [Google Scholar] [CrossRef]
- Weybrecht, G. A case of good pollution in museums. In The Future MBA; Routledge: London, UK, 2019; pp. 59–69. [Google Scholar] [CrossRef]
- Irga, P.J.; Mullen, G.; Fleck, R.; Matheson, S.; Wilkinson, S.J.; Torpy, F.R. Volatile organic compounds emitted by humans indoors–A review on the measurement, test conditions, and analysis techniques. Build. Environ. 2024, 255, 111442. [Google Scholar] [CrossRef]
- Fenech, A. Lifetime of Colour Photographs in Mixed Archival Collections. Ph.D. Thesis, University College London, London, UK, 2011. [Google Scholar]
- Krupińska, B.; Van Grieken, R.; De Wael, K. Air quality monitoring in a museum for preventive conservation: Results of a three-year study in the Plantin-Moretus Museum in Antwerp, Belgium. Microchem. J. 2013, 110, 350–360. [Google Scholar] [CrossRef]
- Gibson, L.T.; Watt, C.M. Acetic and formic acids emitted from wood samples and their effect on selected materials in museum environments. Corros. Sci. 2010, 52, 172–178. [Google Scholar] [CrossRef]
- Schieweck, A.; Salthammer, T. Emissions from construction and decoration materials for museum showcases. Stud. Conserv. 2009, 54, 218–235. [Google Scholar] [CrossRef]
- Lattuati-Derieux, A.; Bonnassies-Termes, S.; Lavédrine, B. Characterisation of compounds emitted during natural and artificial ageing of a book. Use of headspace-solid-phase microextraction/gas chromatography/mass spectrometry. J. Cult. Herit. 2006, 7, 123–133. [Google Scholar] [CrossRef]
- Clark, A.J.; Calvillo, J.L.; Roosa, M.S.; Green, D.B.; Ganske, J.A. Degradation product emission from historic and modern books by headspace SPME/GC-MS: Evaluation of lipid oxidation and cellulose hydrolysis. Anal. Bioanal. Chem. 2011, 399, 3589–3600. [Google Scholar] [CrossRef]
- Gibson, L.T.; Ewlad-Ahmed, A.; Knight, B.; Horie, V.; Mitchell, G.; Robertson, C.J. Measurement of volatile organic compounds emitted in libraries and archives: An inferential indicator of paper decay? Chem. Cent. J. 2012, 6, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.; Higgitt, C.; Gibson, L.T. Emissions from polymeric materials: Characterised by thermal desorption-gas chromatography. Polym. Degrad. Stab. 2014, 107, 328–340. [Google Scholar] [CrossRef]
- Curran, K.; Underhill, M.; Grau-Bové, J.; Fearn, T.; Gibson, L.T.; Strlič, M. Classifying Degraded Modern Polymeric Museum Artefacts by Their Smell. Angew. Chemie-Int. Ed. 2018, 57, 7336–7340. [Google Scholar] [CrossRef]
- Curran, K.; Možir, A.; Underhill, M.; Gibson, L.T.; Fearn, T.; Strlič, M. Cross-infection effect of polymers of historic and heritage significance on the degradation of a cellulose reference test material. Polym. Degrad. Stab. 2014, 107, 294–306. [Google Scholar] [CrossRef]
- Shashoua, Y.R. Effect of indoor climate on the rate and degradation mechanism of plasticized poly (vinyl chloride). Polym. Degrad. Stab. 2003, 81, 29–36. [Google Scholar] [CrossRef]
- Druzik, J.R.; Hatchfield, P.B. Pollutants in the Museum Environment: Practical Strategies for Problem Solving in Design, Exhibition and Storage. J. Am. Inst. Conserv. 2003, 42, 485. [Google Scholar] [CrossRef]
- Portoni, F. Protecting Objects from Themselves. 2018. Available online: https://blog.sciencemuseum.org.uk/protecting-objects-from-themselves/ (accessed on 25 April 2024).
- Schieweck, A.; Salthammer, T. Indoor air quality in passive-type museum showcases. J. Cult. Herit. 2011, 12, 205–213. [Google Scholar] [CrossRef]
- Bertheau, E.; Simon, V.; Raynaud, C.D. Emissions of Volatile Organic Compounds (VOCs) as Safety Indicators in the Development of Wood-Based Binderless Boards. Appl. Sci. 2024, 14, 1266. [Google Scholar] [CrossRef]
- Arni, P.C.; Cochrane, G.C.; Gray, J.D. The emission of corrosive vapours by wood. II. The analysis of the vapours emitted by certain freshly felled hardwoods and softwoods by gas chromato-graphy and spectrophotometry. J. Appl. Chem. 1965, 15, 463–468. [Google Scholar] [CrossRef]
- Simon, A.; Mobasher, B.; Neithalath, N. Post-Consumer Carpet Fibres in Concrete: Fibres Behavior in Alkaline Environments and Concrete Durability. Materials 2024, 17, 977. [Google Scholar] [CrossRef]
- Abbas, N.N.S.; Soma, S.; Philomena, M.B. Impact of Carpets on Indoor Air Quality. Appl. Sci. 2022, 12, 12989. [Google Scholar] [CrossRef]
- Lang, A.; Zimmer, J. (Eds.) Blessing and Curse–Biocides: Application, Analysis, Evaluation; German Historical Museum Foundation: Berlin, Germany, 2016. [Google Scholar]
- Goldberg, L. A history of pest control measures in the anthropology collections, national museum of natural history, smithsonian institution. J. Am. Inst. Conserv. 1996, 35, 23–43. [Google Scholar] [CrossRef]
- Portoni, F.; Grau-Bové, J.; Strlič, M. Application of a non-invasive, non-destructive technique to quantify naphthalene emission rates from museum objects. Herit. Sci. 2019, 7, 1–9. [Google Scholar] [CrossRef]
- Pool, M.; Nancy, O.; Huber, M.J. Identifying the Pesticides: Pesticides Names, Classification, and History of Use. Old Poisons N. Probl. A Mus. Resour. Manag. Contam. Cult. Mater. 2005, 2, 5–31. [Google Scholar]
- Glastrup, J. Insecticide analysis by gas chromatography in the stores of the danish national museum’s ethnographic collection. Stud. Conserv. 1987, 32, 59–64. [Google Scholar] [CrossRef]
- Rushworth, I.D.; Higgitt, C.; Smith, M.; Gibson, L.T. Non-invasive multiresidue screening methods for the determination of pesticides in heritage collections. Herit. Sci. 2014, 2, 3. [Google Scholar] [CrossRef]
- Kaczkowski, R.A.; Makos, K.A.; Hawks, C.; Hunt, M. Investigation of Residual Contamination Inside Storage Cabinets: Collection Care Benefits from an Industrial Hygiene Study. J. Am. Inst. Conserv. 2017, 56, 142–160. [Google Scholar] [CrossRef]
- Kraševec, I.; Nemeček, N.; Štamcar, M.L.; Cigić, I.K.; Prosen, H. Non-destructive detection of pentachlorophenol residues in historical wooden objects. Polymers 2021, 13, 1052. [Google Scholar] [CrossRef] [PubMed]
- Schieweck, A.; Delius, W.; Siwinski, N.; Vogtenrath, W.; Genning, C.; Salthammer, T. Occurrence of organic and inorganic biocides in the museum environment. Atmos. Environ. 2007, 41, 3266–3275. [Google Scholar] [CrossRef]
- Heald, S.; Madden, O. Investigations into naphthalene mitigation on museum objects. In Proceedings of the NATCC Preprints, 8th North American Textile Conservation Conference, Oaxaca, Mexico, 8–11 November 2001; pp. 291–297. [Google Scholar]
- Fenske, J.D.; Paulson, S.E. Human breath emissions of VOCs. J. Air Waste Manag. Assoc. 1999, 49, 594–598. [Google Scholar] [CrossRef]
- Pagonis, D.; Price, D.J.; Algrim, L.B.; Day, D.A.; Handschy, A.V.; Stark, H.; Miller, S.L.; de Gouw, J.A.; Jimenez, J.L.; Ziemann, P.J. Time-Resolved Measurements of Indoor Chemical Emissions, Deposition, and Reactions in a University Art Museum. Environ. Sci. Technol. 2019, 53, 4794–4802. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Tasoglou, A.; Huber, H.; Stevens, P.S.; Boor, B.E. Influence of Mechanical Ventilation Systems and Human Occupancy on Time-Resolved Source Rates of Volatile Skin Oil Ozonolysis Products in a LEED-Certified Office Building. Environ. Sci. Technol. 2021, 55, 16477–16488. [Google Scholar] [CrossRef] [PubMed]
- Stönner, C.; Edtbauer, A.; Williams, J. Real-world volatile organic compound emission rates from seated adults and children for use in indoor air studies. Indoor Air 2018, 28, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Misztal, P.K.; Nazaroff, W.W.; Goldstein, A.H. Volatile organic compound emissions from humans indoors. Environ. Sci. Technol. 2016, 50, 12686–12694. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ernle, L.; Bekö, G.; Wargocki, P.; Williams, J. Emission Rates of Volatile Organic Compounds from Humans. Environ. Sci. Technol. 2022, 56, 4838–4848. [Google Scholar] [CrossRef]
- Steinemann, A.C.; MacGregor, I.C.; Gordon, S.M.; Gallagher, L.G.; Davis, A.L.; Ribeiro, D.S.; Wallace, L.A. Fragranced consumer products: Chemicals emitted, ingredients unlisted. Environ. Impact Assess. Rev. 2011, 31, 328–333. [Google Scholar] [CrossRef]
- Bembibre, C. From Smelly Buildings to the Scented Past: An Overview of Olfactory Heritage. Front. Psychol. 2022, 12, 718287. [Google Scholar] [CrossRef]
- Jacobo, C.B. Smell of Heritage. Ph.D. Thesis, University College London, London, UK, 2020. [Google Scholar]
- Aggleton, J.P.; Waskett, L. The ability of odours to serve as state-dependent cues for real-world memories: Can Viking smells aid the recall of Viking experiences? Br. J. Psychol. 1999, 90, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Verbeek, C. In Search of Lost Scents: The Olfactory Dimension of Italian Futurism. Int. Yearb. Futur. Stud. 2020, 2020, 247–275. [Google Scholar] [CrossRef]
- Verbeek, C. Presenting Volatile Heritage: Two Case Studies on Olfactory Reconstructions in the Museum. Futur. Anterior J. Hist. Preserv. Hist. Theory Crit. 2016, 13, 33–42. [Google Scholar] [CrossRef]
- Fleeting-Scents in Colour. Available online: https://www.mauritshuis.nl/en/press-releases/smell-the-art-fleeting-scents-in-colour/ (accessed on 25 April 2024).
- The Essence of a Painting. An Olfactory Exhibition. Available online: https://www.museodelprado.es/en/whats-on/exhibition/the-essence-of-a-painting-an-olfactory-exhibition/07849a71-d9b0-faeb-94c1-689f2614f8d0 (accessed on 25 April 2024).
- Bartsch, J.; Uhde, E.; Salthammer, T. Analysis of odour compounds from scented consumer products using gas chromatography-mass spectrometry and gas chromatography-olfactometry. Anal. Chim. Acta 2016, 904, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Spectrometry, C.; Rastogi, S.C. Analysis of Fragrances in Cosmetics by Gas. J. High Resolut. Chromatogr. 1995, 18, 653–658. [Google Scholar]
- Winberry, E.A.; William, T.; Linda, F.; Norma, T.M.; Angela, C.; Barbara, P. Compendium of Methods for the Deterimnation of Air Pollutants in Indoor Air; Engineering-Science, Inc.: Cary, NC, USA, 1990. [Google Scholar]
- Spinelle, L.; Gerboles, M.; Kok, G.; Persijn, S.; Sauerwald, T. Review of portable and low-cost sensors for the ambient air monitoring of benzene and other volatile organic compounds. Sensors 2017, 17, 1520. [Google Scholar] [CrossRef] [PubMed]
- Randy, M. Measurement of Passive Uptake Rates for Volatile Organic Compounds on Commercial Thermal Desorption Tubes and the Effect of Ozone on Sampling. 2013. Available online: https://eta-publications.lbl.gov/sites/default/files/lbnl-6257e-measurement_of_passive_uptake_rates.pdf (accessed on 25 April 2024).
- Górecki, T.; Namienik, J. Passive sampling. TrAC-Trends Anal. Chem. 2002, 21, 276–291. [Google Scholar] [CrossRef]
- Pennequin-Cardinal, A.; Plaisance, H.; Locoge, N.; Ramalho, O.; Kirchner, S.; Galloo, J.C. Dependence on sampling rates of Radiello® diffusion sampler for BTEX measurements with the concentration level and exposure time. Talanta 2005, 65, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Pennequin-Cardinal, A.; Plaisance, H.; Locoge, N.; Ramalho, O.; Kirchner, S.; Galloo, J.C. Performances of the Radiello® diffusive sampler for BTEX measurements: Influence of environmental conditions and determination of modelled sampling rates. Atmos. Environ. 2005, 39, 2535–2544. [Google Scholar] [CrossRef]
- Plutowska, B.; Chmiel, T.; Dymerski, T.; Wardencki, W. A headspace solid-phase microextraction method development and its application in the determination of volatiles in honeys by gas chromatography. Food Chem. 2011, 126, 1288–1298. [Google Scholar] [CrossRef]
- Tholl, D.; Boland, W.; Hansel, A.; Loreto, F.; Röse, U.S.R.; Schnitzler, J.P. Practical approaches to plant volatile analysis. Plant J. 2006, 45, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Amenduni, A.; Brattoli, M.; De Gennaro, G.; Massari, F.; Palmisani, J.; Tutino, M. Chemical characterization of ODOR active volatile organic compounds emitted from perfumes by GC/MS-O. Environ. Eng. Manag. J. 2016, 15, 1963–1969. [Google Scholar] [CrossRef]
- Kaiser, R. Trapping, Investigation and Reconstitution of Flower Scents. In Perfumes: Art, Science and Technology; Springer: Dordrecht, The Netherlands, 1994; pp. 213–250. [Google Scholar] [CrossRef]
- Uhde, E.; Borgschulte, A.; Salthammer, T. Characterization of the field and laboratory emission cell-FLEC: Flow field and air velocities. Atmos. Environ. 1998, 32, 773–781. [Google Scholar] [CrossRef]
- Kim, K.W.; Kim, S.; Kim, H.J.; Park, J.C. Formaldehyde and TVOC emission behaviors according to finishing treatment with surface materials using 20L chamber and FLEC. J. Hazard. Mater. 2010, 177, 90–94. [Google Scholar] [CrossRef] [PubMed]
- BS EN ISO 16000-9:2006; Indoor Air—Part 9: Determination of the Emission of Volatile Organic Compounds from Building Products and Furnishing—Emission Test Chamber Method. ISO: Geneva, Switzerland, 2006.
- Ochiai, N.; Tsuji, A.; Nakamura, N.; Daishima, S.; Cardin, D.B. Stabilities of 58 volatile organic compounds in fused-silica-lined and SUMMA polished canisters under various humidified conditions. J. Environ. Monit. 2002, 4, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Villatoro, C.; Vera, L.; Gygax, H. Comparative study of odours present in twin fragrances by GC-sniffing-ToFMS. Chem. Eng. Trans. 2016, 54, 133–138. [Google Scholar] [CrossRef]
- Dincer, F.; Odabasi, M.; Muezzinoglu, A. Chemical characterization of odorous gases at a landfill site by gas chromatography-mass spectrometry. J. Chromatogr. A 2006, 1122, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Vallat, A.; Gu, H.; Dorn, S. How rainfall, relative humidity and temperature influence volatile emissions from apple trees in situ. Phytochemistry 2005, 66, 1540–1550. [Google Scholar] [CrossRef]
- Plutowska, B.; Wardencki, W. Application of gas chromatography-olfactometry (GC-O) in analysis and quality assessment of alcoholic beverages—A review. Food Chem. 2008, 107, 449–463. [Google Scholar] [CrossRef]
- Seisonen, S.; Kivima, E.; Vene, K. Characterisation of the aroma profiles of different honeys and corresponding flowers using solid-phase microextraction and gas chromatography-mass spectrometry/olfactometry. Food Chem. 2015, 169, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Alissandrakis, E.; Tarantilis, P.A.; Harizanis, P.C.; Polissiou, M. Evaluation of four isolation techniques for honey aroma compounds. J. Sci. Food Agric. 2005, 85, 91–97. [Google Scholar] [CrossRef]
- Piasenzotto, L.; Gracco, L.; Conte, L. Solid phase microextraction (SPME) applied to honey quality control. J. Sci. Food Agric. 2003, 83, 1037–1044. [Google Scholar] [CrossRef]
- Bembibre, C.; Strlič, M. Smell of heritage: A framework for the identification, analysis and archival of historic odours. Herit. Sci. 2017, 5, 2. [Google Scholar] [CrossRef]
- Kearney, M.; Parkin, I.; Townsend, J.H.; Hidalgo, M.; Curran, K. Characterisation of VOCs Surrounding Naum Gabo’s Construction in Space ‘Two Cones’, (Tate) by in situ SPME GC-MS Monitoring. Stud. Conserv. 2018, 63, 369–371. [Google Scholar] [CrossRef]
- Shashoua, Y. Inhibiting the Deterioration of Plasticized Poly (Vinyl Chloride): A Museum Perspective. Ph.D. Thesis, Technical University of Denmark, Department of Chemical and Biochemical Engineering, Kongens Lyngby, Denmark, 2001. [Google Scholar]
- Curran, K.; Underhill, M.; Gibson, L.T.; Strlic, M. The development of a SPME-GC/MS method for the analysis of VOC emissions from historic plastic and rubber materials. Microchem. J. 2016, 124, 909–918. [Google Scholar] [CrossRef]
- Thiébaut, B.; Lattuati-Derieux, A.; Hocevar, M.; Vilmont, L.B. Application of headspace SPME-GC-MS in characterisation of odorous volatile organic compounds emitted from magnetic tape coatings based on poly(urethane-ester) after natural and artificial ageing. Polym. Test. 2007, 26, 243–256. [Google Scholar] [CrossRef]
- Lattuati-Derieux, A.; Egasse, C.; Thao-Heu, S.; Balcar, N.; Barabant, G.; Lavédrine, B. What do plastics emit? HS-SPME-GC/MS analyses of new standard plastics and plastic objects in museum collections. J. Cult. Herit. 2013, 14, 238–247. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, J.Y.; Choi, Y.S.; Sung, J.M.; Jang, H.W. Comparison of Different Types of SPME Arrow Sorbents to Analyze Volatile Compounds in Cirsium setidens Nakai. Foods 2020, 9, 785. [Google Scholar] [CrossRef] [PubMed]
- Helin, A.; Rönkkö, T.; Parshintsev, J.; Hartonen, K.; Schilling, B.; Läubli, T.; Riekkola, M.L. Solid phase microextraction Arrow for the sampling of volatile amines in wastewater and atmosphere. J. Chromatogr. A 2015, 1426, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Šikuten, I.; Štambuk, P.; Konti, J.K.; Maleti, E. Optimization of SPME-Arrow-GC/MS Method for Determination of Free and Bound Volatile Organic Compounds from Grape Skins. Molecules 2021, 26, 7409. [Google Scholar] [CrossRef]
- Emmons, R.V.; Tajali, R.; Gionfriddo, E. Development, Optimization and Applications of Thin Film Solid Phase Microextraction (TF-SPME) Devices for Thermal Desorption: A Comprehensive Review. Separations 2019, 6, 39. [Google Scholar] [CrossRef]
- Rom, S.M.; Itziar, S.; Eva, P.P.; Garde-cerd, T. Analytica Chimica Acta Optimization of thin film-microextraction (TF-SPME) method in order to determine musts volatile compounds. Anal. Chim. Acta 2022, 1226, 340254. [Google Scholar]
- La Nasa, J.; Biale, G.; Modugno, F.; Ceccarini, A.; Giannarelli, S. Magic extraction: Solid-phase extraction and analytical pyrolysis to study polycyclic aromatic hydrocarbon and polychlorinated biphenyls in freshwater. Environ. Sci. Pollut. Res. 2022, 29, 64252–64258. [Google Scholar] [CrossRef]
- Online, V.A.; Bohlin, P.; Audy, O. Environmental Science Processes & Impacts Outdoor passive air monitoring of semi volatile organic compounds (SVOCs): A critical evaluation of performance and limitations of polyurethane. Environ. Sci. Process. Impacts 2014, 16, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Melymuk, L.; Nizzetto, P.B.; Harner, T.; White, K.B.; Wang, X.; Tominaga, M.Y.; He, J.; Li, J.; Ma, J.; Ma, W.-L.; et al. Global intercomparison of polyurethane foam passive air samplers evaluating sources of variability in SVOC measurements. Environ. Sci. Policy 2021, 125, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bidleman, T.F.; Melymuk, L. Forty-five Years of Foam: A Retrospective on Air Sampling with Polyurethane Foam. Bull. Environ. Contam. Toxicol. 2019, 102, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Lenka, S.; Petra, P. Environmental Science Processes & Impacts Evaluation and guidelines for using polyurethane chambers to assess semi-volatile organic. Environ. Sci. Process. Impacts 2014, 16, 2617–2626. [Google Scholar] [CrossRef]
- Schilling, B.; Kaiser, R.; Natsch, A.; Gautschi, M. Investigation of odors in the fragrance industry. Chemoecology 2010, 20, 135–147. [Google Scholar] [CrossRef]
- Canosa, E.; Norrehed, S. Strategies for Pollutant Monitoring in Museum Environments; Riksantikvarieämbetet: Stockholm, Sweden, 2019. [Google Scholar]
- da Silva, C.F.F.P.; Rana, C.; Maskell, D.; Dengel, A.; Ansell, M.P.; Ball, R.J. Influence of eco-materials on indoor air quality. Green Mater. 2016, 4, 72–80. [Google Scholar] [CrossRef]
- Ding, L.; Yang, Q.; Liu, J.; Lee, Z. Evaluating volatile organic compounds from Chinese traditional handmade paper by SPME-GC/MS. Herit. Sci. 2021, 9, 1–16. [Google Scholar] [CrossRef]
- Szulczynski, B.; Gebicki, J. Currently Commercially Available Chemical Sensors Employed for Detection of Volatile Organic Compounds in Outdoor and Indoor Air. Environments 2017, 4, 21. [Google Scholar] [CrossRef]
- Xu, W.; Cai, Y.; Gao, S.; Hou, S.; Yang, Y.; Duan, Y.; Fu, Q.; Chen, F.; Wu, J. New understanding of miniaturized VOCs monitoring device: PID-type sensors performance evaluations in ambient air. Sensors Actuators B Chem. 2021, 330, 129285. [Google Scholar] [CrossRef]
- Posudin, Y. Methods of Analysis of Volatile Organic Compounds. In Methods of Measuring Environmental Parameters; Wiley: New York, NY, USA, 2014; pp. 229–243. [Google Scholar] [CrossRef]
- Fenech, A.; Strlič, M.; Cigić, I.K.; Levart, A.; Gibson, L.T.; de Bruin, G.; Ntanos, K.; Kolar, J.; Cassar, M. Volatile aldehydes in libraries and archives. Atmos. Environ. 2010, 44, 2067–2073. [Google Scholar] [CrossRef]
- Grzywacz, C.M.; Tennent, N.H. Pollution monitoring in storage and display cabinets: Carbonyl pollutant levels in relation to artifact deterioration. Stud. Conserv. 1994, 39 (Suppl. S2), 164–170. [Google Scholar] [CrossRef]
- Gibson, L.T.; Cooksey, B.G.; Littlejohn, D. A diffusion tube sampler for the determination of acetic acid and formic acid vapours in museum cabinets. Anal. Chim. Acta 1997, 341, 11–19. [Google Scholar] [CrossRef]
- Kraševec, I.; Menart, E.; Strlič, M.; Cigić, I.K. Validation of passive samplers for monitoring of acetic and formic acid in museum environments. Herit. Sci. 2021, 9, 1–10. [Google Scholar] [CrossRef]
- Arena, E.; Guarrera, N.; Campisi, S.; Asmundo, C.N. Comparison of odour active compounds detected by gas-chromatography- olfactometry between hand-squeezed juices from different orange varieties. Food Chem. 2006, 98, 59–63. [Google Scholar] [CrossRef]
- Perestrelo, R.; Fernandes, A.; Albuquerque, F.F.; Marques, J.C.; Câmara, J.S. Analytical characterization of the aroma of Tinta Negra Mole red wine: Identification of the main odorants compounds. Anal. Chim. Acta 2006, 563, 154–164. [Google Scholar] [CrossRef]
- Chiantore, O.; Riedo, C.; Poli, T.; Cotrufo, G.; Hohenstatt, P. Risk Assessment and Preservative Measures for Volatile Organic Compounds in Museum Showcases. Stud. Conserv. 2018, 63, 58–63. [Google Scholar] [CrossRef]
- Alford, K.L.; Kumar, N. Pulmonary health effects of indoor volatile organic compounds—A meta-analysis. Int. J. Environ. Res. Public Health 2021, 18, 1578. [Google Scholar] [CrossRef]
- PAS 198:2012; Specification for Managing Environmental Conditions for Cultural Collections. British Standards Institution: London, UK, 2012.
- WHO guidelines for air quality. Indian Pediatr. 1998, 35, 812–815.
- De Brouwere, K.; Bautmans, B.; Benoy, S.; Geerts, L.; Stranger, M.; Van Holderbeke, M. Establishing target and intervention guidance values for indoor air in dwellings and publicly accessible buildings: The Flemish approach. Int. J. Hyg. Environ. Health 2020, 230, 113579. [Google Scholar] [CrossRef]
- German Federal Environment Agency, Guide Values I and II. German Committee on Indoor Guide Values. 2018. Available online: www.umweltbundesamt.de/en/topics/health/commissions-working-groups/germancommittee-on-indoor-guide-values (accessed on 25 April 2024).
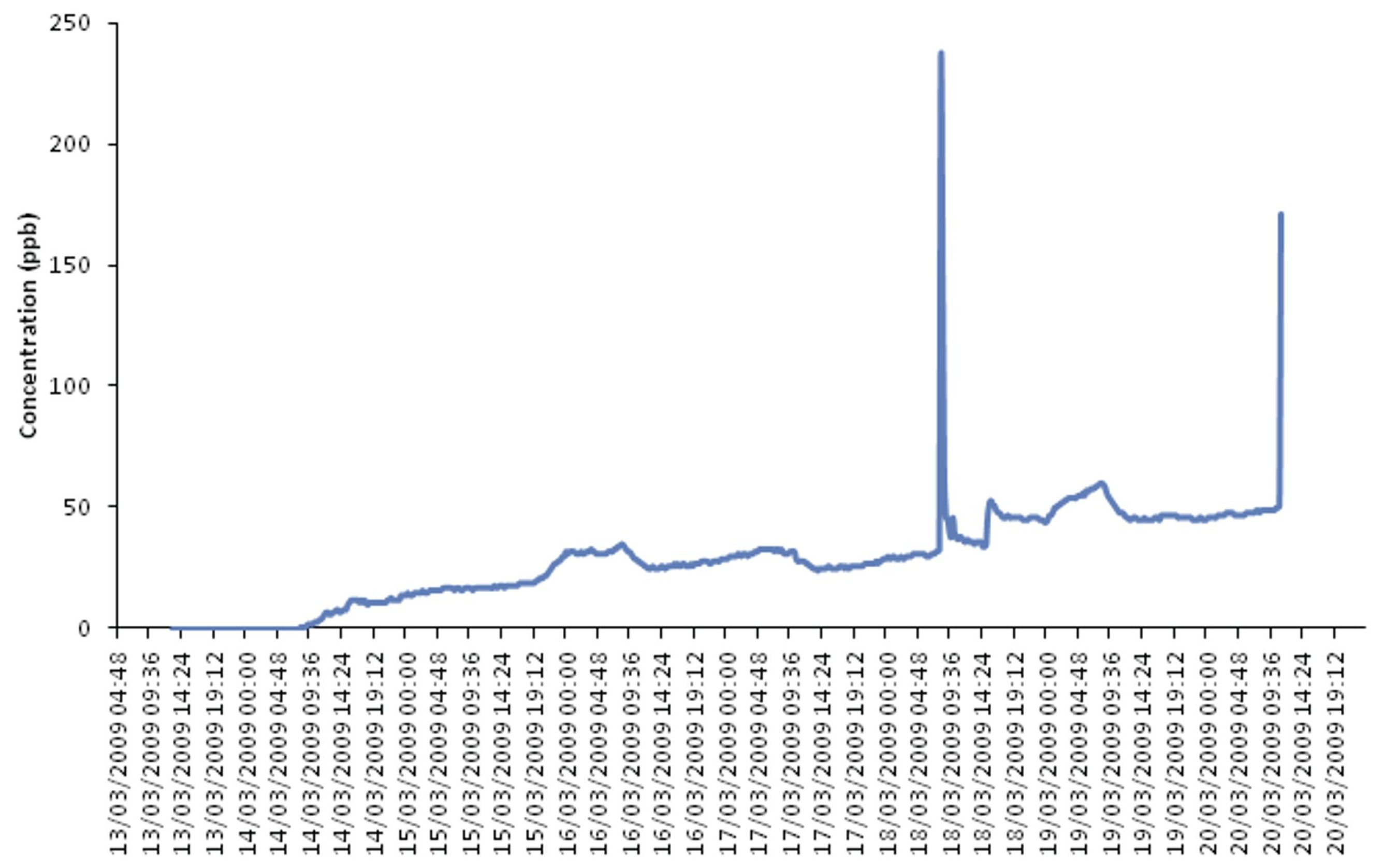
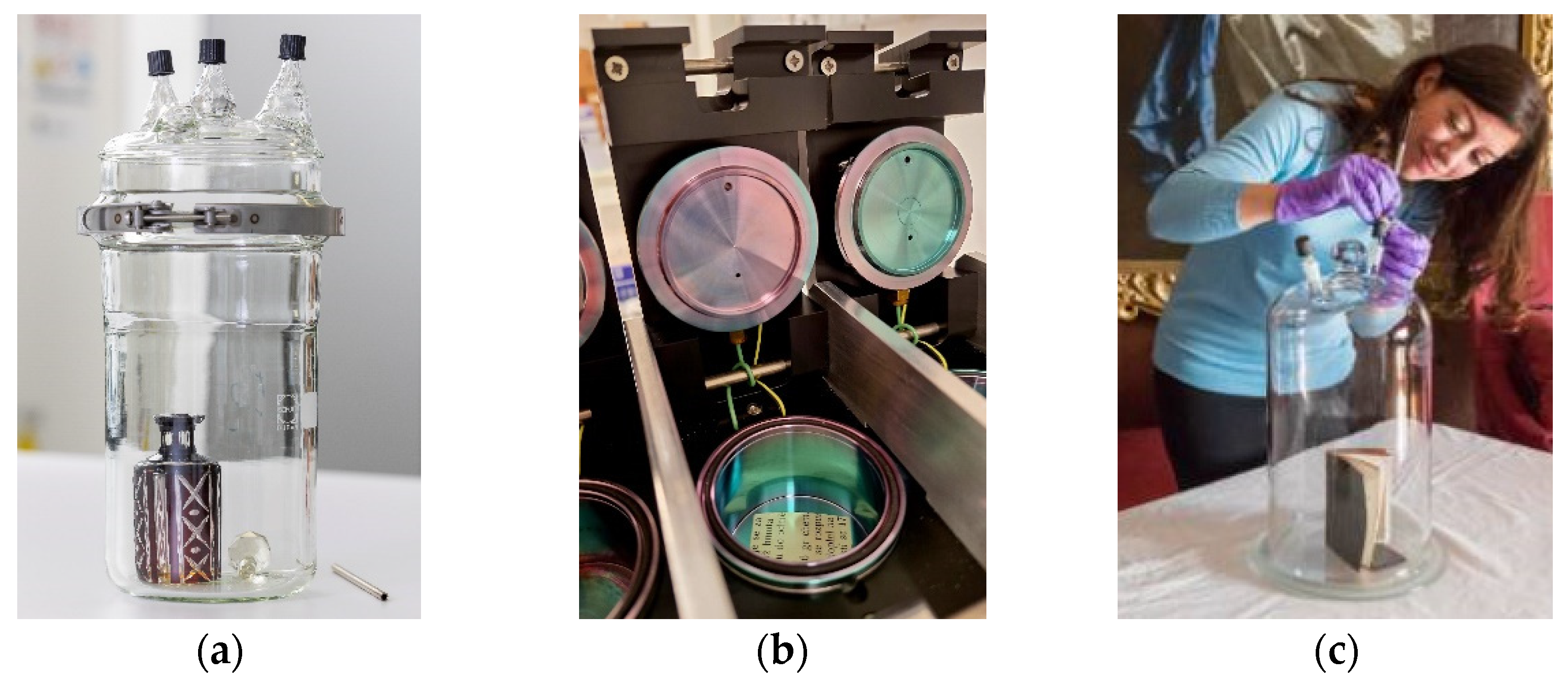

| Compound | Indoor Sources | Effects on the Material | Suggested Threshold (ppb) | ||
|---|---|---|---|---|---|
| Sensitive Material (Grzywacz 2006) | General Collection (Grzywacz 2006) | Long-Term Storage (PAS 198:2012) | |||
| Acetaldehyde | Wood products, polyvinyl acetate adhesive | Oxidation to carboxylic acid in high RH and/or in the presence of oxidants | 1–20 | – | – |
| Formaldehyde | Furnishing, coating for wood products, carpet finishing products | Oxidation to carboxylic acid in high RH and/or in the presence of oxidants, metal corrosion | 0.1–5 | 10–20 | 300 |
| Acetic acid | Wood products, furnishing, cellulose acetate objects (vinegar syndrome), acid-type silicon sealants, degradation of organic materials, emulsion paints, adhesives | Metal corrosion, efflorescence on calcareous materials and soda-rich glass objects, reduction in cellulose degree of polymerization | 5 | 40–280 | 100–1000 |
| Formic acid | Wood products, degradation of organic materials, oil-based paints | Metal corrosion, efflorescence on calcareous materials and soda-rich glass objects, reduction in cellulose degree of polymerization | 5 | 5–21 | 500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paolin, E.; Strlič, M. Volatile Organic Compounds (VOCs) in Heritage Environments and Their Analysis: A Review. Appl. Sci. 2024, 14, 4620. https://doi.org/10.3390/app14114620
Paolin E, Strlič M. Volatile Organic Compounds (VOCs) in Heritage Environments and Their Analysis: A Review. Applied Sciences. 2024; 14(11):4620. https://doi.org/10.3390/app14114620
Chicago/Turabian StylePaolin, Emma, and Matija Strlič. 2024. "Volatile Organic Compounds (VOCs) in Heritage Environments and Their Analysis: A Review" Applied Sciences 14, no. 11: 4620. https://doi.org/10.3390/app14114620
APA StylePaolin, E., & Strlič, M. (2024). Volatile Organic Compounds (VOCs) in Heritage Environments and Their Analysis: A Review. Applied Sciences, 14(11), 4620. https://doi.org/10.3390/app14114620





