Growth, Fatty Acid Profile and Malondialdehyde Concentration of Meagre Argyrosomus regius Fed Diets with Different Lipid Content
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Location
2.2. Experimental Fish and Feeding Trial
2.3. Sample Preparation and Analysis
2.4. Statistical Analysis
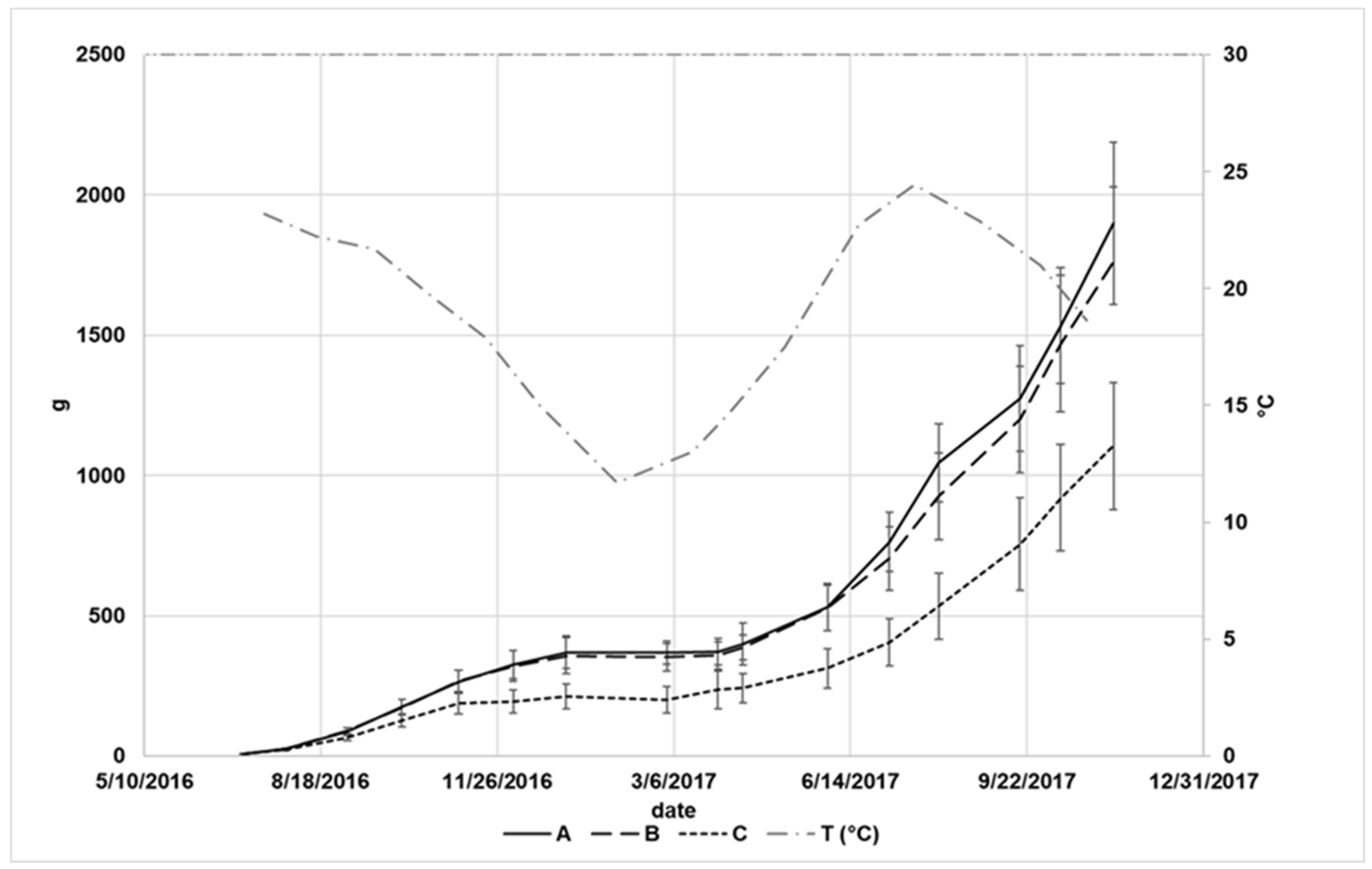
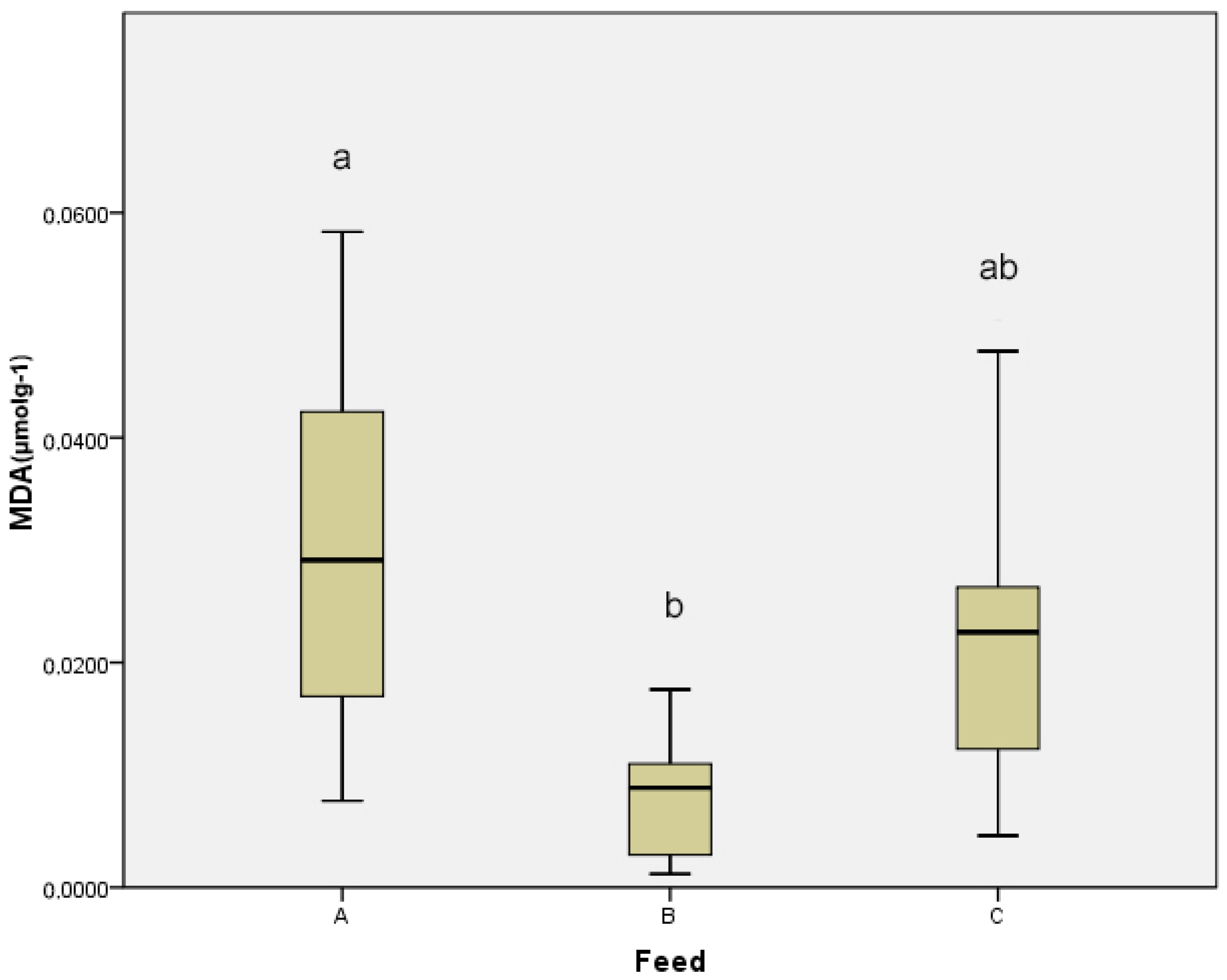
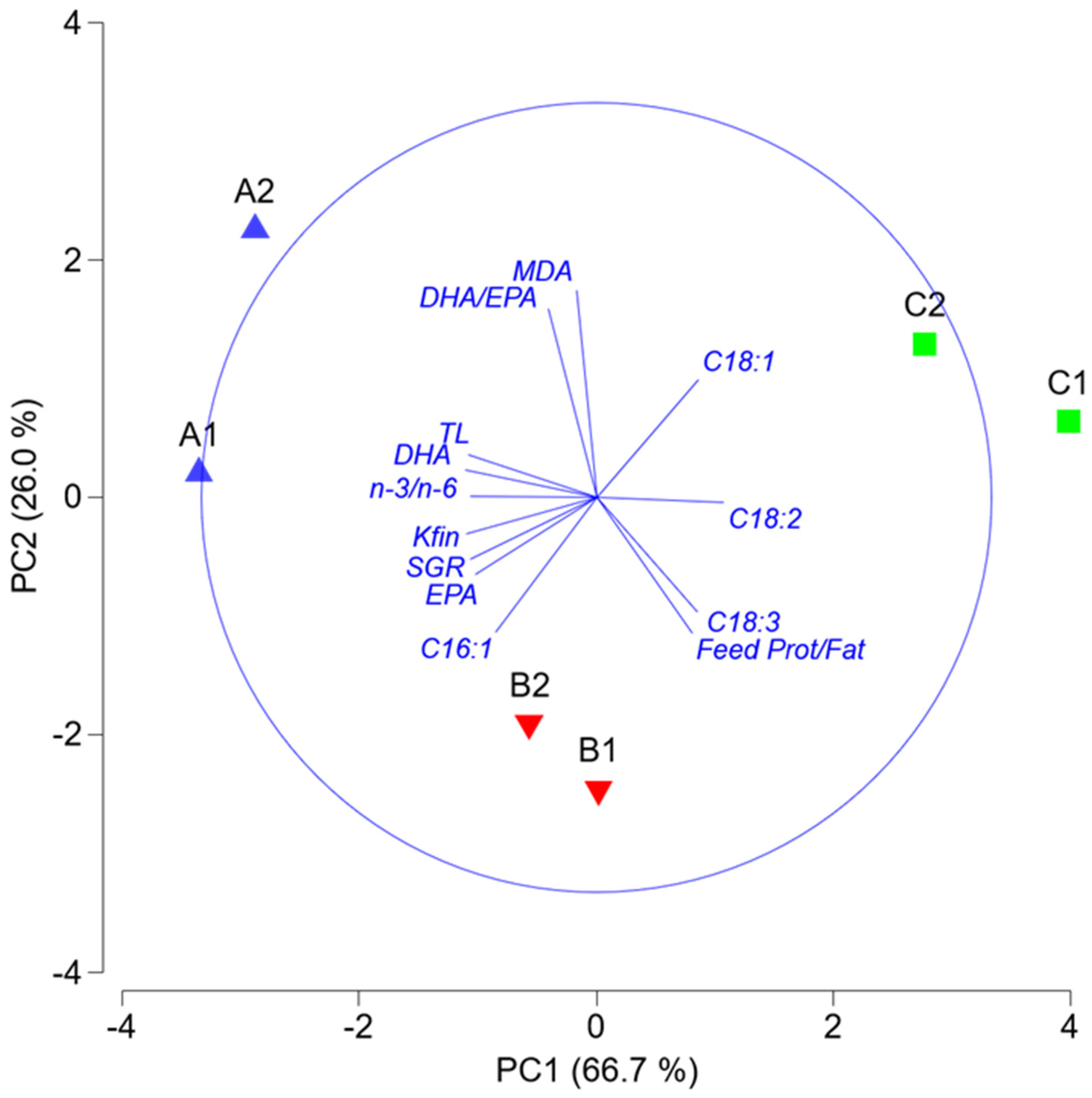
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anastasiades, G. Biodiversity in Eastern Mediterranean Marine Aquaculture: An Approach to New Species. Ph.D. Thesis, University of Insubria, Department of Biotechnology and Molecular Sciences, Varese, Italy, 2010; p. 112. [Google Scholar]
- Mosqueira, M.; Pombo, A.; Borges, C.; Brito, A.C.; Zacarias, N.; Esteves, R.; Palma, C. Potential for Coastal and Offshore Aquaculture in Portugal: Insights from Physico-Chemical and Oceanographic Conditions. Appl. Sci. 2022, 12, 2742. [Google Scholar] [CrossRef]
- Haffray, P.; Malha, R.; Sidi, M.O.T.; Prista, N.; Hassan, M.; Castelnaud, G.; Karahan-Nomm, B.; Gamsiz, K.; Sadek, S.; Bruant, J.S.; et al. Very high genetic fragmentation in a large marine fish, the meagre Argyrosomus regius (Sciaenidae, Perciformes): Impact of reproductive migration, oceanographic barriers and ecological factors. Aquat. Living Resour. 2012, 25, 173–183. [Google Scholar] [CrossRef]
- FAO (2005–2011). Cultured Aquatic Species Information Programme. Argyrosomus regius. Cultured Aquatic Species Information Programme. Text by Stipa, O. Angelini, M In: FAO Fisheries and Aquaculture Department (Online). Rome. Updated 10 February 2005. Available online: http://www.fao.org/fishery/culturedspecies/Argyrosomus_regius/en (accessed on 10 October 2023).
- Papadakis, I.; Kentouri, M.; Divanach, P.; Mylonas, C.C. Ontogeny of the digestive system of meagre Argyrosomus regius reared in a mesocosm, and quantitative changes of lipids in the liver from hatching to juvenile. Aquaculture 2013, 388–391, 76–88. [Google Scholar] [CrossRef]
- Duncan, N.J.; Estévez, A.; Fernández-Palacios, H.; Gairin, I.; Hernández-Cruz, C.M.; Roo, J.; Schuchardt, D.; Vallés, R. Aquaculture production of meagre (Argyrosomus regius): Hatchery techniques, ongrowing and market. In Advances in Aquaculture Hatchery Technology; Alan, G., Gavin, B., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 519–541. [Google Scholar]
- Fountoulaki, E.; Grigorakis, K.; Kounna, C.; Rigos, G.; Papandroulakis, N.; Diakogeorgakis, J.; Kokou, F. Growth performance and product quality of meagre (Argyrosomus regius) fed diets of different protein/lipid levels at industrial scale. Ital. J. Anim. Sci. 2017, 16, 685–694. [Google Scholar] [CrossRef]
- Carvalho, M.; Peres, H.; Saleh, R.; Fontanillas, R.; Rosenlund, G.; Oliva-Teles, A.; Izquierdo, M. Dietary requirement for n-3 long-chain polyunsaturated fatty acids for fast growth of meagre (Argyrosomus regius, Asso 1801) fingerlings. Aquaculture 2018, 488, 105–113. [Google Scholar] [CrossRef]
- Monfort, M.C. Present market situation and prospects of meagre (Argyrosomus regius), as an emerging species in Mediterranean aquaculture. In Studies and Reviews, General Fisheries Commission for the Mediterranean No. 89; FAO: Roma, Italy, 2010; p. 28. [Google Scholar]
- Martinez Llorens, S.; Espert Real, J.; Moya, V.; Moya Salvador, V.J.; Jover Cerda, M.; Tomas Vidal, A. Growth and nutrient efficiency of meagre (Argyrosomus regius, Asso, 1801) fed extruded diets with different protein and lipid levels. Int. J. Fish. Aquac. 2011, 3, 195–203. [Google Scholar]
- Hernandez, M.D.; Lopez, M.B.; Alvarez, A.; Ferrandini, E.; Garcia Garcia, B.; Garrido, M.D. Sensory, physical, chemical and microbiological changes in aquacultured meagre (Argyrosomus regius) fillets during ice storage. Food Chem. 2009, 114, 237–245. [Google Scholar] [CrossRef]
- Grigorakis, K.; Fountoulaki, E.; Vasilaki, A.; Mittakos, I.; Nathanailides, C. Lipid quality and filleting yield of reared meagre (Argyrosomus regius). Int. J. Food Sci. Technol. 2011, 46, 711–716. [Google Scholar] [CrossRef]
- Parisi, G.; Teroa, G.; Gasco, L.; Piccolo, G.; Roncarati, A.; Moretti, V.M.; Centoducati, G.; Gatta, P.P.; Pais, A. Current status and future perspectives of Italian finfish aquaculture. Rev. Fish Biol. Fish. 2014, 24, 15–73. [Google Scholar] [CrossRef]
- Kružić, N.; Mustać, B.; Župan, I.; Čolak, S. Meagre (Argyrosomus regius Asso, 1801) aquaculture in Croatia. Croat. J. Fish. 2016, 74, 14–19. [Google Scholar] [CrossRef]
- Hixson, S.M. Fish nutrition and current issues in aquaculture: The balance in providing safe and nutritious seafood, in an environmentally sustainable manner. J. Aquac. Res. Dev. 2014, 5, 1000234. [Google Scholar] [CrossRef]
- Ayala, M.D.; López-Albors, O.; Blanco, A.; Garcia-Alcázar, A.; Abellán, E.; Ramirez-Zarzosa, G.; Gil, F. Structural and ultrastructural changes on muscle tissue of sea bass, Dicentrarchus labrax L., after cooking and freezing. Aquaculture 2005, 250, 215–231. [Google Scholar] [CrossRef]
- Sargent, J.R.; Tocher, D.R.; Bell, G.J. The lipids. In Fish Nutrition; Halver, J.E., Hardy, R.W., Eds.; Academic Press: Cambridge, MA, USA, 2002; pp. 182–257, An Imprint of Elsevier Science; Available online: http://hdl.handle.net/1893/2926 (accessed on 23 November 2023).
- Glencross, B.D. A feed is still only as good as its ingredients: An update on the nutritional research strategies for the optimal evaluation of ingredients for aquaculture feeds. Aquac. Nutr. 2020, 26, 1871–1883. [Google Scholar] [CrossRef]
- Piccolo, G.; Bovera, F.; De Riu, N.; Marono, S.; Salati, F.; Cappuccinelli, R.; Moniello, G. Effect of two different protein/fat ratios of the diet on meagre (Argyrosomus regius) traits. Ital. J. Anim. Sci. 2008, 7, 363–371. [Google Scholar] [CrossRef]
- Craig, S. Understanding Fish Nutrition, Feeds, and Feeding; College of Agriculture and Life Sciences, Virginia Tech: Blacksburg, VA, USA, 2017; Volume 420, pp. 1–6. [Google Scholar]
- Kaur, G.; Guo, X.; Sinclair, A.J. Short update on docosapentaenoic acid: A bioactive long-chain n-3 fatty acid. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 88–91. [Google Scholar] [CrossRef]
- Senadheera, T.R.L.; Hossain, A.; Shahidi, F. Marine Bioactives and Their Application in the Food Industry: A Review. Appl. Sci. 2023, 13, 12088. [Google Scholar] [CrossRef]
- Auchterlonie, N. The continuing importance of fishmeal and fish oil in aquafeeds. In Proceedings of the Aquafarm Conference, Pordenone, Italy, 15–16 February 2020; Available online: www.iffo.net/iffo-presentations (accessed on 10 November 2023).
- De Silva, S.; Francis, D.S.; Tacon, A.G.J. Fish oil in aquaculture: In retrospect. In Fish Oil Replacement and Alternative Lipid Sources in Aquaculture Feeds; Turchini, G.M., Ng, W.-K., Tocher, D.R., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 1–20. [Google Scholar] [CrossRef]
- Ackman, R.G. Fatty acids in fish and shellfish. In Fatty Acids in Foods and Their Health Implications, 3rd ed.; Chow, C.K., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 155–185. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018; p. 227.
- Sies, H.; Stahl, W.; Sevanian, A. Nutritional, dietary and postprandial oxidative stress. J. Nutr. 2005, 135, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, T.W. The Biochemistry of the Carotenoids, Volume II Animals; Chapman and Hall: London, UK, 1984; p. 224. [Google Scholar] [CrossRef]
- Draper, H.H.; Squires, E.J.; Mahmooch, H.; Wu, J.; Agarwal, S.; Handley, M. A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free Radic. Biol. Med. 1993, 15, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Janero, D.P. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef]
- Cordiano, R.; Di Gioacchino, M.; Mangifesta, R.; Panzera, C.; Gangemi, S.; Minciullo, P.L. Malondialdehyde as a Potential Oxidative Stress Marker for Allergy-Oriented Diseases: An Update. Molecules 2023, 28, 5979. [Google Scholar] [CrossRef]
- Directive 2004/48/EC (2004); Corrigendum to Directive 2004/48/EC of the European Parliament and of the Council of 29 April 2004 on the Enforcement of Intellectual Property Rights. Edward Elgar Publishing: Cheltenham, UK, 2017.
- NN 102/2017 Animal Protection Act—Narodne Novine. Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2017_10_102_2342.html (accessed on 4 November 2023). (In Croatian).
- European Commission. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, 50, 33–79. [Google Scholar]
- Groto, D.; Santa Maria, L.D.; Boeira, S.; Valentini, J.; Charao, M.F.; Moro, A.M.; Nascimento, P.C.; Pomblum, V.J.; Garcia, S.C. Rapid quantification of malondialdehyde in plasma by high performance liquid chromatography-visible detection. J. Pharm. Biomed. Anal. 2007, 42, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.D.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Morrison, W.R.; Smith, L.M. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride-methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [CrossRef]
- Chatzifotis, S.; Panagiotidou, M.; Divanach, P. Effect of protein and lipid dietary levels on the growth of juvenile meagre (Argyrosomus regius). Aquac. Int. 2012, 20, 91–98. [Google Scholar] [CrossRef]
- Fei, S.; Xia, Y.; Chen, Z.; Liu, C.; Liu, H.; Han, D.; Jin, J.; Yang, Y.; Zhu, X.; Xie, S. A high-fat diet alters lipid accumulation and oxidative stress and reduces the disease resistance of overwintering hybrid yellow catfish (Pelteobagrus fulvidraco♀ × P. vachelli♂). Aquac. Rep. 2022, 23, 101043. [Google Scholar] [CrossRef]
- Tocher, D.R. Metabolism and Functions of Lipids and Fatty Acids in Teleost Fish. Rev. Fish. Sci. 2003, 11, 107–184. [Google Scholar] [CrossRef]
- Leaver, M.J.; Bautista, J.M.; Björnsson, B.T.; Jönsson, E.; Krey, G.; Tocher, D.R.; Torstensen, B. Towards Fish Lipid Nutrigenomics: Current State and Prospects for Fin-Fish Aquaculture. Rev. Fish. Sci. 2008, 16, 73–94. [Google Scholar] [CrossRef]
- Castro, C.; Corraze, G.; Panserat, S.; Oliva-Teles, A. Effects of fish oil replacement by a vegetable oil blend on digestibility, postprandial serum metabolite profile, lipid and glucose metabolism of European sea bass (Dicentrarchus labrax) juveniles. Aquac. Nutr. 2014, 21, 592–603. [Google Scholar] [CrossRef]
- Cook, H.W.; McMaster, R.C.R. Biochemistry of Lipids, Lipoproteins, and Membranes; Vance, D.E., Vance, J.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 181–204. [Google Scholar] [CrossRef]
- Poli, B.M.; Parisi, G.; Zampacavallo, G.; Iurzan, F.; Mecatti, M.; Lupi, P.; Bonelli, A. Preliminary results on quality and quality changes in reared meagre (Argyrosomus regius): Body and fillet traits and freshness changes in refrigerated commercial-size fish. Aquac. Int. 2003, 11, 301–311. [Google Scholar] [CrossRef]
- FAO/WHO. Diet, Nutrition, and the Prevention of Chronic Diseases; WHO Technical Reper Series; WHO: Geneva, Switzerland, 2003; p. 916.
- Biandolino, F.; Prato, E.; Grattagliano, A.; Parlapiano, I. Effect of Different Cooking Methods on Lipid Content and Fatty Acid Profile of Red Mullet (Mullus barbatus). Pol. J. Food Nutr. Sci. 2023, 73, 59–69. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: Nutritional implications for chronic diseases. Biomed. Pharmacother. 2006, 60, 502–507. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-3 fatty acids in health and disease and in growth and development, a review. Am. J. Clin. Nutr. 1991, 54, 438–463. [Google Scholar] [CrossRef] [PubMed]
- Jobling, J.; Leknes, O. Cod liver oil: Feed oil influences on fatty acid composition. Aquac. Int. 2010, 18, 223–230. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med. Cell. Longev. 2014, 31, 360438. [Google Scholar] [CrossRef] [PubMed]
- Secci, G.; Parisi, G. From farm to fork: Lipid oxidation in fish products. A review. Ital. J. Anim. Sci. 2016, 15, 124–136. [Google Scholar] [CrossRef]
- Chen, Y.J.; Liu, Y.J.; Yang, H.J.; Yuan, Y.; Liu, F.J.; Tian, L.X.; Liang, G.Y.; Yuan, R.M. Effect of dietary oxidized fish oil on growth performance, body composition, antioxidant defence mechanism and liver histology of juvenile largemouth bass Micropterus salmoides. Aquac. Nutr. 2012, 18, 321–331. [Google Scholar] [CrossRef]
- Halamičkova, A.; Vorlova, L.; Smutna, M.; Svobodova, Z.; Buchtova, H. Comparison of malondialdehyde concentration in different muscle areas of tench Tinca tinca L. Fish Physiol. Biochem. 2003, 29, 305–312. [Google Scholar] [CrossRef]
- Vinagre, C.; Madeira, D.; Narciso, L.; Cabral, H.N.; Diniz, M. Effect of temperature on oxidative stress in fish: Lipid peroxidation and catalase activity in the muscle of juvenile seabass, Dicentrarchus labrax. Ecol. Indic. 2012, 23, 274–279. [Google Scholar] [CrossRef]
- Tocher, D.R. Fatty acid requirements in ontogeny of marine and freshwater fish. Aquac. Res. 2010, 41, 717–732. [Google Scholar] [CrossRef]
- Montero, D.; Mathlouthi, F.; Tort, L.; Afonso, J.M.; Fernández-Vaquero, A.; Izquierdo, M.S. Replacement of dietary fish oil by vegetable oils affects humoral immunity and oxidative stress in gilthead seabream (Sparus aurata). Fish Shellfish Immunol. 2010, 29, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Bou, M.; Berge, G.M.; Baeverfjord, G.; Sigholt, T.; Østbye, T.-K.; Romarheim, O.H.; Hatlen, B.; Lee, Y.-S.; Ruyter, B. Requirement of n-3 very long-chain PUFA in Atlantic salmon (Salmo salar L.) post-smolt fed vegetable oil blends. Aquaculture 2017, 479, 638–649. [Google Scholar] [CrossRef]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef] [PubMed]

| Feed Mixtures | |||
|---|---|---|---|
| Proximal Composition (% of Diet) | A | B | C |
| Crude protein | 52.0 | 56.0 | 48.0 |
| Crude fat | 21.0 | 18.0 | 16.0 |
| Crude fiber | 0.7 | 0.4 | 3.0 |
| Ash | 11.4 | 10.4 | 8.3 |
| P | 1.6 | 1.65 | 1.12 |
| Ca | 2.4 | 2.11 | 1.87 |
| Na | 0.9 | 0.68 | 0.38 |
| Vitamins (UI/kg) * | |||
| Vit. A | 15,000 | 15,000 | 15,000 |
| Vit D3 | 2500 | 800 | 800 |
| Vit C | 500 | 150 | 200 |
| Vit E | 300 | 150 | 250 |
| Microelements (mg/kg) | |||
| Cu | 4.7 | 1.5 | 4.1 |
| Mn | - | 12 | 12 |
| Na (µg/kg) | 438 | - | - |
| Zn | 301 | 75 | 75 |
| I | 4.6 | 1.8 | 1.8 |
| Antioxidants (mg/kg) | |||
| Propyl galate | 22 | 100 | 100 |
| BHA ¥ | - | 100 | 100 |
| Citric acid | 20 | - | - |
| Group A | Group B | Group C | |
|---|---|---|---|
| Initial weight (g) | 6.74 ± 0.99 | 6.91 ± 1.07 | 6.85 ± 1.05 |
| Final weight (g) | 1899.51 a ± 289.10 | 1760.40 b ± 270.04 | 1105.13 c ± 227.16 |
| Final K | 1.28 a ± 0.07 | 1.25 b ± 0.07 | 1.17 c ± 0.10 |
| SGR | 1.184 | 1.177 | 1.137 |
| TGC | 1.223 | 1.216 | 1.118 |
| FCR | 2.04 | 2.25 | 2.41 |
| Feed Mixtures | p Level | ||||||
|---|---|---|---|---|---|---|---|
| A | B | C | |||||
| Fatty Acid | SD | SD | SD | ||||
| C14:0 | 3.03 a | 0.68 | 2.80 b | 0.70 | 1.97 c | 0.413 | *** |
| C15:0 | 0.42 a | 0.11 | 0.34 b | 0.16 | 0.33 b | 0.07 | ** |
| C16:3 (n-3) | 1.00 ab | 0.39 | 1.21 a | 0.35 | 0.79 b | 0.20 | *** |
| C16:1 (n-7) | 4.10 a | 0.89 | 3.95 a | 0.93 | 3.24 b | 0.51 | ** |
| C16:0 | 17.27 a | 1.06 | 17.45 a | 1.19 | 16.03 b | 0.87 | *** |
| C17:1 (n-9) | 0.74 | 0.45 | 0.86 | 0.64 | 0.62 | 0.26 | ns |
| C18:3 (n-3) | 2.20 | 1.18 | 3.72 | 2.11 | 3.05 | 2.24 | ns |
| C18:2 (n-6) | 5.86 b | 2.18 | 7.76 b | 2.23 | 10.48 a | 2.62 | * |
| C18:1 | 17.97 ab | 3.00 | 17.43 b | 2.80 | 21.05 a | 4.77 | * |
| C18:0 | 6.50 b | 0.67 | 6.71 a | 0.59 | 6.66 b | 0.77 | * |
| C20:4 (n-6)_ARA | 0.55 a | 0.07 | 0.53 a | 0.05 | 0.44 b | 0.05 | *** |
| C20:5 (n-3)_EPA | 11.65 a | 1.56 | 11.21 a | 1.10 | 9.34 b | 1.10 | *** |
| C20:3 (n-6) | 1.40 | 0.29 | 1.38 | 0.32 | 1.34 | 0.18 | ns |
| C20:1 (n-7) | 1.56 a | 0.31 | 1.17 b | 0.21 | 1.26 b | 0.16 | *** |
| C20:0 | 0.27 ab | 0.11 | 0.23 b | 0.08 | 0.33 a | 0.06 | *** |
| C22:6 (n-3)_DHA | 21.59 a | 3.46 | 19.69 a | 3.61 | 17.29 b | 3.55 | ** |
| C22:4 (n-6) | 1.92 b | 0.17 | 1.65 a | 0.23 | 1.83 b | 0.25 | * |
| C22:1 (n-9) | 1.98 a | 0.54 | 1.17 b | 0.38 | 1.22 b | 0.29 | *** |
| C22:0 | 0.23 | 0.12 | 0.15 | 0.018 | 0.19 | 0.043 | ns |
| C24:1 | 0.68 a | 0.22 | 0.49 b | 0.13 | 0.46 b | 0.09 | *** |
| SFA | 27.49 a | 1.25 | 27.61 a | 1.50 | 25.43 b | 1.35 | *** |
| UNSFA | 72.51 b | 1.25 | 72.39 b | 1.50 | 74.57 a | 1.35 | *** |
| MUFA | 26.90 | 4.19 | 25.76 | 3.71 | 27.74 | 4.47 | ns |
| PUFA | 45.62 | 4.44 | 46.63 | 4.65 | 46.83 | 5.13 | ns |
| n-6 | 9.19 b | 4.58 | 12.34 ab | 4.42 | 14.50 a | 7.16 | ** |
| n-3 | 35.48 a | 6.12 | 34.56 a | 6.28 | 30.65 b | 6.82 | * |
| n-3/n-6 | 3.86 a | 1.54 | 2.80 b | 1.13 | 2.11 c | 0.86 | ** |
| DHA/EPA | 1.96 | 0.40 | 1.70 | 0.28 | 1.83 | 0.22 | ns |
| Total lipids | 16.81 | 7.84 | 13.04 | 5.78 | 11.18 | 3.59 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matulić, D.; Blažina, M.; Pritišanac, E.; Čolak, S.; Bavčević, L.; Barić, R.; Križanac, S.; Vitlov, B.; Šuran, J.; Strunjak Perović, I.; et al. Growth, Fatty Acid Profile and Malondialdehyde Concentration of Meagre Argyrosomus regius Fed Diets with Different Lipid Content. Appl. Sci. 2024, 14, 4842. https://doi.org/10.3390/app14114842
Matulić D, Blažina M, Pritišanac E, Čolak S, Bavčević L, Barić R, Križanac S, Vitlov B, Šuran J, Strunjak Perović I, et al. Growth, Fatty Acid Profile and Malondialdehyde Concentration of Meagre Argyrosomus regius Fed Diets with Different Lipid Content. Applied Sciences. 2024; 14(11):4842. https://doi.org/10.3390/app14114842
Chicago/Turabian StyleMatulić, Daniel, Maria Blažina, Ena Pritišanac, Slavica Čolak, Lav Bavčević, Renata Barić, Silvia Križanac, Božena Vitlov, Jelena Šuran, Ivančica Strunjak Perović, and et al. 2024. "Growth, Fatty Acid Profile and Malondialdehyde Concentration of Meagre Argyrosomus regius Fed Diets with Different Lipid Content" Applied Sciences 14, no. 11: 4842. https://doi.org/10.3390/app14114842
APA StyleMatulić, D., Blažina, M., Pritišanac, E., Čolak, S., Bavčević, L., Barić, R., Križanac, S., Vitlov, B., Šuran, J., Strunjak Perović, I., & Tomljanović, T. (2024). Growth, Fatty Acid Profile and Malondialdehyde Concentration of Meagre Argyrosomus regius Fed Diets with Different Lipid Content. Applied Sciences, 14(11), 4842. https://doi.org/10.3390/app14114842







