Retained Placenta as a Potential Source of Mastitis Pathogens in Dairy Cows
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Study Design and Sample Collection
2.2. Gene Detection by Polymerase Chain Reaction
2.3. Bacterial Culture
2.4. Statistical Analyses
3. Results
3.1. Identification of Mastitis Pathogens and BlaZ Gene via RT-PCR and Their Association with Parity and Fever
3.2. Identification of Mastitis Pathogens with Bacterial Culture and Agreement between Methodologies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, W.N.; Han, S.G. Bovine Mastitis: Risk Factors, Therapeutic Strategies, and Alternative Treatments—A Review. Asian-Australas. J. Anim. Sci. 2020, 33, 1699–1713. [Google Scholar] [CrossRef] [PubMed]
- Wagener, K.; Grunert, T.; Prunner, I.; Ehling-Schulz, M.; Drillich, M. Dynamics of Uterine Infections with Escherichia coli, Streptococcus uberis and Trueperella pyogenes in Post-Partum Dairy Cows and Their Association with Clinical Endometritis. Vet. J. 2014, 202, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Lewis, G.S.; LeBlanc, S.; Gilbert, R.O. Defining Postpartum Uterine Disease in Cattle. Theriogenology 2006, 65, 1516–1530. [Google Scholar] [CrossRef] [PubMed]
- Dohmen, M.J.W.; Joop, K.; Sturk, A.; Bols, P.E.J.; Lohuis, J.A.C.M. Relationship between Intra-Uterine Bacterial Contamination, Endotoxin Levels and the Development of Endometritis in Postpartum Cows with Dystocia or Retained Placenta. Theriogenology 2000, 54, 1019–1032. [Google Scholar] [CrossRef]
- Silva, J.C.C.; Siqueira, L.C.; Rodrigues, M.X.; Zinicola, M.; Wolkmer, P.; Pomeroy, B.; Bicalho, R.C. Intrauterine Infusion of a Pathogenic Bacterial Cocktail Is Associated with the Development of Clinical Metritis in Postpartum Multiparous Holstein Cows. J. Dairy Sci. 2023, 106, 607–623. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Cronin, J.; Goetze, L.; Donofrio, G.; Schuberth, H.-J. Defining Postpartum Uterine Disease and the Mechanisms of Infection and Immunity in the Female Reproductive Tract in Cattle1. Biol. Reprod. 2009, 81, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Mahnani, A.; Sadeghi-Sefidmazgi, A.; Ansari-Mahyari, S.; Ghorbani, G.-R. Assessing the Consequences and Economic Impact of Retained Placenta in Holstein Dairy Cattle. Theriogenology 2021, 175, 61–68. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, S.J. Postpartum Uterine Disease and Dairy Herd Reproductive Performance: A Review. Vet. J. 2008, 176, 102–114. [Google Scholar] [CrossRef]
- Han, Y.K.; Kim, I.H. Risk Factors for Retained Placenta and the Effect of Retained Placenta on the Occurrence of Postpartum Diseases and Subsequent Reproductive Performance in Dairy Cows. J. Vet. Sci. 2005, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Ballas, P.; Gabler, C.; Wagener, K.; Drillich, M.; Ehling-Schulz, M. Streptococcus uberis Strains Originating from Bovine Uteri Provoke Upregulation of Pro-Inflammatory Factors mRNA Expression of Endometrial Epithelial Cells In Vitro. Vet. Microbiol. 2020, 245, 108710. [Google Scholar] [CrossRef] [PubMed]
- Paiano, R.B.; Moreno, L.Z.; Gomes, V.T.M.; Parra, B.M.; Barbosa, M.R.; Sato, M.I.Z.; Bonilla, J.; Pugliesi, G.; Baruselli, P.S.; Moreno, A.M. Assessment of the Main Pathogens Associated with Clinical and Subclinical Endometritis in Cows by Culture and MALDI-TOF Mass Spectrometry Identification. J. Dairy Sci. 2022, 105, 3367–3376. [Google Scholar] [CrossRef] [PubMed]
- Kaeberlein, T.; Lewis, K.; Epstein, S.S. Isolating “Uncultivable” Microorganisms in Pure Culture in a Simulated Natural Environment. Science 2002, 296, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.M.A.; Gilbert, R.O.; Bicalho, R.C. Metagenomic Analysis of the Uterine Bacterial Microbiota in Healthy and Metritic Postpartum Dairy Cows. J. Dairy Sci. 2011, 94, 291–302. [Google Scholar] [CrossRef]
- Compton, S.R. PCR and RT-PCR in the Diagnosis of Laboratory Animal Infections and in Health Monitoring. J. Am. Assoc. Lab. Anim. Sci. 2020, 59, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Vidal, S.; Kegler, K.; Posthaus, H.; Perreten, V.; Rodriguez-Campos, S. Amplicon Sequencing of Bacterial Microbiota in Abortion Material from Cattle. Vet. Res. 2017, 48, 64. [Google Scholar] [CrossRef] [PubMed]
- Machado, V.S.; Oikonomou, G.; Bicalho, M.L.S.; Knauer, W.A.; Gilbert, R.; Bicalho, R.C. Investigation of Postpartum Dairy Cows’ Uterine Microbial Diversity Using Metagenomic Pyrosequencing of the 16S rRNA Gene. Vet. Microbiol. 2012, 159, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.J.; Vieira-Neto, A.; Gobikrushanth, M.; Daetz, R.; Mingoti, R.D.; Parize, A.C.B.; de Freitas, S.L.; da Costa, A.N.L.; Bicalho, R.C.; Lima, S.; et al. Uterine Microbiota Progression from Calving until Establishment of Metritis in Dairy Cows. Appl. Environ. Microbiol. 2015, 81, 6324–6332. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Li, H.; Fu, K.; Pang, B.; Yang, Y.; Liu, Y.; Tian, W.; Cao, R. Characterization of the Cervical Bacterial Community in Dairy Cows with Metritis and during Different Physiological Phases. Theriogenology 2018, 108, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Çömlekcioğlu, U.; Jezierska, S.; Opsomer, G.; Pascottini, O.B. Uterine Microbial Ecology and Disease in Cattle: A Review. Theriogenology 2024, 213, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Savov, N.; Buchvarova, I. Relationship between infections of the genitalia and udder in cows. Vet. Med. Nauki 1977, 14, 43–46. [Google Scholar]
- Itoh, M.; Furuoka, M.; Baba, Y.; Saitoh, T.; Hata, E.; Hirano, Y.; Senna, K.; Yamada, K. Relationship between Genital Carriage and Udder Infection with Mycoplasma bovigenitalium in Dairy Farms. Jpn. J. Vet. Res. 2021, 69, 83–87. [Google Scholar] [CrossRef]
- Bicalho, R.C.; Cheong, S.H.; Galvão, K.N.; Warnick, L.D.; Guard, C.L. Effect of Twin Birth Calvings on Milk Production, Reproductive Performance, and Survival of Lactating Cows. J. Am. Vet. M. Ass. 2007, 231, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Azawi, O.; Rahawy, M.; Hadad, J. Bacterial Isolates Associated with Dystocia and Retained Placenta in Iraqi Buffaloes. Reprod. Domest. Anim. 2008, 43, 286–292. [Google Scholar] [CrossRef] [PubMed]
- de Koff, E.M.; van Baarle, D.; van Houten, M.A.; Reyman, M.; Berbers, G.A.M.; van den Ham, F.; Chu, M.L.J.N.; Sanders, E.A.M.; Bogaert, D.; Fuentes, S. Mode of Delivery Modulates the Intestinal Microbiota and Impacts the Response to Vaccination. Nat. Commun. 2022, 13, 6638. [Google Scholar] [CrossRef] [PubMed]
- Wallet, F.; Loïez, C.; Renaux, E.; Lemaitre, N.; Courcol, R.J. Performances of VITEK 2 Colorimetric Cards for Identification of Gram-Positive and Gram-Negative Bacteria. J. Clin. Microbiol. 2005, 43, 4402–4406. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.L. Interrater Reliability: The Kappa Statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Várhidi, Z.; Csikó, G.; Bajcsy, Á.C.; Jurkovich, V. Uterine Disease in Dairy Cows: A Comprehensive Review Highlighting New Research Areas. Vet. Sci. 2024, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Rzewuska, M.; Kwiecień, E.; Chrobak-Chmiel, D.; Kizerwetter-Świda, M.; Stefańska, I.; Gieryńska, M. Pathogenicity and Virulence of Trueperella pyogenes: A Review. Int. J. Mol. Sci. 2019, 20, 2737. [Google Scholar] [CrossRef] [PubMed]
- Cobirka, M.; Tancin, V.; Slama, P. Epidemiology and Classification of Mastitis. Animals 2020, 10, 2212. [Google Scholar] [CrossRef] [PubMed]
- Hazelton, M.S.; Morton, J.M.; Bosward, K.L.; Sheehy, P.A.; Parker, A.M.; Dwyer, C.J.; Niven, P.G.; House, J.K. Mycoplasma Species in Vaginas of Dairy Cows before and after Exposure to Bulls and Their Association with Conception. J. Dairy Sci. 2020, 103, 11795–11805. [Google Scholar] [CrossRef] [PubMed]
- Gioia, G.; Addis, M.F.; Santisteban, C.; Gross, B.; Nydam, D.V.; Sipka, A.S.; Virkler, P.D.; Watters, R.D.; Wieland, M.; Zurakowski, M.J.; et al. Mycoplasma Species Isolated from Bovine Milk Collected from US Dairy Herds between 2016 and 2019. J. Dairy Sci. 2021, 104, 4813–4821. [Google Scholar] [CrossRef] [PubMed]
- Eiler, H.; Fecteau, K.A. Retained Placenta. In Current Therapy in Large Animal Theriogenology; Elsevier: Amsterdam, The Netherlands, 2007; pp. 345–354. ISBN 978-0-7216-9323-1. [Google Scholar]
- Saini, P.; Singh, M.; Kumar, P. Fungal Endometritis in Bovines. Open Vet. J. 2019, 9, 94. [Google Scholar] [CrossRef] [PubMed]
- Bicalho, M.L.S.; Santin, T.; Rodrigues, M.X.; Marques, C.E.; Lima, S.F.; Bicalho, R.C. Dynamics of the Microbiota Found in the Vaginas of Dairy Cows during the Transition Period: Associations with Uterine Diseases and Reproductive Outcome. J. Dairy Sci. 2017, 100, 3043–3058. [Google Scholar] [CrossRef] [PubMed]
- Bicalho, M.L.S.; Machado, V.S.; Oikonomou, G.; Gilbert, R.O.; Bicalho, R.C. Association between Virulence Factors of Escherichia coli, Fusobacterium necrophorum, and Arcanobacterium pyogenes and Uterine Diseases of Dairy Cows. Vet. Microbiol. 2012, 157, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Khatun, M.; Sørensen, P.; Ingvartsen, K.L.; Bjerring, M.; Røntved, C.M. Effects of Combined Liver and Udder Biopsying on the Acute Phase Response of Dairy Cows with Experimentally Induced E. coli Mastitis. Animal 2013, 7, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- Aditya, A.; Julianingsih, D.; Tabashsum, Z.; Alvarado-Martinez, Z.; Tung, C.-W.; Wall, M.; Biswas, D. Dominance of Diarrheagenic E. coli Virulent Types in Integrated Crop–Livestock Farms and Their Antibiotic Resistance Patterns. Zoonotic Dis. 2024, 4, 11–21. [Google Scholar] [CrossRef]
- Hansen, A.-M.; Chaerkady, R.; Sharma, J.; Díaz-Mejía, J.J.; Tyagi, N.; Renuse, S.; Jacob, H.K.C.; Pinto, S.M.; Sahasrabuddhe, N.A.; Kim, M.S.; et al. The Escherichia coli Phosphotyrosine Proteome Relates to Core Pathways and Virulence. PLoS Pathog. 2013, 9, e1003403. [Google Scholar] [CrossRef] [PubMed]
- Dervishi, E.; Zhang, G.; Hailemariam, D.; Dunn, S.M.; Ametaj, B.N. Occurrence of Retained Placenta Is Preceded by an Inflammatory State and Alterations of Energy Metabolism in Transition Dairy Cows. J. Animal Sci. Biotechnol. 2016, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Moyes, K.M.; Drackley, J.K.; Morin, D.E.; Rodriguez-Zas, S.L.; Everts, R.E.; Lewin, H.A.; Loor, J.J. Mammary Gene Expression Profiles during an Intramammary Challenge Reveal Potential Mechanisms Linking Negative Energy Balance with Impaired Immune Response. Physiol. Genom. 2010, 41, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Hillreiner, M.; Flinspach, C.; Pfaffl, M.W.; Kliem, H. Effect of the Ketone Body Beta-Hydroxybutyrate on the Innate Defense Capability of Primary Bovine Mammary Epithelial Cells. PLoS ONE 2016, 11, e0157774. [Google Scholar] [CrossRef] [PubMed]
- Swartz, T.H.; Bradford, B.J.; Mamedova, L.K. Connecting Metabolism to Mastitis: Hyperketonemia Impaired Mammary Gland Defenses during a Streptococcus uberis Challenge in Dairy Cattle. Front. Immunol. 2021, 12, 700278. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Kawahara, N.; Kim, Y.-H.; Ichijo, T.; Sato, S. Changes in Oxidative Stress Parameters in Healthy and Diseased Holstein Cows during the Transition Period in Yamagata Prefecture, Japan. J. Vet. Med. Sci. 2020, 82, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Malledevarahalli Chandrappa, S.; Pascottini, O.B.; Opsomer, G.; Meineri, G.; Martino, N.A.; Banchi, P.; Vincenti, L.; Ricci, A. Circulating and Endometrial Cell Oxidative Stress in Dairy Cows Diagnosed with Metritis. Theriogenology 2023, 198, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Turk, R.; Koledić, M.; Maćešić, N.; Benić, M.; Dobranić, V.; Đuričić, D.; Cvetnić, L.; Samardžija, M. The Role of Oxidative Stress and Inflammatory Response in the Pathogenesis of Mastitis in Dairy Cows. Mljekarstvo 2017, 67, 91–101. [Google Scholar] [CrossRef]
- Zdunczyk, S.; Ahlers, D.; Grunert, E. The relationship between bovine clinical mastitis at the time of parturition and retained placenta. Dtsch. Tierarztl. Wochenschr. 1992, 99, 386–389. [Google Scholar] [PubMed]
- Schukken, Y.H.; Erb, H.N.; David Smith, R. The Relationship between Mastitis and Retained Placenta in a Commercial Population of Holstein Dairy Cows. Prev. Vet. Med. 1988, 5, 181–190. [Google Scholar] [CrossRef]
- Suriyasathaporn, W.; Schukken, Y.H.; Nielen, M.; Brand, A. Low Somatic Cell Count: A Risk Factor for Subsequent Clinical Mastitis in a Dairy Herd. J. Dairy Sci. 2000, 83, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Ghavi Hossein-Zadeh, N.; Ardalan, M. Cow-Specific Risk Factors for Retained Placenta, Metritis and Clinical Mastitis in Holstein Cows. Vet. Res. Commun. 2011, 35, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Vanderhaeghen, W.; Piepers, S.; Leroy, F.; Van Coillie, E.; Haesebrouck, F.; De Vliegher, S. Identification, Typing, Ecology and Epidemiology of Coagulase Negative Staphylococci Associated with Ruminants. Vet. J. 2015, 203, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Addis, M.F.; Maffioli, E.M.; Ceciliani, F.; Tedeschi, G.; Zamarian, V.; Tangorra, F.; Albertini, M.; Piccinini, R.; Bronzo, V. Influence of Subclinical Mastitis and Intramammary Infection by Coagulase-Negative Staphylococci on the Cow Milk Peptidome. J. Proteom. 2020, 226, 103885. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.P.; Ramos, S.Á.; Cavalcanti Neto, C.C.; Dos Santos, T.M.C.; De Oliveira, J.A.C.; Dos Santos, M.T.; Da Silva, J.M.; Brito Neto, J.S.; Montaldo, Y.C. Vaginal Microbiota of Nulliparous and Multiparous Cows and Their Resistance to Antimicrobials. Med. Vet. 2019, 13, 474. [Google Scholar] [CrossRef]
- Garoussi, M.T.; Khosrave, A.R.; Havareshti, P. Mycoflora of Cervicovaginal Fluids in Dairy Cows with or without Reproductive Disorders. Mycopathologia 2007, 164, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza, C.; Cervantes Olivares, R.A.; Ducoing Watty, A.E.; de la Peña Moctezuma, A.; Villa Tanaca, L. Yeasts Isolation from Bovine Mammary Glands under Different Mastitis Status in the Mexican High Plateu. Rev. Iberoam. Micol. 2011, 28, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Ksouri, S.; Djebir, S.; Hadef, Y.; Benakhla, A. Survey of Bovine Mycotic Mastitis in Different Mammary Gland Statuses in Two North-Eastern Regions of Algeria. Mycopathologia 2015, 179, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Toyotome, T.; Matsui, S. Analysis of Prototheca and Yeast Species Isolated from Bulk Tank Milk Collected in Tokachi District, Japan. J. Dairy Sci. 2022, 105, 8364–8370. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, D.; Mizomoto, T.; Ueda, C.; Takagi, N.; Shimizu, N.; Matsuura, Y.; Makuuchi, Y.; Watanabe, A.; Shinozuka, Y.; Kawai, K. Factors Affecting the Incidence and Outcome of Trueperella pyogenes Mastitis in Cows. J. Vet. Med. Sci. 2017, 79, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Chirico, J.; Jonsson, P.; Kjellberg, S.; Thomas, G. Summer Mastitis Experimentally Induced by Hydrotaea irritans Exposed to Bacteria. Med. Vet. Entomol. 1997, 11, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Wald, R.; Baumgartner, M.; Gutschireiter, J.; Bazzanella, B.; Lichtmannsperger, K.; Wagner, M.; Wittek, T.; Stessl, B. Comparison of the Population Structure of Streptococcus uberis Mastitis Isolates from Austrian Small-Scale Dairy Farms and a Slovakian Large-Scale Farm. J. Dairy Sci. 2020, 103, 1820–1830. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.; Rodriguez-Lecompte, J.C.; Blanchard, A.; McClure, J.T.; Sánchez, J. Molecular Variability of Streptococcus uberis Isolates from Intramammary Infections in Canadian Dairy Farms from the Maritime Region. Can. J. Vet. Res. 2019, 83, 168–176. [Google Scholar] [PubMed]
- Fessia, A.S.; Odierno, L.M. Potential Factors Involved in the Early Pathogenesis of Streptococcus uberis Mastitis: A Review. Folia Microbiol. 2021, 66, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Blowey, R.; Edmondson, P. Bactoscan and Total Bacterial Count (TBC). In Mastitis Control in Dairy Herds; CABI: Wallingford, UK, 2010; pp. 171–183. [Google Scholar] [CrossRef]
- Abd El-Aziz, N.K.; Ammar, A.M.; El Damaty, H.M.; Abd Elkader, R.A.; Saad, H.A.; El-Kazzaz, W.; Khalifa, E. Environmental Streptococcus uberis Associated with Clinical Mastitis in Dairy Cows: Virulence Traits, Antimicrobial and Biocide Resistance, and Epidemiological Typing. Animals 2021, 11, 1849. [Google Scholar] [CrossRef] [PubMed]
- Bannerman, D.D.; Paape, M.J.; Goff, J.P.; Kimura, K.; Lippolis, J.D.; Hope, J.C. Innate Immune Response to Intramammary Infection with Serratia marcescens and Streptococcus uberis. Vet. Res. 2004, 35, 681–700. [Google Scholar] [CrossRef] [PubMed]
- Tassi, R.; McNeilly, T.N.; Fitzpatrick, J.L.; Fontaine, M.C.; Reddick, D.; Ramage, C.; Lutton, M.; Schukken, Y.H.; Zadoks, R.N. Strain-Specific Pathogenicity of Putative Host-Adapted and Nonadapted Strains of Streptococcus uberis in Dairy Cattle. J. Dairy Sci. 2013, 96, 5129–5145. [Google Scholar] [CrossRef]
- Muskens, J.; van Maanen, C.; Mars, M.H. Dairy Cows with Metritis: Coxiella burnetii Test Results in Uterine, Blood and Bulk Milk Samples. Vet. Microbiol. 2011, 147, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Kronfeld, H.; Kemper, N.; Hölzel, C.S. Vaginal and Uterine Microbiomes during Puerperium in Dairy Cows. Agriculture 2022, 12, 405. [Google Scholar] [CrossRef]
- Ashraf, A.; Imran, M.; Yaqub, T.; Tayyab, M.; Shehzad, W.; Thomson, P.C. A Novel Multiplex PCR Assay for Simultaneous Detection of Nine Clinically Significant Bacterial Pathogens Associated with Bovine Mastitis. Mol. Cell. Probes 2017, 33, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Imam, T.; Horsman, S.; Wood, B.; Grewar, J.D.; Langhorne, C.; Price, R.; Wood, C.; Henning, J.; Gibson, J.S. Assessment of Sensitivity and Specificity of Bacterial Culture and the VetMAXTM Mastitype Multi Kit in Detecting Streptococcus uberis and Escherichia coli in Milk Samples from Dairy Cows with Clinical Mastitis in Subtropical Australia. Soc. Sci. Res. Netw. 2024. preprint. [Google Scholar] [CrossRef]
| Bacteria Identified by RT-PCR | % Infected Cows (x/X) | 95% CI | Parity | Fever |
|---|---|---|---|---|
| Escherichia coli | 93.3 (14/15) | 70.2–98.9 | 0.12 | 0.28 |
| Staphylococcus spp. | 93.3 (14/15) | 70.2–98.9 | 0.12 | 0.28 |
| Yeasts * | 92.9 (13/14) | 68.5–98.7 | 0.34 | 0.18 |
| Trueperella pyogenes/Peptoniphilus indolicus | 80.0 (12/15) | 54.8–93.0 | 1.00 | 0.30 |
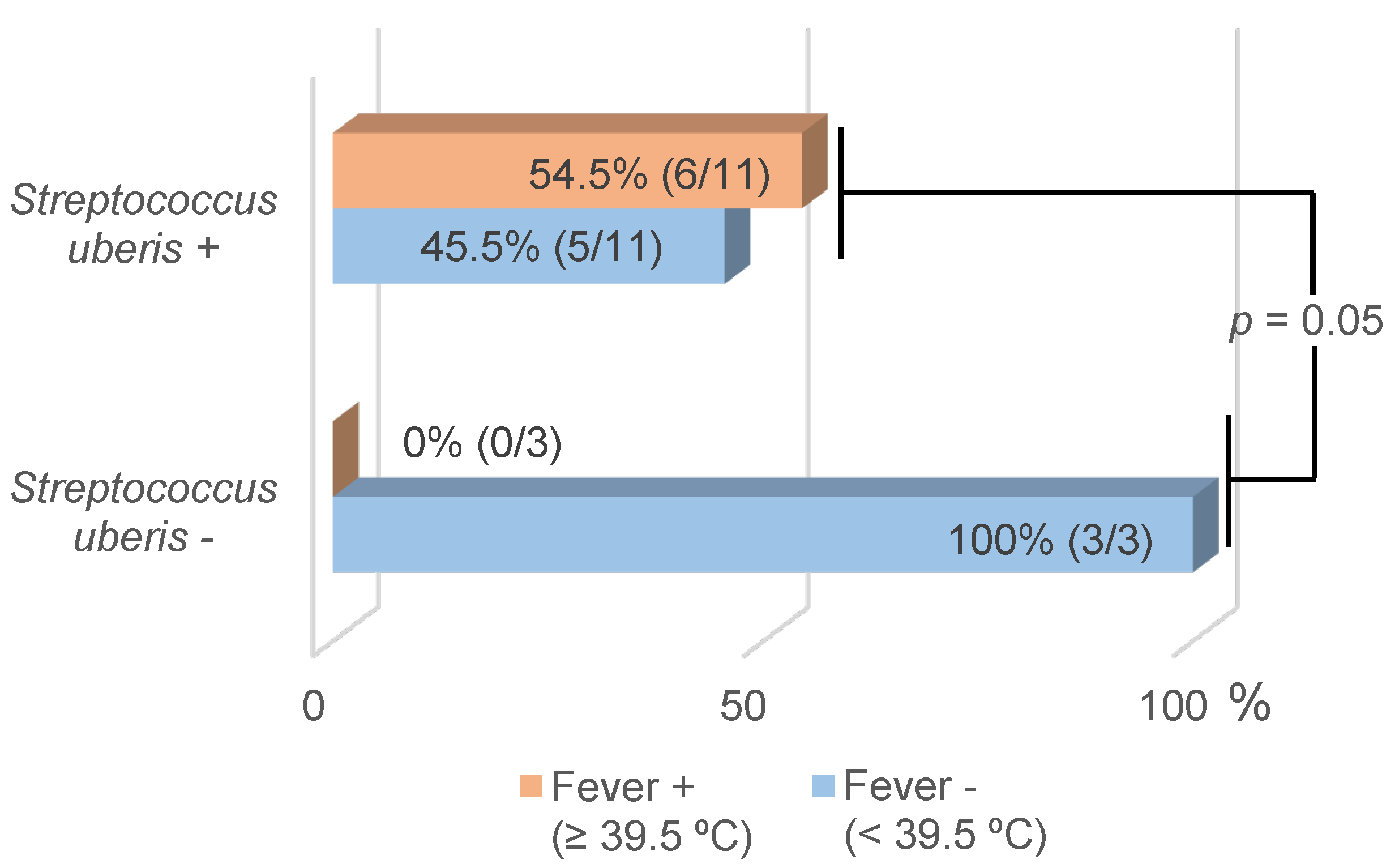
| Streptococcus uberis * | 78.6 (11/14) | 52.4–92.4 | 0.85 | 0.05 |
| Streptococcus dysgalactiae * | 57.1 (8/14) | 32.6–78.6 | 0.62 | 0.30 |
| Enterococcus spp. | 46.7 (7/15) | 24.8–69.9 | 0.71 | 0.83 |
| Mycoplasma spp. | 40.0 (6/15) | 19.8–64.3 | 0.26 | 0.50 |
| BlaZ gene * | 28.6 (4/14) | 11.7–54.7 | 0.60 | 0.75 |
| Streptococcus agalactiae | 6.7 (1/15) | 1.2–29.8 | 0.11 | 0.14 |
| Bacteria | Agreement between Methods | Cohen’s Kappa Coefficient | Number of Isolates BC- and RT-PCR+ (1) |
|---|---|---|---|
| E. coli (n = 15) | 93.3% | 0.64 (substantial) ** | 1 |
| Strep. uberis (n = 14) | 64.3% | 0.34 (fair) * | 5 |
| Enterococcus spp. (n = 15) | 60.0% | 0.15 (slight) | 6 |
| Strep. dysgalactiae (n = 14) | 50.0% | 0.11 (slight) | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, D.; Astiz, S.; Fernandez-Novo, A.; Margatho, G.; Simões, J. Retained Placenta as a Potential Source of Mastitis Pathogens in Dairy Cows. Appl. Sci. 2024, 14, 4986. https://doi.org/10.3390/app14124986
Ribeiro D, Astiz S, Fernandez-Novo A, Margatho G, Simões J. Retained Placenta as a Potential Source of Mastitis Pathogens in Dairy Cows. Applied Sciences. 2024; 14(12):4986. https://doi.org/10.3390/app14124986
Chicago/Turabian StyleRibeiro, Diana, Susana Astiz, Aitor Fernandez-Novo, Gisele Margatho, and João Simões. 2024. "Retained Placenta as a Potential Source of Mastitis Pathogens in Dairy Cows" Applied Sciences 14, no. 12: 4986. https://doi.org/10.3390/app14124986
APA StyleRibeiro, D., Astiz, S., Fernandez-Novo, A., Margatho, G., & Simões, J. (2024). Retained Placenta as a Potential Source of Mastitis Pathogens in Dairy Cows. Applied Sciences, 14(12), 4986. https://doi.org/10.3390/app14124986







