Cytotoxicity and Antioxidant Defences in Euplotes aediculatus Exposed to Single and Binary Mixtures of Heavy Metals and Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ciliate Strain and Culture Conditions
2.2. Metal Salts (Chemicals)
2.3. Single HM and NP Toxicity Tests at 1 h and 24 h
2.4. Bimetallic Mixture (Cd + Zn, Cd + ZnO) Toxicity Tests at 1 h and 24 h
2.5. Total Phenolic Content and Antioxidant Activity Assays
2.6. Statistical Analysis
3. Results
3.1. Acute and Chronic Cytotoxicity of Single HMs (Cd, Cu, and Zn) and NPs (CuO, ZnO, TiO2, and SiO2)
3.2. 1 h Binary Mixture of Cd + Zn—MixTOX Analysis
3.3. 24 h Binary Mixture of Cd + Zn—MixTOX Analysis
3.4. 1 h and 24 h Binary Mixture of Cd + ZnO—MixTOX Analysis
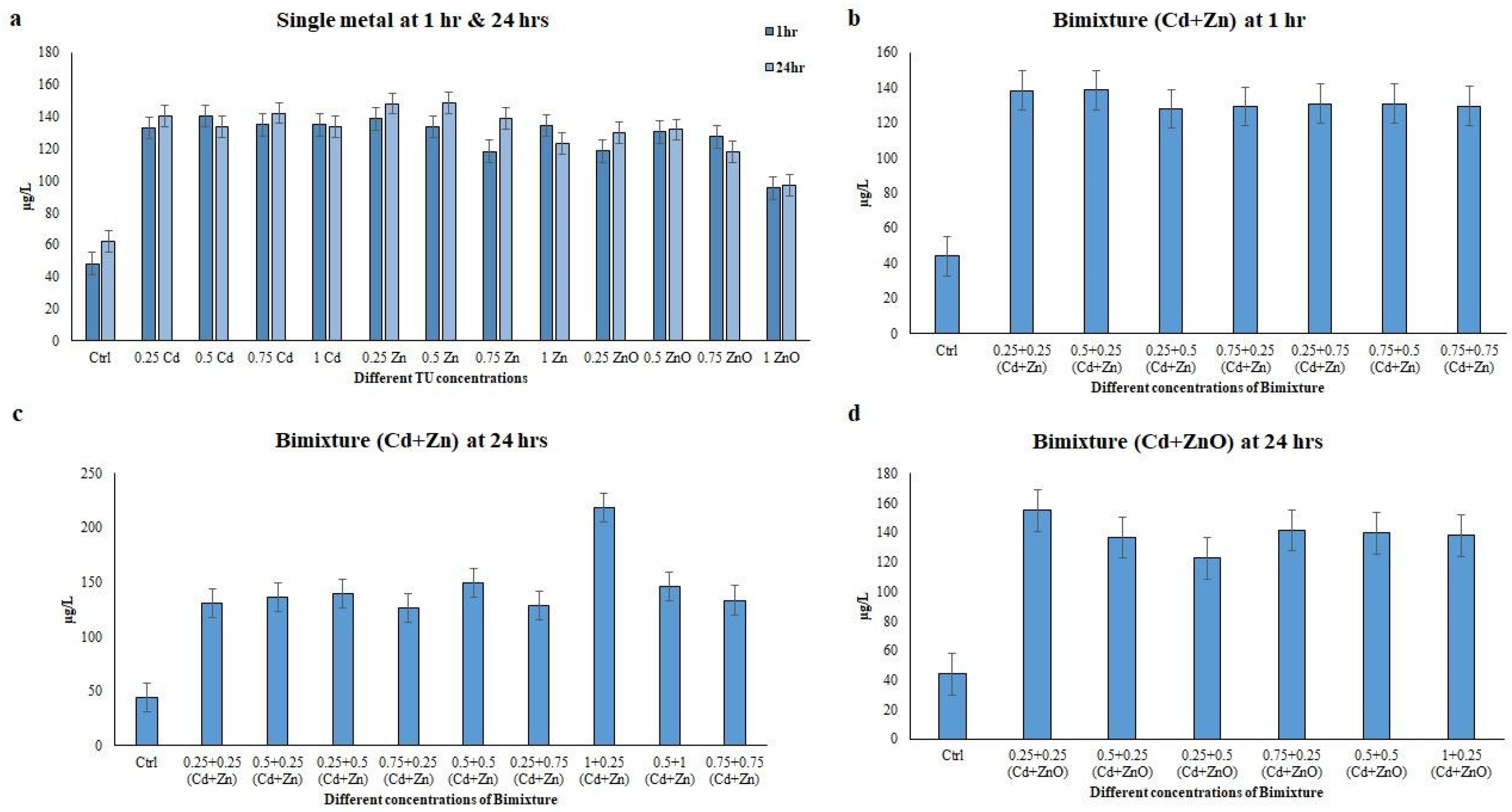
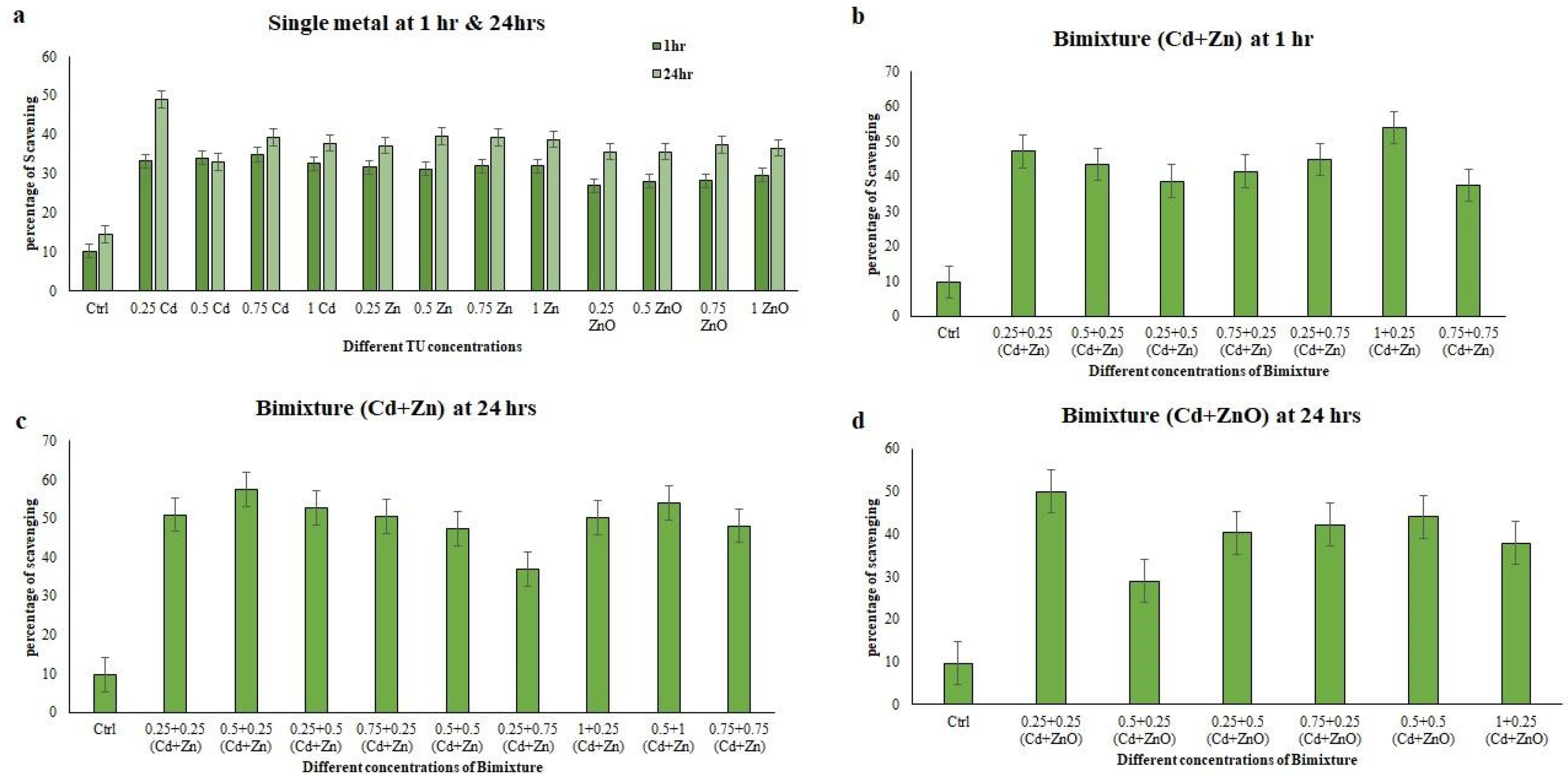
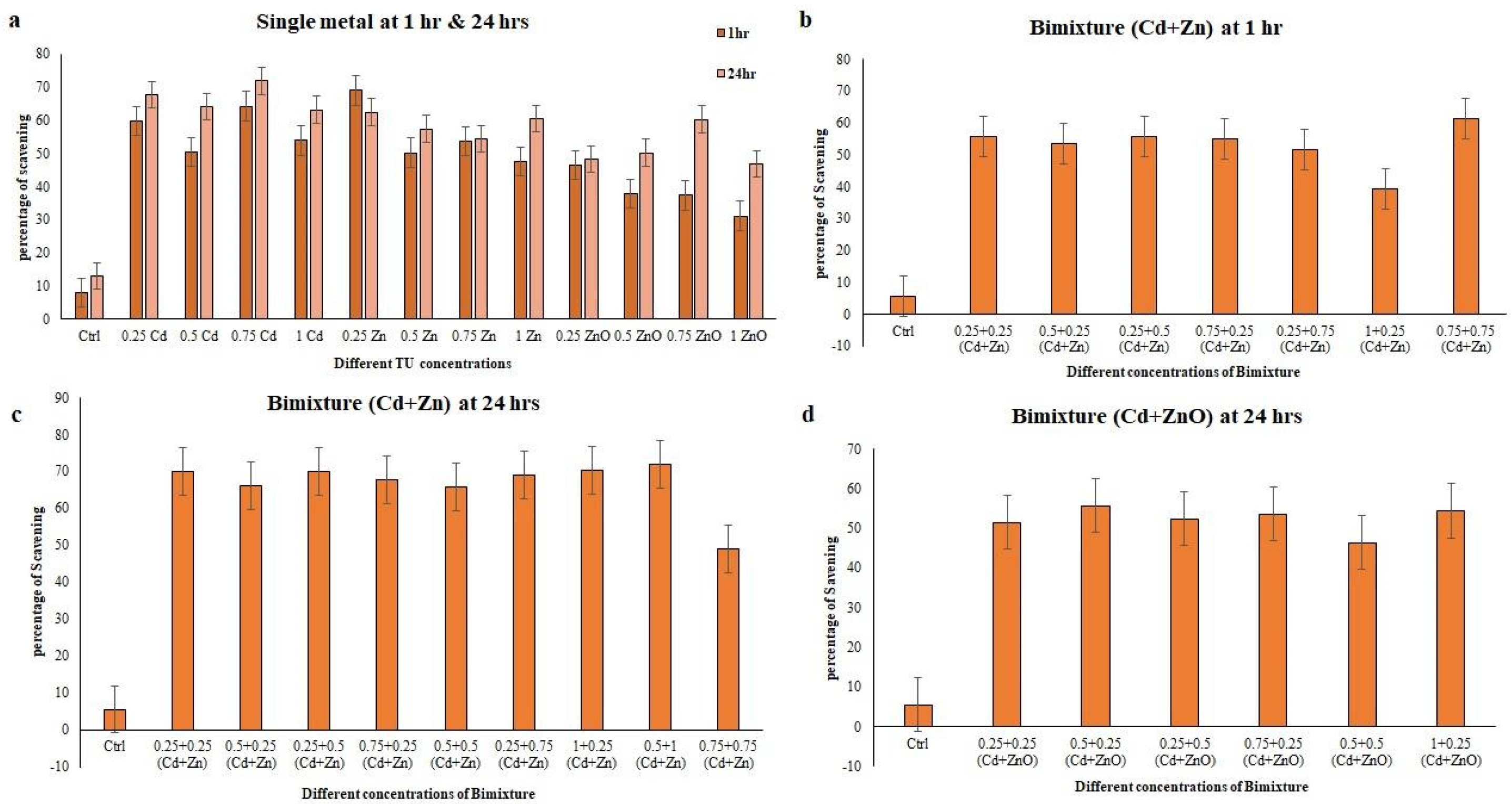
3.5. Antioxidant Properties of E. aediculatus Extracts Treated with Single and Binary Mixture at 1 h and 24 h
4. Discussion
5. Conclusions
- E. aediculatus shows different sensitivities to the two types of pollutants, with a higher resistance to HMs than to NPs. Furthermore, both HMs and NPs can induce effective antioxidant responses in E. aediculatus, as evidenced by an increase in antioxidant properties and activity.
- Despite being exposed to oxidative stress, E. aediculatus exhibits a strong response with greater effectiveness.
- Various antioxidant assays reveal a significantly increased level of antioxidant activity and free radical scavenging activity in E. aediculatus when exposed to both single and bimetallic mixtures.
- In conclusion, our results suggest that E. aediculatus can be used as a bioindicator organism for the detection of the bioavailable fraction of various toxic pollutants, such as HMs and NPs, in real environmental samples. The observed differential sensitivity of this species to the toxic effects of HMs and NPs suggests that they can be used in the development of rapid, simple, and cost-effective ciliate test arrays for the detection of specific pollutants in different environmental matrices.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amoatey, P.; Baawain, M.S. Effects of pollution on freshwater aquatic organisms. Water Environ. Res. 2019, 91, 1272–1287. [Google Scholar] [CrossRef] [PubMed]
- Kadim, M.K.; Risjani, Y. Biomarker for monitoring heavy metal pollution in aquatic environment: An overview toward molecular perspectives. Emerg. Contam. 2022, 8, 195–205. [Google Scholar] [CrossRef]
- Wang, T.; Liu, W. Emerging investigator series: Metal nanoparticles in freshwater: Transformation, bioavailability and effects on invertebrates. Environ. Sci. Nano 2022, 9, 2237–2263. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.E.; Thomas, S.M.; Bodour, A.A. Prioritizing research for trace pollutants and emerging contaminants in the freshwater environment. Environ. Pollut. 2010, 158, 3462–3471. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhai, M.; Liu, Q.; Sun, J.; Guo, J. Residues of organochlorine pesticides (OCPs) in upper reach of the Huaihe River, East China. Ecotoxicol. Environ. Saf. 2011, 74, 2252–2259. [Google Scholar] [CrossRef] [PubMed]
- Puri, M.; Gandhi, K.; Kumar, M.S. Emerging environmental contaminants: A global perspective on policies and regulations. J. Environ. Manag. 2023, 332, 117344. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Deng, Q.; Bai, J.; Li, Y.; Wang, Y.; Liu, N.; Gong, Y. Management system for engineering and decoration waste: An exploratory study in Shenzhen. J. Environ. Manag. 2022, 314, 115085. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, S.; Abraham, J.S.; Maurya, S.; Toteja, R.; Gupta, R.; Makhija, S. Molecular characterization and transcriptional modulation of stress-responsive genes under heavy metal stress in freshwater ciliate, Euplotes aediculatus. Ecotoxicology 2022, 31, 271–288. [Google Scholar] [CrossRef]
- Lovley, D.R. Fe (III) and Mn (IV) reduction. In Environmental Microbe-Metal Interactions; Wiley: Hoboken, NJ, USA, 2000; pp. 1–30. [Google Scholar]
- Beveridge, T.; Hughes, M.; Lee, H.; Leung, K.; Poole, R.; Savvaidis, I.; Silver, S.; Trevors, J. Metal-microbe interactions: Contemporary approaches. Adv. Microb. Physiol. 1996, 38, 177–243. [Google Scholar]
- Chen, S.; Chen, B.; Fath, B.D. Ecological risk assessment on the system scale: A review of state-of-the-art models and future perspectives. Ecol. Model. 2013, 250, 25–33. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, C.; Zhao, Y.; Shan, B.; Song, Z. Pollution, toxicity, and ecological risk of heavy metals in surface river sediments of a large basin undergoing rapid economic development. Environ. Toxicol. Chem. 2017, 36, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, R.; Rajeswari, N. ZnO/PBAT nanocomposite films: Investigation on the mechanical and biological activity for food packaging. Polym. Adv. Technol. 2017, 28, 20–27. [Google Scholar] [CrossRef]
- Corsi, I.; Cherr, G.N.; Lenihan, H.S.; Labille, J.; Hassellov, M.; Canesi, L.; Dondero, F.; Frenzilli, G.; Hristozov, D.; Puntes, V. Common strategies and technologies for the ecosafety assessment and design of nanomaterials entering the marine environment. ACS Nano 2014, 8, 9694–9709. [Google Scholar] [CrossRef] [PubMed]
- Libralato, G.; Devoti, A.C.; Zanella, M.; Sabbioni, E.; Mičetić, I.; Manodori, L.; Pigozzo, A.; Manenti, S.; Groppi, F.; Ghirardini, A.V. Phytotoxicity of ionic, micro-and nano-sized iron in three plant species. Ecotoxicol. Environ. Saf. 2016, 123, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Vale, G.; Mehennaoui, K.; Cambier, S.; Libralato, G.; Jomini, S.; Domingos, R.F. Manufactured nanoparticles in the aquatic environment-biochemical responses on freshwater organisms: A critical overview. Aquat. Toxicol. 2016, 170, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Q.; Yousaf, B.; Ullah, H.; Ali, M.U.; Ok, Y.S.; Rinklebe, J. Environmental transformation and nano-toxicity of engineered nano-particles (ENPs) in aquatic and terrestrial organisms. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2523–2581. [Google Scholar] [CrossRef]
- Azhar, W.; Khan, A.R.; Salam, A.; Ulhassan, Z.; Qi, J.; Shah, G.; Liu, Y.; Chunyan, Y.; Yang, S.; Gan, Y. Ethylene accelerates copper oxide nanoparticle-induced toxicity at physiological, biochemical, and ultrastructural levels in rice seedlings. Environ. Sci. Pollut. Res. 2023, 30, 26137–26149. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, X.; Hoang, S.A.; Bolan, N.S.; Kirkham, M.; Liu, J.; Xia, X.; Li, Y. Silver nanoparticles in aquatic sediments: Occurrence, chemical transformations, toxicity, and analytical methods. J. Hazard. Mater. 2021, 418, 126368. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Castro, I.; Molina, L.; Prieto-Fernández, M.-Á.; Segura, A. Past, present and future trends in the remediation of heavy-metal contaminated soil-Remediation techniques applied in real soil-contamination events. Heliyon 2023, 9, e16692. [Google Scholar] [CrossRef] [PubMed]
- Garg, V.; Arora, A.; Prakash, A. A Review on Bioremediation Using Nanobiotechnology and Microbial Heavy Metal Resistance Mechanisms. Curr. Mater. Sci. Former. Recent Pat. Mater. Sci. 2024, 17, 289–303. [Google Scholar] [CrossRef]
- Valbonesi, A.; Luporini, P. Description of two new species of Euplotes and Euplotes rariseta from Antarctica. Polar Biol. 1990, 11, 47–53. [Google Scholar] [CrossRef]
- Warren, A.; Patterson, D.J.; Dunthorn, M.; Clamp, J.C.; Achilles-Day, U.E.; Aescht, E.; Al-Farraj, S.A.; Al-Quraishy, S.; Al-Rasheid, K.; Carr, M.; et al. Beyond the “Code”: A guide to the description and documentation of biodiversity in ciliated protists (Alveolata, Ciliophora). J. Eukaryot. Microbiol. 2017, 64, 539–554. [Google Scholar] [CrossRef] [PubMed]
- Bharti, D.; Santosh, K.; Federico, B.; Ortenzi, C.; Montanari, A.; Pablo, Q.-A.; La Terza, A. Free living ciliated protists from the chemoautotrophic cave ecosystem of Frasassi (Italy). Subterr. Biol. 2022, 44, 167–198. [Google Scholar] [CrossRef]
- Madoni, P.; Davoli, D.; Gorbi, G.; Vescovi, L. Toxic effect of heavy metals on the activated sludge protozoan community. Water Res. 1996, 30, 135–141. [Google Scholar] [CrossRef]
- Fulgentini, L.; Passini, V.; Colombetti, G.; Miceli, C.; La Terza, A.; Marangoni, R. UV radiation and visible light induce hsp70 gene expression in the antarctic psychrophilic ciliate euplotes focardii. Microb. Ecol. 2015, 70, 372–379. [Google Scholar] [CrossRef] [PubMed]
- La Terza, A.; Papa, G.; Miceli, C.; Luporini, P. Divergence between two Antarctic species of the ciliate Euplotes, E. focardii and E. nobilii, in the expression of heat-shock protein 70 genes. Mol. Ecol. 2001, 10, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.C.; Martín-González, A.; Díaz, S.; Ortega, R. Ciliates as a potential source of cellular and molecular biomarkers/biosensors for heavy metal pollution. Eur. J. Protistol. 2003, 39, 461–467. [Google Scholar] [CrossRef]
- Martín-González, A.; Díaz, S.; Borniquel, S.; Gallego, A.; Gutiérrez, J.C. Cytotoxicity and bioaccumulation of heavy metals by ciliated protozoa isolated from urban wastewater treatment plants. Res. Microbiol. 2006, 157, 108–118. [Google Scholar] [CrossRef] [PubMed]
- La Terza, A.; Barchetta, S.; Buonanno, F.; Ballarini, P.; Miceli, C. The protozoan ciliate Tetrahymena thermophila as biosensor of sublethal levels of toxicants in the soil. Fresenius Environ. Bull. 2008, 17, 1144–1150. [Google Scholar]
- Gertler, C.; Näther, D.J.; Gerdts, G.; Malpass, M.C.; Golyshin, P.N. A mesocosm study of the changes in marine flagellate and ciliate communities in a crude oil bioremediation trial. Microb. Ecol. 2010, 60, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, C.; Calisi, A.; Sanchez-Hernandez, J.C.; Varodi, C.; Pogăcean, F.; Pruneanu, S.; Dondero, F. Carbon nanomaterial functionalization with pesticide-detoxifying carboxylesterase. Chemosphere 2022, 309, 136594. [Google Scholar] [CrossRef] [PubMed]
- La Terza, A.; Miceli, C.; Luporini, P. The gene for the heat-shock protein 70 of Euplotes focardii, an Antarctic psychrophilic ciliate. Antarct. Sci. 2004, 16, 23–28. [Google Scholar] [CrossRef]
- Baun, A.; Hartmann, N.B.; Grieger, K.; Kusk, K.O. Ecotoxicity of engineered nanoparticles to aquatic invertebrates: A brief review and recommendations for future toxicity testing. Ecotoxicology 2008, 17, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Kahru, A.; Dubourguier, H.-C.; Blinova, I.; Ivask, A.; Kasemets, K. Biotests and biosensors for ecotoxicology of metal oxide nanoparticles: A minireview. Sensors 2008, 8, 5153–5170. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-W.; Wang, Y.; Huang, B.; Wang, N.-X.; Wei, Z.-B.; Luo, J.; Miao, A.-J.; Yang, L.-Y. TiO2 nanoparticles act as a carrier of Cd bioaccumulation in the ciliate Tetrahymena thermophila. Environ. Sci. Technol. 2014, 48, 7568–7575. [Google Scholar] [CrossRef] [PubMed]
- Djibril Sekou, K.; Hannah Akasi, A.; Bachir Yaou, B.; Irédon, A.; Manka, M.; Patel, H. A Review on the Impact of Nanoparticles on Heavy Metals in the Soils. SSRG Int. J. Agric. Environ. Sci. 2023, 10, 16–28. [Google Scholar]
- Mortimer, M.; Kasemets, K.; Vodovnik, M.a.; Marinšek-Logar, R.; Kahru, A. Exposure to CuO nanoparticles changes the fatty acid composition of protozoa Tetrahymena thermophila. Environ. Sci. Technol. 2011, 45, 6617–6624. [Google Scholar] [CrossRef] [PubMed]
- Djibril Sekou, K.; Patel, H. A Review on the interaction between Nanoparticles and Toxic metals in Soil: Meta-analysis of their effects on soil, plants and human health. Soil Sediment Contam. Int. J. 2023, 32, 417–447. [Google Scholar] [CrossRef]
- Kim, S.-H.; Jung, M.-Y.; Lee, Y.-M. Effect of heavy metals on the antioxidant enzymes in the marine ciliate Euplotes crassus. Toxicol. Environ. Health Sci. 2011, 3, 213–219. [Google Scholar] [CrossRef]
- Gomiero, A.; Dagnino, A.; Nasci, C.; Viarengo, A. The use of protozoa in ecotoxicology: Application of multiple endpoint tests of the ciliate E. crassus for the evaluation of sediment quality in coastal marine ecosystems. Sci. Total Environ. 2013, 442, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Kim, S.-J.; Lee, J.-S.; Lee, Y.-M. Acute effects of heavy metals on the expression of glutathione-related antioxidant genes in the marine ciliate Euplotes crassus. Mar. Pollut. Bull. 2014, 85, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Marín-Leal, J.C.; Rincón-Miquilena, N.J.; Díaz-Borrego, L.C.; Pire-Sierra, M.C. Acute toxicity of potentially toxic elements on ciliated protozoa from Lake Maracaibo (Venezuela). Acta Limnol. Bras. 2022, 34, e21. [Google Scholar] [CrossRef]
- Zhao, X.; Fan, X.; Gong, Z.; Gao, X.; Wang, Y.; Ni, B. The toxic effects of Cu and CuO nanoparticles on Euplotes aediculatus. Microb. Ecol. 2023, 85, 544–556. [Google Scholar] [CrossRef]
- Miyake, A. Physiology and biochemistry of conjugation in ciliates. Biochem. Physiol. Protozoa 1981, 2, 125–198. [Google Scholar]
- Clark, C.; Lee, J.; Soldo, A. Protocols in Protozoology; Society of Protozoology: Lawrence, KS, USA, 1992. [Google Scholar]
- Sprague, J.B. Measurement of pollutant toxicity to fish, I.I. Utilizing and applying bioassay results. Water Res. 1970, 4, 3–32. [Google Scholar] [CrossRef]
- Ravindran, C.; Varatharajan, G.R.; Rajasabapathy, R.; Vijayakanth, S.; Kumar, A.H.; Meena, R.M. A role for antioxidants in acclimation of marine derived pathogenic fungus (NIOCC 1) to salt stress. Microb. Pathog. 2012, 53, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Vattem, D.A.; Shetty, K. Solid-state production of phenolic antioxidants from cranberry pomace by Rhizopus oligosporus. Food Biotechnol. 2002, 16, 189–210. [Google Scholar] [CrossRef]
- Yıldırım, A.; Mavi, A.; Kara, A.A. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J. Agric. Food Chem. 2001, 49, 4083–4089. [Google Scholar] [CrossRef] [PubMed]
- Kunchandy, E.; Rao, M. Oxygen radical scavenging activity of curcumin. Int. J. Pharm. 1990, 58, 237–240. [Google Scholar] [CrossRef]
- Jonker, M.J.; Svendsen, C.; Bedaux, J.J.; Bongers, M.; Kammenga, J.E. Significance testing of synergistic/antagonistic, dose level-dependent, or dose ratio-dependent effects in mixture dose-response analysis. Environ. Toxicol. Chem. Int. J. 2005, 24, 2701–2713. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Vijver, M.G.; Qiu, H.; Baas, J.; Peijnenburg, W.J. Statistically significant deviations from additivity: What do they mean in assessing toxicity of mixtures? Ecotoxicol. Environ. Saf. 2015, 122, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Belz, R.G.; Cedergreen, N.; Sørensen, H. Hormesis in mixtures—Can it be predicted? Sci. Total Environ. 2008, 404, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, D.; Lin, Z.; An, Q.; Yin, C.; Huang, Q. Prediction of mixture toxicity from the hormesis of a single chemical: A case study of combinations of antibiotics and quorum-sensing inhibitors with gram-negative bacteria. Chemosphere 2016, 150, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Xiao, X.; He, Y.; Hu, L.; Hu, C.; Huang, X. Hormetic effects of metal ions upon V. fischeri and the application of a new parameter for the quantitative assessment of hormesis. J. Hazard. Mater. 2017, 322, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Flores, S.; Santos-Medrano, G.E.; Rico-Martínez, R. Integral Study of Paramecium caudatum Acute and Chronic Toxicity, Sites of Entry and Distribution, Bioconcentration and Body Burdens of Five Metals. Bull. Environ. Contam. Toxicol. 2023, 111, 19. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.-W.; An, Y.-J. Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO, and Sb2O3) to Escherichia coli, Bacillus subtilis, and Streptococcus aureus. Sci. Total Environ. 2011, 409, 1603–1608. [Google Scholar] [CrossRef] [PubMed]
- Maurer-Jones, M.A.; Gunsolus, I.L.; Murphy, C.J.; Haynes, C.L. Toxicity of engineered nanoparticles in the environment. Anal. Chem. 2013, 85, 3036–3049. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liao, A.; Hu, S.; Zheng, Y.; Liang, S.; Han, S.; Lin, Y. Acute and chronic toxicity of binary mixtures of bisphenol A and heavy metals. Toxics 2022, 10, 255. [Google Scholar] [CrossRef] [PubMed]
- Karri, V.; Kumar, V.; Ramos, D.; Oliveira, E.; Schuhmacher, M. An in vitro cytotoxic approach to assess the toxicity of heavy metals and their binary mixtures on hippocampal HT-22 cell line. Toxicol. Lett. 2018, 282, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, A.; Liu, Y. Combined biological effects of silver nanoparticles and heavy metals in different target cell lines. Environ. Sci. Pollut. Res. 2022, 29, 16324–16331. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Alaizeri, Z.M.; Alhadlaq, H.A. TiO2 nanoparticles potentiated the cytotoxicity, oxidative stress and apoptosis response of cadmium in two different human cells. Environ. Sci. Pollut. Res. 2020, 27, 10425–10435. [Google Scholar] [CrossRef] [PubMed]
- Mansour, W.A.; Abdelsalam, N.R.; Tanekhy, M.; Khaled, A.A.; Mansour, A.T. Toxicity, inflammatory and antioxidant genes expression, and physiological changes of green synthesis silver nanoparticles on Nile tilapia (Oreochromis niloticus) fingerlings. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 247, 109068. [Google Scholar] [CrossRef] [PubMed]
- Wells, P.G.; Depledge, M.H.; Butler, J.N.; Manock, J.J.; Knap, A.H. Rapid toxicity assessment and biomonitoring of marine contaminants—Exploiting the potential of rapid biomarker assays and microscale toxicity tests. Mar. Pollut. Bull. 2001, 42, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Pudpong, S.; Chantangsi, C. Effects of Four Heavy Metals on Cell Morphology and Survival Rate of the Ciliate Bresslauides sp`. Trop. Nat. Hist. 2015, 15, 117–125. [Google Scholar]
- Díaz, S.; Martín-González, A.; Gutiérrez, J.C. Evaluation of heavy metal acute toxicity and bioaccumulation in soil ciliated protozoa. Environ. Int. 2006, 32, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Rico, D.; Martín-González, A.; Díaz, S.; de Lucas, P.; Gutiérrez, J.-C. Heavy metals generate reactive oxygen species in terrestrial and aquatic ciliated protozoa. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 149, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Blinova, I.; Ivask, A.; Heinlaan, M.; Mortimer, M.; Kahru, A. Ecotoxicity of nanoparticles of CuO and ZnO in natural water. Environ. Pollut. 2010, 158, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Rainbow, P.; Amiard-Triquet, C.; Amiard, J.; Smith, B.; Langston, W. Observations on the interaction of zinc and cadmium uptake rates in crustaceans (amphipods and crabs) from coastal sites in UK and France differentially enriched with trace metals. Aquat. Toxicol. 2000, 50, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Barata, C.; Markich, S.J.; Baird, D.J.; Taylor, G.; Soares, A.M. Genetic variability in sublethal tolerance to mixtures of cadmium and zinc in clones of Daphnia magna Straus. Aquat. Toxicol. 2002, 60, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.D.; Lydy, M.J. Increased toxicity to invertebrates associated with a mixture of atrazine and organophosphate insecticides. Environ. Toxicol. Chem. Int. J. 2002, 21, 1507–1514. [Google Scholar] [CrossRef]
- Cedergreen, N.; Christensen, A.M.; Kamper, A.; Kudsk, P.; Mathiassen, S.K.; Streibig, J.C.; Sørensen, H. A review of independent action compared to concentration addition as reference models for mixtures of compounds with different molecular target sites. Environ. Toxicol. Chem. Int. J. 2008, 27, 1621–1632. [Google Scholar] [CrossRef] [PubMed]
- Cedergreen, N.; Kamper, A.; Streibig, J.C. Is prochloraz a potent synergist across aquatic species? A study on bacteria, daphnia, algae and higher plants. Aquat. Toxicol. 2006, 78, 243–252. [Google Scholar] [CrossRef] [PubMed]
- He, E.; Baas, J.; Van Gestel, C.A. Interaction between nickel and cobalt toxicity in Enchytraeus crypticus is due to competitive uptake. Environ. Toxicol. Chem. 2015, 34, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Franklin, N.M.; Stauber, J.L.; Lim, R.P.; Petocz, P. Toxicity of metal mixtures to a tropical freshwater alga (Chlorella sp.): The effect of interactions between copper, cadmium, and zinc on metal cell binding and uptake. Environ. Toxicol. Chem. Int. J. 2002, 21, 2412–2422. [Google Scholar] [CrossRef]
- Farley, K.J.; Meyer, J.S. Metal mixture modeling evaluation project: 3. Lessons learned and steps forward. Environ. Toxicol. Chem. 2015, 34, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Kuda, T.; Tsunekawa, M.; Goto, H.; Araki, Y. Antioxidant properties of four edible algae harvested in the Noto Peninsula, Japan. J. Food Compos. Anal. 2005, 18, 625–633. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Wianowska, D.; Olszowy, M. On practical problems in estimation of antioxidant activity of compounds by DPPH method (Problems in estimation of antioxidant activity). Food Chem. 2012, 131, 1037–1043. [Google Scholar] [CrossRef]
- Sanaye, S.; Pise, N.; Pawar, A.; Parab, P.; Sreepada, R.; Pawar, H.; Murugan, A. Total phenolic Content and In-Vitro Antioxidant Activities from Methanolic Extract of Alligator Pipefish, Syngnathoides biaculeatus (Bloch, 1785). 2015. Available online: http://drs.nio.org/drs/handle/2264/7679 (accessed on 20 February 2015).
- Rabiei, K.; Bekhradnia, S.; Nabavi, S.; Nabavi, S.; Ebrahimzadeh, M. Antioxidant activity of polyphenol and ultrasonic extracts from fruits of Crataegus pentagyna subsp. elburensis. Nat. Prod. Res. 2012, 26, 2353–2357. [Google Scholar] [CrossRef] [PubMed]
| HMs | 1 h—Euplotes | 24 h—Euplotes | ||
|---|---|---|---|---|
| LC20 (mg L−1) | LC50 (mg L−1) | LC20 (mg L−1) | LC50 (mg L−1) | |
| CdCl2 | 42.53 | 91.80 | 19.85 | 53.72 |
| CuSo4 | 34.78 | 76.41 | 0.18 | 0.43 |
| ZnSo4 | 282.41 | 283.95 | 76.47 | 137.65 |
| NPs | 1 h—Euplotes | 24 h—Euplotes | ||
| LC20 (mg L−1) | LC50 (mg L−1) | LC20 (mg L−1) | LC50 (mg L−1) | |
| CuO | 5922.50 | 6535.03 | 3.10 | 4.93 |
| ZnO | 77.32 | 139.99 | 30.93 | 56.16 |
| TiO2 | 7042.94 | 7361.62 | 2608.55 | 4439.97 |
| SiO2 | Up to 60,080 mg L−1, there is no effects to the cells | |||
| CdCl2 + ZnSo4Exposure | Parameter | The Concentration Addition (CA)-Based Module | |||
|---|---|---|---|---|---|
| CA | S/A | DR | DL | ||
| 1 h | a | – | 1.405 | 0.917 | 0.106 |
| b | – | – | 1.018 | −7.852 | |
| R2 | 0.34 | 0.85 | 0.85 | 0.87 | |
| p(χ2) CA vs. | – | 1.8 × 10−232 * | 2.3 × 10−232 * | 2.98 × 10−238 * | |
| S/A vs. | – | – | 0.008 * | 5.247 × 10−09 * | |
| 24 h | a | – | 1.54 | 0.724 | −0.362 |
| b | – | – | 0.414 | 2.058 | |
| R2 | 0.72 | 0.77 | 0.77 | 0.77 | |
| p(χ2) CA vs. | – | 7.04 × 10−21 * | 4.86 × 10−08 * | 3.61 × 10−18 * | |
| S/A vs. | – | – | 0.486 × 10−08 * | 3.206 × 10−05 * | |
| Parameter | The Independent Action (IA)-Based Module | ||||
| IA | S/A | DR | DL | ||
| 1 h | a | – | −1.282 | −4.864 | −3.020 |
| b | – | – | 6.742 | 1.371 | |
| R2 | 0.82 | 0.86 | 0.88 | 0.87 | |
| p(χ2) IA vs. | – | 7.42 × 10−17 * | 6.38 × 10−25 * | 2.65 × 10−24 * | |
| S/A vs. | – | – | 9.78 × 10−11 * | 4.2 × 10−10 * | |
| 24 h | a | – | −0.378 | −0.744 | −1.871 |
| b | – | – | −0.680 | 1.482 | |
| R2 | 0.77 | 0.77 | 0.77 | 0.78 | |
| p(χ2) IA vs. | – | 0.17 | 0.331 | 0.003 * | |
| S/A vs. | – | – | 0.533 | 0.002 * | |
| CdCl2 + ZnO Exposure | Parameter | The Concentration Addition (CA)-Based Module | |||
|---|---|---|---|---|---|
| CA | S/A | DR | DL | ||
| 1 h | a | – | −5.325 | −3.654 | −15.34 |
| b | – | – | −5.365 | −8.324 | |
| R2 | 0.47 | 0.97 | 0.98 | 0.99 | |
| p(χ2) CA vs. | – | 0.000 * | 0.000 * | 0.000 * | |
| S/A vs. | – | – | 7.4 × 10−11 * | 5.17 × 10−14 * | |
| 24 h | a | – | −0.043 | −2.805 | −0.205 |
| b | – | – | 5.643 | 0.362 | |
| R2 | 0.87 | 0.87 | 0.91 | 0.87 | |
| p(χ2) CA vs. | – | 0.587 | 5.49 × 10−14 * | 0.768 | |
| S/A vs. | – | – | 6.42 × 10−15 * | 0.631 | |
| Parameter | The Independent Action (IA)-Based Module | ||||
| IA | S/A | DR | DL | ||
| 1 h | a | – | −5.325 | −3.654 | −15.34 |
| b | – | – | −5.365 | −8.324 | |
| R2 | 0.35 | 0.78 | 0.85 | 0.99 | |
| p(χ2) IA vs. | – | 1.5 × 10−282 * | 0.000 * | 0.000 * | |
| S/A vs. | – | – | 1.72 × 10−45 * | 1.52 × 10−138 * | |
| 24 h | a | – | −1.478 | −7.544 | −1.812 |
| b | – | – | 10.366 | 0.312 | |
| R2 | 0.86 | 0.97 | 0.92 | 0.87 | |
| p(χ2) IA vs. | – | 1.1 × 10−07 * | 3.89 × 10−23 * | 6.48 × 10−7 * | |
| S/A vs. | – | – | 4.66 × 10−18 * | 0.574 | |
| Single Metal Treated 1 h | Single Metal Treated 24 h | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DPPH | HRSA | TPC | DPPH | HRSA | TPC | ||||||
| DPPH | 1 | DPPH | 1 | ||||||||
| HRSA | 0.86 | 1 | HRSA | 0.86 | 1 | ||||||
| TPC | 0.90 | 0.85 | 1 | TPC | 0.80 | 0.86 | 1 | ||||
| Bimixture (Cd + Zn) 1 h | Bimixture (Cd + Zn) 24 h | Bimixture (Cd + ZnO) 24 h | |||||||||
| DPPH | HRSA | TPC | DPPH | HRSA | TPC | DPPH | HRSA | TPC | |||
| DPPH | 1 | DPPH | 1 | DPPH | 1 | ||||||
| HRSA | 0.93 | 1 | HRSA | 0.7 | 1 | HRSA | 0.91 | 1 | |||
| TPC | 0.93 | 0.74 | 1 | TPC | 0.77 | 0.89 | 1 | TPC | 0.95 | 0.81 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varatharajan, G.R.; Calisi, A.; Kumar, S.; Bharti, D.; Dondero, F.; La Terza, A. Cytotoxicity and Antioxidant Defences in Euplotes aediculatus Exposed to Single and Binary Mixtures of Heavy Metals and Nanoparticles. Appl. Sci. 2024, 14, 5058. https://doi.org/10.3390/app14125058
Varatharajan GR, Calisi A, Kumar S, Bharti D, Dondero F, La Terza A. Cytotoxicity and Antioxidant Defences in Euplotes aediculatus Exposed to Single and Binary Mixtures of Heavy Metals and Nanoparticles. Applied Sciences. 2024; 14(12):5058. https://doi.org/10.3390/app14125058
Chicago/Turabian StyleVaratharajan, Govindhasamay R., Antonio Calisi, Santosh Kumar, Daizy Bharti, Francesco Dondero, and Antonietta La Terza. 2024. "Cytotoxicity and Antioxidant Defences in Euplotes aediculatus Exposed to Single and Binary Mixtures of Heavy Metals and Nanoparticles" Applied Sciences 14, no. 12: 5058. https://doi.org/10.3390/app14125058






