Numerical Simulation of Hydrodynamics and Heat Transfer in a Reactor with a Fluidized Bed of Catalyst Particles in a Three-Dimensional Formulation
Abstract
1. Introduction
- Intensive mixing of particles provides high mass and heat transfer rates between the medium and the particles (for instance, in the case of an exothermic reaction occurring on the surface of the particles), and, as a result, improves heat removal through the reactor wall.
- Since the fluidized particle bed has some liquid properties, it is possible to design apparatuses with a circulating particle bed and use various remote devices.
- The simple hardware design of the fluidization allows for their use in many industries.
- The modules of geometry and computational mesh construction and visualization of results built into ANSYS Workbench are well synchronized with each other, allowing one to quickly make changes to the model, if necessary, and to process the simulation results without the use of other programs.
- ANSYS Fluent provides both a wide choice of models and a wide possibility of their parameterization, which is a key factor in this work. OpenFOAM has even more flexibility due to its open-source nature, but the requirement to know the C++ programming language and the theoretical foundations of computational fluid dynamics can become a significant obstacle. In the case of FlowVision, the software package does not have the ability to describe particles using the Lagrange approach (i.e., there is no discrete element model).
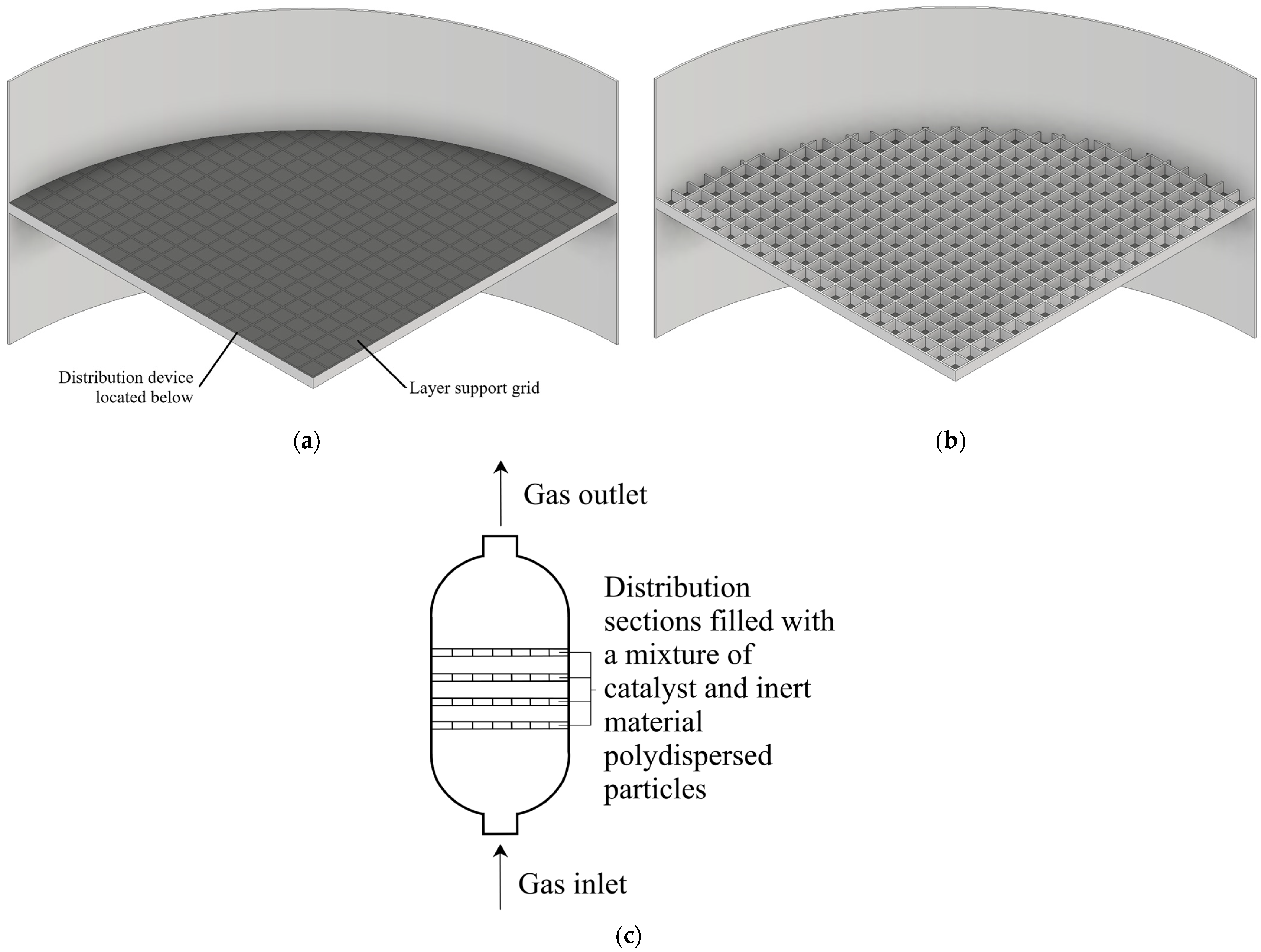
2. Materials and Methods
2.1. Object of Study
2.2. Research Methods
- to conduct three-dimensional simulation, since the reduced scale frees up a significant amount of computing power;
- to use more precise models, such as the Unresolved Discrete Particle Model (UDPM), and Resolved Discrete Particle Model (RDPM), which are not capable of simulating objects larger than the laboratory size. In this work, preference was given to UDPM, since, according to the assumptions obtained, the interaction of particles between themselves and the walls, and not between gas and particles, plays a decisive role in the behavior of the particle bed;
- to reduce the total time of each calculation, which allows one to make more calculations to collect more detailed information about the system.
- for DPM: the rheological “spring-damper” scheme determines the nature of collisions of the particles with objects. In this case it implies an inelastic collision; for the particle–plane symmetry pair, an ideally elastic collision was chosen. The lateral faces of the upper part of the simulated cell (Figure 4) were set as planes of symmetry, since they represent the gas–gas interface planes, i.e., there is a gas phase in front of and behind the plane;
- a turbulence model: the realizable k-ε model with restriction of the turbulence kinetic energy generation term and viscous heating to make calculations taking heating into account [49].
2.2.1. Gas and Particle Motion Models
2.2.2. Turbulence Model
- the turbulent viscosity formula now includes the variable originally proposed by Reynolds;
- the dissipation equation has been changed, based on the dynamic equation of the mean-square vorticity fluctuation.
2.2.3. Heat Transfer
3. Results
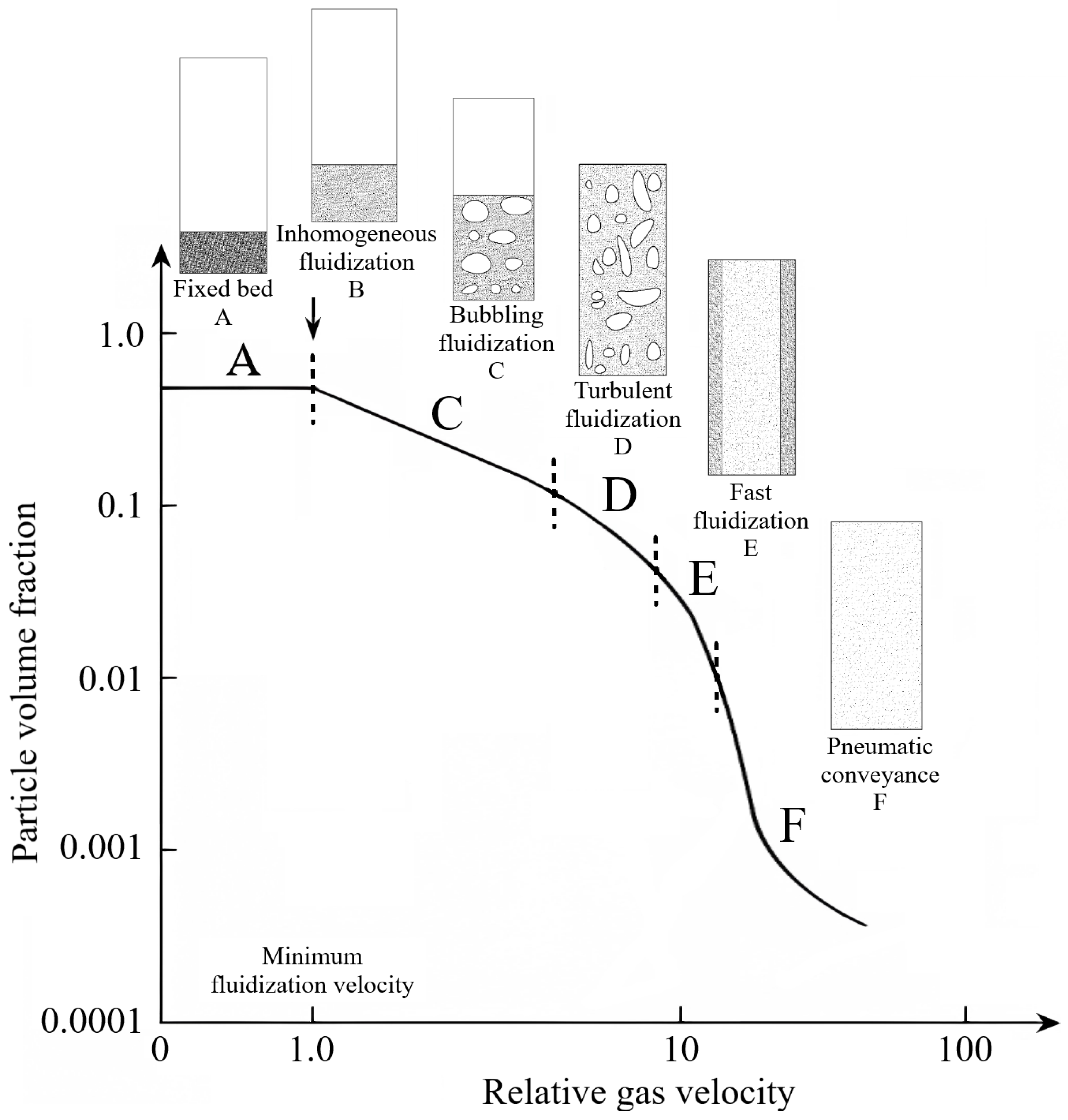
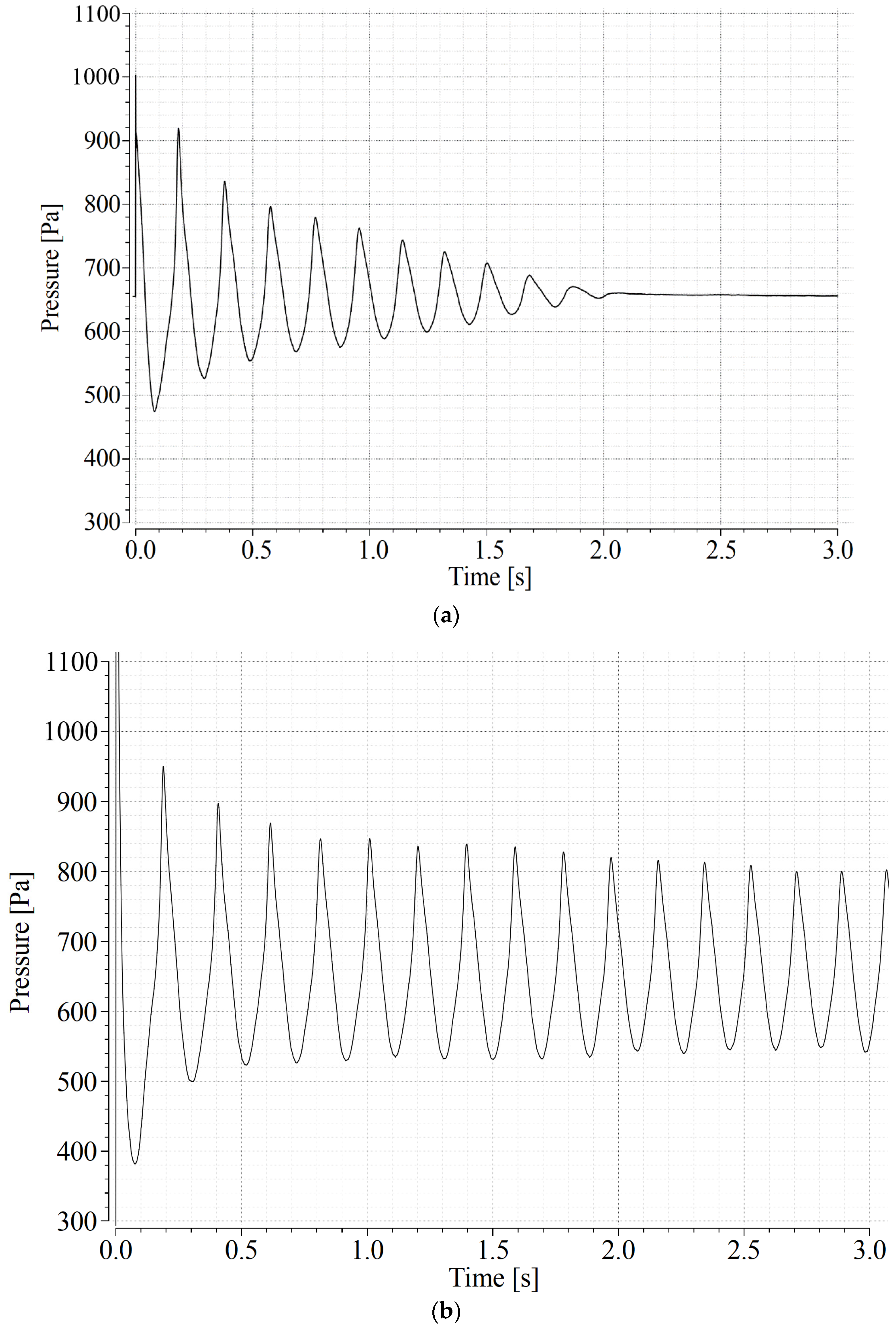
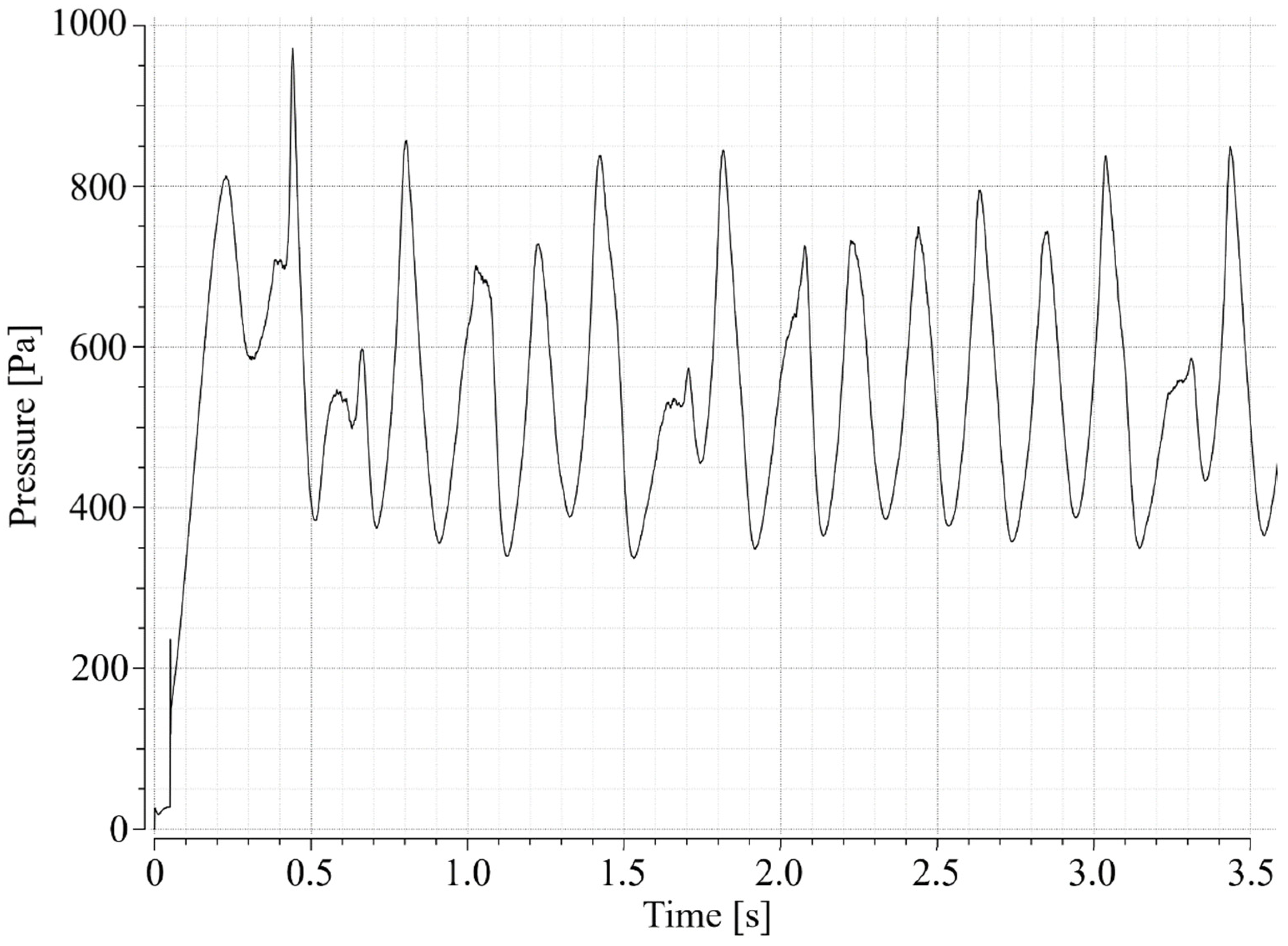
3.1. Determination of the Minimum Fluidization Velocity
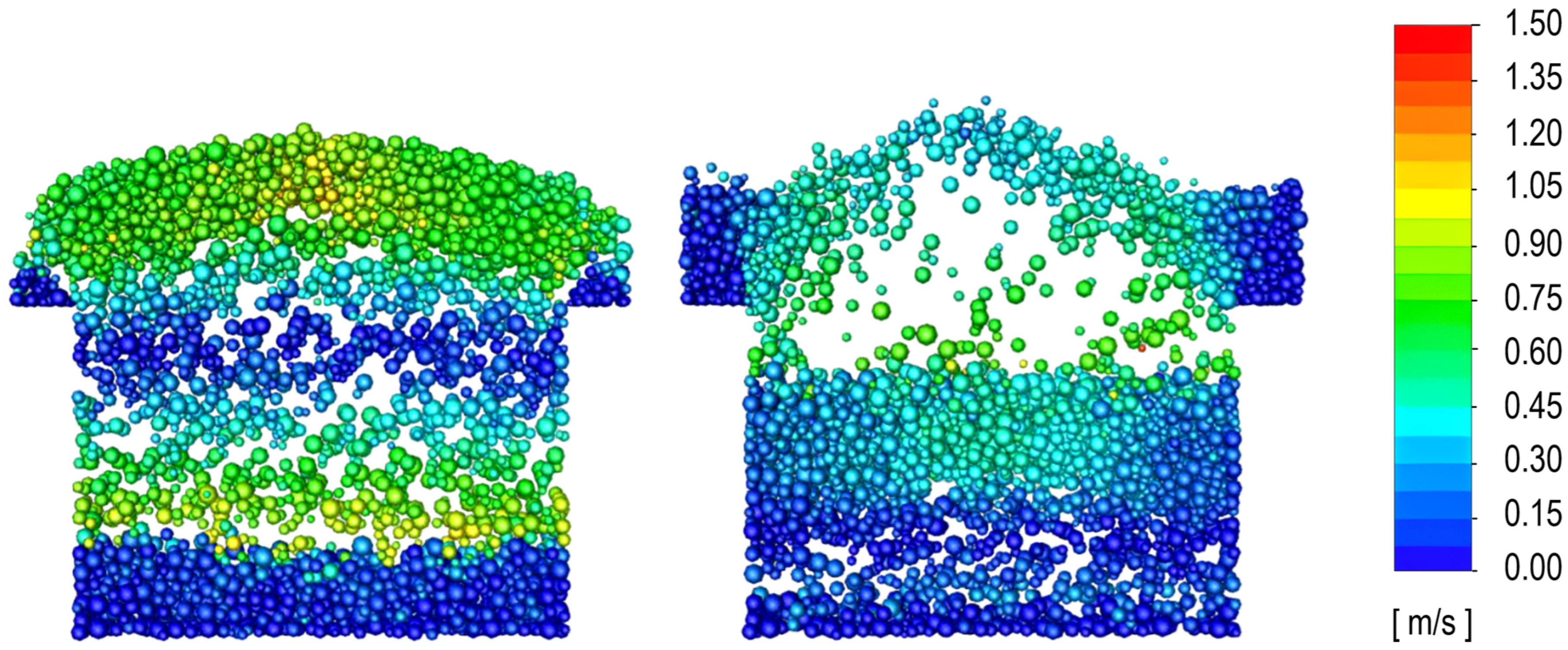
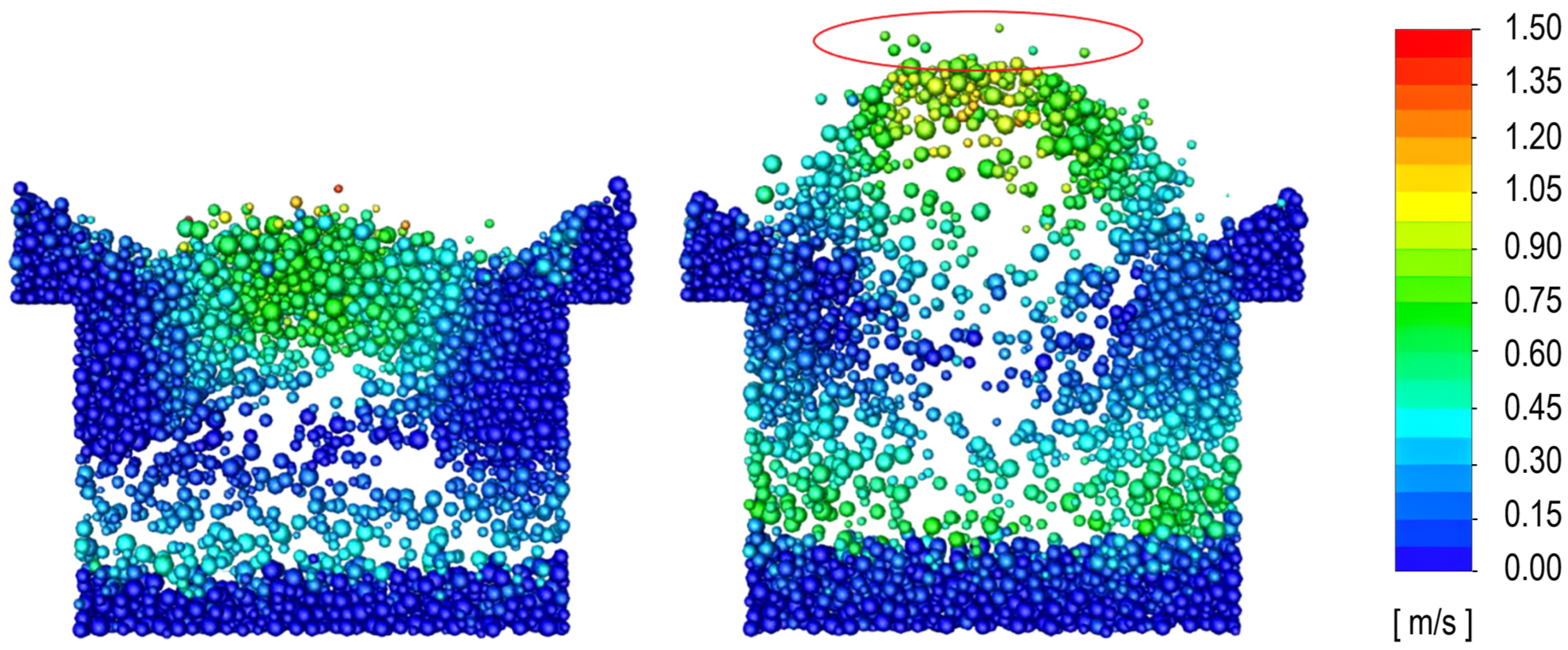
3.2. Determination of the Minimum Velocity of Bubbling Regime
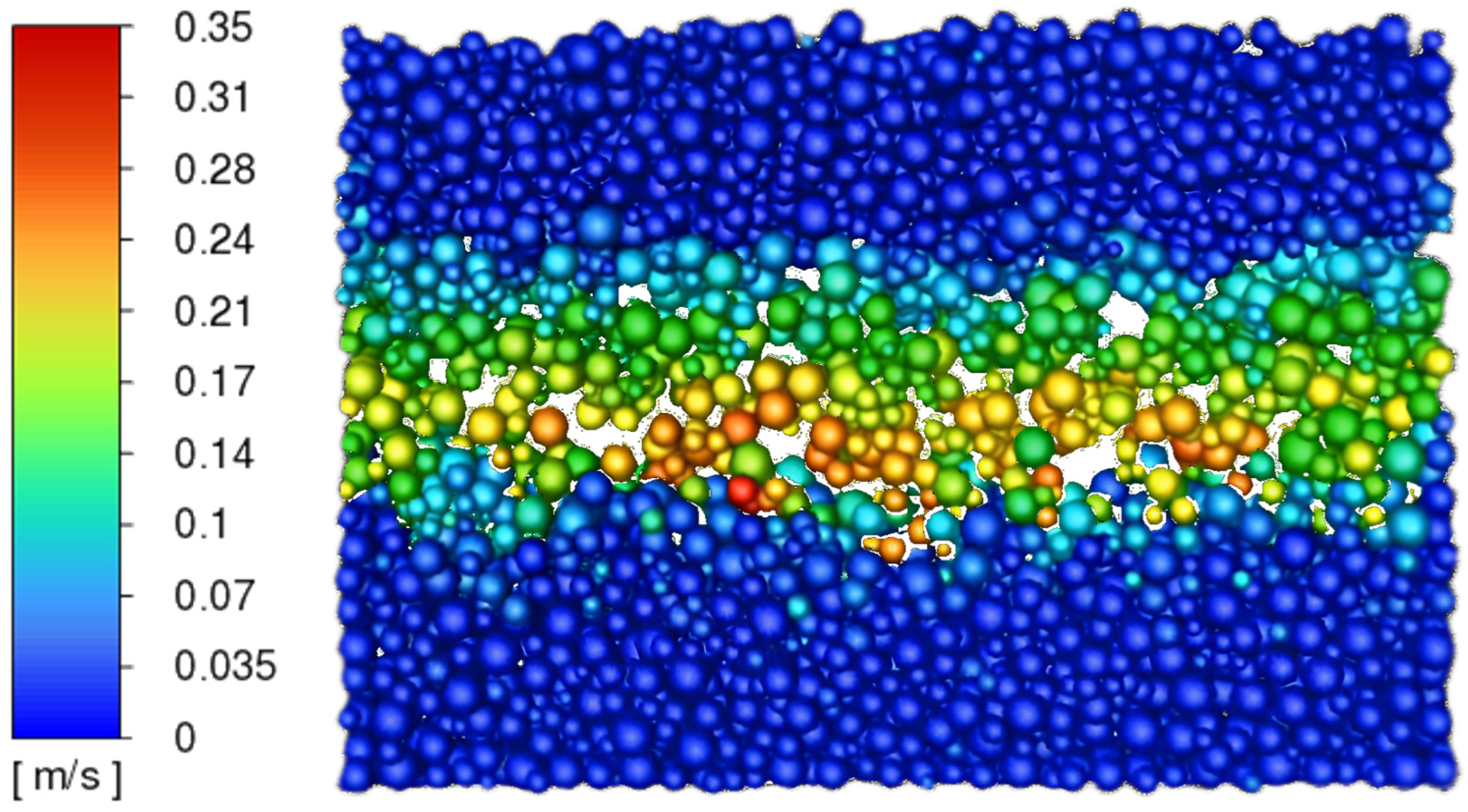
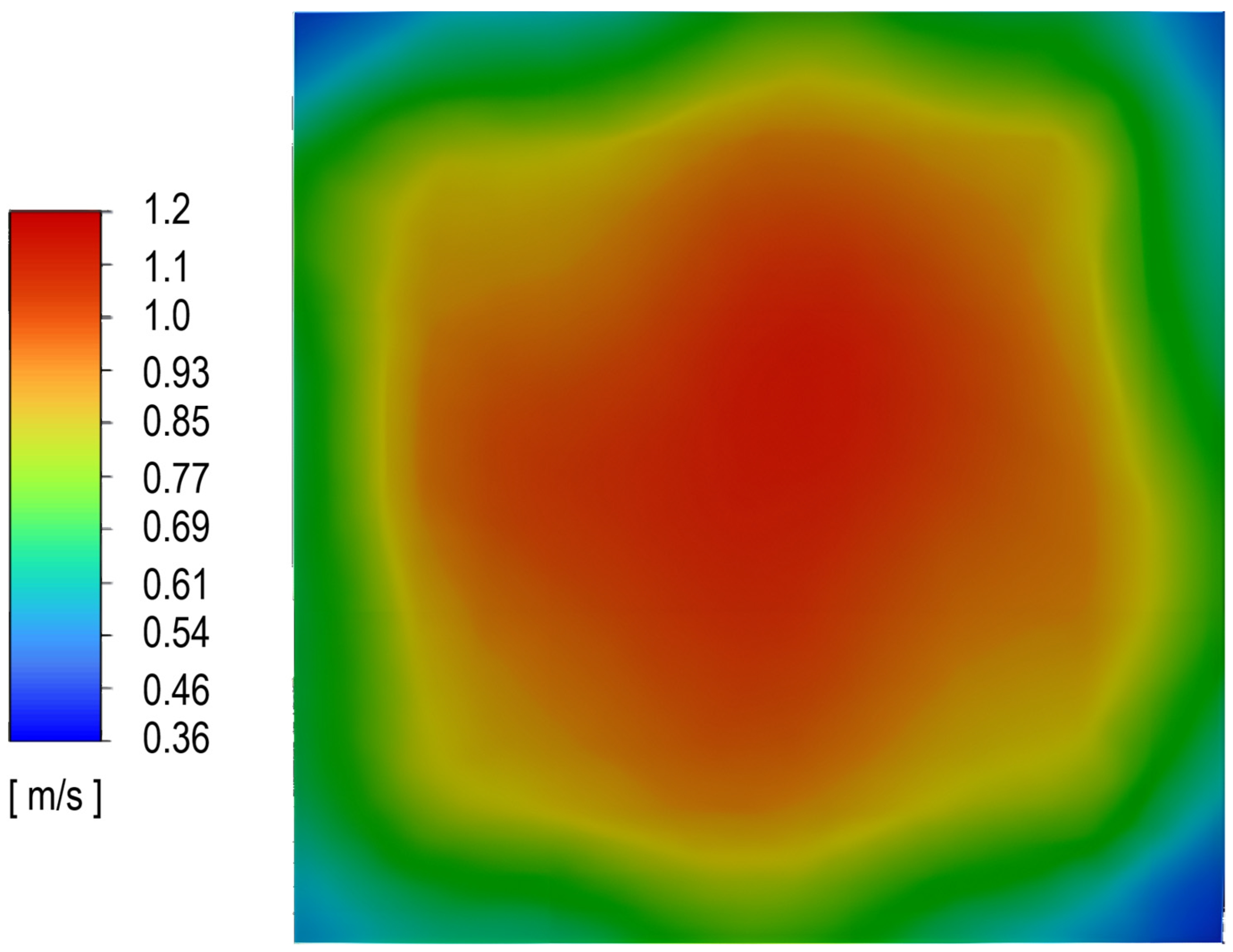
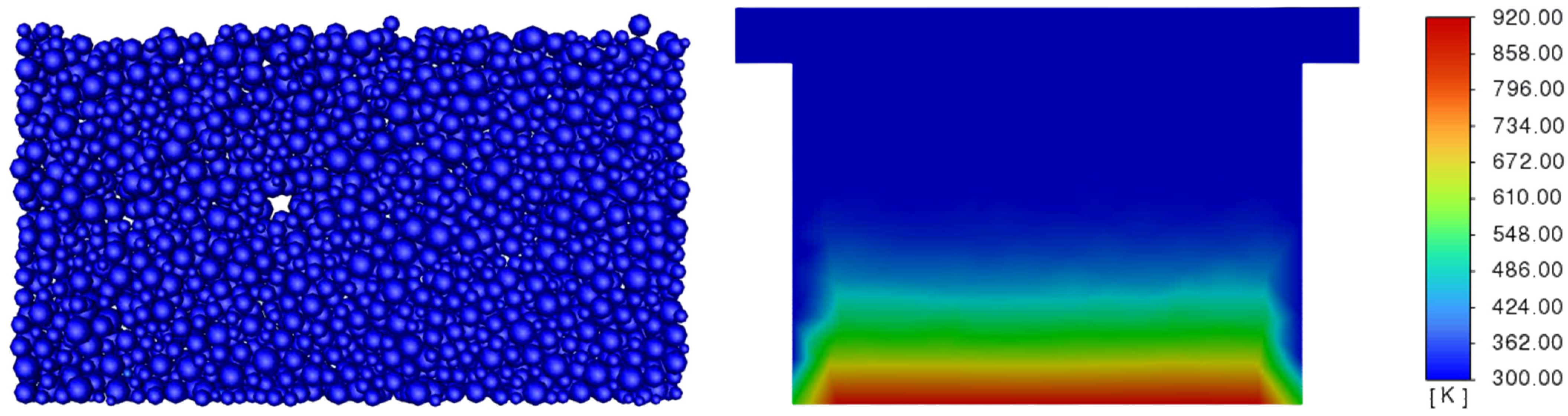
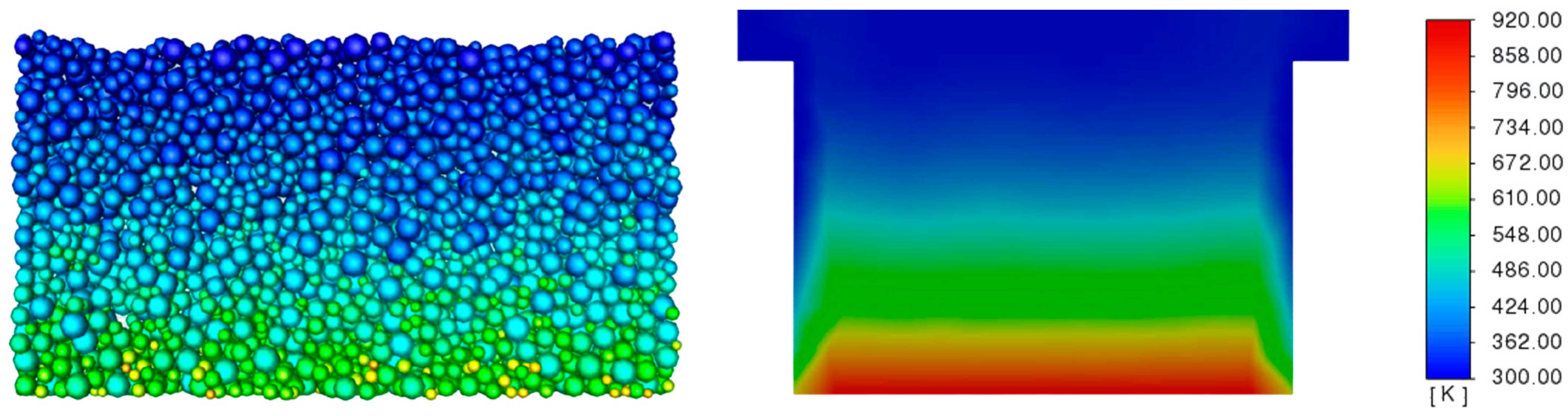
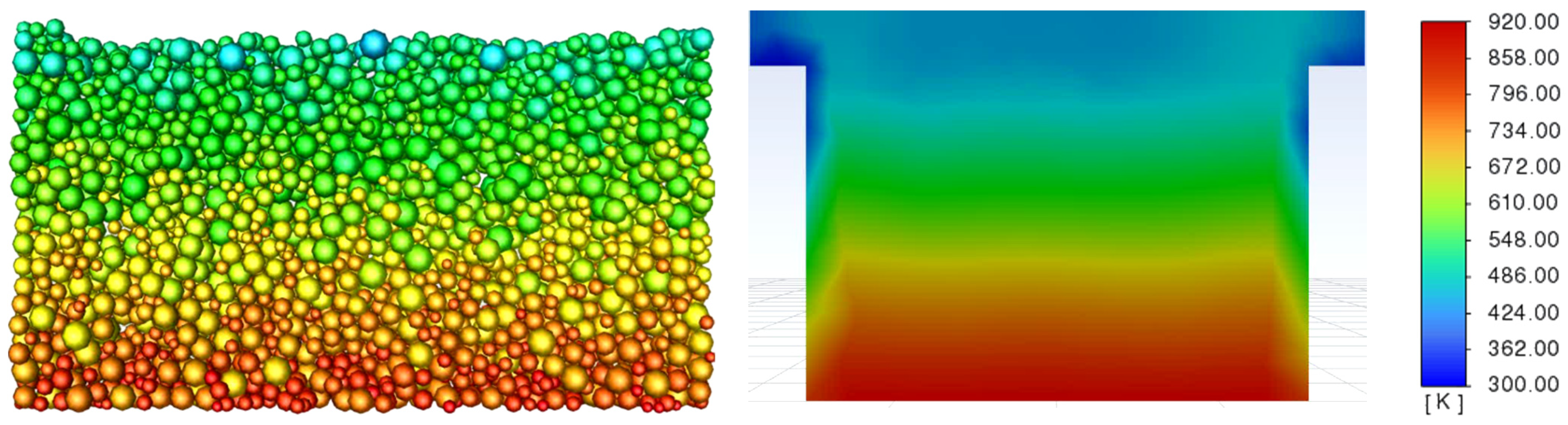
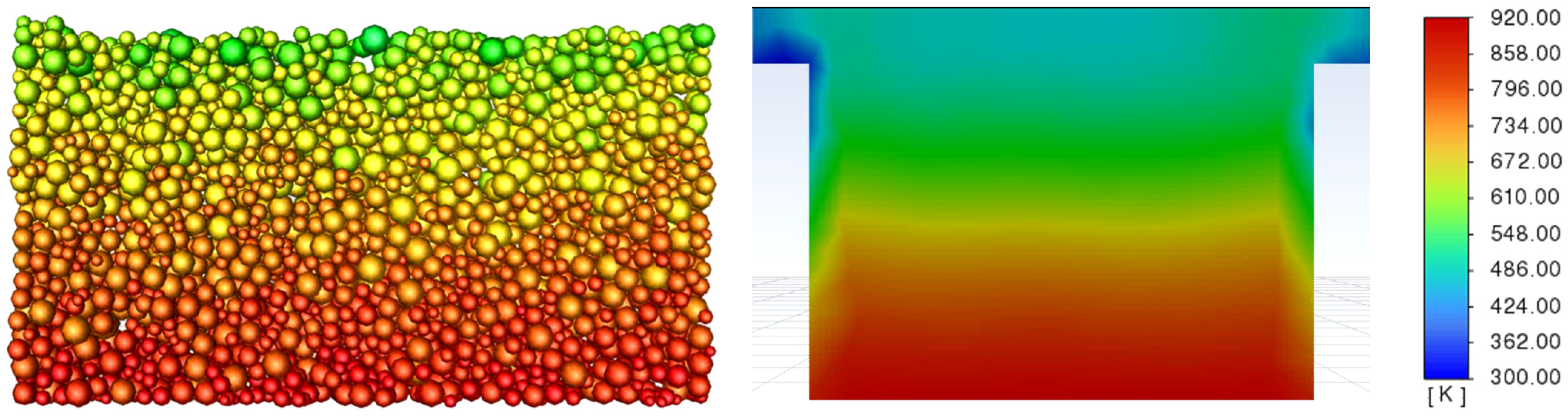
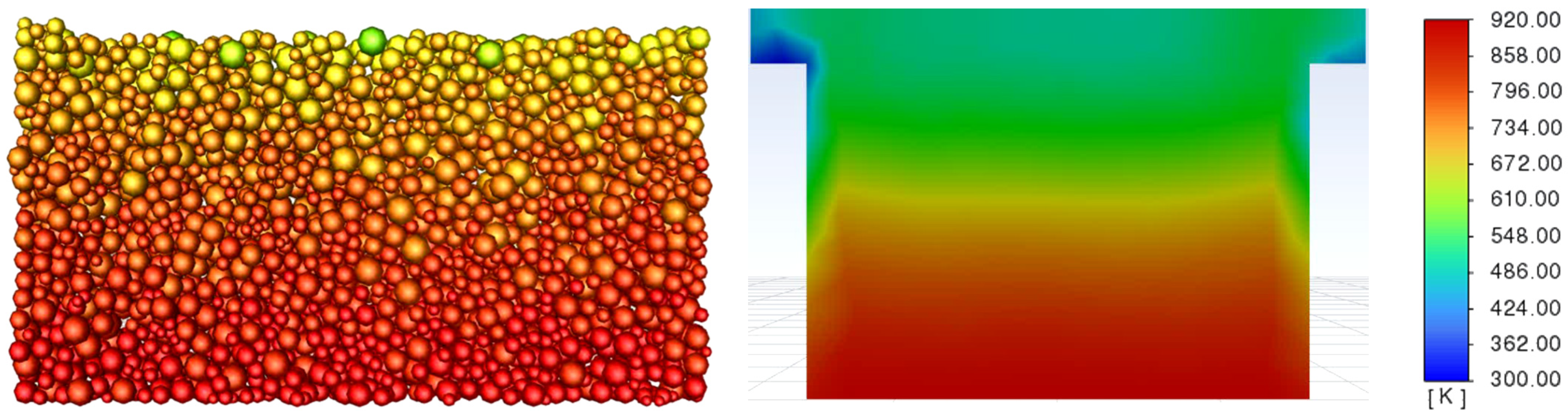
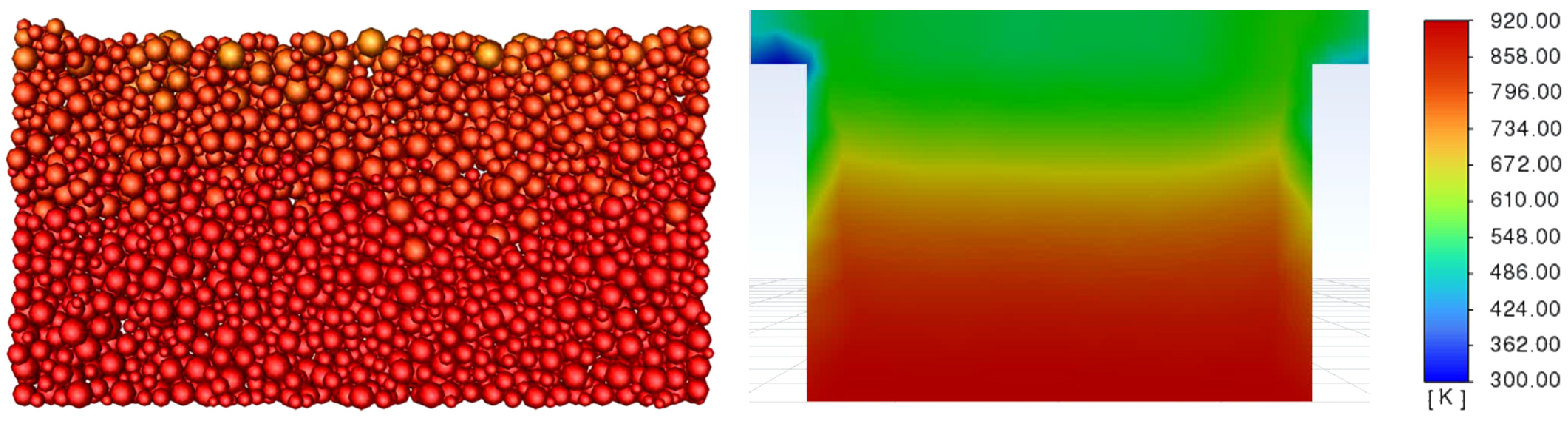
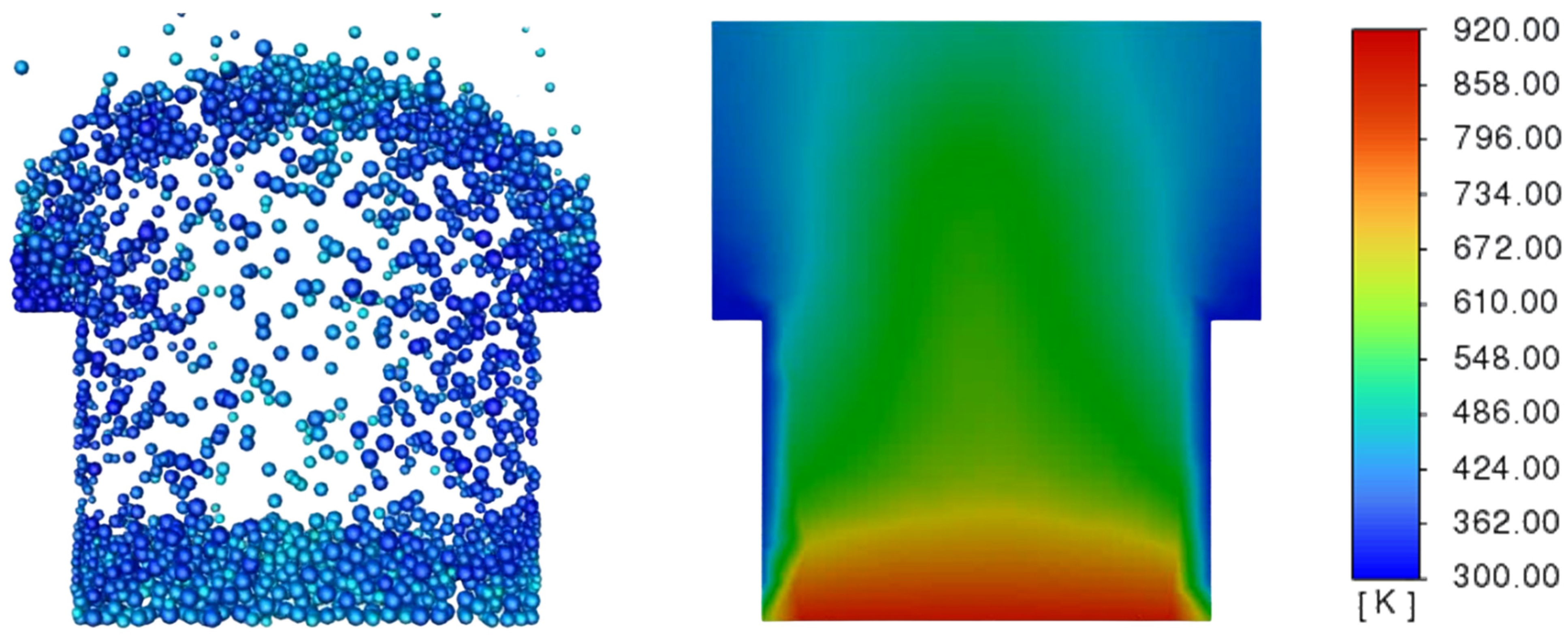
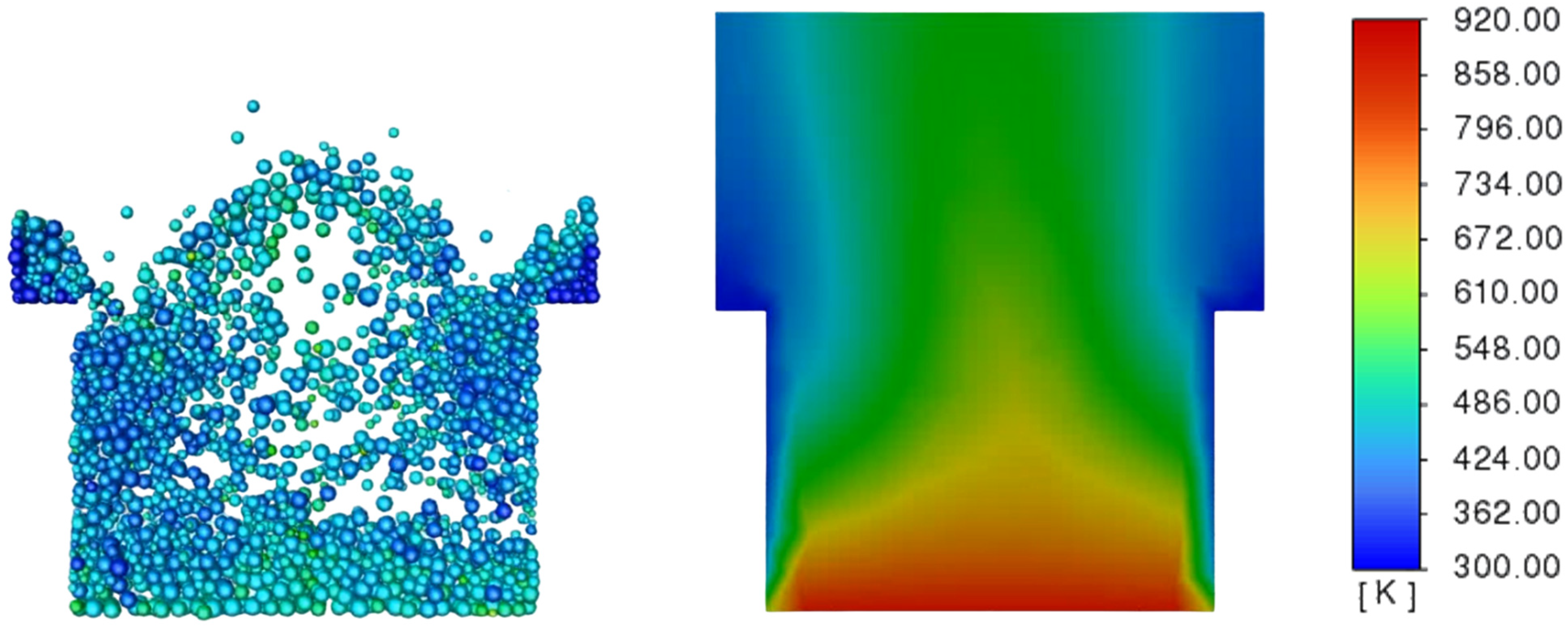
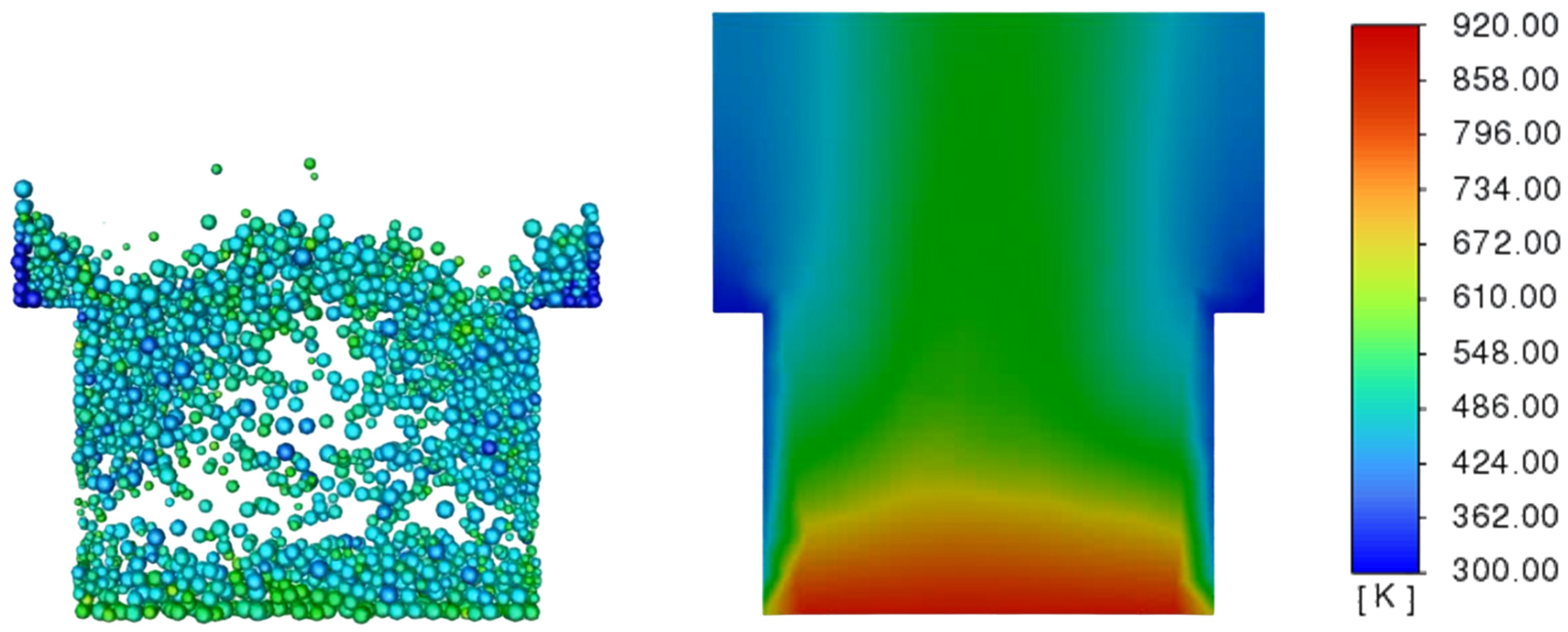
3.3. Determination of the Effect of the Fluidization Regime on Particle Heating and the Effect of High Gas Temperature on the Fluidization Regime Itself
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Menard, J.-C.; Thibault, N.W. Abrasives. In Ullmann’s Encyclopedia of Industrial Chemistry; Electronic Release; Wiley-VCH: Weinheim, Germany, 2000; pp. 28–45. [Google Scholar] [CrossRef]
- Bello, M.M.; Raman, A.A.A.; Purushothaman, M. Applications of fluidized bed reactors in wastewater treatment—A review of the major design and operational parameters. J. Clean. Prod. 2017, 141, 1492–1514. [Google Scholar] [CrossRef]
- Yates, J.G.; Lettieri, P. Fluidized-Bed Reactors: Processes and Operating Conditions; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Khawaja, H.A.; Boiger, G.; Messahel, R.; Moatamedi, M.; Zisis, I. Multiphysics Modelling of Fluid-Particulate Systems; Academic Press: London, UK, 2020. [Google Scholar] [CrossRef]
- Grace, J.R.; Bi, X.; Ellis, N. Essentials of Fluidization Technology; Wiley-VCH Verlag GmbH&Co., KgaA: Weinheim, Germany, 2020. [Google Scholar] [CrossRef]
- Kunii, D.; Levenspiel, O. Fluidization Engineering; Butterworth-Heinemann: Oxford, UK, 1991. [Google Scholar] [CrossRef]
- McKetta, J.J. Encyclopedia of Chemical Processing and Design, V. 48—Residual Refining and Processing to Safety, Operating Discipline; Marcel Dekker. Inc.: New York, NY, USA, 1994. [Google Scholar] [CrossRef]
- Gibilaro, L.G. Fluidization Dynamics, 1st ed.; Butterworth-Heinemann: Oxford, UK, 2001. [Google Scholar] [CrossRef]
- Basu, P. Circulating Fluidized Bed Boilers: Design, Operation and Maintenance; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Li, S. Fluidized Bed Reactor. In Reaction Engineering; Li, S., Ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 369–403. [Google Scholar] [CrossRef]
- Arastoopour, H.; Gidaspow, D.; Lyczkowski, R.W. Transport Phenomena in Multiphase Systems; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Chen, X.-Z.; Shi, D.-P.; Gao, X.; Luo, Z.-H. A fundamental CFD study of the gas–solid flow field in fluidized bed polymerization reactors. Powder Technol. 2011, 205, 276–288. [Google Scholar] [CrossRef]
- Li, T.; Grace, J.; Bi, X. Study of wall boundary condition in numerical simulations of bubbling fluidized beds. Powder Technol. 2010, 203, 447–457. [Google Scholar] [CrossRef]
- Akbari, V.; Borhani, T.N.G.; Aramesh, R.; Hamid, M.K.A.; Shamiri, A.; Hussain, M.A. Evaluation of hydrodynamic behavior of the perforated gas distributor of industrial gas phase polymerization reactor using CFD-PBM coupled model. Comput. Chem. Eng. 2015, 82, 344–361. [Google Scholar] [CrossRef]
- Akbari, V.; Borhani, T.N.G.; Shamiri, A.; Hamid, M.K.A. A CFD–PBM coupled model of hydrodynamics and mixing/segregation in an industrial gas-phase polymerization reactor. Chem. Eng. Res. Des. 2015, 96, 103–120. [Google Scholar] [CrossRef]
- Soloveva, O.V.; Solovev, S.A.; Egorova, S.R.; Lamberov, A.A.; Antipin, A.V.; Shamsutdinov, E.V. CFD modeling a fluidized bed large scale reactor with various internal elements near the heated particles feeder. Chem. Eng. Res. Des. 2018, 138, 212–228. [Google Scholar] [CrossRef]
- Bi, H.T.; Grace, J.R. Flow regime diagrams for gas-solid fluidization and upward transport. Int. J. Multiph. Flow 1995, 21, 1229–1236. [Google Scholar] [CrossRef]
- Werther, J. Fluidized-Bed Reactors. In Ullmann’s Encyclopedia of Industrial Chemistry, 7th ed.; Electronic Release; Wiley-VCH: Weinheim, Germany, 2012; pp. 319–366. [Google Scholar] [CrossRef]
- Gordon, A.L.; Amundson, N.R. Modelling of fluidized bed reactors—IV: Combustion of carbon particles. Chem. Eng. Sci. 1976, 31, 1163–1178. [Google Scholar] [CrossRef]
- Grace, J.R. Fluidized-bed catalytic reactors. In Multiphase Catalytic Reactors: Theory, Design, Manufacturing, and Applications; Önsan, Z.L., Avci, A.K., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 80–94. [Google Scholar] [CrossRef]
- Leckner, B. Hundred years of fluidization for the conversion of solid fuels. Powder Technol. 2022, 411, 117935. [Google Scholar] [CrossRef]
- Mishra, S.; Baliarsingh, M.; Mahanta, J.; Beuria, P.C. Batch scale study on magnetizing roasting of low-grade iron ore tailings using fluidized bed roaster. Mater. Today Proc. 2022, 62, 5856–5860. [Google Scholar] [CrossRef]
- de Munck, M.J.A.; Peters, E.A.J.F.; Kuipers, J.A.M. Experimental investigation of monodisperse solids drying in a gas-fluidized bed. Chem. Eng. Sci. 2022, 259, 117783. [Google Scholar] [CrossRef]
- Lv, B.; Dong, B.; Zhang, C.; Chen, Z.; Zhao, Z.; Deng, X.; Fang, C. Effective adsorption of methylene blue from aqueous solution by coal gangue-based zeolite granules in a fluidized bed: Fluidization characteristics and continuous adsorption. Powder Technol. 2022, 408, 117764. [Google Scholar] [CrossRef]
- Singh, S.K.D.; Lu, K. SiOC coatings on yttria stabilized zirconia microspheres using a fluidized bed coating process. Powder Technol. Part A 2022, 396, 158–166. [Google Scholar] [CrossRef]
- Le, V.G.; Luu, T.A.; Bui, N.T.; Mofijur, M.; Van, H.T.; Lin, C.; Tran, H.T.; Bahari, M.B.; Vu, C.T.; Huang, Y.H. Fluidized-bed homogeneous granulation for potassium and phosphorus recovery: K-struvite release kinetics and economic analysis. J. Taiwan Inst. Chem. Eng. 2022, 139, 104494. [Google Scholar] [CrossRef]
- Pujan, R.; Hauschild, S.; Gröngröft, A. Process simulation of a fluidized-bed catalytic cracking process for the conversion of algae oil to biokerosene. Fuel Process. Technol. 2017, 167, 582–607. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, F.; Sun, Y.; Zhang, J.; Tang, S.; Xu, G. Characteristics of tar abatement by thermal cracking and char catalytic reforming in a fluidized bed two-stage reactor. Fuel 2018, 231, 18–25. [Google Scholar] [CrossRef]
- Ahn, H.; Kim, D.; Lee, Y. Combustion characteristics of sewage sludge solid fuels produced by drying and hydrothermal carbonization in a fluidized bed. Renew. Energy 2020, 147, 957–968. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, S.; Li, D.; Zhao, C.; Han, C.; Yu, G.; Song, M. Effect of the blend ratio on the co-gasification of biomass and coal in a bubbling fluidized bed with CFD-DEM. Int. J. Hydrogen Energy 2022, 47, 22328–22339. [Google Scholar] [CrossRef]
- Zhang, X.; Qian, W.; Zhang, H.; Sun, Q.; Ying, W. Effect of the operation parameters on the Fischer–Tropsch synthesis in fluidized bed reactors. Chin. J. Chem. Eng. 2018, 26, 245–251. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, L.; Liu, C.; Wang, J.; Xing, X.; Liu, Y.; Chen, Y.; Chao, L.; Dai, J.; Zhang, Y.; et al. Interconnected pyrolysis and gasification of typical biomass in a novel dual fluidized bed. Energy Convers. Manag. 2022, 271, 116323. [Google Scholar] [CrossRef]
- Wang, J.; van der Hoef, M.A.; Kuipers, J.A.M. Coarse grid simulation of bed expansion characteristics of industrial-scale gas-solid bubbling fluidized beds. Chem. Eng. Sci. 2010, 65, 2125–2131. [Google Scholar] [CrossRef]
- Sanfilippo, D. Dehydrogenations in fluidized bed: Catalysis and reactor engineering. Catal. Today 2011, 178, 142–150. [Google Scholar] [CrossRef]
- van Ommen, J.R.; Schouten, J.C.; vander Stappen, M.L.M.; van den Bleek, C.M. Response characteristics of probe-transducer systems for pressure measurements in gas-solid fluidized beds: How to prevent pitfalls in dynamic pressure measurements. Powder Technol. 1999, 106, 199–218. [Google Scholar] [CrossRef]
- Cundall, P.A.; Strack, O.D. A discrete numerical model for granular assemblies. Géotechnique 1979, 29, 47–65. [Google Scholar] [CrossRef]
- Gui, N.; Jiang, S.; Tu, J.; Yang, X. Gas-Particle and Granular Flow Systems: Coupled Numerical Methods and Applications; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Li, B.; Jia, Z.; Jiao, S.; Ma, H. Study on the Influence of Different Factors on Pneumatic Conveying in Horizontal Pipe. Appl. Sci. 2023, 13, 5483. [Google Scholar] [CrossRef]
- Wang, X.; Chen, K.; Kang, T.; Ouyang, J. A Dynamic Coarse Grain Discrete Element Method for Gas-Solid Fluidized Beds by Considering Particle-Group Crushing and Polymerization. Appl. Sci. 2020, 10, 1943. [Google Scholar] [CrossRef]
- Hoomans, B.P.B.; Kuipers, J.A.M.; Briels, W.J.; van Swaaij, W.P.M. Discrete particle simulation of bubble and slug formation in a two-dimensional gas-fluidised bed: A hard-sphere approach. Chem. Eng. Sci. 1996, 51, 99–118. [Google Scholar] [CrossRef]
- Xu, B.H.; Yu, A.B. Numerical simulation of the gas-solid flow in a fluidized bed by combining discrete particle method with computational fluid dynamics. Chem. Eng. Sci. 1997, 52, 2785–2809. [Google Scholar] [CrossRef]
- van der Hoef, M.A.; Ye, M.; van Sint Annaland, M.; Andrews, A.T.; Sundaresan, S.; Kuipers, J.A.M. Multiscale modeling of gas-fluidized beds. Adv. Chem. Eng. 2006, 31, 65–149. [Google Scholar] [CrossRef]
- Deen, N.G.; van Sint Annaland, M.; van der Hoef, M.A.; Kuipers, J.A.M. Review of discrete particle modeling of fluidized beds. Chem. Eng. Sci. 2007, 62, 28–44. [Google Scholar] [CrossRef]
- Zhu, H.P.; Zhou, Z.Y.; Yang, R.Y.; Yu, A.B. Discrete particle simulation of particulate systems: Theoretical developments. Chem. Eng. Sci. 2007, 62, 3378–3396. [Google Scholar] [CrossRef]
- Ge, W.; Wang, L.; Xu, J.; Chen, F.; Zhou, G.; Lu, L.; Chang, Q.; Li, J. Discrete simulation of granular and particle-fluid flows: From fundamental study to engineering application. Rev. Chem. Eng. 2017, 33, 551–623. [Google Scholar] [CrossRef]
- Golshan, S.; Sotudeh-Gharebagh, R.; Zarghami, R.; Mostoufi, N.; Blais, B.; Kuipers, J.A.M. Review and implementation of CFD-DEM applied to chemical process systems. Chem. Eng. Sci. 2020, 221, 115646. [Google Scholar] [CrossRef]
- Constant, M.; Coppin, N.; Dubois, F.; Vidal, V.; Legat, V.; Lambrechts, J. Simulation of air invasion in immersed granular beds with an unresolved FEM-DEM model. Comput. Part. Mech. 2021, 8, 535–560. [Google Scholar] [CrossRef]
- OpenFOAM User Guide, Version 10; The OpenFOAM Foundation, Ltd.: London, UK, 2022. Available online: https://www.openfoam.com/documentation/user-guide (accessed on 6 May 2024).
- ANSYS Fluent Theory Guide; Release 20.1. ANSYS, Inc.: Canonsburg, PA, USA, 2020. Available online: https://www.ansys.com/products/fluids/ansys-fluent (accessed on 6 May 2024).
- FlowVision User’s Guide; TESIS, Ltd., Russian Federation: Moscow, Russia, 2023. Available online: https://flowvisioncfd.com/webhelp/fven_31302/index.html?vvedenie.htm (accessed on 6 May 2024).
- Tsuji, Y.; Kawaguchi, T.; Tanaka, T. Discrete particle simulation of two-dimensional fluidized bed. Powder Technol. 1993, 77, 79–87. [Google Scholar] [CrossRef]
- van der Hoef, M.A.; van Sint Annaland, M.; Deen, N.G.; Kuipers, J.A.M. Numerical simulation of dense gas-solid fluidized beds: A multiscale modeling strategy. Annu. Rev. Fluid Mech. 2008, 40, 47–70. [Google Scholar] [CrossRef]
- Servin, M.; Wang, D.; Lacoursière, C.; Bodin, K. Examining the smooth and nonsmooth discrete element approaches to granular matter. Int. J. Numer. Methods 2014, 97, 878–902. [Google Scholar] [CrossRef]
- Yang, S.; Li, H.; Zhu, Q. Experimental study and numerical simulation of baffled bubbling fluidized beds with Geldart A particles in three dimensions. Chem. Eng. J. 2015, 259, 338–347. [Google Scholar] [CrossRef]
- Yang, S.; Peng, L.; Liu, W.; Zhao, H.; Lv, X.; Li, H.; Zhu, Q. Simulation of hydrodynamics in gas-solid bubbling fluidized bed with louver baffles in three dimensions. Powder Technol. 2016, 296, 37–44. [Google Scholar] [CrossRef]
- Raza, N.; Mehdi, R.; Ahsan, M.; Mehran, M.T.; Naqvi, S.R.; Uddin, E. Optimizing Design and Operational Parameters for Enhanced Mixing and Hydrodynamics in Bubbling Fluidized Bed Gasifiers: An Experimental and CFD-Based Approach. Appl. Sci. 2023, 13, 9317. [Google Scholar] [CrossRef]
- Rennebaum, H.S.; Brummerloh, D.L.; Benders, S.; Penn, A. The effect of baffles on the hydrodynamics of a gas-solid fluidized bed studied using real-time magnetic resonance imaging. Powder Technol. 2024, 432, 119114. [Google Scholar] [CrossRef]
- Zhu, L.-T.; Ouyang, B.; Luo, Z.-H. Using mesoscale drag model-augmented coarse-grid simulation to design fluidized bed reactor: Effect of bed internals and sizes. Chem. Eng. J. 2022, 253, 117547. [Google Scholar] [CrossRef]
- Phuakpunk, K.; Chalermsinsuwan, B.; Assabumrungrat, S. Reduction of bubble coalescence by louver baffles in fluidized bed gasifier. Energy Rep. 2022, 8, 96–106. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Y.; Zhang, S.; Oloruntoba, A. Effect of structure parameters on forces acting on baffles used in gas–solids fluidized beds. Particuology 2022, 61, 111–119. [Google Scholar] [CrossRef]
- Zimmerman, V.; McBrien, R.; Quaiattini, R.; Ker, V.; Jiang, Y. A Bed Plate with Supporting Grid for a Fluidized Bed Reactor and Its Modeling. Patent No. WO 2016/181238, 15 April 2016. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2016181238 (accessed on 6 May 2024).
- Lussier, R.J.; Wallace, M.D. Alumina Trihydrate Derived High Pore Volume, High Surface Area Aluminum Oxide Composites and Methods of Their Preparation and Use. Patent No. WO 2001/045838, 28 June 2001. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2001045838 (accessed on 6 May 2024).
- Anderson, J.D., Jr. Computational Fluid Dynamics: The Basics with Applications; McGraw-Hill, Inc.: New York, NY, USA, 1995. [Google Scholar]
- Patankar, S.V. Numerical Heat Transfer and Fluid Flow; Hemisphere Publishing Corporation: London, UK, 1980. [Google Scholar] [CrossRef]
- Batchelor, G.K. An Introduction to Fluid Dynamics; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar] [CrossRef]
- Skodras, G.; Kaldis, S.P.; Sakellaropoulos, G.P.; Sofialidis, D.; Faltsi, O. Simulation of a molten bath gasifier by using a CFD code. Fuel 2003, 82, 2033–2044. [Google Scholar] [CrossRef]
- Christensen, D.; Vervloet, D.; Nijenhuis, J.; van Wachem, B.G.M.; van Ommen, J.R.; Coppens, M.-O. Insights in distributed secondary gas injection in a bubbling fluidized bed via discrete particle simulations. Powder Technol. 2008, 183, 454–466. [Google Scholar] [CrossRef]
- Jackson, R. The Dynamics of Fluidized Particles; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Zwinkels, M.F.M.; Järås, S.G.; Menon, P.G.; Griffin, T.A. Catalytic materials for high-temperature combustion. Catal. Rev. Sci. Eng. 1993, 35, 319–358. [Google Scholar] [CrossRef]
- Cui, J.; Hou, Q.; Shen, Y. CFD-DEM study of coke combustion in the raceway cavity of an ironmaking blast furnace. Powder Technol. 2020, 362, 539–549. [Google Scholar] [CrossRef]
- Xie, J.; Zhoung, W.; Shao, Y.; Li, K. Coupling of CFD-DEM and reaction model for 3D fluidized beds. Powder Technol. 2019, 353, 72–83. [Google Scholar] [CrossRef]
- Xie, J.; Zhoung, W.; Shao, Y. Study on the char combustion in a fluidized bed by CFD-DEM simulations: Influences of fuel properties. Powder Technol. 2021, 394, 20–34. [Google Scholar] [CrossRef]




| Performance Comparison | Computational Fluid Dynamics Software Packages | ||
|---|---|---|---|
| OpenFOAM (v11) | ANSYS Fluent (2021R1) | FlowVision (3.13.03) | |
| 1 | 2 | 3 | 4 |
| Manufacturer | The OpenFOAM Foundation, Ltd., London, UK | ANSYS, Inc., USA, Ansys Drive Canonsburg, PA USA | LLC “TESIS”, Moscow, Russia |
| The need for additional software tools for the creation of geometry of the computational domain and for the visualization of calculations | The program is provided together with a set of independent utilities for the creation of geometry, calculation area and the visualization of results | ANSYS Fluent is part of the ANSYS Workbench platform, which includes not only the necessary geometry and visualization modules, but also other software packages | The program has separate modules for the creation of geometry of the computational domain and the visualization of the resulting solution |
| Numerical method | Final volumes | Final volumes | Final volumes |
| Built-in turbulence models | Raynolds Averaged Simulation (RAS), Detached Eddy Simulation (DES), Large Eddy Simulation (LES) | Spalart–Allmaras model, k-ε, k-ω, Reynolds stress, Detached Eddy Simulation (DES), Large Eddy Simulation (LES) | k-ε, k-ω, Spalart–Allmares, Large Eddy Simulation (LES) |
| Possibility of compressible flow simulation | Possible (including supersonic) | Possible (including supersonic) | Possible (including supersonic) |
| Possibility of multiphase flow simulation | Possible | Possible | Possible only through Euler’s approach |
| Computational mesh | Structured and non-structured mesh | Structured and non-structured mesh | Adaptive mesh structured locally |
| Possibility of chemical reaction simulation | Possible | Possible | Possible |
| Possibility of combustion simulation | Combustion with chemical reaction | Combustion with chemical reaction | Combustion with chemical reaction |
| Intended use | General purpose | General purpose | Aero- and hydrodynamics |
| Source code expandability | Open source code (C++ language) allows one to modify any module or any utility of the program | It is possible to add custom-made models directly to the source code | The source code is not expanded |
| License type | Free | Commercial | Commercial |
| Name of the Parameter | Parameter Value |
|---|---|
| Catalyst particle size, mm | 1.5–3.0 * |
| Apparent density of catalyst particles, kg/m3 | 2500 |
| Heat capacity of catalyst particles, J/(kg∙K) | 962.6 |
| Volume fraction of catalyst in the catalyst–inert material mixture, % | 20 |
| Particle size of inert material, mm | 2–4 * |
| Apparent density of inert material particle, kg/m3 | 2200 |
| Heat capacity of particles of inert material, J/(kg∙K) | 879.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulitin, N.V.; Tereshchenko, K.A.; Rodionov, I.S.; Alekseev, K.A.; Shiyan, D.A.; Kharlampidi, K.E.; Mezhuev, Y.O. Numerical Simulation of Hydrodynamics and Heat Transfer in a Reactor with a Fluidized Bed of Catalyst Particles in a Three-Dimensional Formulation. Appl. Sci. 2024, 14, 5009. https://doi.org/10.3390/app14125009
Ulitin NV, Tereshchenko KA, Rodionov IS, Alekseev KA, Shiyan DA, Kharlampidi KE, Mezhuev YO. Numerical Simulation of Hydrodynamics and Heat Transfer in a Reactor with a Fluidized Bed of Catalyst Particles in a Three-Dimensional Formulation. Applied Sciences. 2024; 14(12):5009. https://doi.org/10.3390/app14125009
Chicago/Turabian StyleUlitin, Nikolai V., Konstantin A. Tereshchenko, Ilya S. Rodionov, Konstantin A. Alekseev, Daria A. Shiyan, Kharlampii E. Kharlampidi, and Yaroslav O. Mezhuev. 2024. "Numerical Simulation of Hydrodynamics and Heat Transfer in a Reactor with a Fluidized Bed of Catalyst Particles in a Three-Dimensional Formulation" Applied Sciences 14, no. 12: 5009. https://doi.org/10.3390/app14125009
APA StyleUlitin, N. V., Tereshchenko, K. A., Rodionov, I. S., Alekseev, K. A., Shiyan, D. A., Kharlampidi, K. E., & Mezhuev, Y. O. (2024). Numerical Simulation of Hydrodynamics and Heat Transfer in a Reactor with a Fluidized Bed of Catalyst Particles in a Three-Dimensional Formulation. Applied Sciences, 14(12), 5009. https://doi.org/10.3390/app14125009









