Physiological Indices for the Selection of Drought-Tolerant Safflower Genotypes for Cultivation in Marginal Areas
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Physiological Characteristics, Seed Yield and Yield Components
3.2. Correlation among Traits
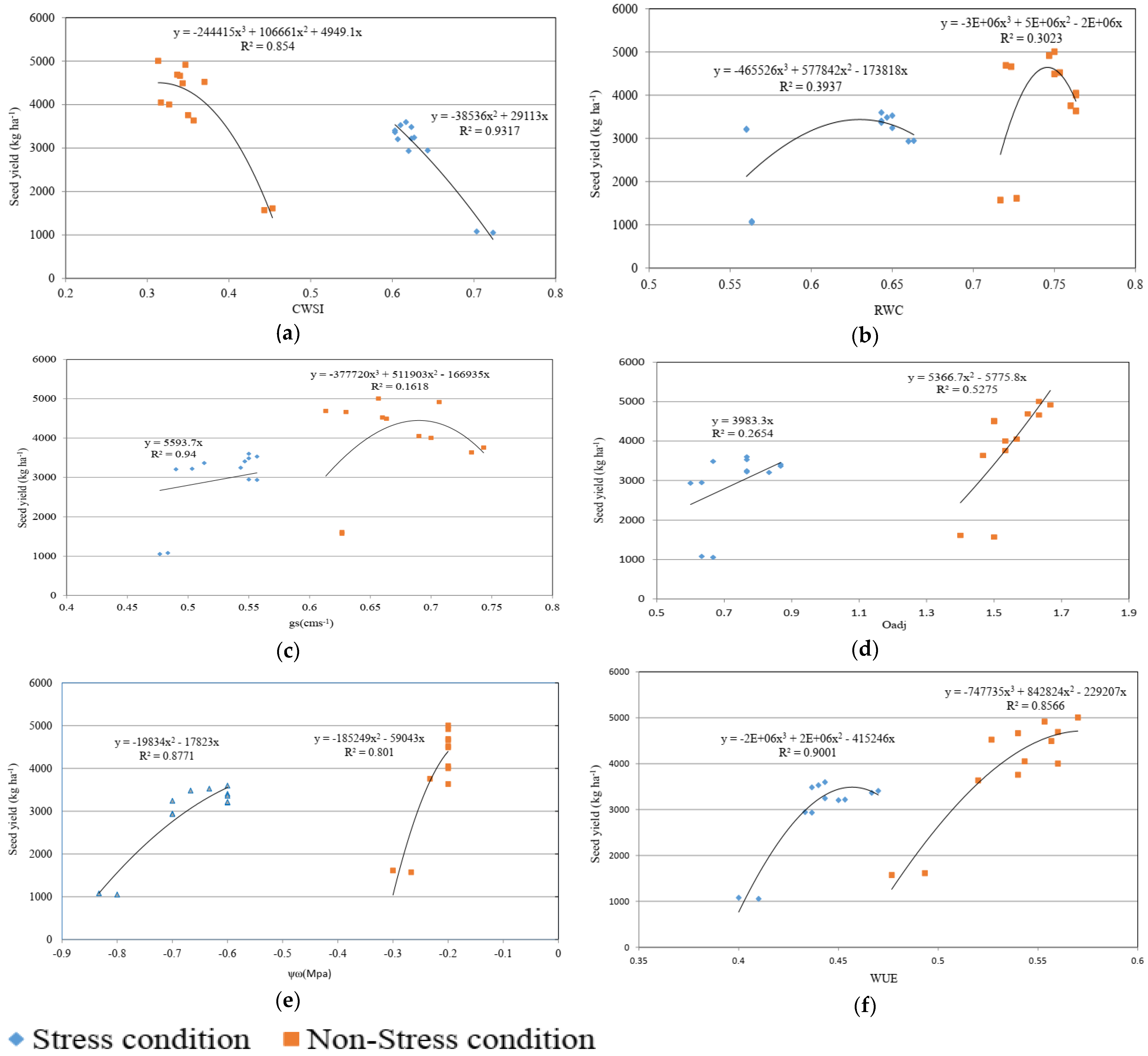
3.3. Regression Relationship between Seed Yield and Physiological Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bassil, E.S.; Kaffka, S.R. Response of safflower (Carthamus tinctorius L.) to saline soils and irrigation. I. Consumptive water use. Agric. Water Manag. J. 2002, 54, 67–80. [Google Scholar] [CrossRef]
- Pabuayon, I.L.B.; Singh, S.; Ritchie, G.L. Effects of deficit irrigation on yield and oil content of sesame, safflower, and sunflower. Agron. J. 2019, 111, 3091–3098. [Google Scholar] [CrossRef]
- Ozturk, E.; Ozer, H.; Polat, T. Growth and yield of safflower genotypes grown under irrigation and non-irrigated condition in a highland environment. Plant Soil Environ. J. 2008, 54, 453–460. [Google Scholar] [CrossRef]
- Pasban Eslam, B. Evaluation of physiological indices for improving water deficit tolerance in spring safflower. J. Agric. Sci. Technol. 2011, 13, 327–338. [Google Scholar]
- Sánchez-Blanco, M.J.; Rodríguez, P.; Morales, M.A.; Ortuño, M.F.; Torrecillas, A. Comparative growth and water relation of Cistus albidus and Cistus monspeliensis plants during water deficit conditions and recovery. Plant Sci. 2002, 162, 107–113. [Google Scholar] [CrossRef]
- Irani, S.; Majidi, M.M.; Mirlohi, A.; Zargar, M.; Karami, M. Assessment of drought tolerance in sainfoin: Physiological and Drought Tolerance Indices. Agron. J. 2015, 107, 1771–1781. [Google Scholar] [CrossRef]
- Lovelli, S.M.; Perniola, A.F.; Di-Tommaso, T. Yield response factor to water (Ky) and water use efficiency of Carthamus tinctorius L. and Solanum melongena L. Agric. Water Manag. 2007, 92, 73–80. [Google Scholar] [CrossRef]
- Liu, E.K.; Mei, X.R.; Yan, C.R.; Gong, D.Z.; Zhang, Y.Q. Effects of water stress on photosynthetic characteristics, dry matter translocation and WUE in two winter wheat genotypes. Agric. Water Manag. 2016, 167, 75–85. [Google Scholar] [CrossRef]
- Motzo, R.; Pruneddu, G.; Giunta, F. The role of stomatal conductance for water and radiation use efficiency of durum wheat and triticale in a Mediterranean environment. Eur. J. Agron. 2013, 44, 87–97. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Chauhan, Y.S.; Johansen, C. Patterns of osmotic adjustment in pigeon pea and its importance as a mechanism of drought resistance. Eur. J. Agron. 2000, 12, 239–249. [Google Scholar] [CrossRef]
- Turner, N.C.; Abbo, S.; Berger, J.D.; Chaturvedi, S.K.; French, R.J.; Ludwing, C.; Mannur, D.M.; Singh, S.J.; Yadava, H.S. Osmotic adjustment in chickpea (Cicer arietinum L.) results in no yield benefit under terminal drought. J. Exp. Bot. 2007, 58, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Kar, G.; Kumar, A.; Martha, M. Water use efficiency and crop coefficients of dry-season oilseed crops. Agric. Water Manag. 2007, 87, 73–82. [Google Scholar] [CrossRef]
- Omidi, A.H.; Khazaei, H.; Monneveux, P.; Stoddard, F. Effect of cultivar and water regime on yield and yield components in safflower (Carthamus tinctorius L.). Turk. J. Field Crops 2012, 17, 10–15. [Google Scholar]
- Faraji, A.; Latifi, N.; Soltani, A.; Shirani-Rad, A.H. Seed yield and water use efficiency of canola (Brassica napus L.) as affected by high temperature stress and supplemental irrigation. Agric. Water Manag. J. 2009, 96, 132–140. [Google Scholar] [CrossRef]
- Bhattarai, B.; Singh, S.; Angadi, S.V.; Begna, S.; Saini, R.; Auld, D. Spring safflower water use patterns in response to preseason and in-season irrigation applications. Agric. Water Manag. J. 2020, 228, 276–286. [Google Scholar] [CrossRef]
- Saini, H.S.; Westgate, M.E. Reproductive development in grain crops during drought. Adv. Agron. J. 2000, 68, 59–96. [Google Scholar]
- Salem, N.; Msaada, K.; Dhifi, W.; Sriti, J.; Mejri, H.; Limam, F.; Marzouk, B. Effect of drought on safflower natural dyes and their biological activities. Exp. Clin. Sci. J. 2014, 13, 1–18. [Google Scholar]
- Bortolheiro, F.P.A.P.; Silva, M.A. Physiological response and productivity of safflower lines under water deficit and rehydration. An. Braz. Acad. Sci. J. 2017, 89, 3051–3066. [Google Scholar] [CrossRef] [PubMed]
- Koutroubas, S.D.; Papakosta, D.K.; Doitsinis, A. Cultivar and seasonal effects on the contribution of pre-anthesis assimilates to safflower yield. Field Crops Res. 2004, 90, 263–274. [Google Scholar] [CrossRef]
- Joshan, Y.; Sani, B.; Jabbari, H.; Mozafari, H.; Moaveni, P. Effect of drought stress on oil content and fatty acids composition of some safflower genotypes. Plant Soil Environ. J. 2019, 65, 563–567. [Google Scholar] [CrossRef]
- Shahrokhnia, M.H.; Sepaskhah, A.R. Physiologic and agronomic traits in safflower under various irrigation strategies, planting methods and nitrogen fertilization. Ind. Crops Prod. J. 2017, 95, 126–139. [Google Scholar] [CrossRef]
- Harish-Babu, B.N.; Rudra Naih, V.; Hanumantharaya, L.; Raju, S.G.; Yara-Goppa, S.D. Evaluation of promising breeding lines of safflower for Alternaria tolerance, seed yield and its components. Karnataka Agric. Sci. J. 2005, 18, 803–806. [Google Scholar]
- Mozaffari, K.; Asadi, A.A. Relationship among traits using correlation, principal component and path analysis in safflower mutants sown in irrigated and drought stress condition. Asian J. Plant Sci. 2006, 5, 977–983. [Google Scholar]
- Ebrahimian, E.; Seyyedi, S.M.; Bybordi, A.; Damalas, C.A. Seed yield and oil quality of sunflower, safflower and sesam under different levels of irrigation water availability. Agric. Water Manag. J. 2019, 218, 149–157. [Google Scholar] [CrossRef]
- Yeilaghi, H.; Arzani, A.; Ghaderian, M. Evaluating the contribution of ionic and agronomic components toward salinity tolerance in safflower. Agron. J. 2015, 107, 2205–2212. [Google Scholar] [CrossRef]
- Jensen, C.R.; Mogensen, V.O.; Mortensen, G.; Fieldsedn, J.K.; Milford, G.F.J.; Andersen, M.N.; Thage, J.H. Seed glucosinolate, oil and protein contents of field-grown rape (Brassica napus L.) affected by soil drying and evaporative demand. Field Crops Res. 1996, 47, 93–105. [Google Scholar] [CrossRef]
- Lazcano-ferrat, I.; Lovatt, C.J. Relationship between relative water content, nitrogen pools and growth of Phaseolus vulgaris L. and P. acutifolius A. during water deficit. Crop Sci. 1999, 39, 467–475. [Google Scholar] [CrossRef]
- Dejonge, K.C.; Taghvaeian, S.; Trout, T.J.; Comas, L.H. Comparison of canopy temperature-based water stress indices for maize. Agric. Water Manag. J. 2015, 156, 51–62. [Google Scholar] [CrossRef]
- Takele, A. Canopy temperature and excised leaf water loss of tef (Eragrostis tef [Zucc.] Trotter.) cultivars under water deficit conditions at anthesis. Acta Agron. Hung. J. 2001, 49, 109–117. [Google Scholar] [CrossRef]
- Echeverria, C.P.; Espinace, D.; Sepulveda Reyes, D.; Zuniga, M.; Sanchez, M. Analysis of crop water stress index (CWSI) for estimating stem water potential in grapevines: Comparison between natural reference and baseline approaches. Acta Hort. J. 2017, 1150, 189–193. [Google Scholar] [CrossRef]
- Jones, H.G.; Stoll, M.; Santos, T.; Souse, C.D.; Chaves, M.M.; Grant, O.M. Use of infrared thermography for monitoring stomatal closure in the field: Application to grapevine. J. Exp. Bot. 2002, 53, 2249–2260. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C. Osmotic adjustment osmoregulation. In Encyclopedia of Plant and Crop Science; Goodman, R.M., Ed.; Marcel Dekker: New York, NY, USA, 2004; pp. 850–853. [Google Scholar]
- Alizadeh-Yeloojeh, K.; Saeidi, G. Genetic analysis of safflower populations under water stress and non-stress conditions. Agron. J. 2020, 112, 9341–9347. [Google Scholar] [CrossRef]
- El-ferjani, R.; Soolanayakanahally, R. Canola responses to drought, heat, and combined stress: Shared and specific effects on carbon assimilation, seed yield, and oil composition. Front. Plant Sci. 2018, 9, 1224–1241. [Google Scholar] [CrossRef] [PubMed]
- Gholami-Baseri, S.; Honar, T.; Heidari, B.; Salami, M.; Richards, C.M. Oil and seed yields affected by sowing dates and irrigation regimes applied in growth phenological stages of safflower. Crop Sci. 2022, 62, 1967–1980. [Google Scholar] [CrossRef]
- Pradawet, C.; Khongdee, N.; Pansak, W.; Spreer, W.; Hilger, T.; Cadisch, G. Thermal imaging for assessment of maize water stress and yield prediction under drought conditions. J. Agron. Crop Sci. 2022, 209, 56–70. [Google Scholar] [CrossRef]
- Grab, R.; Bottcher, U.; Lilienthal, H.; Wilde, P.; Kage, H. Is canopy temperature suitable for high throughput field phenotyping of drought resistance of winter rye in temperate climate? Eur. J. Agron. 2020, 120, 126–138. [Google Scholar]
- Bahrami, F.; Arzani, A.; Karimi, V. Evaluation of yield-based drought tolerance Indices for screening safflower genotypes. Agron. J. 2014, 106, 1219–1224. [Google Scholar] [CrossRef]
| FC (%) | PWP (%) | AWC (%) | |||||
|---|---|---|---|---|---|---|---|
| Soil Depth (cm) | Year | 2017–2018 | 2018–2019 | 2017–2018 | 2018–2019 | 2017–2018 | 2018–2019 |
| 0–30 | 26.7 | 25.2 | 12.6 | 12.8 | 14.1 | 12.4 | |
| 30–60 | 20.8 | 20.4 | 10.0 | 11.4 | 10.8 | 9.0 | |
| 60–90 | 13.7 | 13.1 | 7.5 | 8.0 | 6.2 | 5.1 | |
| Year | Month | Mean Minimum Air Temperature (°C) | Mean Maximum Air Temperature (°C) | Mean of Total Air Temperature (°C) | Sum of Rainfall (mm) | Sum of Evaporation from Class A Pan (mm) |
|---|---|---|---|---|---|---|
| 2017 | September | 17.1 | 34.2 | 25.7 | 0.0 | 311.2 |
| October | 7.6 | 22.7 | 15.2 | 10.5 | 170.1 | |
| November | 5.8 | 17.5 | 11.7 | 16.6 | 89.2 | |
| December | −4.3 | 6.5 | 1.1 | 26.4 | 1.7 | |
| 2018 | January | −1.0 | 9.1 | 4.1 | 21.5 | 0.0 |
| February | −2.3 | 7.8 | 2.8 | 80.0 | 0.0 | |
| March | 2.8 | 13.6 | 2.8 | 33.0 | 0.0 | |
| April | 5.8 | 19.2 | 12.5 | 48.9 | 120.0 | |
| May | 8.4 | 20.4 | 14.4 | 78.3 | 167.5 | |
| June | 13.6 | 28.5 | 21.0 | 27.2 | 260.0 | |
| July | 21.2 | 37.4 | 29.3 | 0.0 | 409.6 | |
| August | 21.3 | 37.0 | 29.2 | 0.0 | 385.0 | |
| September | 17.4 | 32.3 | 24.8 | 3.1 | 296.5 | |
| October | 11.6 | 26.2 | 18.9 | 5.5 | 167.0 | |
| November | −0.3 | 22.8 | 9.5 | 15.6 | 78.0 | |
| December | 1.7 | 9.2 | 5.5 | 80.3 | 0.0 | |
| 2019 | January | −2.1 | 6.2 | 2.1 | 15.8 | 0.0 |
| February | −0.8 | 7.2 | 3.2 | 62.3 | 0.0 | |
| March | 1.1 | 10.5 | 5.8 | 29.5 | 0.0 | |
| April | 5.1 | 14.7 | 9.9 | 96.3 | 9.6 | |
| May | 8.4 | 20.9 | 14.6 | 39.5 | 180.4 | |
| June | 16.3 | 30.8 | 23.6 | 4.6 | 300.9 | |
| July | 19.9 | 34.5 | 27.2 | 0.0 | 411.4 | |
| August | 20.4 | 35.1 | 27.7 | 0.0 | 407.7 |
| Mean Squares | ||||||
|---|---|---|---|---|---|---|
| Source | df | Crop Water Stress Index | Relative Water Content | Stomatal Conductance | Osmotic Adjustment | Water Potential |
| Year (Y) | 1 | 0.001 | 0.0009 | 0.0008 | 0.009 | 0.0009 |
| Replication/Y | 4 | 0.001 | 0.0010 | 0.0010 | 0.002 | 0.0009 |
| Stress (S) | 1 | 1.376 ** | 0.2780 ** | 0.3740 ** | 12.417 ** | 3.6990 ** |
| S × Y | 1 | 0.001 | 0.0009 | 0.0010 | 0.009 | 0.0007 |
| Error1 | 4 | 0.001 | 0.0003 | 0.0008 | 0.003 | 0.0009 |
| Genotype (G) | 5 | 0.022 ** | 0.0120 ** | 0.0160 ** | 0.077 ** | 0.0310 ** |
| G × Y | 5 | 0.001 | 0.0007 | 0.0007 | 0.002 | 0.0003 |
| S × G | 5 | 0.001 | 0.0020 ** | 0.0020 ** | 0.003 | 0.0110 ** |
| S ×G × Y | 5 | 0.001 | 0.00009 | 0.0010 | 0.002 | 0.0004 |
| Error2 | 40 | 0.001 | 0.0005 | 0.0005 | 0.004 | 0.0003 |
| C.V (%) | 4.28 | 2.12 | 3.26 | 5.74 | 3.96 | |
| Source | df | Water Use Efficiency | Capitula per Plant | Seeds in a Capitulum | 1000-Seed Weight | Seed Yield |
| Year (Y) | 1 | 0.0003 | 0.500 | 3.920 | 0.245 | 8320.500 |
| Replication/Y | 4 | 0.0010 | 1.778 | 10.891 | 12.757 | 185,288.153 |
| Stress (S) | 1 | 0.1670 ** | 53.389 ** | 1226.776 ** | 64.980 * | 17,586,406.556 ** |
| S × Y | 1 | 0.0004 | 2.000 | 0.376 | 7.736 | 5904.222 |
| Error1 | 4 | 0.0002 | 1.111 | 3.869 | 6.038 | 77,433.264 |
| Genotype (G) | 5 | 0.0070 ** | 48.822 ** | 897.379 ** | 76.505 ** | 13,498,965.356 ** |
| G × Y | 5 | 0.0001 | 0.233 | 23.367 | 12.311 | 16,587.733 |
| S × G | 5 | 0.0005 | 3.722 ** | 97.742 ** | 32.080 ** | 684,142.322 ** |
| S ×G × Y | 5 | 0.0004 | 0.667 | 32.809 | 3.749 | 8738.922 |
| Error2 | 40 | 0.0003 | 0.644 | 13.193 | 8.153 | 65,866.975 |
| C.V (%) | 3.35 | 9.20 | 7.55 | 8.50 | 7.51 | |
| Stress Levels | Genotype | Crop Water Stress Index | Relative Water Content | Stomatal Conductance (cm s−1) | Osmotic Adjustment (MPa) | Water Potential (MPa) |
|---|---|---|---|---|---|---|
| Non-stressed | Padideh | 0.353 d | 0.76 a | 0.738 a | 1.508 bc | −0.24 bc |
| Golemehr | 0.338 d | 0.72 b | 0.622 c | 1.633 a | −0.21 a | |
| Mec.14 | 0.322 d | 0.76 a | 0.695 b | 1.592 ab | −0.21 a | |
| Mec.248 | 0.357 d | 0.75 a | 0.662 b | 1.525 bc | −0.23 ab | |
| Mec.295 | 0.330 d | 0.74 a | 0.682 b | 1.658 a | −0.20 a | |
| Parnian | 0.448 c | 0.72 b | 0.627 c | 1.450 c | −0.26 c | |
| Stressed | Padideh | 0.632 b | 0.66 c | 0.553 d | 0.642 f | −0.72 g |
| Golemehr | 0.615 b | 0.56 d | 0.497 ef | 0.783 de | −0.63 e | |
| Mec.14 | 0.613 b | 0.64 c | 0.553 d | 0.750 de | −0.64 ef | |
| Mec.248 | 0.625 b | 0.65 c | 0.547 d | 0.716 ef | −0.66 f | |
| Mec.295 | 0.603 b | 0.64 c | 0.530 de | 0.850 d | −0.59 d | |
| Parnian | 0.713 a | 0.56 d | 0.480 f | 0.642 f | −0.815 h | |
| LSD 5% | 0.0466 | 0.0203 | 0.0338 | 0.0987 | 0.0279 | |
| Stress Levels | Genotype | Water Use Efficiency (kg m−3) | Capitula per Plant | Seeds in a Capitulum | 1000-Seed Weight (g) | Seed Yield (kg h−1) |
| Non-stressed | Padideh | 0.53 b | 11.3 a | 52.4 b | 31.6 b-e | 3697 cd |
| Golemehr | 0.55 ab | 8.7 cd | 51.7 b | 31.1 c-e | 4676 ab | |
| Mec.14 | 0.55 ab | 10.7 ab | 51.8 b | 37.4 a | 4026 c | |
| Mec.248 | 0.54 ab | 11.5 a | 61.0 a | 34.1 a-d | 4508 b | |
| Mec.295 | 0.56 a | 9.8 bc | 60.3 a | 35.7 a-c | 4961 a | |
| Parnian | 0.49 c | 5.5 f | 36.3 e | 37.5 a | 1593 g | |
| Stressed | Padideh | 0.44 e | 9.0 cd | 41.4 de | 28.1 e | 2939 f |
| Golemehr | 0.45 de | 8.2 de | 50.9 b | 30.4 e | 3211 ef | |
| Mec.14 | 0.44 de | 7.2 e | 50.0 bc | 29.8 de | 3564 de | |
| Mec.248 | 0.44 de | 9.5 b-d | 43.9 cd | 32.5 e | 3364 de | |
| Mec.295 | 0.47 cd | 9.0 cd | 52.7 b | 34.5 a-d | 3385 de | |
| Parnian | 0.41 f | 4.3 f | 27.1 f | 36.4 ab | 1068 h | |
| LSD 5% | 0.026 | 1.253 | 5.671 | 4.458 | 400.7 | |
| Trait | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | |
|---|---|---|---|---|---|---|---|---|---|---|
| (1) | Crop Water Stress Index | −0.91 ** | −0.93 ** | −0.98 ** | −0.98 ** | −0.98 ** | −0.60 * | −0.65 * | −0.21 | −0.66 * |
| (2) | Relative water content | 0.95 ** | 0.88 ** | 0.90 ** | 0.87 ** | 0.63 * | 0.55 | 0.15 | 0.58 * | |
| (3) | Stomatal conductance | 0.88 ** | 0.90 ** | 0.87 ** | 0.65 * | 0.55 | 0.15 | 0.59 * | ||
| (4) | Osmotic adjustment | 0.99 ** | 0.95 ** | 0.46 | 0.55 | 0.32 | 0.55 | |||
| (5) | Water potential | 0.95 ** | 0.51 | 0.60 * | 0.28 | 0.58 * | ||||
| (6) | Water use efficiency | 0.65 * | 0.74 ** | 0.20 | 0.75 ** | |||||
| (7) | Capitula per plant | 0.82 ** | −0.17 | 0.86 ** | ||||||
| (8) | Seeds in a capitulum | −0.13 | 0.92 ** | |||||||
| (9) | 1000-seed weight | −0.23 | ||||||||
| (10) | Seed yield | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasban Eslam, B.; Chenari Bouket, A.; Oszako, T.; Belbahri, L. Physiological Indices for the Selection of Drought-Tolerant Safflower Genotypes for Cultivation in Marginal Areas. Appl. Sci. 2024, 14, 5106. https://doi.org/10.3390/app14125106
Pasban Eslam B, Chenari Bouket A, Oszako T, Belbahri L. Physiological Indices for the Selection of Drought-Tolerant Safflower Genotypes for Cultivation in Marginal Areas. Applied Sciences. 2024; 14(12):5106. https://doi.org/10.3390/app14125106
Chicago/Turabian StylePasban Eslam, Bahman, Ali Chenari Bouket, Tomasz Oszako, and Lassaad Belbahri. 2024. "Physiological Indices for the Selection of Drought-Tolerant Safflower Genotypes for Cultivation in Marginal Areas" Applied Sciences 14, no. 12: 5106. https://doi.org/10.3390/app14125106
APA StylePasban Eslam, B., Chenari Bouket, A., Oszako, T., & Belbahri, L. (2024). Physiological Indices for the Selection of Drought-Tolerant Safflower Genotypes for Cultivation in Marginal Areas. Applied Sciences, 14(12), 5106. https://doi.org/10.3390/app14125106







