Abstract
Ensuring precise segmentation of colorectal polyps holds critical importance in the early diagnosis and treatment of colorectal cancer. Nevertheless, existing deep learning-based segmentation methods are fully supervised, requiring extensive, precise, manual pixel-level annotation data, which leads to high annotation costs. Additionally, it remains challenging to train large-scale segmentation models when confronted with limited colonoscopy data. To address these issues, we introduce the general segmentation foundation model—the Segment Anything Model (SAM)—into the field of medical image segmentation. Fine-tuning the foundation model is an effective approach to tackle sample scarcity. However, current SAM fine-tuning techniques still rely on precise annotations. To overcome this limitation, we propose WSPolyp-SAM, a novel weakly supervised approach for colonoscopy polyp segmentation. WSPolyp-SAM utilizes weak annotations to guide SAM in generating segmentation masks, which are then treated as pseudo-labels to guide the fine-tuning of SAM, thereby reducing the dependence on precise annotation data. To improve the reliability and accuracy of pseudo-labels, we have designed a series of enhancement strategies to improve the quality of pseudo-labels and mitigate the negative impact of low-quality pseudo-labels. Experimental results on five medical image datasets demonstrate that WSPolyp-SAM outperforms current fully supervised mainstream polyp segmentation networks on the Kvasir-SEG, ColonDB, CVC-300, and ETIS datasets. Furthermore, by using different amounts of training data in weakly supervised and fully supervised experiments, it is found that weakly supervised fine-tuning can save 70% to 73% of annotation time costs compared to fully supervised fine-tuning. This study provides a new perspective on the combination of weakly supervised learning and SAM models, significantly reducing annotation time and offering insights for further development in the field of colonoscopy polyp segmentation.
1. Introduction
Polyps are abnormal growths in the colon. They may worsen over time and potentially lead to colorectal cancer [1], a life-threatening disease. Currently, colonoscopy using medical imaging technology is considered one of the most effective screening and diagnostic methods, playing a pivotal role in early prevention [2]. Considering that the shape and size of polyps in colonoscopy images are highly correlated with polyp types, development stage, and ongoing treatment, accurately delineating the shape of polyps rather than just identifying their presence is essential. However, the precise identification and delineation of polyp contours in colonoscopy images, known as the instance segmentation process, often heavily rely on the expertise and experience of medical professionals. The complexity and variability of colonoscopy images often make traditional segmentation methods challenging to apply. The diversity of polyps (in terms of shape, texture, and size) poses significant challenges for the design and implementation of automatic segmentation algorithms.
In recent years, substantial advancements have been achieved in both semantic segmentation [3,4] and medical image segmentation [5,6] through the utilization of deep learning methodologies. Among these advancements, methods for colonoscopy polyp segmentation have also been widely explored [7,8]. Nevertheless, existing segmentation models often focus on specific imaging modalities and segmentation targets [9]. Constructing large-scale segmentation models necessitates a considerable volume of medical data. However, acquiring and annotating such data poses significant challenges and expenses, as they demand skilled physicians to conduct meticulous pixel-level annotations. To mitigate this challenge, fine-tuning of foundational models has emerged as an effective approach, particularly in scenarios with limited data availability [10]. In recent times, the Segment Anything Model (SAM) [11] has appeared as a noteworthy segmentation model. The SAM trained on a large-scale visual segmentation dataset exhibits robust generalization capabilities and adaptability to previously unseen data. Composed of an image encoder, a prompt encoder, and a mask decoder, SAM is adept at creating precise object masks derived from a variety of input cues, including points, bounding boxes, and existing masks. Considering its outstanding zero-shot segmentation performance, numerous researchers have begun fine-tuning SAM to tailor it for specific medical image segmentation domains. For instance, Hu et al. [12] directly fine-tuned SAM (excluding the image encoder) for skin cancer segmentation tasks, while Wu et al. [13] presented the Medical SAM Adapter (MSA), which embeds medical domain expertise by interspersing new components within the SAM’s encoder–decoder framework. While these approaches have achieved notable advancements, their reliance primarily on fully supervised learning entails a considerable investment of time and manpower for precise pixel-level annotations.
To reduce the dependence of SAM fine-tuning on precise pixel-level annotation data, this study proposes a SAM method for colonoscopy polyp segmentation called WSPolyp-SAM, based on weak supervision and self-guided fine-tuning. Specifically, the SAM method is guided to generate segmentation masks based on weak annotations (bounding box annotations in this study), which are then treated as pseudo-labels to guide the fine-tuning of SAM. Utilizing self-generated pseudo-labels for fine-tuning not only decreases the dependence on precisely annotated data but also avoids the need to introduce additional segmentation models. High-quality pseudo-labels provide additional information, helping the model to better capture specific features. To enhance the reliability and accuracy of pseudo-labels, this study designs three enhancement strategies. Firstly, employing a multi-augmentation fusion strategy involves generating multiple augmented views for each image and then fusing their corresponding segmentation masks. This approach highlights reliable prediction results and counteracts biases introduced by image augmentation, thereby enhancing the quality of the final fused mask. Secondly, incorporating a pixel-level weighting strategy involves assigning higher weights to pixels with high certainty predictions through an entropy-based pixel-level weighting mechanism, further improving the accuracy of the segmentation mask. Finally, we introduce mask post-processing techniques that can diminish potential noise and inaccuracies in the masks generated by SAM. These three strategies aim to optimize the details of the segmentation masks and improve their consistency with ground-truth labels.
The main contributions of this study include:
- The proposal of a novel weakly supervised and self-guided fine-tuning method of SAM for colonoscopy polyp segmentation (abbreviated as WSPolyp-SAM). This method reduces the dependence on precise annotations by fully utilizing SAM’s zero-shot ability to use weak annotations for guiding the generation of segmentation masks, which are then utilized as pseudo-labels for self-guided fine-tuning, avoiding the need to introduce additional segmentation models.
- The introduction of a series of pseudo-label enhancement strategies to generate high-quality pseudo-labels. These enhancement strategies, including multi-enhancement fusion, pixel-level weighting, and mask post-processing techniques, enable the acquisition of more accurate pseudo-labels.
- Experimental results on five medical image datasets demonstrate that WSPolyp-SAM outperforms current fully supervised mainstream polyp segmentation networks on the Kvasir-SEG, ColonDB, CVC-300, and ETIS datasets. Particularly, on the ColonDB dataset, our method demonstrated an improvement of 9.4% in mDice score and 9.6% in mIoU score compared to state-of-the-art networks, representing a significant breakthrough in the field of colonoscopy polyp segmentation. Furthermore, by using different amounts of training data in weakly supervised and fully supervised experiments, it is found that weakly supervised fine-tuning can save 70% to 73% of the cost of annotation time compared to fully supervised fine-tuning.
2. Related Work
2.1. Polyp Segmentation
Medical image segmentation continues to be a prominent area of interest in the realm of medical image analysis and processing. The ongoing advancements in deep learning have consistently spurred innovation, resulting in a proliferation of segmentation methodologies underpinned by neural networks. The fully convolutional neural network (FCN) [14] is the first end-to-end pixel-level classification method, which has opened a new paradigm in the segmentation field. UNet [15], a prominent variant of fully convolutional neural network (FCN), is renowned for its symmetric U-shaped encoder–decoder architecture, integrating skip connections to fuse deep and shallow features across different scales, making it a standard benchmark architecture for polyp segmentation methods. Successive adaptations, exemplified by UNet++ [16], ResUNet [17] and U2-Net [18] have aimed to enhance feature extraction, receptive field, and multi-scale information integration. Despite the progress in polyp segmentation, there remains a need for comprehensive environmental context around polyps. Various strategies have been explored to enhance polyp segmentation and boundary recognition, including dilated convolutions [19], integration of ASPP modules [20], and utilization of the RFB module [21]. Approaches like SFA [22] introduced a shared encoder branch and two decoder branches, along with an innovative edge-sensitive loss to enhance the precision of polyp segmentation and the clarity of boundary delineation. ACSNet [23] leveraged both local and global contextual features to guide encoder modules, with a particular emphasis on the edge region. CCBANet [24] obtained more comprehensive context awareness through cascaded context and balanced attention to improve segmentation performance.
Although these methods have made significant progress, they often require a large amount of training data to produce desirable results. This requirement is challenging for specific tasks, such as colonoscopy polyp segmentation, where the dataset is limited. To confront this challenge, this work fine-tunes the SAM model pre-trained on large-scale datasets so as to exploit its general knowledge for the specific task.
2.2. Segment Anything Model (SAM) Related Work
The Segment Anything Model (SAM), introduced by Kirillov et al. [11], acts as a foundational model for universal image segmentation and has been trained on an expansive dataset exceeding one billion unique masks. While SAM demonstrates impressive capabilities in generating accurate object masks using prompts or autonomously, its efficacy for segmenting medical images is constrained by the significant disparities that exist between natural images and those found in the medical domain. SAM may encounter challenges, particularly in tasks with faintly defined boundaries commonly found in medical images [25,26,27,28,29,30,31,32,33,34,35]. Recognizing this limitation, Ma et al. [9] introduced MedSAM, which leverages a diverse array of medical image datasets and employs fine-tuning techniques to adjust SAM specifically for medical image segmentation tasks. Cheng et al. [36] presented SAM-Med2D, a comprehensive exploration of applying SAM to 2D medical images. This approach incorporates adaptive layers in the image encoder, fine-tunes the prompt encoder, and updates the mask decoder through interactive training. Furthermore, SAM has been applied in weakly supervised segmentation tasks. For example, Jiang et al. [37] introduced using SAM to generate pseudo-labels, which were subsequently utilized in training other weakly supervised semantic segmentation models. In addition, He et al. [38] used sparse annotations as prompts to generate segmentation masks, enhancing the training of other hidden target segmentation models.
Compared to previous studies [9,36], which required full supervision during fine-tuning and necessitated precise pixel-level annotations, our method circumvents this requirement by using weak annotations to guide SAM in generating pseudo-labels as supervision masks. In contrast to [37,38], which introduced additional segmentation models, we chose to use pseudo-labels to guide the fine-tuning training of SAM.
3. Methods
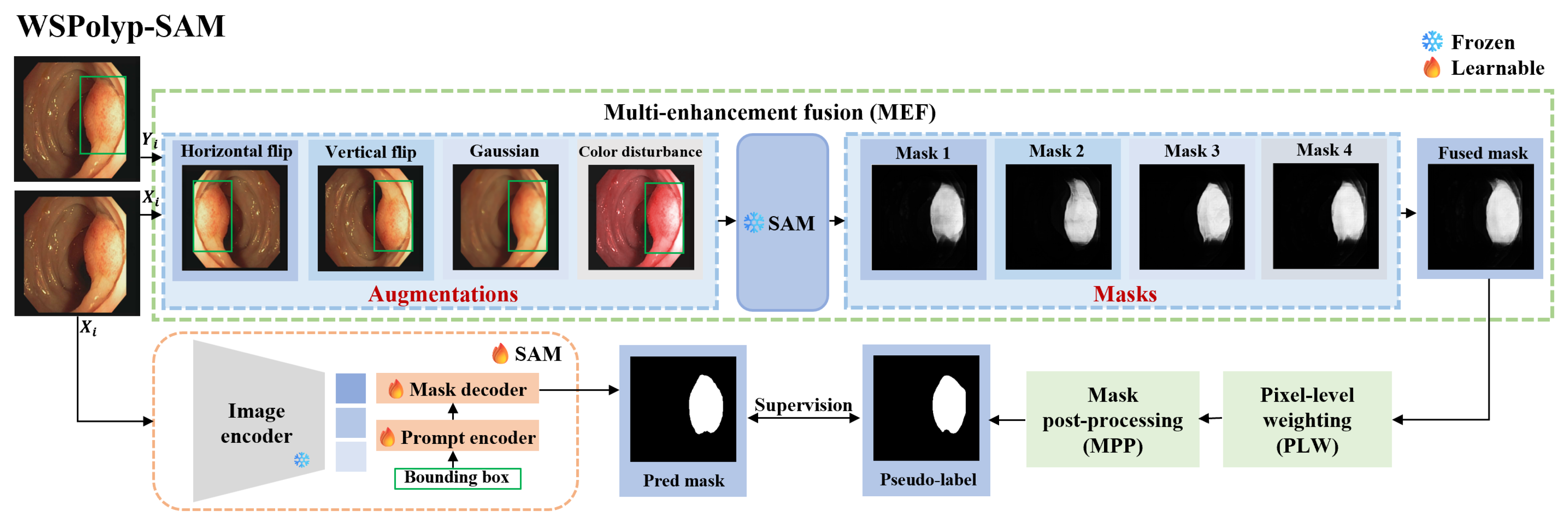
Weakly supervised and Self-guided Fine-tuning of SAM for Colonoscopy Polyp Segmentation (WSPolyp-SAM) aims to fine-tune the SAM model from a weakly annotated training dataset and to test it on testing dataset , where represents the ith testing image, represents the ith training image, and represents weak annotations, i.e., the bounding box annotations used in this study. S and T respectively represent the numbers of training and testing data in the dataset. Particularly, the training image first undergoes a series of augmentation operations, and the augmented images along with their weak annotations are then input into SAM to obtain the corresponding segmentation masks. Multiple masks are subsequently fused, followed by pixel-level weighting and mask post-processing, to obtain pseudo-labels. Finally, these pseudo-labels serve as supervision masks, guiding the fine-tuning training of SAM. During this process, only the prompt encoder and mask decoder undergo fine-tuning.
3.1. Background
The Segment Anything Model (SAM) exploits a transformer-based encoder–decoder architecture [39] to achieve excellent performance in natural language processing [40] and image recognition tasks [41]. This model is equipped with a visual transformer to encode and extract image features, a prompt encoder that facilitates the incorporation of user inputs, and a mask decoder designed to produce segmentation outcomes alongside their associated confidence metrics, all derived from a synthesis of image, prompt, and output token embeddings. The image encoder features a visual transformer component that is pre-trained using a masked autoencoder modeling method [42], enabling it to handle high-resolution images (e.g., 1024 × 1024). The prompt encoder is adaptable and accommodates a variety of user inputs, including key points, bounding boxes, prompt text, and object masks. SAM features an efficient mask decoder, which integrates two layers of transformers equipped with dynamic mask projection heads and mechanisms for calculating the Intersection over Union (IoU) score. The mask projection heads are designed to generate three reduced-resolution masks that correspond to the entirety, components, and sub-components of the objects.
3.2. Prompt Selection
SAM offers three primary segmentation modes: segment everything mode, bounding box mode, and point mode. However, each of these modes exhibits distinct characteristics, leading to certain biases in their segmentation outcomes. Ma et al. [9] explored the efficacy of three distinct segmentation approaches on standard abdominal CT scans and proved that the bounding box mode has broader practical value than the segment everything mode and point mode.
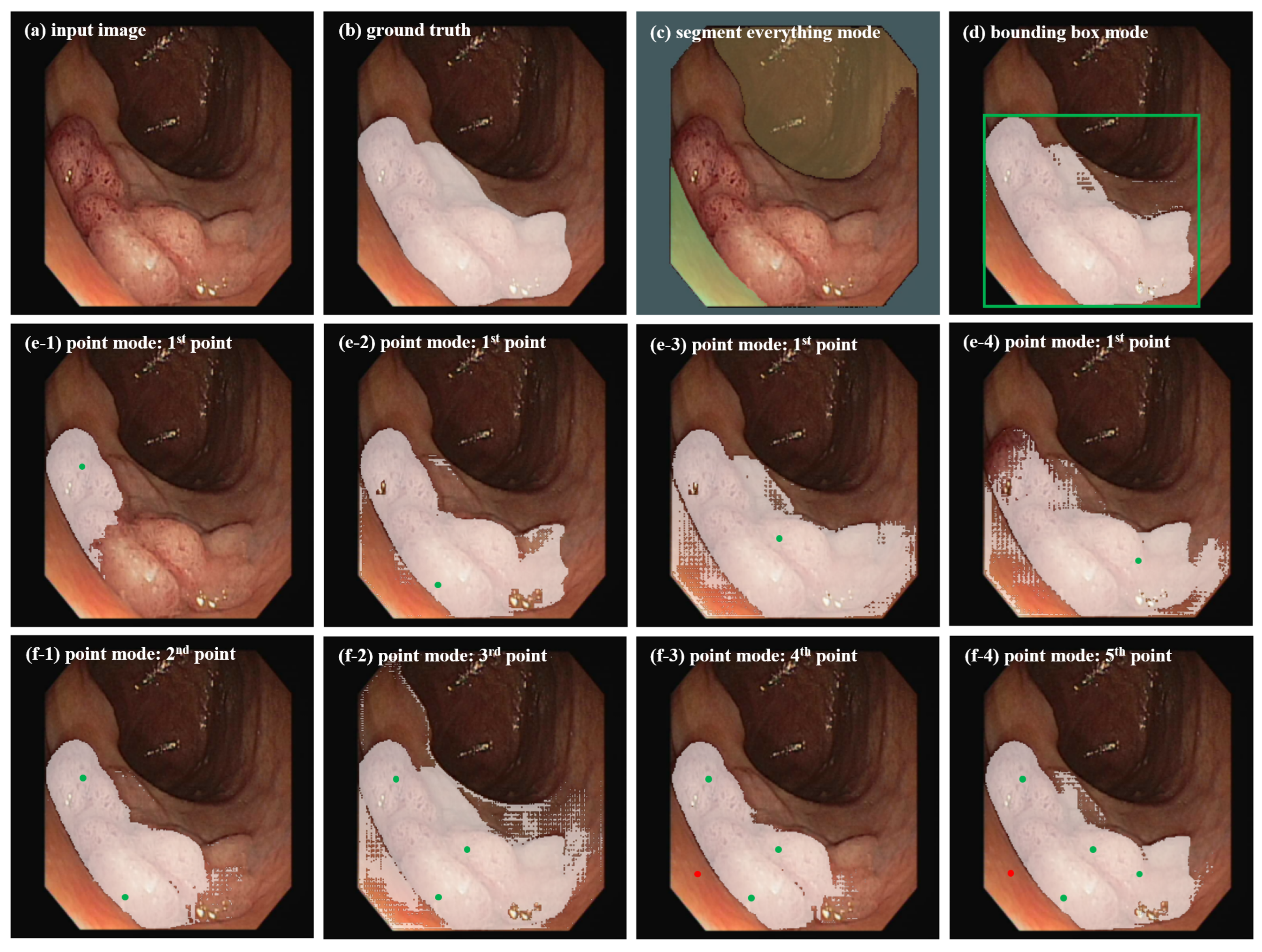
Considering the significant differences in target morphology, image format, and target region features between abdominal organ images and colonoscopy polyp images, we apply SAM with these three segmentation modes to colonoscopy polyp data to investigate whether the bounding box mode is the optimal choice for our segmentation task. Experimental results are shown in Figure 1. (1) Segment everything mode: This mode partitions the entire image into multiple regions based on image intensity by defining segmentation grid points (here using 9 × 9), but the segmentation results often lack semantic labels and cannot clearly focus on the ROI that clinical doctors care about, limiting its utility. In Figure 1c, it can be seen that, due to indistinguishable features between polyps and surrounding skin tissue, it is easy to regard the entire polyp area as background in this mode. (2) Bounding box mode: This mode segments the target objects by providing points for the lower right corner and upper left corner of the bounding box (in green in Figure 2d). In Figure 1d, it can be seen that this mode can better identify the polyp area and is more similar to the ground truth in Figure 1b. (3) Point mode: This mode generates the required mask by continuously adding foreground or background points. In Figure 1e1–e4, it can be seen that the segmented area with one point-prompt under this mode will change with the position of the point, leading to segmentation instability, and the contour of the target area does not converge. The target area will only approach the ground truth after repeatedly adding foreground and background points. In which, the green represents the foreground points, and the red represents the background points. Overall, the segmentation results with multiple point-prompts in Figure 1f1–f4 under the point mode are not as good as that in the box mode in Figure 1d. The point mode usually requires multiple prediction corrections and iterations, which is consistent with the conclusions of the study [9].
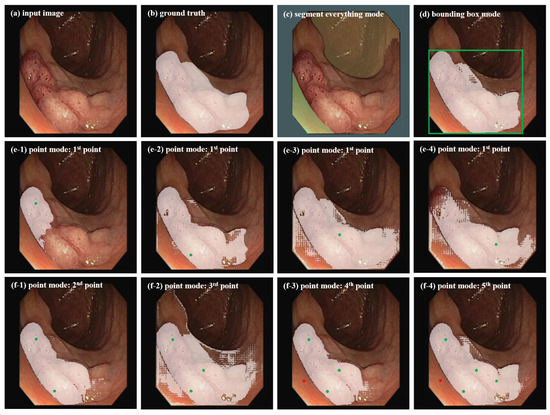
Figure 1.
Segmentation results of SAM on a colonoscopy polyp image for different segmentation modes.
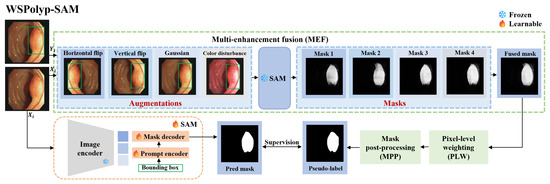
Figure 2.
WSPolyp-SAM polyp framework under bounding box guidance. Note that the masks corresponding to flipping augmentation should be flipped back before engaging in the fusion process.
In summary, when applying SAM to colonoscopy polyp segmentation, the segment everything mode often leads to irrelevant region divisions, while the point mode’s ambiguity often requires several rounds of adjustments for accurate predictions. Conversely, the bounding box mode precisely delineates the region of interest (ROI), yielding satisfactory segmentation outcomes with fewer iterative corrections. Therefore, in subsequent experiments, bounding boxes are utilized as prompt information for both pseudo-label generation and fine-tuning in the SAM model.
3.3. Self-Guided Pseudo-Label Generation
As a general segmentation foundation model, SAM faces challenges when employed in medical image segmentation, particularly in colonoscopy polyp segmentation. The inherent similarity between polyps and surrounding skin tissues, coupled with their indistinct boundaries, exacerbates the complexity of the segmentation task. Consequently, when weak annotation guides pretrained SAM in generating segmentation masks as pseudo-labels for the next precise segmentation, the resulting segmentation mask lacks precision due to these complexities. Employing imprecise segmentation masks as pseudo-labels not only fails to provide additional information but also may mislead the model and therefore could impede SAM’s fine-tuning. To address this challenge, we designed three techniques, i.e., multi-augmentation fusion, pixel-level weighting, and mask post-processing, to enhance the accuracy of ‘pseudo-labels’, especially in complex scenarios. For a detailed overview of the model architecture, refer to Figure 2.
Multi-enhancement fusion (MEF). The information content of a single segmentation mask is often insufficient and inaccurate. To boost the information capacity and accuracy of the segmentation mask, we adopt multiple enhancement fusion techniques. For each image in the training set , random augmentation is applied, including color jittering, Gaussian blurring, vertical flipping, and horizontal flipping. Prompt information also changes accordingly after flipping operations, resulting in the generation of K augmented images and corresponding prompts . Specifically, color jittering follows the method proposed in [43], where the brightness factor is uniformly sampled from the range , contrast is set to 0.2, the saturation factor to 0.1, and the hue factor to 0.01. Subsequently, we feed into SAM to generate the corresponding segmentation masks , where
Note that and have the same shape but differ from the shape of . To ensure consistency between the mask and the original image, obtained after flipping will be flipped back.
Due to using different augmented images for segmentation, the generated masks may exhibit some shape differences, but they often overlap in certain regions. SAM reliably predicts these overlapping regions, remains unaffected by image transformations, and usually corresponds to the correct foreground areas. Considering this complementarity, we propose a method to fuse the segmentation masks of different augmented images. The fusion process is described as follows:
where denotes the fused mask. We expected the fused mask to be more dependable than individual masks due to its ability to comprehensively reflect the features of the colonoscopy image.
Pixel-level weighting (PLW). The confidence of predictions may fluctuate across pixels. To accentuate the most reliable predictions, we suggest employing entropy for weighting. We compute the entropy of each pixel to generate an entropy map:
The entropy map is computed from the fused mask and quantifies the prediction uncertainty for each pixel across all augmented images. Pixels with high confidence and consistent predictions across all augmented images exhibit low entropy. Thus, we can utilize the entropy map to weight the fused mask, assigning greater weights to more reliable pixels. By applying entropy weights on the fused mask, we obtain a pixel-level weighted mask as follows:
This strategy considers the prediction uncertainty of pixels, allowing more reliable pixels in the fused mask to receive higher weights. As a result, it enhances the segmentation accuracy of the SAM model in critical regions.
Mask post-processing (MPP). The pixel-level weighted mask may have some issues, such as insufficiently smooth edges or disconnected pathological areas. To address these problems, we perform some post-processing operations. First, the pixel-level weighted mask is binarized as follows:
represents the binarized mask, with representing the threshold, which is set to 0.2. Next, we employ Gaussian filtering techniques to smooth images and diminish noise, along with morphological closing to connect adjacent regions, thereby ensuring the contiguity of pathological areas and smoother edges. Through these post-processing operations, we reduce noise interference and extraneous information, yielding high-quality pseudo-labels as follows:
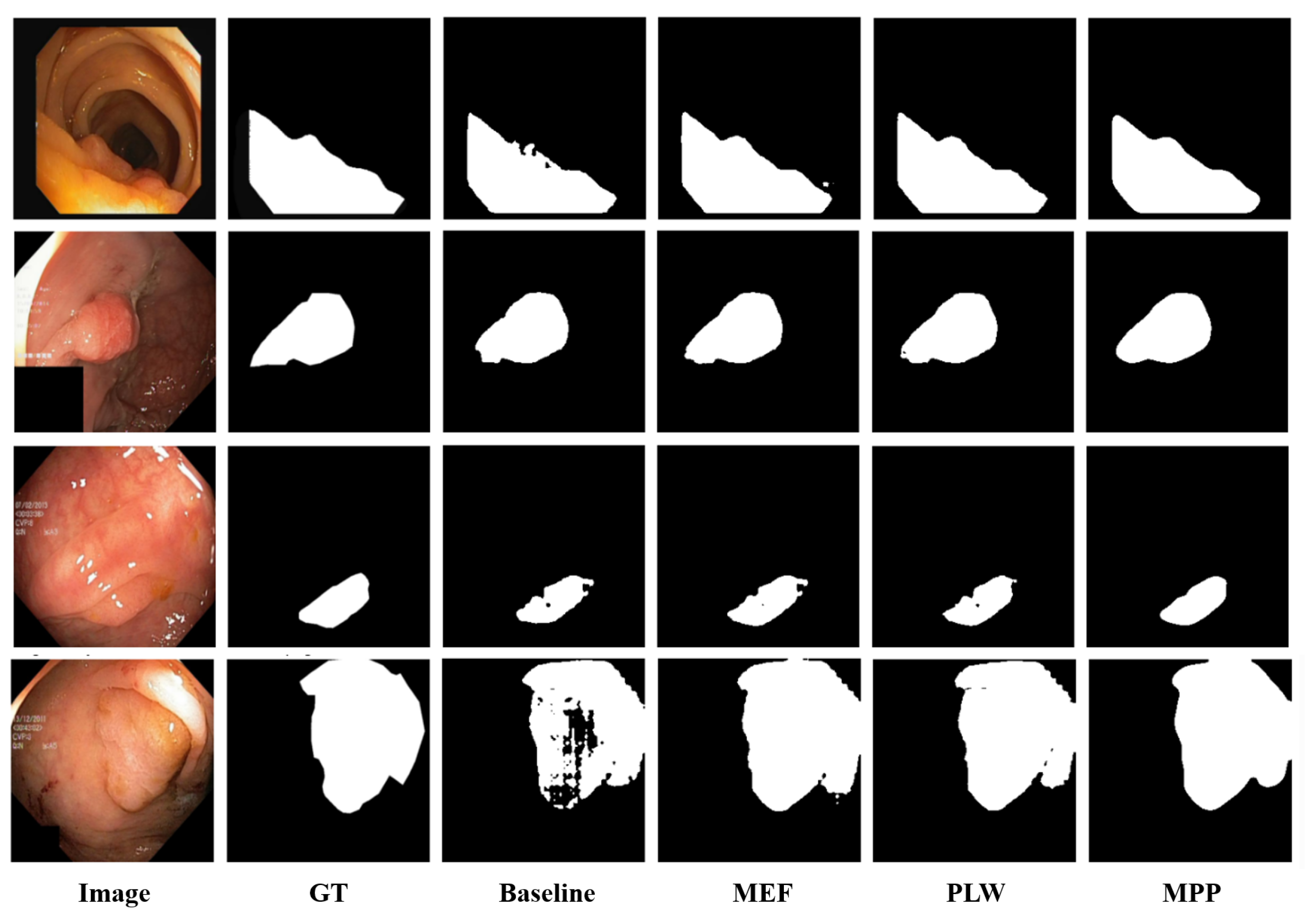
Note that, as shown in Figure 3, multi-enhancement fusion, pixel-level weighting, and mask post-processing operations are applied sequentially to make the generated pseudo-labels more accurate.
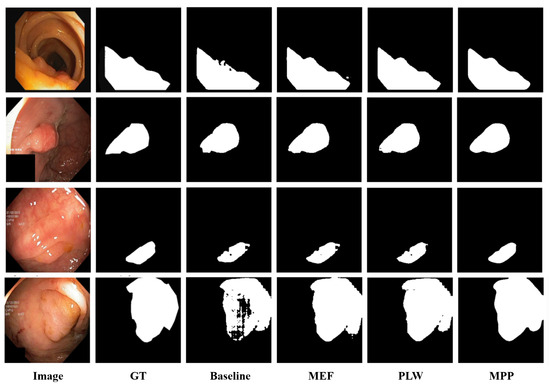
Figure 3.
Visualization of the ablation study results.
3.4. Weakly Supervised Fine-Tuning
In order to adapt SAM to specific colonoscopy images, it is necessary to select appropriate prompt information and network components for fine-tuning. Based on the results in Figure 1, the bounding box is the optimal choice as the prompt information for generating segmentation masks. It is crucial to highlight that the bounding box is derived from the ground-truth label, with each polyp region aligning with a specific bounding box. The architectural framework of SAM is constituted by three principal elements: an image encoder for feature extraction, a prompt encoder to integrate user inputs, and a mask decoder that generates the segmentation outputs. Due to the significant computational load primarily on the image encoder, which is built on visual transformers, we maintain the image encoder’s frozen state and solely fine-tune the prompt encoder and mask decoder components.
Moreover, the choice of loss function is crucial during model training due to the disparities between pseudo-labels and ground-truth labels. We incorporate the weighted Intersection over Union (IoU) loss from the original SAM loss function, alongside popular segmentation loss functions such as the binary cross-entropy loss and dice coefficient loss, which have demonstrated robustness across various segmentation tasks [44,45]. Figure 2 supplies an overview of the WSPolyp-SAM framework, where the SAM model’s parameters remain frozen during the generation of pseudo-label . Subsequently, we guide SAM’s fine-tuning process using as the supervision mask, focusing solely on fine-tuning the prompt encoder and mask decoder. The total loss function is presented in the formula below:
where represents the predicted mask, i.e., . Fine-tuning the SAM model helps it better understand and segment the colonoscopy polyp regions, adapting to the image features generated by the pseudo-labels.
4. Experiments
4.1. Datasets
To facilitate a fair comparison, we use the following five frequently adopted benchmark datasets:
- 1.
- Kvasir-SEG [46]: This dataset, gathered by the Vestre Viken Health Trust in Norway, comprises 1000 colonoscopy video sequences showcasing polyp images, each accompanied by its respective annotations. The image resolution ranges from 332 × 487 to 1920 × 1072 pixels. Annotations are meticulously labeled by medical professionals and validated by seasoned gastroenterologists.
- 2.
- ClinicDB [47]: The collection of images within this dataset, totaling 612 and sourced from 29 distinct colonoscopy procedures, features a pixel dimension of 288 × 384. This initiative was developed collaboratively with the renowned Hospital Clinic of Barcelona, located in Spain.
- 3.
- ColonDB [48]: The dataset encompasses 380 images of polyps, complete with their respective annotations, all captured at a resolution of 500 × 570 pixels. These images are extracted from 15 distinct videos, with frames meticulously chosen by experts to showcase diverse perspectives. The annotations are manually crafted with precision.
- 4.
- CVC-300 [49]: The dataset includes 60 images of polyps, each with a resolution of 500 × 574 pixels.
- 5.
- ETIS [50]: This dataset was released by the MIC-CAI Polyp Detection Subchallenge in 2017, encompassing 196 polyp images extracted from colonoscopy videos, each paired with its respective label. The images boast a resolution of 966 × 1225 pixels.
These datasets provide polyp images of varying quantities and resolutions, covering diverse scenarios and perspectives. All images have been manually annotated and verified by medical professionals, serving as benchmarks for evaluating algorithm performance and conducting fair comparisons.
4.2. Implementation Details
Data Split. To ensure an equitable comparison, we employ a data partitioning methodology identical to that utilized in the experiments conducted by PraNet [51]. Specifically, the Kvasir-SEG dataset comprises 1000 polyp images, while ClinicDB comprises 612 images. From the Kvasir-SEG and ClinicDB datasets, we allocate 900 and 550 images, respectively, for the training set. The remaining 100 and 62 images are allocated for the test set. Furthermore, our model undergoes evaluation on three additional datasets not previously encountered: ColonDB, CVC-300, and ETIS.
Data Augmentation. The colonoscopy polyp datasets have the following three characteristics: (1) significant variations in polyp size, including both large and small polyps; (2) close similarity in color between most polyps and the background leads to a diminished contrast between foreground and background, making segmentation of some samples challenging; (3) blurry imaging resulting from the rotating movement of the camera within the intestine, significantly increasing the difficulty of polyp detection. To tackle these issues, we introduced a series of random augmentation operations during model training: (1) random scaling by a factor of 0.7 to 1.3; (2) color augmentation following the method in [32]; (3) contrast enhancement sampled randomly within the range of [0.8, 1.2]; (4) Gaussian filtering using a 5 × 5 convolutional kernel. The purpose of Gaussian filtering is to simulate the blurriness caused by lens rotation, aiming to improve the model’s ability to adapt to blurred samples. It is worth noting that random scaling involves operations on the shape of the image, so the corresponding pseudo-labels and bounding boxes also follow this operation.
Training Details. Our algorithm is trained using the PyTorch framework version 2.3.0. For hardware, we leverage an NVIDIA Tesla A100 GPU equipped with 40 GB of GPU memory to train the segmentation model. Throughout the network training phase, mini-batch training iterations are conducted with a batch size set to 4. The entirety of the training process spans a total of 25 epochs. The model parameters are optimized utilizing the Adam [9] optimization algorithm, initialized with a learning rate of 0.0001 and a weight decay of 0.00001.
Evaluation Metrics. In line with the evaluation metrics used in Polyp-PVT [52] and HSNet [43], we employ the mean Intersection over Union (mIoU) and mean dice score (mDice) to assess the segmentation results in our experiments.
4.3. Results
4.3.1. Quantitative Results
In this section, we assessed our model’s learning capacity using the Kvasir-SEG and ClinicDB datasets. To evaluate the model’s generalization ability, we conducted evaluations on three additional datasets: ColonDB, CVC-300, and ETIS. We conducted a comparison between our model and the current mainstream fully supervised polyp segmentation networks.
Table 1 presents the comparative findings of our method with fully supervised networks (including UNet [15], UNet++ [16], SFA [22], PraNet [51], C2FNet [53], Polyp-PVT [52], and HSNet [43]). From the comparison in Table 1, WSPolyp-SAM exhibits superior performance over existing mainstream baseline networks across the Kvasir-SEG, ColonDB, CVC-300, and ETIS datasets. Specifically, our approach demonstrates a 0.7% enhancement in mDice score and a 0.2% boost in mIoU score compared to HSNet on the Kvasir-SEG dataset. In ColonDB, it achieves a notable 9.4% improvement in mDice score and a substantial 9.6% increase in mIoU score relative to HSNet. Furthermore, on the CVC-300 dataset, it delivers a 3% increase in mDice score and a 3.9% elevation in mIoU score compared to HSNet. On ETIS, it achieves an 8% improvement in mDice score and a 7.9% improvement in mIoU score compared to HSNet. Compared to the original SAM, our method demonstrates significant improvements after fine-tuning on all five datasets, demonstrating that SAM is more adaptable to specific domains after fine-tuning.

Table 1.
Quantitative results of WSPolyp-SAM and SAM on Kvasir-SEG, ClinicDB, ColonDB, CVC-300 and ETIS datasets. Here, ‘B’ represents the use of ViT-B, and ‘’ represents the increase relative to SAM. The bold indicates the highest scores.
4.3.2. Qualitative Results
To visually illustrate the superiority of our proposed method, Figure 4 illustrates the predicted outcomes of our model in comparison to the rival models. From Figure 4, our method more accurately locates and identifies the contours of polyps, generating prediction masks that closely resemble ground-truth labels compared to fully supervised models. This improvement can be attributed to the enhanced learning of complex polyp features in SAM after fine-tuning, with the incorporation of prompts helping to reduce misjudgments in erroneous areas. Additionally, we provide visualizations of the original prediction masks by SAM, revealing issues such as misjudgments, jagged contours, and unclear edges before fine-tuning. Overall, our method effectively captures global contextual information and restores detailed appearance features, thereby better delineating the polyp regions.

Figure 4.
Visualization results with different models.
4.3.3. Ablation Study
Our method includes a key component, which is pseudo-label generation based on SAM. It consists of three parts: multi-enhancement fusion (MEF), pixel-level weighting (PLW), and mask post-processing (MPP). In this section, we conduct comprehensive ablation experiments on five colonoscopy polyp datasets, with results shown in Table 2. The first row presents the results of directly using SAM to generate segmentation masks on the five datasets. As for the baseline, we used SAM to generate a segmentation mask for training images and fine-tuned SAM (second row) as the baseline without data augmentation. We then gradually added MEF (third row), PLW (fourth row), and MPP (fifth row) on top of the baseline, ultimately achieving the best evaluation scores.

Table 2.
Ablation study of WSPolyp-SAM’s pseudo-labels. The bold indicates the highest scores.
Furthermore, we performed visual analysis to evaluate the influence of each module on the quality of model predictions (see Figure 3). It can be observed that, in the baseline scenario, due to unclear boundaries between polyps and surrounding mucosa and low contrast between polyp foreground and background information, ‘Baseline’ exhibits issues such as unclear predicted edges and missed detections. After introducing ‘MEF’, the fusion of multiple enhancement results provided additional predictive information, significantly reducing instances of missed detections. Subsequently, with the introduction of ‘PLW’, assigning higher weights to reliable pixels further reduced the probability of error detection. Finally, with the inclusion of ‘MPP’, better noise reduction during prediction and improvement in edge clarity were achieved, further enhancing the segmentation results. In summary, our method enhances the detection coverage of polyp regions, reduces segmentation errors, and minimizes missed detections.
4.3.4. Further Analysis
Different Visual Backbones. We also investigated the segmentation capabilities of the SAM model with a different Vision Transformer (ViT) for encoding image features, as shown in Table 3. Table 3 (first, second, and third rows) shows the predictions of the original SAM model without fine-tuning. It can be observed that, on the ETIS dataset, although SAM-H and SAM-L are more computationally complex, their performance is not as good as SAM-B. To fully explore the transfer learning capabilities of SAM on different ViTs, we conducted fine-tuning. Considering memory limitations, in this work, we only fine-tuned SAM-B and SAM-L. From Table 3 (fourth row and fifth row), it can be observed that, under weakly supervised strategies, SAM-B outperforms SAM-L on the Kvasir-SEG, ClinicDB, and ETIS datasets, while performing less effectively on ColonDB and CVC-300 compared to SAM-L. This indicates that the lightweight SAM-B demonstrates better learning capabilities compared to the higher-complexity SAM-L.

Table 3.
Results of SAM with different visual backbones. Model-ViT represents SAM with different ViTs. Mask indicates the supervision mask used during fine-tuning, where ‘Pseudo’ represents pseudo-labels and ‘GT’ represents ground-truth labels.
To verify this point, we performed full supervision fine-tuning on SAM-B and SAM-L using ground-truth labels. As shown in Table 3 (sixth row and seventh row), under full supervision, the trends across datasets align with those observed under weak supervision. In general, although SAM-L entails significantly greater computational complexity, it does not surpass the lightweight SAM-B model on three out of five public datasets. This finding suggests that the lightweight SAM-B model is well-suited for adoption in medical image applications. Additionally, we observed that, under the same ViT, weak supervision performs only 0.3% lower in average mDice than full supervision on the Kvasir-SEG, ColonDB, and CVC-300 datasets. This result indicates that, while weakly supervised methods reduce dependence on precise annotations, they can still maintain a segmentation precision comparable to fully supervised methods.
Pseudo-label generation vs. WSPolyp-SAM. We conducted a series of experiments (see Table 4) comparing our method, WSPolyp-SAM, directly with pseudo-label generation, aiming to evaluate whether our fine-tuning approach is superior to the direct adoption of pseudo-label generation. The experimental results from five test datasets indicate that the mDice and mIoU scores obtained through the pseudo-label generation strategy are between the original predictions of SAM and those after fine-tuning, and they are significantly lower than the results after fine-tuning. This indicates that, while the pseudo-label generation method can optimize the predictions of the original SAM to some extent, there is still a certain gap compared to the predictions after fine-tuning. Additionally, the pseudo-label generation process requires multiple invocations of SAM, incurring high time costs, making it challenging for direct use in predictions. In comparison, using WSPolyp-SAM is more feasible.

Table 4.
Comparison between pseudo-label generation and WSPolyp-SAM.
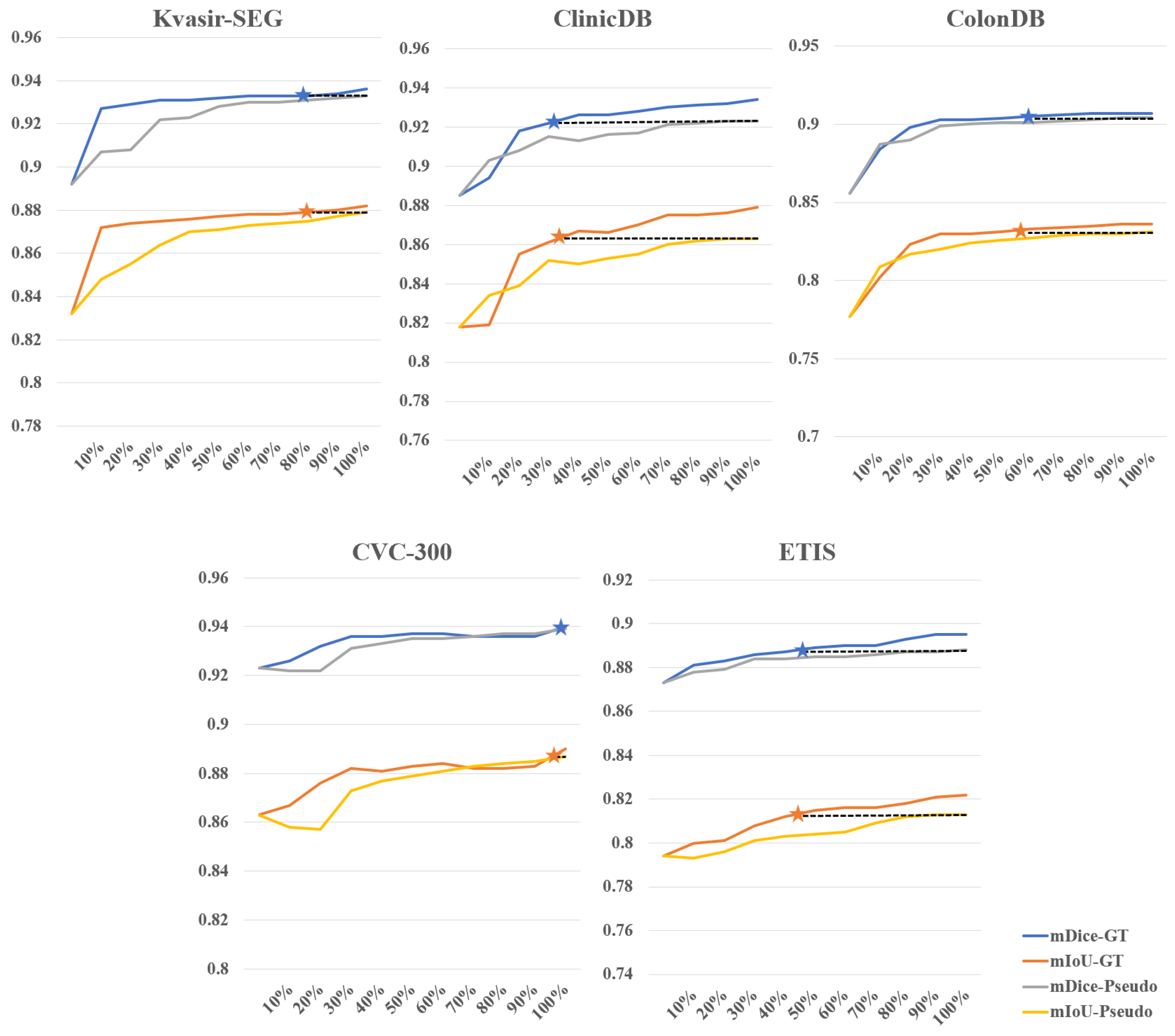
Effectiveness of weakly supervised fine-tuning in reducing annotation costs. We further investigated the impact of different amounts of training data for supervision masks on the results of fine-tuned SAM, fully demonstrating SAM’s efficiency in utilizing fine-tuning data. Figure 5 illustrates the comparison between weakly supervised and fully supervised approaches when the training data amount to between 10% and 100%, using the SAM-B model for fine-tuning. As the amount of data increases, the overall accuracy gradually improves. On the CVC-300 dataset, a weakly supervised strategy with 100% training data achieves results comparable to a fully supervised strategy with 90% to 100% training data. On the Kvasir-SEG dataset, a weakly supervised strategy with 100% training data achieves results comparable to a fully supervised strategy with 80% training data. On the ColonDB dataset, a weakly supervised strategy with 100% training data achieves results comparable to a fully supervised strategy with 50% training data. However, performance is relatively poorer on the other two datasets. On the ETIS dataset, a weakly supervised strategy with 100% training data achieves results comparable to a fully supervised strategy with 40% to 50% training data. On the ClinicDB dataset, a weakly supervised strategy with 100% training data only achieves results comparable to a fully supervised strategy with 20% to 30% training data. Overall, using 100% training data under the weakly supervised strategy achieves 56% to 62% of the training results of fully supervised learning. We simulated the scenario of annotating polyp data and found that the time ratio between bounding box annotation and rough pixel-level annotation is approximately 1:6 (which would be even higher if precise pixel-level annotation is done by professional annotators). Based on this calculation, for example, if bounding box annotation takes 1 s and pixel-level annotation takes 6 s, to achieve the training data results of 56% to 62% under fully supervised learning with 100 images, the annotation time ratio would be approximately 27% to 30%, saving 70% to 73% of the time cost. This demonstrates the effectiveness of our method in reducing annotation costs.
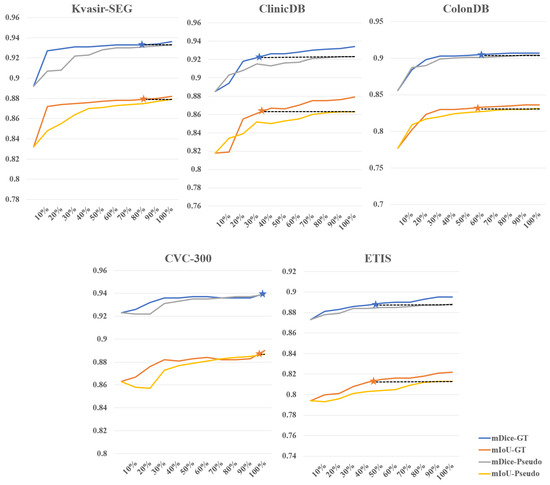
Figure 5.
Comparison between weakly supervised and fully supervised fine-tuning for different percentages of supervision mask. The stars indicate that the performance of the weakly supervised strategy with 100% of the training data is comparable to that of the fully supervised strategy.
5. Conclusions
This study proposes a novel method for colon polyp segmentation called WSPolyp-SAM, which utilizes weak annotations to guide SAM in generating pseudo-labels for self-guided fine-tuning, reducing the dependence on precise annotation data. The experimental results demonstrate the competitive performance of WSPolyp-SAM in colon polyp segmentation compared to current fully supervised learning methods, bringing a new technological breakthrough to this field. Additionally, through experiments on the segmentation performance of the SAM model under different versions of the ViT and comparing different amounts of training data under different supervision strategies (weakly supervised vs. fully supervised), several key findings can be obtained.
Firstly, despite the higher computational complexity of SAM-L, its performance on most public datasets is not superior to the lightweight SAM-B. This finding demonstrates that SAM-B is more suitable for efficient deployment in medical imaging applications.
Secondly, under the weakly supervised strategy, using 100% of the training data can achieve training results of 56% to 62% compared to fully supervised learning. This means that 70% to 73% of the annotation time costs can be saved. These findings not only affirm the technical efficacy of our proposed approach but also emphasize its potential value in reducing annotation costs and improving work efficiency.
However, our research is not without its limitations. (1) Pseudo-label quality control: The qualities of the pseudo-labels used in the model fine-tuning process of WSPolyp-SAM are inevitably affected by the coarse segmentation masks. In the future, we will study more robust methods to identify error-prone areas and then refine the poor masks, which are expected to alleviate this problem. (2) Multi-class polyp identification: As WSPolyp-SAM uses the same mask decoder as SAM, it is currently unable to distinguish between different types of polyps, which is crucial for clinical management strategies. We hope to expand our method to recognize different types of polyps by introducing more information like the polyp’s shape and visual appearance into WSPolyp-SAM and designing a new mask decoder supporting multi-class prediction in the future. (3) Optimization of real-time segmentation performance: In clinical colonoscopy, real-time segmentation is essential for quick diagnosis and treatment. Actually, the proposed WSPolyp-SAM is flexible to fulfill this demand by adopting more lightweight visual backbones, such as the tiny ViT used in MobileSAM. Additionally, in practical applications, the inference speed can be further boosted by exploiting low-bit inference, model compression, and quantization techniques. We believe that these improvements will make WSPolyp-SAM a more comprehensive tool, further enhancing its performance and functionality in the task of polyp segmentation.
Author Contributions
Conceptualization, T.C., H.Y. and K.D.; methodology, T.C., H.Y. and K.D.; software, T.C.; validation, T.C.; resources, T.C.; data curation, T.C.; supervision, H.Y. and K.D.; writing—original draft preparation, T.C.; writing—review and editing, T.C., H.Y., K.D., Y.Z. (Yan Zhang) and Y.Z. (Yueyue Zhou); funding acquisition, K.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China under Grant 62306310.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets analyzed during the current study are available at the link https://github.com/DengPingFan/PraNet (accessed on 9 August 2023).
Acknowledgments
The authors wish to thank the reviewers for their valuable comments and suggestions concerning this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gschwantler, M.; Kriwanek, S.; Langner, E.; Göritzer, B.; Schrutka-Kölbl, C.; Brownstone, E.; Feichtinger, H.; Weiss, W. High-grade dysplasia and invasive carcinoma in colorectal adenomas: A multivariate analysis of the impact of adenoma and patient characteristics. Eur. J. Gastroenterol. Hepatol. 2002, 14, 183. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut J. Br. Soc. Gastroenterol. 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Shi, J.; Qi, X.; Wang, X.; Jia, J. Pyramid scene parsing network. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Honolulu, HI, USA, 21–26 July 2017; pp. 2881–2890. [Google Scholar]
- Chen, L.C.; Papandreou, G.; Kokkinos, I.; Murphy, K.; Yuille, A.L. Deeplab: Semantic image segmentation with deep convolutional nets, atrous convolution, and fully connected crfs. IEEE Trans. Pattern Anal. Mach. Intell. 2017, 40, 834–848. [Google Scholar] [CrossRef] [PubMed]
- Litjens, G.; Kooi, T.; Bejnordi, B.E.; Setio, A.A.A.; Ciompi, F.; Ghafoorian, M.; Van Der Laak, J.A.; Van Ginneken, B.; Sánchez, C.I. A survey on deep learning in medical image analysis. Med. Image Anal. 2017, 42, 60–88. [Google Scholar] [CrossRef]
- Asgari Taghanaki, S.; Abhishek, K.; Cohen, J.P.; Cohen-Adad, J.; Hamarneh, G. Deep semantic segmentation of natural and medical images: A review. Artif. Intell. Rev. 2021, 54, 137–178. [Google Scholar] [CrossRef]
- Zhang, R.; Lai, P.; Wan, X.; Fan, D.J.; Gao, F.; Wu, X.J.; Li, G. Lesion-aware dynamic kernel for polyp segmentation. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Singapore, 18–22 September 2022; Springer: Cham, Switzerland, 2022; pp. 99–109. [Google Scholar]
- Zhou, T.; Zhou, Y.; Gong, C.; Yang, J.; Zhang, Y. Feature aggregation and propagation network for camouflaged object detection. IEEE Trans. Image Process. 2022, 31, 7036–7047. [Google Scholar] [CrossRef]
- Ma, J.; He, Y.; Li, F.; Han, L.; You, C.; Wang, B. Segment anything in medical images. Nat. Commun. 2024, 15, 654. [Google Scholar] [CrossRef]
- Ding, N.; Qin, Y.; Yang, G.; Wei, F.; Yang, Z.; Su, Y.; Hu, S.; Chen, Y.; Chan, C.M.; Chen, W.; et al. Parameter-efficient fine-tuning of large-scale pre-trained language models. Nat. Mach. Intell. 2023, 5, 220–235. [Google Scholar] [CrossRef]
- Kirillov, A.; Mintun, E.; Ravi, N.; Mao, H.; Rolland, C.; Gustafson, L.; Xiao, T.; Whitehead, S.; Berg, A.C.; Lo, W.Y.; et al. Segment anything. In Proceedings of the IEEE/CVF International Conference on Computer Vision, Paris, France, 2–3 October 2023; pp. 4015–4026. [Google Scholar]
- Hu, M.; Li, Y.; Yang, X. Skinsam: Empowering skin cancer segmentation with segment anything model. arXiv 2023, arXiv:2304.13973. [Google Scholar]
- Wu, J.; Fu, R.; Fang, H.; Liu, Y.; Wang, Z.; Xu, Y.; Jin, Y.; Arbel, T. Medical sam adapter: Adapting segment anything model for medical image segmentation. arXiv 2023, arXiv:2304.12620. [Google Scholar]
- Long, J.; Shelhamer, E.; Darrell, T. Fully convolutional networks for semantic segmentation. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Boston, MA, USA, 7–12 June 2015; pp. 3431–3440. [Google Scholar]
- Ronneberger, O.; Fischer, P.; Brox, T. U-net: Convolutional networks for biomedical image segmentation. In Proceedings of the 18th International Conference of Medical Image Computing and Computer-Assisted Intervention (MICCAI 2015), Munich, Germany, 5–9 October 2015; Proceedings—Part III 18; Springer: Cham, Switzerland, 2015; pp. 234–241. [Google Scholar]
- Zhou, Z.; Siddiquee, M.M.R.; Tajbakhsh, N.; Liang, J. Unet++: Redesigning skip connections to exploit multiscale features in image segmentation. IEEE Trans. Med. Imaging 2019, 39, 1856–1867. [Google Scholar] [CrossRef]
- Yang, X.; Li, X.; Ye, Y.; Lau, R.Y.; Zhang, X.; Huang, X. Road detection and centerline extraction via deep recurrent convolutional neural network U-Net. IEEE Trans. Geosci. Remote. Sens. 2019, 57, 7209–7220. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, Z.; Huang, C.; Dehghan, M.; Zaiane, O.R.; Jagersand, M. U2-Net: Going deeper with nested U-structure for salient object detection. Pattern Recognit. 2020, 106, 107404. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, P.; Wang, D.; Cao, Y.; Liu, B. Colorectal polyp segmentation by U-Net with dilation convolution. In Proceedings of the 2019 18th IEEE International Conference on Machine Learning and Applications (ICMLA), Boca Raton, FL, USA, 16–19 December 2019; pp. 851–858. [Google Scholar]
- Chen, L.C.; Zhu, Y.; Papandreou, G.; Schroff, F.; Adam, H. Encoder-decoder with atrous separable convolution for semantic image segmentation. In Proceedings of the European Conference on Computer Vision (ECCV), Munich, Germany, 8–14 September 2018; pp. 801–818. [Google Scholar]
- Liu, S.; Huang, D. Receptive field block net for accurate and fast object detection. In Proceedings of the European Conference on Computer Vision (ECCV), Munich, Germany, 8–14 September 2018; pp. 385–400. [Google Scholar]
- Fang, Y.; Chen, C.; Yuan, Y.; Tong, K.y. Selective feature aggregation network with area-boundary constraints for polyp segmentation. In Proceedings of the 22nd International Conference of Medical Image Computing and Computer Assisted Intervention (MICCAI 2019), Shenzhen, China, 13–17 October 2019; Proceedings—Part I 22; Springer: Cham, Switzerland, 2019; pp. 302–310. [Google Scholar]
- Zhang, R.; Li, G.; Li, Z.; Cui, S.; Qian, D.; Yu, Y. Adaptive context selection for polyp segmentation. In Proceedings of the 23rd International Conference of Medical Image Computing and Computer Assisted Intervention (MICCAI 2020), Lima, Peru, 4–8 October 2020; Proceedings—Part VI 23; Springer: Cham, Switzerland, 2020; pp. 253–262. [Google Scholar]
- Nguyen, T.C.; Nguyen, T.P.; Diep, G.H.; Tran-Dinh, A.H.; Nguyen, T.V.; Tran, M.T. CCBANet: Cascading context and balancing attention for polyp segmentation. In Proceedings of the 24th International Conference of Medical Image Computing and Computer Assisted Intervention (MICCAI 2021), Strasbourg, France, 27 September–1 October 2021; Proceedings—Part I 24; Springer: Cham, Switzerland, 2021; pp. 633–643. [Google Scholar]
- Deng, R.; Cui, C.; Liu, Q.; Yao, T.; Remedios, L.W.; Bao, S.; Landman, B.A.; Wheless, L.E.; Coburn, L.A.; Wilson, K.T.; et al. Segment anything model (sam) for digital pathology: Assess zero-shot segmentation on whole slide imaging. arXiv 2023, arXiv:2304.04155. [Google Scholar]
- Hu, C.; Li, X. When sam meets medical images: An investigation of segment anything model (sam) on multi-phase liver tumor segmentation. arXiv 2023, arXiv:2304.08506. [Google Scholar]
- He, S.; Bao, R.; Li, J.; Grant, P.E.; Ou, Y. Accuracy of segment-anything model (sam) in medical image segmentation tasks. arXiv 2023, arXiv:2304.09324. [Google Scholar]
- Roy, S.; Wald, T.; Koehler, G.; Rokuss, M.R.; Disch, N.; Holzschuh, J.; Zimmerer, D.; Maier-Hein, K.H. Sam. md: Zero-shot medical image segmentation capabilities of the segment anything model. arXiv 2023, arXiv:2304.05396. [Google Scholar]
- Zhou, T.; Zhang, Y.; Zhou, Y.; Wu, Y.; Gong, C. Can sam segment polyps? arXiv 2023, arXiv:2304.07583. [Google Scholar]
- Mohapatra, S.; Gosai, A.; Schlaug, G. Brain extraction comparing segment anything model (sam) and fsl brain extraction tool. arXiv 2023, arXiv:2304.04738. [Google Scholar]
- Mazurowski, M.A.; Dong, H.; Gu, H.; Yang, J.; Konz, N.; Zhang, Y. Segment anything model for medical image analysis: An experimental study. Med. Image Anal. 2023, 89, 102918. [Google Scholar] [CrossRef]
- Chen, J.; Bai, X. Learning to “segment anything” in thermal infrared images through knowledge distillation with a large scale dataset satir. arXiv 2023, arXiv:2304.07969. [Google Scholar]
- Tang, L.; Xiao, H.; Li, B. Can sam segment anything? When sam meets camouflaged object detection. arXiv 2023, arXiv:2304.04709. [Google Scholar]
- Ji, G.P.; Fan, D.P.; Xu, P.; Cheng, M.M.; Zhou, B.; Van Gool, L. SAM Struggles in Concealed Scenes–Empirical Study on “Segment Anything”. arXiv 2023, arXiv:2304.06022. [Google Scholar] [CrossRef]
- Ji, W.; Li, J.; Bi, Q.; Li, W.; Cheng, L. Segment anything is not always perfect: An investigation of sam on different real-world applications. arXiv 2023, arXiv:2304.05750. [Google Scholar] [CrossRef]
- Cheng, J.; Ye, J.; Deng, Z.; Chen, J.; Li, T.; Wang, H.; Su, Y.; Huang, Z.; Chen, J.; Jiang, L.; et al. Sam-med2d. arXiv 2023, arXiv:2308.16184. [Google Scholar]
- Jiang, P.T.; Yang, Y. Segment anything is a good pseudo-label generator for weakly supervised semantic segmentation. arXiv 2023, arXiv:2305.01275. [Google Scholar]
- He, C.; Li, K.; Zhang, Y.; Xu, G.; Tang, L.; Zhang, Y.; Guo, Z.; Li, X. Weakly-supervised concealed object segmentation with sam-based pseudo labeling and multi-scale feature grouping. In Proceedings of the Advances in Neural Information Processing Systems, Vancouver, BC, Canada, 13 February 2024; Volume 36. [Google Scholar]
- Reedha, R.; Dericquebourg, E.; Canals, R.; Hafiane, A. Transformer neural network for weed and crop classification of high resolution UAV images. Remote. Sens. 2022, 14, 592. [Google Scholar] [CrossRef]
- Brown, T.; Mann, B.; Ryder, N.; Subbiah, M.; Kaplan, J.D.; Dhariwal, P.; Neelakantan, A.; Shyam, P.; Sastry, G.; Askell, A.; et al. Language models are few-shot learners. Adv. Neural Inf. Process. Syst. 2020, 33, 1877–1901. [Google Scholar]
- Dosovitskiy, A.; Beyer, L.; Kolesnikov, A.; Weissenborn, D.; Zhai, X.; Unterthiner, T.; Dehghani, M.; Minderer, M.; Heigold, G.; Gelly, S.; et al. An image is worth 16x16 words: Transformers for image recognition at scale. arXiv 2020, arXiv:2010.11929. [Google Scholar]
- He, K.; Chen, X.; Xie, S.; Li, Y.; Dollár, P.; Girshick, R. Masked autoencoders are scalable vision learners. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, New Orleans, LA, USA, 18–24 June 2022; pp. 16000–16009. [Google Scholar]
- Zhang, W.; Fu, C.; Zheng, Y.; Zhang, F.; Zhao, Y.; Sham, C.W. HSNet: A hybrid semantic network for polyp segmentation. Comput. Biol. Med. 2022, 150, 106173. [Google Scholar] [CrossRef]
- Isensee, F.; Jaeger, P.F.; Kohl, S.A.; Petersen, J.; Maier-Hein, K.H. nnU-Net: A self-configuring method for deep learning-based biomedical image segmentation. Nat. Methods 2021, 18, 203–211. [Google Scholar] [CrossRef]
- Ma, J.; Chen, J.; Ng, M.; Huang, R.; Li, Y.; Li, C.; Yang, X.; Martel, A.L. Loss odyssey in medical image segmentation. Med. Image Anal. 2021, 71, 102035. [Google Scholar] [CrossRef] [PubMed]
- Jha, D.; Smedsrud, P.H.; Riegler, M.A.; Halvorsen, P.; De Lange, T.; Johansen, D.; Johansen, H.D. Kvasir-seg: A segmented polyp dataset. In Proceedings of the 26th International Conference of MultiMedia Modeling (MMM 2020), Daejeon, Republic of Korea, 5–8 January 2020; Proceedings—Part II 26; Springer: Cham, Switzerland, 2020; pp. 451–462. [Google Scholar]
- Bernal, J.; Sánchez, F.J.; Fernández-Esparrach, G.; Gil, D.; Rodríguez, C.; Vilariño, F. WM-DOVA maps for accurate polyp highlighting in colonoscopy: Validation vs. saliency maps from physicians. Comput. Med. Imaging Graph. 2015, 43, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Tajbakhsh, N.; Gurudu, S.R.; Liang, J. Automated polyp detection in colonoscopy videos using shape and context information. IEEE Trans. Med. Imaging 2015, 35, 630–644. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, D.; Bernal, J.; Sánchez, F.J.; Fernández-Esparrach, G.; López, A.M.; Romero, A.; Drozdzal, M.; Courville, A. A benchmark for endoluminal scene segmentation of colonoscopy images. J. Healthc. Eng. 2017, 2017, 4037190. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Histace, A.; Romain, O.; Dray, X.; Granado, B. Toward embedded detection of polyps in wce images for early diagnosis of colorectal cancer. Int. J. Comput. Assist. Radiol. Surg. 2014, 9, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.P.; Ji, G.P.; Zhou, T.; Chen, G.; Fu, H.; Shen, J.; Shao, L. Pranet: Parallel reverse attention network for polyp segmentation. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Lima, Peru, 4–8 October 2020; Springer: Cham, Switzerland, 2020; pp. 263–273. [Google Scholar]
- Dong, B.; Wang, W.; Fan, D.P.; Li, J.; Fu, H.; Shao, L. Polyp-pvt: Polyp segmentation with pyramid vision transformers. arXiv 2021, arXiv:2108.06932. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, G.; Zhou, T.; Zhang, Y.; Liu, N. Context-aware cross-level fusion network for camouflaged object detection. arXiv 2021, arXiv:2105.12555. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).