The Influence of Cytokinin on the Multiplication Efficiency and Genetic Stability of Scutellaria baicalensis Regenerants in In Vitro Culture Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Culture Conditions
2.2. DNA Extraction
2.3. SCoT Analysis
2.4. DNA Data Analysis
3. Results and Discussion
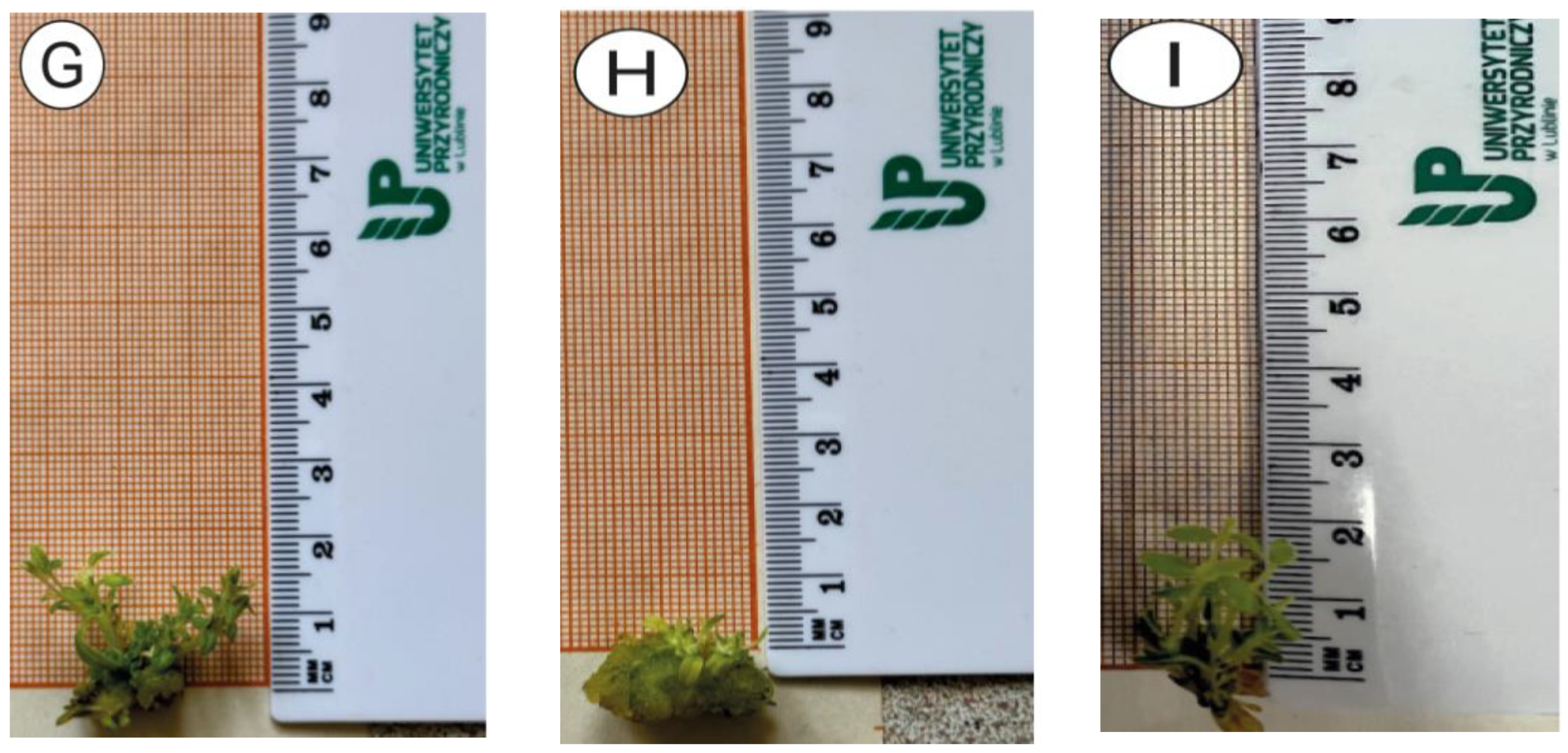
3.1. In Vitro Culture Analysis
3.2. Molecular Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.-L.; Wang, S.; Kuang, Y.; Hu, Z.-M.; Qiao, X.; Ye, M. A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm. Biol. 2018, 56, 465–484. [Google Scholar] [CrossRef]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, X. Scutellaria baicalensis Georgi. (Lamiaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 2019, 71, 1353–1369. [Google Scholar] [CrossRef]
- Song, J.-W.; Long, J.-Y.; Xie, L.; Zhang, L.-L.; Xie, Q.-X.; Chen, H.-J.; Deng, M.; Li, X.-F. Applications, phytochemistry, pharmacological effects, pharmacokinetics, toxicity of Scutellaria baicalensis Georgi. And its probably potential therapeutic effects on COVID-19: A review. Chin. Med. 2020, 15, 102. [Google Scholar] [CrossRef]
- Liu, H.; Ye, F.; Sun, Q.; Liang, H.; Li, C.; Lu, R.; Huang, B.; Tan, W.; Lai, L. Scutellaria baicalensis extract and baicalein inhibit replication of SARS-CoV-2 and its 3C-like protease in vitro. J. Enzym. Inhib. Med. Chem. 2021, 36, 497–503. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, X.-Y.; Martin, C. Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants. Sci. Bull. 2016, 61, 1391–1398. [Google Scholar] [CrossRef]
- Wang, D.; Luo, X.; Huang, Y.; Wang, M.; Xia, Z. Combined magnetic molecularly imprinted polymers with a ternary deep eutectic solvent to purify baicalein from the Scutellaria baicalensis Georgi by magnetic separation. Microchem. J. 2020, 157, 105–109. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K.; Tankhaeva, L.M. Phenolic compounds of Scutellaria baicalensis Georgi. Russ. J. Bioorg. Chem. 2010, 37, 816–842. [Google Scholar] [CrossRef]
- Karpińska, E. Anti-inflammatory and anticancer properties of Scutellaria baicalensis Georgi. Post Fitoter. 2010, 4, 215–223. [Google Scholar]
- Debnath, S.C. Zeatin overcomes thidiazuron-induced inhibition of shoot elongation and promotes rooting in strawberry culture in vitro. J. Hortic. Sci. Biotech. 2006, 81, 349–354. [Google Scholar] [CrossRef]
- Efferth, T. Biotechnology applications of plant callus cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In vitro plant tissue culture: Means for production of biological active compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kikowska, M.; Turowska, N.; Thiem, B. Czy kultury in vitro gatunków roślin chronionych mogą być źródłem surowców do badań fitochemicznych i biologicznych? Farm. Współczesna 2019, 12, 210–217. [Google Scholar]
- Coelho, C.; Bocca, A.L.; Casadevall, A. The tools for virulence of Cryptococcus neoformans. Adv. Appl. Microbiol. 2014, 87, 1–41. [Google Scholar] [CrossRef]
- Ozdemir, F.A.; Yildirim, M.U.; Kahriz, P.P. Micropropagation of endemic Scutellaria orientalis L. subsp. bicolor using modified MS medium & TDZ. Emir. J. Food Agric. 2015, 27, 818–824. [Google Scholar] [CrossRef]
- Ozdemir, F.A.; Kilic, O.; Atalan, E. In vitro callus propagation and antibacterial activities of callus an edible endemic and medicinal plant Scutellaria orientalis L. subsp. bicolor. Prog. Nutr. 2016, 18, 81–86. [Google Scholar]
- Zakaria, I.A.; Sherman, S.; Vaidya, B.; Joshee, N. In vitro propagation and synseed mediated short-term conservation of Scutellaria alpine L. and Scutellaria altissima L. Plant Cell Cult. Micropropag. 2020, 16, e163. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Kuźma, Ł.; Wysokińska, H. In vitro cultures of Scutellaria alpina as a source of pharmacologically active metabolites. Acta Physiol. Plant. 2016, 38, 7. [Google Scholar] [CrossRef]
- Gharari, Z.; Bagheri, K.; Sharafi, A. High-frequency adventitious shoot organogenesis from in vitro stem explants of Scutellaria araxensis Grossh. BioTechnologia 2022, 103, 143–151. [Google Scholar] [CrossRef]
- Brearley, T.; Vaidya, B.; Joshee, N. Cytokinin, Carbon Source, and Acclimatization Requirements for In Vitro Propagation of Scutellaria barbata D. Don and Scutellaria racemose Pers. Am. J. Plant Sci. 2014, 5, 3662–3672. [Google Scholar] [CrossRef]
- Stojakowska, A.; Malarz, J.; Kohlmünzer, J. Micropropagation of Scutellaria baicalensis Georgi. Acta Soc. Bot. Pol. 1999, 68, 103–107. [Google Scholar] [CrossRef]
- Hwang, I.-T.; Lee, J.-J.; Lee, J.; Paik, S.-W.; Kim, Y.-H. Production of Baicalin, Baicalein, and Wogonin on Plant Tissue Culture of Scutellaria baicalensis. Korean J. Plant Resour. 2015, 28, 526–532. [Google Scholar] [CrossRef]
- Trivedi, M.; Trivedi, R.K.; Guag, Z.C.; Guo, G.; Zheng, G. Hormone-Induced Indirect Regeneration Protocol for Scutellaria baicalensis Georgi (Huang-qin). J. Crop Improv. 2011, 25, 550–559. [Google Scholar] [CrossRef]
- Li, H.; Murch, S.J.; Saxena, P.K. Thidiazuron-induced de novo shoot organogenesis on seedlings, etiolated hypocotyls and stem segments of Huang-qin. Plant Cell Tissue Organ Cult. (PCTOC) 2000, 62, 169–173. [Google Scholar] [CrossRef]
- Bairu, M.W.; Kane, M.E. Physiological and developmental problems encountered by in vitro cultured plants. Plant Growth Regul. 2011, 63, 101–103. [Google Scholar] [CrossRef]
- Ferreira, M.d.S.; Rocha, A.d.J.; Nascimento, F.d.S.; Oliveira, W.D.d.S.; Soares, J.M.d.S.; Rebouças, T.A.; Morais Lino, L.S.; Haddad, F.; Ferreira, C.F.; Santos-Serejo, J.A.d.; et al. The Role of Somaclonal Variation in Plant Genetic Improvement: A Systematic Review. Agronomy 2023, 13, 730. [Google Scholar] [CrossRef]
- Marakli, S. A Brief Review of Molecular Markers to Analyse Medically Important Plants. Int. J. Life Sci. Biotechnol. 2018, 1, 29–36. [Google Scholar] [CrossRef]
- Clapa, D.; Hârța, M. Establishment of an Efficient Micropropagation System for Humulus lupulus L. cv. Cascade and Confirmation of Genetic Uniformity of the Regenerated Plants through DNA Markers. Agronomy 2021, 11, 2268. [Google Scholar] [CrossRef]
- Thakur, J.; Dwivedi, M.D.; Sourabh, P.; Uniyal, P.L.; Pandey, A.K. Genetic Homogeneity Revealed Using SCoT, ISSR and RAPD Markers in Micropropagated Pittosporum eriocarpum Royle—An Endemic and Endangered Medicinal Plant. PLoS ONE 2016, 11, e0159050. [Google Scholar] [CrossRef] [PubMed]
- Sathish, D.; Vasudevan, V.; Theboral, J.; Elayaraja, D.; Appunu, C.; Siva, R.; Manickavasagam, M. Efficient direct plant regeneration from immature leaf roll explants of sugarcane (Saccharum officinarum L.) using polyamines and assessment of genetic fidelity by SCoT markers. In Vitro Cell. Dev. Biol.-Plant 2018, 54, 399–412. [Google Scholar] [CrossRef]
- Krishna, H.; Alizadeh, M.; Singh, D.; Singh, U.; Chauhan, N.; Eftekhari, M.; Sadh, R.K. Somaclonal variations and their applications in horticultural crops improvement. 3 Biotech 2016, 6, 54. [Google Scholar] [CrossRef]
- Rai, M.K. Somaclonal variation in improvement of agricultural crops: Recent progress. In Agricultural Biotechnology: Latest Research and Trends; Srivastava, D.K., Thakur, A.K., Kumar, P., Eds.; Springer: Singapore, 2021; pp. 129–146. [Google Scholar]
- Arora, K.; Rai, M.K.; Sharma, A.K. Tissue culture mediated biotechnological interventions in medicinal trees: Recent progress. Plant Cell Tissue Organ Cult. (PCTOC) 2022, 150, 267–287. [Google Scholar] [CrossRef]
- Su, S.; He, C.-M.; Li, L.-C.; Chen, K.-J.; Zhou, T.-S. Genetic characterization and phytochemical analysis of wild and cultivated populations of Scutellaria baicalensis. Chem. Biodivers. 2008, 5, 1353–1363. [Google Scholar] [CrossRef]
- Shao, A.-J.; Li, X.; Huang, L.-Q.; Lin, S.-F.; Chen, J. RAPD analysis of Scutellaria baicalensis from different germplasms. Zhongguo Zhong Yao Za Zhi 2006, 31, 452–455. [Google Scholar]
- Bai, C.; Wen, M.; Zhang, L.; Li, G. Genetic diversity and sampling strategy of Scutellaria baicalensis germplasm resources based on ISSR. Genet. Resour. Crop Evol. 2013, 60, 1673–1685. [Google Scholar] [CrossRef]
- Yuan, Y.; Long, P.; Jiang, C.; Li, M.; Huang, L. Development and characterization of simple sequence repeat (SSR) markers based on a full-length cDNA library of Scutellaria baicalensis. Genomics 2015, 105, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F.A. Revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Gawroński, J.; Dyduch-Siemińska, M. Potential of In Vitro Culture of Scutellaria baicalensis in the Formation of Genetic Variation Confirmed by ScoT Markers. Genes 2022, 13, 2114. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J.; Doyle, J.L. A Rapid DNA Isolation Procedure for Small Quantities of Fresh Leaf Tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Nei, M.; Li, W. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc. Natl. Acad. Sci. USA 1979, 76, 5269–5273. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Elect. 2001, 4, 9. [Google Scholar]
- Kwiecień, I.; Łukaszyk, A.; Miceli, N.; Taviano, M.F.; Davì, F.; Kędzia, E.; Ekiert, H. In Vitro Cultures of Scutellaria brevibracteata subsp. subvelutina as a Source of Bioactive Phenolic Metabolites. Molecules 2023, 28, 1785. [Google Scholar] [CrossRef]
- Hwang, H.D.; Kwon, S.H.; Murthy, H.N.; Yun, S.W.; Pyo, S.S.; Park, S.Y. Temporary immersion bioreactor system as an efficient method for mass production of in vitro plants in horticulture and medicinal plants. Agronomy 2022, 12, 346. [Google Scholar] [CrossRef]
- Petrasek, J.; Hoyerova, K.; Motyka, V.; Hejatko, J.; Dobrev, P.; Kaminek, M.; Vankova, R. Auxins and Cytokinins in Plant Development 2018. Int. J. Mol. Sci. 2019, 20, 909. [Google Scholar] [CrossRef]
- Dyduch-Siemińska, M. A fast and effective protocol for obtaining genetically diverse stevia (Stevia rebaudiana Bertoni) regenerants through indirect organogenesis. Agron. Sci. 2021, 76, 47–62. [Google Scholar] [CrossRef]
- Gharari, Z.; Bagheri, K.; Sharafi, A.; Danafar, H. Thidiazuron induced efficient in vitro organogenesis and regeneration of Scutellaria bornmuelleri: An important medicinal plant. In Vitro Cell. Dev. Biol.-Plant 2019, 55, 133–138. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Kuźma, Ł.; Wysokińska, H. The influence of cytokinins on proliferation and polyphenol accumulation in shoot cultures of Scutellaria altissima L. Phytochem. Lett. 2017, 20, 449–455. [Google Scholar] [CrossRef]
- Rai, M.K. Start codon targeted (SCoT) polymorphism marker in plant genome analysis: Current status and prospects. Planta 2023, 257, 34. [Google Scholar] [CrossRef] [PubMed]
- Rathore, M.S.; Chikara, J.; Mastan, S.G.; Rahman, H.; Anand, K.G.V.; Shekhawat, N.S. Assessment of genetic stability and instability of tissue culture-propagated plantlets of Aloe vera L. by RAPD and ISSR markers. Appl. Biochem. Biotechnol. 2011, 165, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Rawat, J.M.; Rawat, B.; Mehrotra, S.; Chandra, A.; Nautiyal, S. ISSR and RAPD based evaluation of genetic fidelity and active ingredient analysis of regenerated plants of Picrorhiza kurroa. Acta Physiol Plant 2013, 35, 1797–1805. [Google Scholar] [CrossRef]
- Mo, X.-Y.; Long, T.; Liu, Z.; Lin, H.; Liu, X.-Z.; Yang, Y.-M.; Zhang, H.-Y. AFLP analysis of somaclonal variations in Eucalyptus globulus. Biol. Plant. 2009, 53, 741–744. [Google Scholar] [CrossRef]
- Mizia, P.; Kwolek, D.; Ilnicki, T. DNA stability contrasts with chromosome variability in Allium fistulosum calli. Acta Biol. Cracoviensia Ser. Bot. 2014, 56, 66–72. [Google Scholar] [CrossRef]
- Zayova, E.; Vassilevska, I.R.; Kraptchev, B.; Stoeva, D. Somaclonal variations through indirect organogenesis in eggplant (Solanum melongena L.). Biol. Divers. Conserv. 2010, 3, 1–5. [Google Scholar] [CrossRef]
- Saravanan, S.; Sarvesan, R.; Vinod, M.S. Identification of DNA elements involved in somaclonal variants of Rauvolfia serpentine (L.) arising from indirect organogenesis as evaluated by ISSR analysis. Indian J. Sci. Technol. 2011, 4, 1241–1245. [Google Scholar] [CrossRef]
- Sun, S.; Zhong, J.; Li, S.; Wang, X. Tissue culture-induced somaclonal variation of decreased pollen viability in torenia (Torenia fournieri Lind.). Bot. Stud. 2013, 54, 36. [Google Scholar] [CrossRef] [PubMed]
- Sales, E.K.; Butardo, N.G. Molecular analysis of somaclonal variation in tissue culture derived bananas using MSAP and SSR markers. Int. J. Biol. Vet. Agric. Food Eng. 2014, 8, 63–610. [Google Scholar]
- Corpes, R.S.; Santos, A.S. Influência dos reguladores de crescimento 2, 4-D e BAP associados aos agentes solidificantes ágar e Phytagel na indução de calos de Crinum americanum L. (Amaryllidaceae). Res. Soc. Dev. 2021, 10, e299101220378. [Google Scholar] [CrossRef]
- Ramírez-Mosqueda, M.A.; Iglesias-Andreu, L.G. Indirect organogenesis and assessment of somaclonal variation in plantlets of Vanilla planifolia Jacks. Plant Cell Tissue Organ Cult. (PCTOC) 2015, 123, 657–664. [Google Scholar] [CrossRef]
- Liberatore, C.M.; Rodolfi, M.; Beghè, D.; Fabbri, A.; Ganino, T.; Chiancone, B. In vitro leaf-derived organogenesis and somaclonal variant detection in Humulus lupulus L. In Vitro Cell. Dev. Biol. Plant 2020, 56, 865–874. [Google Scholar] [CrossRef]
- Novikova, T.I.; Asbaganov, S.V.; Ambros, E.V.; Zaytseva, Y.G. TDZ-induced axillary shoot proliferation of Rhododendron mucronulatum Turcz and assessment of clonal fidelity using DNA-based markers and flow cytometry. In Vitro Cell. Dev. Biol. Plant 2020, 56, 307–317. [Google Scholar] [CrossRef]
- Roux, N.; Chase, R.; Van den Houwe, I.; Chao, C.P.; Perrier, X.; Jacquemoud-Collet, J.P.; Sardos, J.; Rouard, M. Somaclonal variation in clonal crops: Containing the bad, exploring the good. In Mutation Breeding, Genetic Diversity and Crop Adaptation to Climate Change; Sivasankar, S., Ellis, N., Jankuloski, L., Eds.; CABI: Wallingford, UK, 2021; pp. 355–365. [Google Scholar]
- Gao, X.; Yang, D.; Cao, D.; Ao, M.; Sui, X.; Wang, Q.; Kimatu, J.N.; Wang, L. In Vitro Micropropagation of Freesia hybrida and the Assessment of Genetic and Epigenetic Stability in Regenerated Plantlets. J. Plant Growth Regul. 2010, 29, 257–267. [Google Scholar] [CrossRef]




| No. | Starter Numer | Sequence (5′-3′) |
|---|---|---|
| 1 | SCoT-4 | CAACAATGGCTACCACCT |
| 2 | SCoT-12 | ACGACATGGCGACCAACG |
| 3 | SCoT-14 | ACGACATGGCGACCACGC |
| 4 | SCoT-16 | ACCATGGCTACCACCGAC |
| 5 | SCoT-24 | CACCATGGCTACCACCAT |
| 6 | SCoT-28 | CCATGGCTACCACCGCCA |
| 7 | SCoT-30 | CCATGGCTACCACCGGCG |
| 8 | SCoT-33 | CCATGGCTACCACCGCAG |
| 9 | SCoT-46 | ACAATGGCTACCACTGAG |
| 10 | SCoT-50 | ACAATGGCTACCACTGGG |
| 11 | SCoT-71 | CCATGGCTACCACCGCCG |
| 12 | SCoT-75 | CCATGGCTACCACCGGAG |
| Step | Temperature | Time |
|---|---|---|
| Initial denaturation | 94 °C | 3 min |
| No. of cycles = 35 cycles | ||
| Denaturation | 94 °C | 1 min |
| Annealing | 50 °C | 1 min |
| Extension | 72 °C | 2 min |
| Final extension | 72 °C | 5 min |
| Medium Variant | Mean Number of Shoots per Explant | Mean Shoot Length (mm) | Mean Number of Nodes per Shoot | Calli Formation ** | Average Weight of Regenerants (g) |
|---|---|---|---|---|---|
| MS “0” | 2.4 D * | 22.0 CD | 2.4 C | 1 | 0.09 E |
| MS + KIN 0.25 | 3.6 B–D | 13.1 DE | 2.1 C | 1 | 0.18 DE |
| MS + KIN 0.5 | 2.4 D | 29.6 BC | 3.5 BC | 1 | 0.14 E |
| MS + KIN 1 | 3.0 B–D | 25.9 CD | 3.0 C | 1 | 0.19 DE |
| MS + KIN 2 | 2.1 D | 40.3 AB | 4.9 AB | 1 | 0.22 DE |
| MS + BAP 0.25 | 2.7 CD | 50.0 A | 6.0 A | 2 | 0.35 CD |
| MS + BAP 0.5 | 2.7 CD | 25.5 CD | 2.9 C | 3 | 0.43 C |
| MS + BAP 1 | 5.8 A | 15.0 DE | 2.6 C | 4 | 0.88 B |
| MS + BAP 2 | 4.3 *** A–C | 5.0 *** E | 0.0 D | 4 | 1.18 A |
| Characteristics | Mean Number of Shoots per Explant | Mean Shoot Length | Mean Number of Nodes per Shoot | Calli Formation | Average Weight of Regenerants |
|---|---|---|---|---|---|
| Mean number of shoots per explant | 1.00 | −0.59 ns | −0.49 ns | 0.71 * | 0.30 ns |
| Mean shoot Length | - | 1.00 | 0.97 * | −0.28 ns | −0.09 ns |
| Mean number of nodes per shoot | 1.00 | −0.26 ns | −0.19 ns | ||
| Calli formation | 1.00 | 0.78 * | |||
| Average weight of regenerants | 1.00 |
| Name of Starter | Number of PCR Product | % of Polymorphism | Size Range (bp) | |||
|---|---|---|---|---|---|---|
| Total | Polymorphic Bands | Monomorphic Bands | Specyfic Bands | |||
| SCoT-4 | 15 | 2 | 11 | 2 | 13.3 | 600–7600 |
| SCoT-12 | 11 | 3 | 8 | 0 | 27.3 | 200–4200 |
| SCoT-14 | 10 | 1 | 9 | 0 | 10.0 | 410–4000 |
| SCoT-16 | 6 | 4 | 2 | 0 | 66.7 | 930–6700 |
| SCoT-24 | 7 | 2 | 5 | 0 | 28.6 | 770–6000 |
| SCoT-28 | 11 | 1 | 10 | 0 | 9.0 | 470–7600 |
| SCoT-30 | 5 | 2 | 3 | 0 | 40.0 | 400–2100 |
| SCoT-33 | 9 | 3 | 5 | 1 | 33.3 | 750–3300 |
| SCoT-46 | 11 | 4 | 7 | 0 | 36.3 | 450–6200 |
| SCoT-50 | 8 | 4 | 4 | 0 | 50.0 | 300–5800 |
| SCoT-71 | 7 | 5 | 2 | 0 | 71.4 | 450–4100 |
| SCoT-75 | 11 | 6 | 4 | 1 | 54.5 | 300–8000 |
| Total | 111 | 37 | 61 | 4 | - | 200–8000 |
| Mean | 9.25 | 3.08 | 5.08 | 0.33 | 36.7 | - |
| DP | MS “0” | MS + KIN | MS + BAP | |
|---|---|---|---|---|
| DP | 1.0 | 0.84 | 0.76 | 0.58 |
| MS “0” | 1.0 | 0.80 | 0.47 | |
| MS + KIN | 1.0 | 0.67 | ||
| MS + BAP | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyduch-Siemińska, M.; Gawroński, J. The Influence of Cytokinin on the Multiplication Efficiency and Genetic Stability of Scutellaria baicalensis Regenerants in In Vitro Culture Conditions. Appl. Sci. 2024, 14, 4791. https://doi.org/10.3390/app14114791
Dyduch-Siemińska M, Gawroński J. The Influence of Cytokinin on the Multiplication Efficiency and Genetic Stability of Scutellaria baicalensis Regenerants in In Vitro Culture Conditions. Applied Sciences. 2024; 14(11):4791. https://doi.org/10.3390/app14114791
Chicago/Turabian StyleDyduch-Siemińska, Magdalena, and Jacek Gawroński. 2024. "The Influence of Cytokinin on the Multiplication Efficiency and Genetic Stability of Scutellaria baicalensis Regenerants in In Vitro Culture Conditions" Applied Sciences 14, no. 11: 4791. https://doi.org/10.3390/app14114791







