Hematological and Hemorheological Parameters of Blood Platelets as Biomarkers in Diabetes Mellitus Type 2: A Comprehensive Review
Abstract
1. Introduction
2. Topics and Results
2.1. Enhanced PLT Reactivity in Diabetic Patients Has Been Considered a “Pro-Thrombotic State”
2.2. Platelet Signaling, Hyperaggregation, and Abnormalities in Patients with DM2 Play a Crucial Role in Thrombotic (Clot Formation) Complications and Thromboembolism during DM2 Micro- and Macroangiopathies
2.3. Hyperglycemia Contributes to Elevated PLT Reactivity (Hyperactivation)
2.4. Platelets to Lymphocytes Ratio (PLT/Ly)
The Effect of Oleic Acid on PLT Homeostasis
2.5. Hematological/Hematometric Investigations Related to Metabolic/Glycemic Control of DM2
2.6. Recent Advances in Modeling Diabetic Blood Platelets
2.6.1. Computational Platelet Modeling in DM2
2.6.2. Potential Platelet Biomarkers Related to T2DM
2.7. Combined Properties of Diabetic RBC and PLT Indices
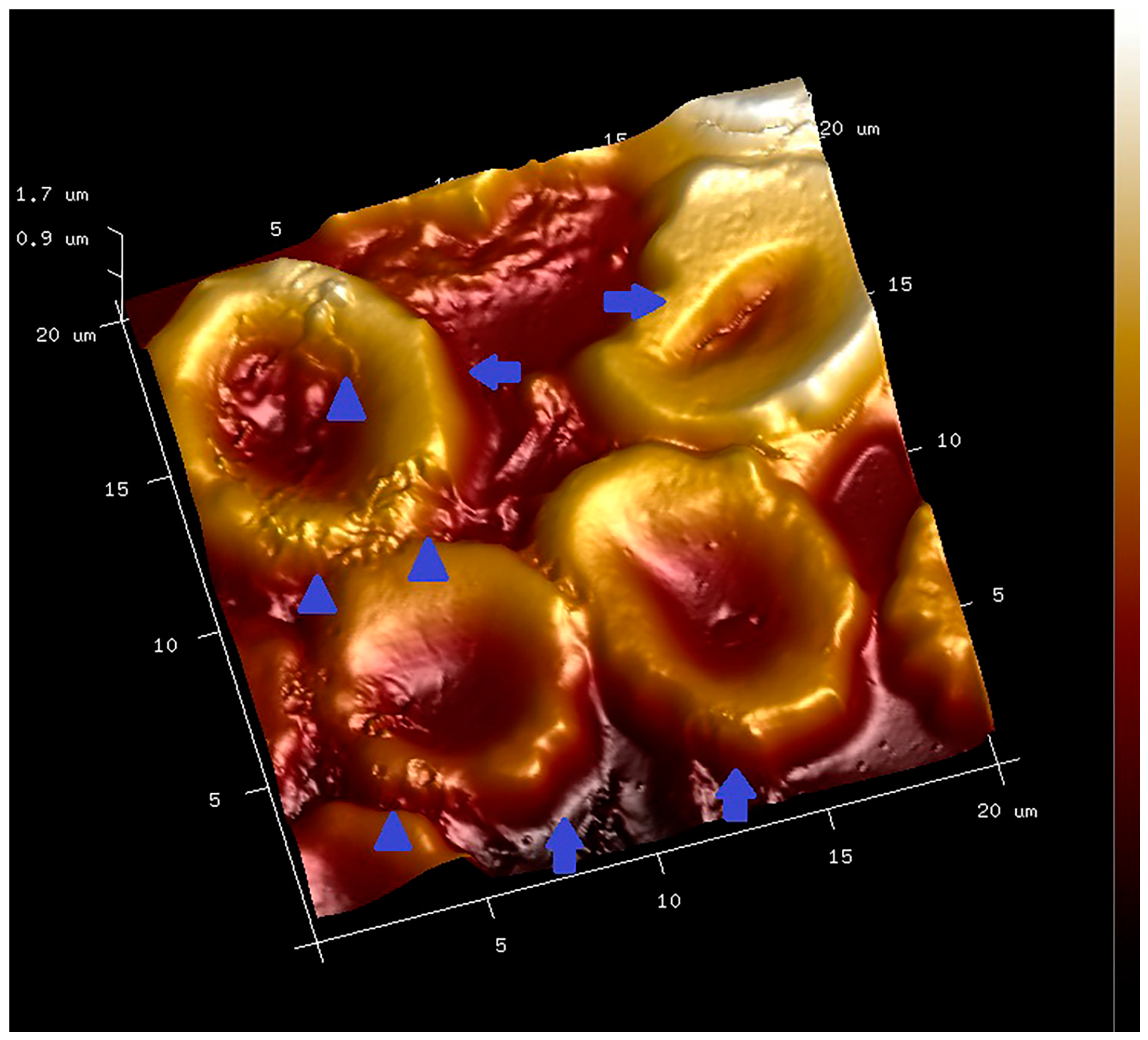
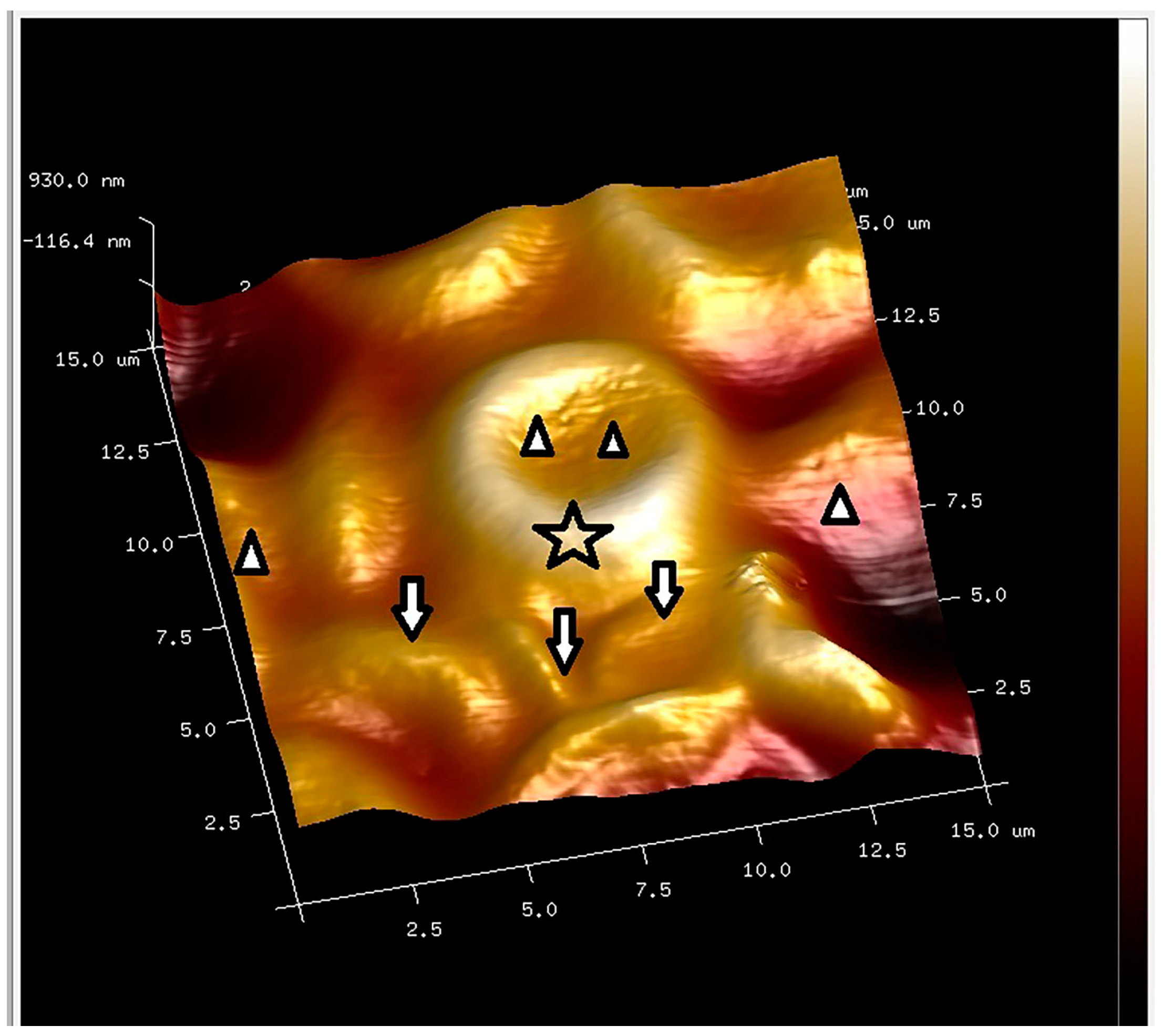
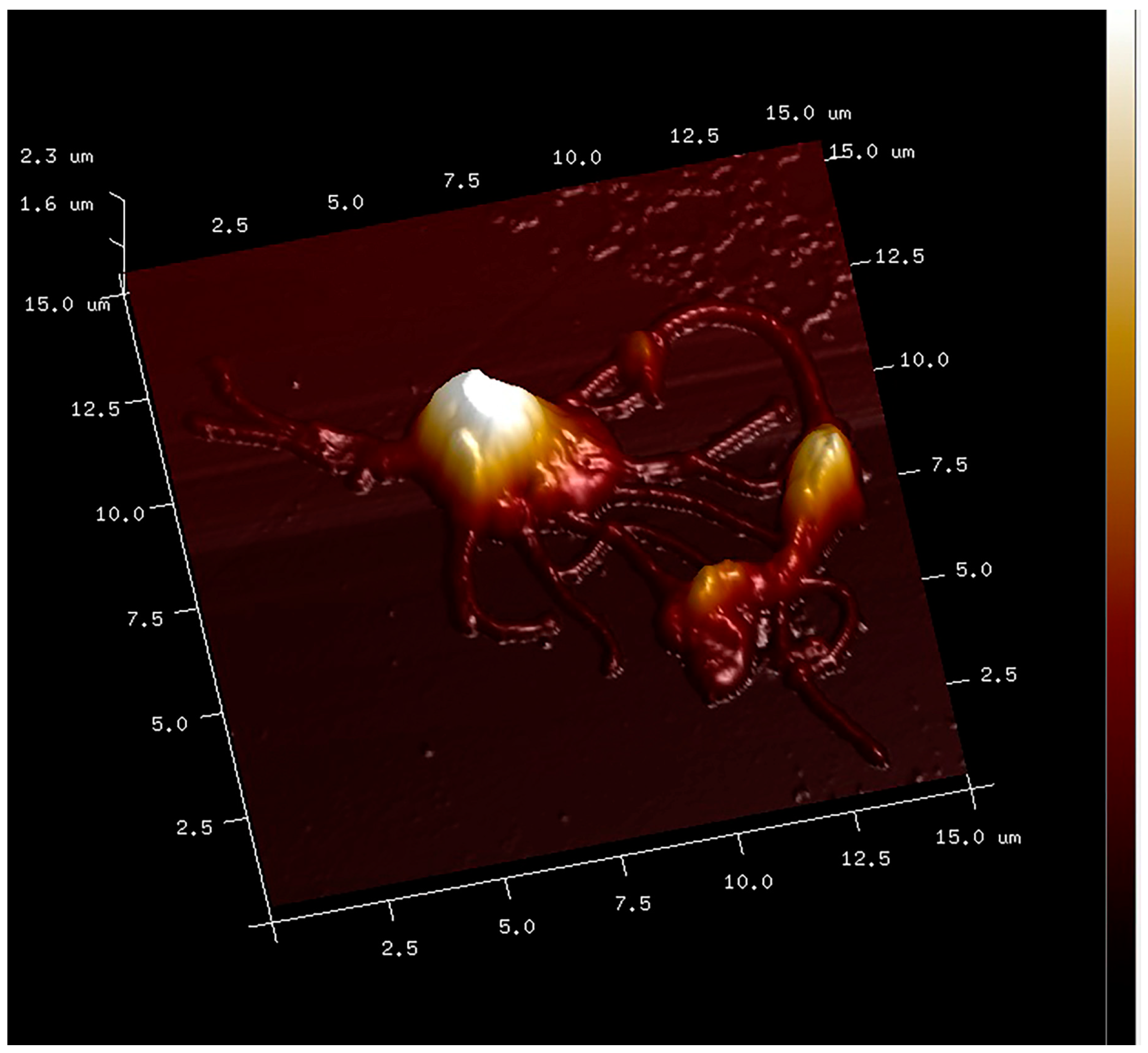

2.8. PLT-Derived microRNAs Are Novel Biomarkers for Early Diagnosis and Prognosis of Type 2 Diabetes Mellitus
2.9. Limitations
2.10. New Pharmacological Strategies Related to Natural Anti-Platelet Agents
3. Conclusions
Funding
Conflicts of Interest
References
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A. Molecular Mechanism of Metformin in DM2—A New Hypothesis. Int. J. Biochem. Res. Rev. 2018, 20, 1–13. [Google Scholar] [CrossRef]
- Elblbesy, M.A.; Kandil, B.A. Hemorheologicsl measurements over the shear-thinning regime. In vitro comparative study for hyperglycemia. AIMD Biophys. 2024, 11, 121–129. [Google Scholar] [CrossRef]
- Sun, J.; Han, K.; Xu, M.; Li, L.; Qian, J.; Li, L.; Li, X. Blood viscosity in subjects with type 2 Diabetes mellitus: Roles of hyperglycemia and elevated plasma fibrinogen. Front. Physiol. 2022, 13, 827428. [Google Scholar] [CrossRef]
- Windberger, U.; Dibiasi, C.; Lotz, E.M.; Scharbert, G.; Reinbacher-Koestinger, A.; Ivanov, I.; Ploszczanski, L.; Antonova, N.; Lichtenegger, H. The effect of hematocrit, fibrinogen concentration and temperature on the kinetics of clot formation of whole blood. Clin. Hemorheol. Microcirc. 2020, 75, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, W.H. Platelets in vascular disease. Clin. Hemorheol. Microcirc. 2013, 53, 71–79. [Google Scholar] [CrossRef]
- Battinelli, E.M.; Markens, B.A.; Italiano, J.E., Jr. Release of angiogenesis regulatory proteins from platelet alpha granules: Modulation of physiologic and pathologic angiogenesis. Blood 2011, 118, 1359–1369. [Google Scholar] [CrossRef]
- Zvetkova, E.; Antonova, N.; Ivanov, I.; Savov, Y.; Gluhcheva, Y. Platelet morphological, functional and rheological properties attributable to addictions. Clin. Hemorheol. Microcirc. 2010, 45, 245–251. [Google Scholar] [CrossRef]
- Munnix, I.C.; Cosemans, J.M.M.; Auger, J.M.; Heemskerk, J.W. Platelet response heterogeneity in thrombus formation. Thromb. Haemost. 2009, 102, 1149–1156. [Google Scholar] [CrossRef]
- Petrochenko, E.P.; Tikhomirova, I.A.; Ryabov, M.M.; Kislov, N.V.; Petrochenko, A.S. Platelet hemostasis in patients with non-myeloid cancer. Ser. Biomech. 2015, 29, 2015. [Google Scholar]
- Nomura, S. Function and clinical significance of platelet-derived microparticles. Int. J. Hematol. 2001, 74, 397–404. [Google Scholar] [CrossRef] [PubMed]
- George, J.N.; Thoi, L.L.; McManus, L.M. Isolation of human platelet membrane microparticles from plasma and serum. Blood 1982, 60, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Alexandrova-Watanabe, A.; Antonova, N.; Kyulavska, M.; Velcheva, I.; Ivanov, I.; Zvetkova, E. Hemorheological and atomic force microscopy studies on the experimental clot formation in patients with type 2 diabetes mellitus. Ser. Biomech. 2018, 32, 63–73. [Google Scholar]
- Pretorius, E.; Bester, J.; Vermeulen, N.; Alummoottil, S.; Soma, P.; Buys, A.V.; Kell, D.B. Poorly controlled type 2 diabetes is accompanied by significant morphological and ultrastructural changes in both erythrocytes and in thrombin-generated fibrin: Implications for diagnostics. Cardiovasc. Diabetol. 2015, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, T.; Komsa-Penkova, R.; Langari, A.; Krumova, S.; Golemanov, G.; Georgieva, G.B.; Taneva, S.G.; Giosheva, I.; Mihaylova, N.; Tchorbanov, A.; et al. Morphometric and nanomechanical features of platelets from women with early pregnancy loss provide new evidence of the impact of inherited thrombophilia. Int. J. Mol. Sci. 2021, 22, 7778. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Liu, Y.; Zhang, J.; Dong, J.F.; Zhao, Z. Transcription factors in megakaryocytes and platelets. Front. Immunol. 2023, 14, 1140501. [Google Scholar] [CrossRef] [PubMed]
- El Haouari, M.; Rosado, J.A. Platelet signalling abnormalities in patients with type 2 diabetes mellitus: A review. Blood Cells Mol. Dis. 2008, 41, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Kubisz, P.; Stančiaková, L.; Staško, J.; Galajda, P.; Mokáň, M. Endothelial and platelet markers in diabetes mellitus type 2. World J. Diabetes 2015, 6, 423. [Google Scholar] [CrossRef]
- Swathi, M.; Ramya, T.; Gangaram, U.; Kumar, K.K.; Sandeep, B. A study of neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) with glycosylated hemoglobin (HBA1C) among type 2 diabetic patients. Int. J. Acad. Med. Pharm. 2023, 5, 1533–1537. [Google Scholar] [CrossRef]
- Xu, F.; Qu, S.; Wang, L.; Qin, Y. Mean platelet volume (MPV) new diagnostic indices for co-morbidity of tuberculosis and diabetis mellitus. BMC Infect. Dis. 2021, 21, 461. [Google Scholar] [CrossRef]
- Pujani, M.; Gahlawat, H.; Agarwal, C.; Chauhan, V.; Singh, K.; Lukhmana, S. Platelet parameters: Can they serve as biomarkers of glycemic control or development of complications in evaluation of type 2 diabetes mellitus. Iraqi J. Hematol. 2018, 7, 72–78. [Google Scholar] [CrossRef]
- Alhadas, K.R.; Santos, S.N.; Freitas, M.M.S. Are platelet indices useful in the evaluation of type2 diabetic patients? J. Bras. Pathol. Med. Lab. 2016, 52, 96–102. [Google Scholar] [CrossRef]
- Joshi, A.A.; Jaison, J. The study of platelet parameters—Mean platelet volume (MPV) and platelet distribution width (PDW) in type 2 Diabetes mellitus. Lab. Med. 2019, 6, A407–A413. [Google Scholar] [CrossRef]
- Olana, C.; Seifu, D.; Menon, M.K.C.; Natesan, G. Abnormal hematological indices and anthropometric parameters associated with type 2 Diabetes. Int. J. Biomed. Adv. Res. 2019, 10, e5296. [Google Scholar]
- Shilpi, K.; Potekar, R.M. A study of platelet indices in Type 2 diabetes mellitus patients. Indian J. Hematol. Blood Transfus. 2018, 34, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Forum, M.H.; Kara, M.Z.; Egilmez, O.B.; Kalender Oglu, A. Complete bood count alterations due to the opioid use: What about the lymphocyte-related ratios, especially in monocyte to lymphocyte ratio and platelet to lymphocyte ratio? J. Immunoass. Immunochem. 2018, 39, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Chen, M.; Feng, Q.; Wan, H.; Wang, J.; Yang, F.; Cao, H. The Platelet-to-Lymphocyte Ratio Predicts Diabetic Retinopathy in Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. 2022, 15, 3617–3626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, H.; Cui, S.; Lin, Y.; Liang, Y.; Zhao, P.; Wang, C.; Xu, S.; Peng, X.; Chen, H.; et al. Platelet-Lymphocyte Ratio, Neutrophil-Lymphocyte Ratio and Their Dynamic Changes with Type 2 Diabetes Mellitus: A Cohort Study in China. Endocr. Res. 2022, 47, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Alhalwani, A.Y.; Jambi, S.; Borai, A.; Khan, M.A.; Almarzouki, H.; Elsayid, M.; Aseri, A.F.; Taher, N.O.; Alghamdi, A.; Alshehri, A. Assessment of the systemic immune-inflammation index in type 2 diabetic patients with and without dry eye disease: A case-control study. Health Sci. Rep. 2024, 7, e1954. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, X.; Zhu, R.; Ming, Z.; Cheng, Z.; Hu, Y. The mechanism of oleic acid inhibiting platelet activation stimulated by collagen. Cell Commun. Signal. 2023, 21, 278. [Google Scholar] [CrossRef]
- Ji, S.; Zhang, J.; Fan, X.; Wang, X.; Ning, X.; Zhang, B.; Shi, H.; Yan, H. The relationship between mean platelet volume and diabetic retinopathy: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2019, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, D.; Panin, V.L. Relationship between hematological parameters and glycemic control in type 2 Diabetes mellitus patients. J. Med. Biochem. 2019, 38, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, A.; Amitkumar, K.; Ganapathy, S.; Ayyavoo, S. Evaluation of mean platelet volume and other platelet parameters in subjects with Type-2 diabetes mellitus. Natl. J. Physiol. Pharm. Pharmacol. 2017, 7, 51. [Google Scholar] [CrossRef]
- Zvetkova, E.; Fuchs, D. Medical significance of simultaneous application of red blood cell distribution width (RDW) and neopterin as diagnostic/prognostic biomarkers in clinical practice. Pteridines 2017, 28, 133–140. [Google Scholar] [CrossRef]
- Zvetkova, E.; Savov, Y.; Gluhcheva, Y.; Ilieva, I.; Bichkidjieva, E. Simultaneous Anisocytosis of Red Blood Cells and Platelets in Chronic Heroin Addicts, Bloodmed–Slide Atlas, Red Cells—Acquired Disorders 2006; Blackwell Publishing: Oxford, UK, 2006; Available online: https://www.bloodmed.com/home/slide-atlas (accessed on 13 March 2024).
- Nada, A.M. Red cell distribution width in type 2 diabetic patients. Diabetes Metab. Syndr. Obes. Targets Ther. 2015, 8, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Antonova, N.; Veltcheva, I.; Paskova, V. Hemorheological and microvascular disturbances in patients with type 2 diabetes mellitus. Clin. Hemorheol. Microcirc. 2022, 81, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.X.; Chang, H.-Y.; Li, H. Recent Advances in Computational Modeling of Biomechanics and Biorheology of Red Blood Cells in Diabetes. Biomimetics 2022, 7, 15. [Google Scholar] [CrossRef]
- Bouchnita, A.; Volpert, V. A multiscale model of platelet-fibrin thrombus growth in the flow. Comput. Fluids 2019, 184, 10–20. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, N.; Deng, Y.; Bluestein, D. A multiple time stepping algorithm for efficient multiscale modeling of platelets flowing in blood plasma. J. Comput. Phys. 2015, 284, 668–686. [Google Scholar] [CrossRef]
- Li, H.; Sampani, K.; Zheng, X.; Papageorgiou, D.P.; Yazdani, A.; Bernabeu, M.O.; Karniadakis, G.E.; Sun, J.K. Predictive modeling of thrombus formation in diabetic retinal microaneurysms. R. Soc. Open Sci. 2020, 7, 201102. [Google Scholar] [CrossRef]
- Chang, H.Y.; Yazdani, A.; Li, X.; Douglas, K.A.; Mantzoros, C.S.; Karniadakis, G.E. Quantifying platelet margination in diabetic blood flow. Biophys. J. 2018, 115, 1371–1382. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Lee, M.Y.; Zhu, S.; Sinno, T.; Diamond, S.L. Multiscale simulation of thrombus growth and vessel occlusion triggered by collagen/tissue factor using a data-driven model of combinatorial platelet signalling. Math. Med. Biol. J. IMA 2017, 34, 523–546. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, A.; Karniadakis, G.E. Sub-cellular modeling of platelet transport in blood flow through microchannels with constriction. Soft Matter 2016, 12, 4339–4351. [Google Scholar] [CrossRef] [PubMed]
- Anand, M.; Rajagopal, K.; Rajagopal, K. A model for the formation, growth, and lysis of clots in quiescent plasma. A comparison between the effects of antithrombin III deficiency and protein C deficiency. J. Theor. Biol. 2008, 253, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Li, H.; Zheng, F.; Kong, F.; Dao, M.; Karniadakis, G.E.; Suresh, S. Artificial intelligence velocimetry and microaneurysmona- chip for three-dimensional analysis of blood flow in physiology and disease. Proc. Natl. Acad. Sci. USA 2021, 118, e2100697118. [Google Scholar] [CrossRef] [PubMed]
- Tosenberger, A.; Ataullakhanov, F.; Bessonov, N.; Panteleev, M.; Tokarev, A.; Volpert, V. Modelling of platelet–fibrin clot formation in flow with a DPD–PDE method. J. Math. Biol. 2016, 72, 649–681. [Google Scholar] [CrossRef] [PubMed]
- Heemskerk, J.W.; Siljander, P.R.; Bevers, E.M.; Farndale, R.W.; Lindhout, T. Receptors and signalling mechanisms in the procoagulant response of platelets. Platelets 2000, 11, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Cakir, E.; Turan Özkoçak, I. Predictive effects of first erythrocyte and thrombocyte volume indices on mortality of geriatric patients with sepsis hospitalized in intensive care units. Turk. J. Geriatr./Türk Geriatr. Derg. 2021, 24, 134–142. [Google Scholar] [CrossRef]
- Ulutas, K.T.; Dokuyucu, R.; Sefil, F.; Yengil, E.; Sumbul, A.T.; Rizaoglu, H.; Ustun, I.; Yula, E.; Sabuncu, T.; Gokce, C. Evaluation of mean platelet volume in patients with type-2 diabetes mellitus and blood glucose regulation: A marker for atherosclerosis? Int. J. Clin. Exp. Med. 2014, 7, 955–961. [Google Scholar] [PubMed] [PubMed Central]
- Aktas, F.; Aktuglu, M.B. Evaluation of the relation between HbA1c and MPV and PDW levels of patients with Type 2 Diabetes admitted in Internal Medicine Polyclinics. North Clin. Istanb. 2023, 10, 681–686. [Google Scholar] [CrossRef]
- Elsayed, A.M.; Araby, E. Neutrophil-Lymphocyte and Platelet-Lymphocyte ratious as a marker for diabetes control and complications. Benha Med. J. 2021, 38, 984–995. [Google Scholar] [CrossRef]
- Hajek, A.S.; Joist, J.H.; Baker, R.K.; Jarett, L.E.O.N.A.R.D.; Daughaday, W.H. Demonstration and partial characterization of insulin receptors in human platelets. J. Clin. Investig. 1979, 63, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Kelem, S.; Adane, T.; Shiferaw, E. Insulin resistance-induced platelet hyperactivity and a potential biomarker role of platelet parameters, A narrave Review. Diabetes Metab. Sydrome Obes. 2023, 16, 2843–3853. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Khalid, M.A.; Munir, M.; Arshad, I.; Maaz, M. Association of Neutrophil to Lymphocyte and Platelet to Lymphocyte ratio with glucose regulation in type 2 diabetes patients. J. Rawalpindi Med. Coll. 2021, 25, 540–543. [Google Scholar] [CrossRef]
- Sobczak, A.I.S.; Stewart, S.J. Coagulatory defects in type-1 and type-2 Diabetes. Int. J. Mol. Sci. 2019, 20, 6345. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart disease and stroke statistics—2018. Update: A report from the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, B.A.T.; Johnson, A.D. Platelet measurements and type 2 Diabetes: Investigations in two population-based Cohorts. Front. Cardiovasc. Med. 2020, 7, 118. [Google Scholar] [CrossRef] [PubMed]
- Dolasık, I.; Sener, S.Y.; Celebı, K.; Aydın, Z.M.; Korkmaz, U.; Canturk, Z. The effect of metformin on mean platelet volume in diabetic patients. Platelets 2013, 24, 118–121. [Google Scholar] [CrossRef]
- Magri, C.J.; Xuereb, S.; Huereb, R.A. Sleep measures and cardiovascular disease in type 2 diabetes mellitus. Clin. Med. 2023, 23, 380–386. [Google Scholar] [CrossRef]
- Chkhitauri, L.; Sanikidze, T.; Giorgadze, E.; Asatiani, K.; Kipiani, N.; Momtselidze, N.; Mantskava, M. Comprehensive study of the rheological status and intensity of oxidative stress during the progression of type 2 Diabetes mellitus to prevent its complications. Clin. Hemorheol. Microcirc. 2023, 83, 69–79. [Google Scholar] [CrossRef]
- Alexandrova-Watanabe, A. Study of Rheological, Mechanical and Morphological Properties of Blood, Its Formal Elements and Parameters of Hemocoagulation in Type 2 Diabetes Mellitus. Ph.D. Thesis, Institute of Mechanics, Bulgarian Academy of Sciences, Sofia, Bulgaria, 2021. [Google Scholar]
- Liu, Y.; Gao, G.; Yang, C.; Zhou, K.; Shen, B.; Liang, H.; Jiang, X. The role of circulating microRNA-126 (miR-126): A novel biomarker for screening prediabetes and newly diagnosed type 2 diabetes mellitus. Int. J. Mol. Sci. 2014, 15, 10567–10577. [Google Scholar] [CrossRef] [PubMed]
- Afsharmanesh, M.R.; Mohammadi, Z.; Mansourian, A.R.; Jafari, S.M. A Review of micro RNAs changes in T2DM in animals and humans. J. Diabetes 2023, 15, 649–664. [Google Scholar] [CrossRef] [PubMed]
- de Boer, H.C.; van Solingen, C.; Prins, J.; Duijs, J.M.; Huisman, M.V.; Rabelink, T.J.; van Zonneveld, A.J. Aspirin treatment hampers the use of plasma microRNA-126 as a biomarker for the progression of vascular disease. Eur. Heart J. 2013, 34, 3451–3457. [Google Scholar] [CrossRef]
- Zhu, H.; Leung, S.W. MicroRNA biomarkers of type 2 diabetes: Evidence synthesis from meta-analyses and pathway modelling. Diabetologia 2023, 66, 288–299. [Google Scholar] [CrossRef]
- Shantikumar, S.; Caporali, A.; Emanueli, C. Role of microRNAs in diabetes and its cardiovascular complications. Cardiovasc. Res. 2012, 93, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Zvetkova, E.B.; Zvetkov, I.B. A cytological method for the simultaneous staining of nucleoproteids and some cathionic proteins. Acta Histochem. 1976, 57, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zvetkova, E.; Kostov, G. Microdensitometrical studies on tumour-induced programmed cell death of peripheral blood tumour-infiltrating lymphocytes (TIL) in cancer patients: Possible applications for early tumour diagnosis. Acta Morphol. Anthropol. 2000, 5, 3–10. [Google Scholar]
- Zvetkova, E.B.; Kostov, G.S.; Gluhcheva, Y.G. 30 Years from Creation of the First Cytochemical Method Analysing RNP, DNP and Cationic Proteins in Circulating Leukocytes of Cancer Patients as a Tool for Early Diagnostics of Malignancies. Clin. Immunol. 2007, 123, S111. [Google Scholar] [CrossRef]
- Zhang, Q.-y.; Han, Y.-l. Opioid peptides and P2Y12 receptor signling pathway. Prog. Mod. Biomed. 2011, 11, 3787–3790. [Google Scholar]
- Sobol, A.B.; Watala, C. The role of platelets in diabetes-related vascular complications. Diabetes Res. Clin. Pract. 2000, 50, 1–16. [Google Scholar] [CrossRef]
- Ashby, B.; Daniel, J.L.; Smith, J.B. Mechanisms of platelet activation and inhibition. Hematol. Oncol. Clin. N. Am. 1990, 4, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Vinik, A.I.; Erbas, T.; Park, T.S.; Nolan, R.; Piterenger, G.L. Platelet dysfunction in type-2-Diabetes. Diabetes Care 2001, 24, 1476–1485. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.E. Diabetes mellitus: A hypercoagulable state. J. Diabetes Its Complicat. 2001, 15, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Vinik, A.; Flemmer, M. Diabetes and macrovascular disease. J. Diabetes Its Complicat. 2002, 16, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Vinik, A.; Vinik, E. Prevention of the complications of Diabetes. Am. J. Manag. Care 2003, 9 (Suppl. S3), S63–S80; quiz S81–S84. [Google Scholar] [PubMed]
- Hirsch, G.E.; Viecili, P.R.N.; de Almeida, A.S.; Nascimento, S.; Porto, F.G.; Otero, J.; Schmidt, A.; da Silva, B.; Parisi, M.M.; Klafke, J.Z. Natural Products with Antiplatelet Action. Curr. Pharm. Des. 2017, 23, 1228–1246. [Google Scholar] [CrossRef] [PubMed]
- Mhatre, K.J.; Patil, J.R.; Nikalje, G.C. Role of Flavonoids in vasodilation. In The Flavonoids, 1st ed.; Apple Academic Press: Palm Bay, FL, USA, 2024; p. 17. [Google Scholar]
- Freedman, J.E.; Parker, C., 3rd; Li, L.; Perlman, J.A.; Frei, B.; Ivanov, V.; Deak, L.R.; Iafrati, M.D.; Folts, J.D. Select flavonoids and whole juice from purple grapes inhibit platelet function and enhance nitric oxide release. Circulation 2001, 103, 2792–2798. [Google Scholar] [CrossRef]
- Olas, B. The multifunctionality of berries toward blood platelets and the role of berry phenolics in cardiovascular disorders. Platelets 2017, 28, 540–549. [Google Scholar] [CrossRef]

| PLTs—platelets count | 140–440 × 109/L |
| MPV—mean platelet volume (MPV) | 7.80–11.0 fL |
| PDW—platelet distribution width | 15.5–30.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zvetkova, E.; Ivanov, I.; Koytchev, E.; Antonova, N.; Gluhcheva, Y.; Alexandrova-Watanabe, A.; Kostov, G. Hematological and Hemorheological Parameters of Blood Platelets as Biomarkers in Diabetes Mellitus Type 2: A Comprehensive Review. Appl. Sci. 2024, 14, 4684. https://doi.org/10.3390/app14114684
Zvetkova E, Ivanov I, Koytchev E, Antonova N, Gluhcheva Y, Alexandrova-Watanabe A, Kostov G. Hematological and Hemorheological Parameters of Blood Platelets as Biomarkers in Diabetes Mellitus Type 2: A Comprehensive Review. Applied Sciences. 2024; 14(11):4684. https://doi.org/10.3390/app14114684
Chicago/Turabian StyleZvetkova, Elissaveta, Ivan Ivanov, Eugeni Koytchev, Nadia Antonova, Yordanka Gluhcheva, Anika Alexandrova-Watanabe, and Georgi Kostov. 2024. "Hematological and Hemorheological Parameters of Blood Platelets as Biomarkers in Diabetes Mellitus Type 2: A Comprehensive Review" Applied Sciences 14, no. 11: 4684. https://doi.org/10.3390/app14114684
APA StyleZvetkova, E., Ivanov, I., Koytchev, E., Antonova, N., Gluhcheva, Y., Alexandrova-Watanabe, A., & Kostov, G. (2024). Hematological and Hemorheological Parameters of Blood Platelets as Biomarkers in Diabetes Mellitus Type 2: A Comprehensive Review. Applied Sciences, 14(11), 4684. https://doi.org/10.3390/app14114684








