Enhancing Lettuce Drought Tolerance: The Role of Organic Acids in Photosynthesis and Oxidative Defense
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vegetation Experiment
2.2. Chlorophyll Fluorescence Measurements
2.3. Biometrical Measurements
2.4. Analysis of Macroelements in Plants
2.5. Physiological Parameters
2.5.1. Superoxide Anion (O2•−)
2.5.2. Hydrogen Peroxide (H2O2)
2.5.3. Malondialdehyde (MDA)
2.5.4. Ascorbic Acid (AsA)
2.5.5. Proline (Pro)
2.5.6. The Total Phenolic Content (TPC)
2.5.7. Flavonols (Fla)
2.5.8. Photosynthetic Pigments
2.6. Statistical Analysis
3. Results
3.1. Plant Yielding
3.2. Nutrient Status
3.3. Chlorophyll Fluorescence
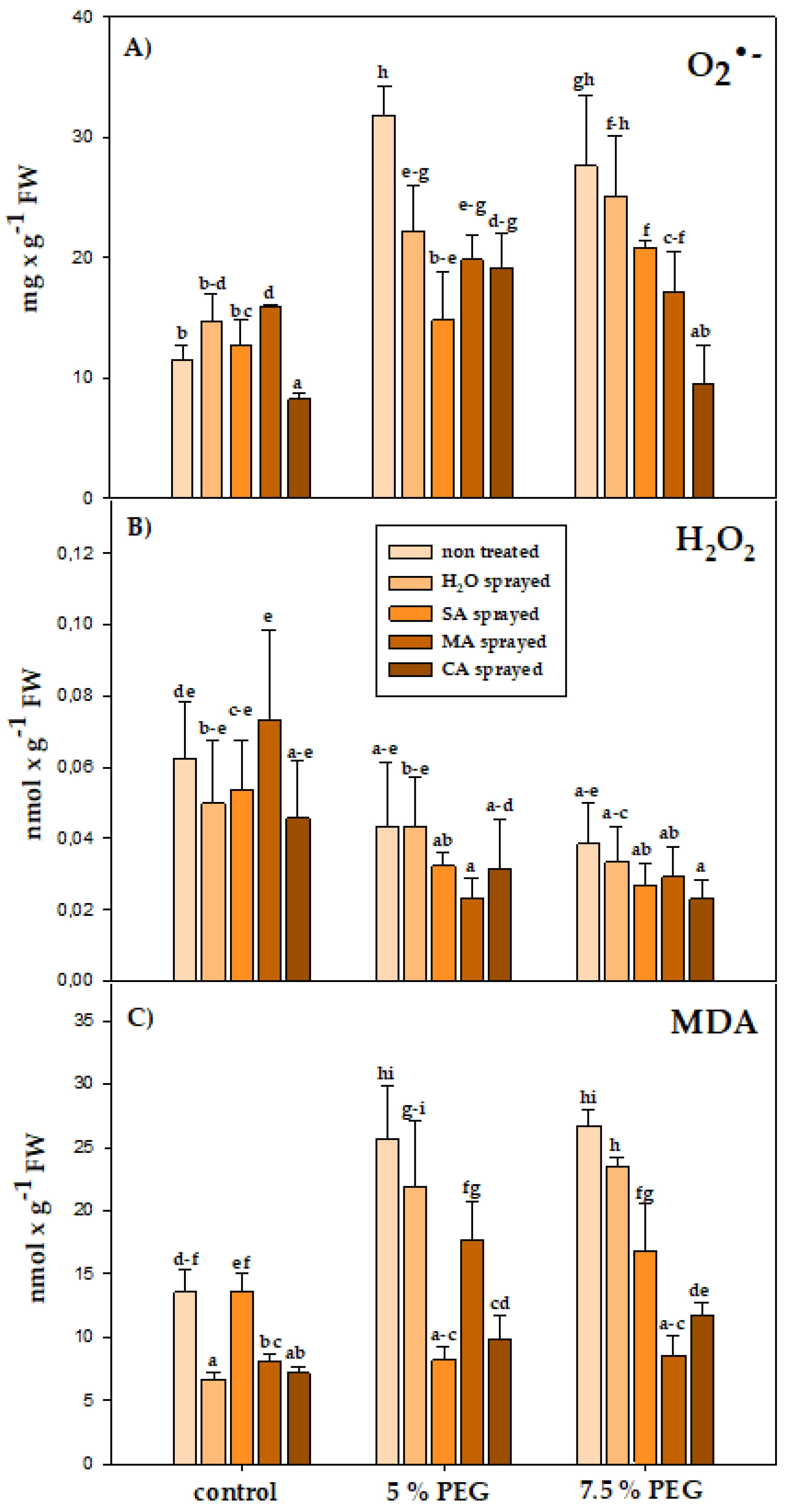
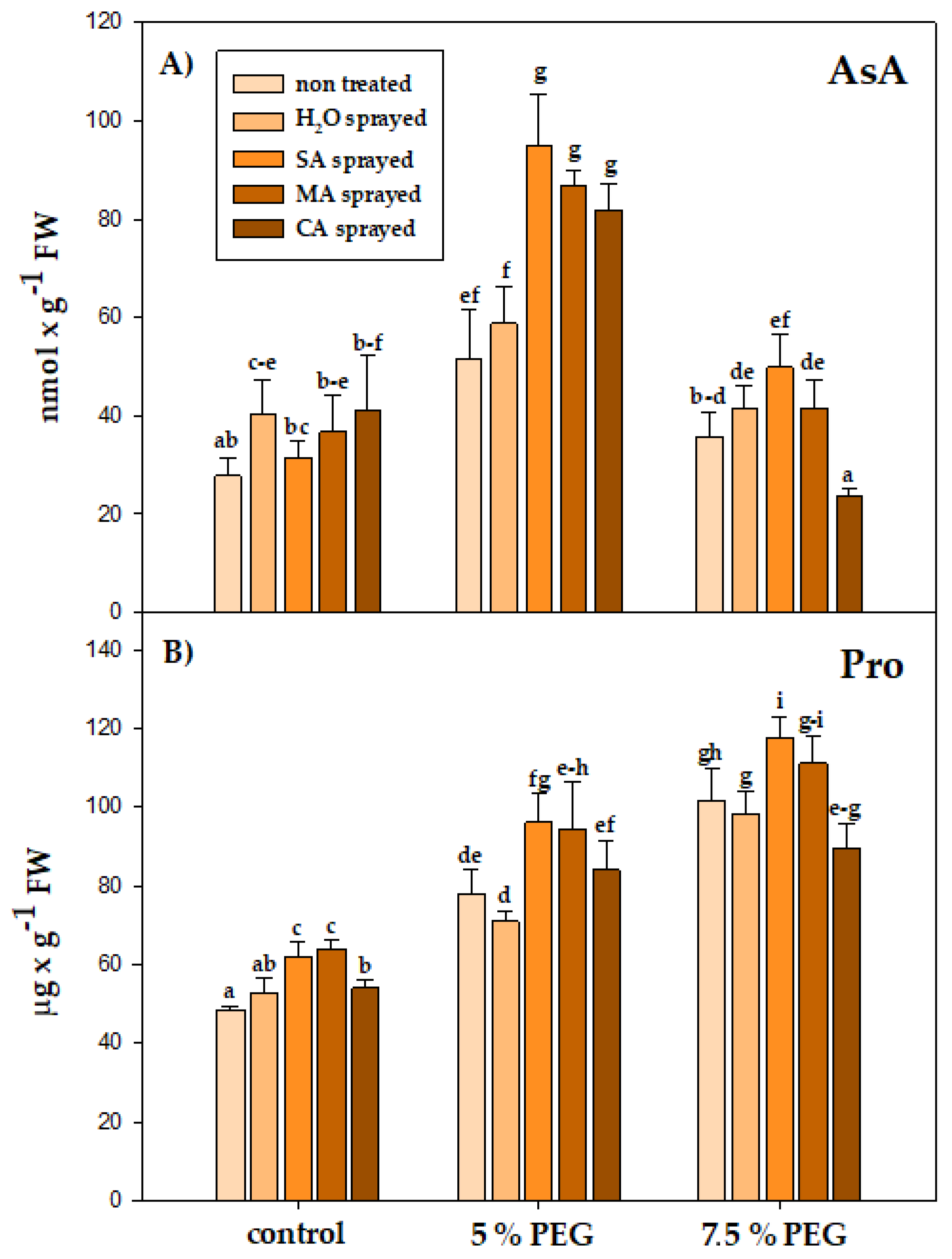
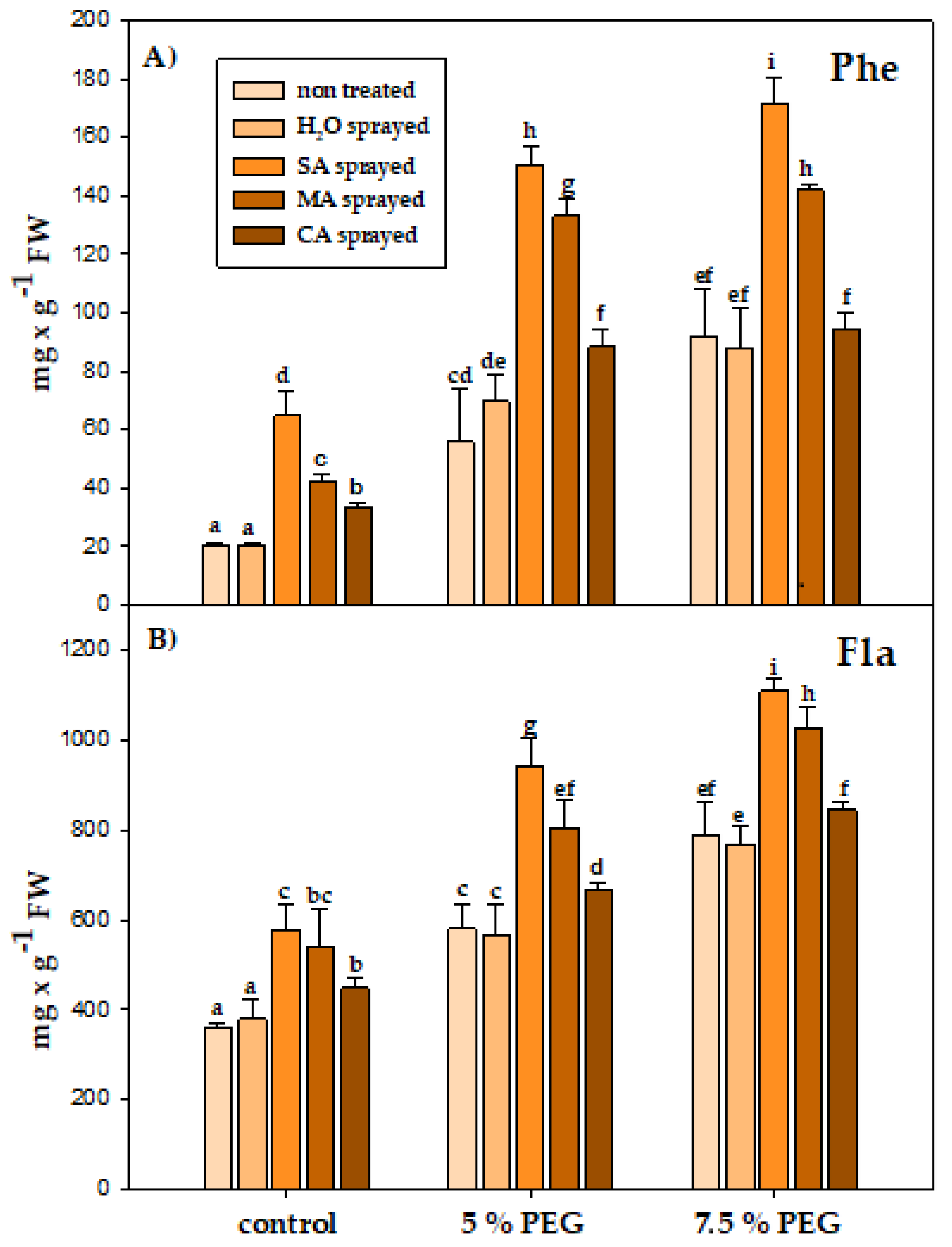
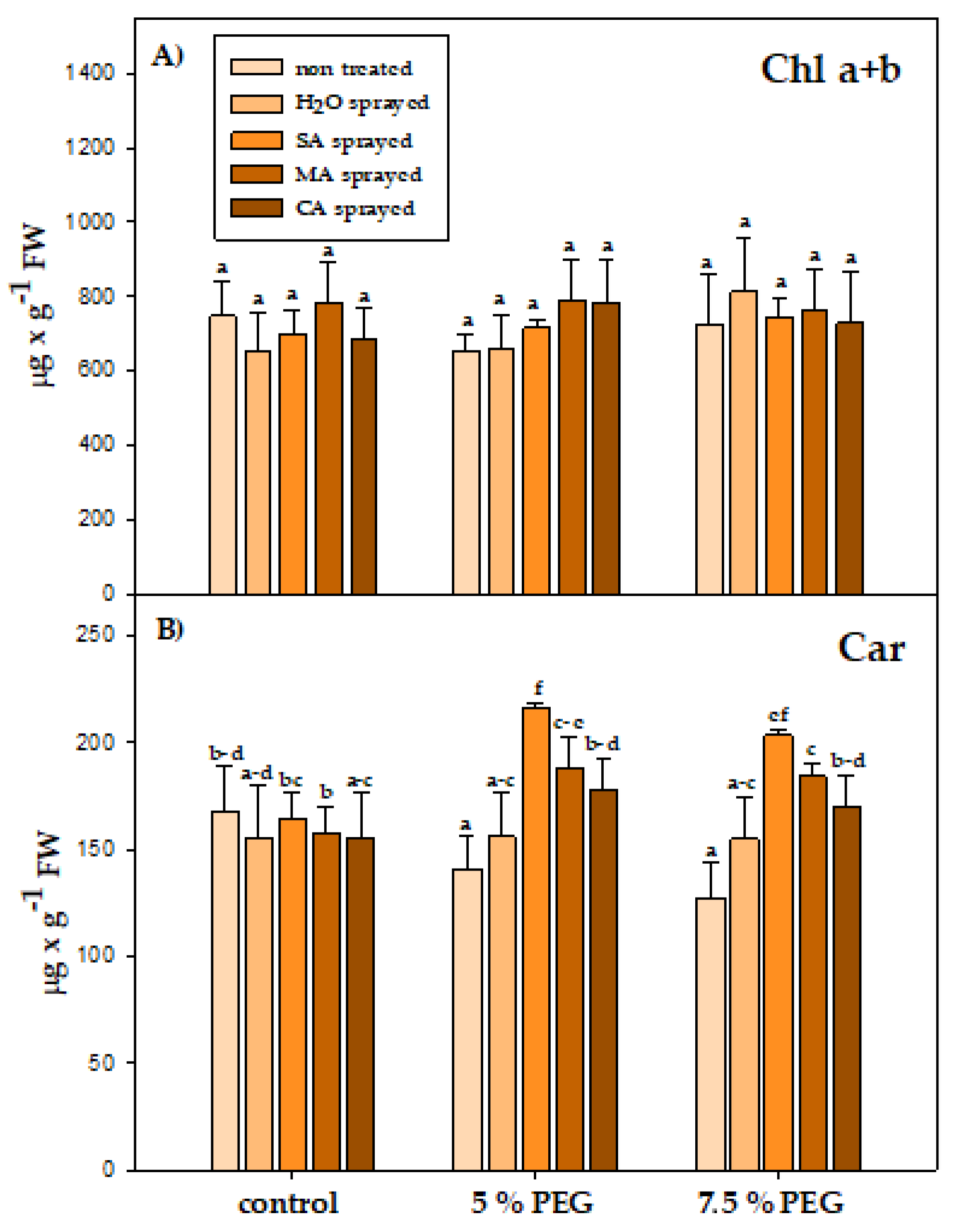
3.4. Physiological Parameters
4. Discussion
4.1. Plant Yielding
4.2. Nutrient Uptake
4.3. Chlorophyll Fluorescence
4.4. Physiological Parameters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The state of the world’s land and water resources for food and agriculture–Systems at breaking point. In Synthesis Report 2021; FAO: Rome, Italy, 2021; Available online: https://www.fao.org/land-water/solaw2021/en/ (accessed on 9 June 2024).
- Orimoloye, I.R. Agricultural Drought and Its Potential Impacts: Enabling Decision-Support for Food Security in Vulnerable Regions. Front. Sustain. Food Syst. 2022, 6, 838824. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, X.; Pan, Y.; Li, S.; Liu, Y.; Ma, Y. Agricultural drought monitoring: Progress, challenges, and prospects. J. Geogr. Sci. 2016, 26, 750–767. [Google Scholar] [CrossRef]
- Salehi-Lisar, S.Y.; Bakhshayeshan-Agdam, H. Drought stress in plants: Causes, consequences, and tolerance. In Drought Stress Tolerance in Plants; Hossain, M., Wani, S., Bhattacharjee, S., Burritt, D., Tran, L.S., Eds.; Springer: Cham, Switzerland, 2016; Volume 1. [Google Scholar]
- Luo, L.; Xia, H.; Lu, B.-R. Editorial: Crop breeding for drought resistance. Front. Plant Sci. 2019, 10, 314. [Google Scholar] [CrossRef] [PubMed]
- Panchal, P.; Miller, A.J.; Giri, J. Organic acids: Versatile stress-response roles in plants. J. Exp. Bot. 2021, 72, 4038–4052. [Google Scholar] [CrossRef] [PubMed]
- Igamberdiev, A.U.; Bykova, N.V. Role of organic acids in the integration of cellular redox metabolism and mediation of redox signalling in photosynthetic tissues of higher plants. Free Radic. Biol. Med. 2018, 22, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Vallarino, J.G.; Osorio, S. Organic acids. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Woodhead Publishing: Sawston, UK, 2019; pp. 207–224. [Google Scholar]
- Zhang, S.; Chen, H.; He, D.; He, X.; Yan, Y.; Wu, K.; Wei, H. Effects of exogenous organic acids on Cd tolerance mechanism of Salix Variegata Franch. under Cd stress. Front. Plant Sci. 2020, 11, 1594. [Google Scholar] [CrossRef] [PubMed]
- Araujo, W.L.; Nunes-Nesi, A.; Nikoloski, Z.; Sweetlove, L.J.; Fernie, A.R. Metabolic control and regulation of the tricarboxylic acid cycle in photosynthetic and heterotrophic plant tissues. Plant Cell Environ. 2012, 35, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Prince, S.; Valliyodan, B.; Joshi, T.; Maldonado dos Santos, J.V.; Wang, J.; Lin, L.; Wan, J.; Wang, Y.; Xu, D.; et al. Genome-wide transcriptome analysis of soybean primary root under varying water-deficit conditions. BMC Genom. 2016, 17, 57. [Google Scholar] [CrossRef]
- Khan, N.; Ali, S.; Zandi, P.; Mehmood, A.; Ullah, S.; Ikram, M.; Ismail, M.A.S.; Babar, M.A. Role of sugars, amino acids and organic acids in improving plant abiotic stress tolerance. Pak. J. Bot. 2020, 52, 355–363. [Google Scholar] [CrossRef]
- Nakandala, S.A.; Weerasinghe, K.D.N.; Senevirathne, P.; Iqbal, S.M.M.; Lakmini, W.G.D.; Vijithasiri, P.M.P.S. Exogenous application of Salicylic Acid alleviates drought stress of rubber nursery plants in the Intermediate Zone of Sri Lanka. J. Rubber Res. Inst. Sri Lanka 2016, 96, 50–58. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Matin, M.A.; Fardus, J.; Hasanuzzaman, M.; Hossain, M.S.; Parvin, K. Foliar application of salicylic acid improves growth and yield attributes by upregulating the antioxidant defense system in Brassica campestris plants grown in lead-amended soils. Acta Agrobot. 2019, 72, 1765. [Google Scholar] [CrossRef]
- Wang, S.; Ma, L.; Xu, Y.; Wang, Y.; Zhu, N.; Liu, J.; Dolfing, J.; Kerr, P.; Wu, Y. The unexpected concentration-dependent response of periphytic biofilm during indole acetic acid removal. Bioresour. Technol. 2020, 303, 122–922. [Google Scholar] [CrossRef] [PubMed]
- Kotapati, K.P.; Bhagath, K.P.; Dinakara, R.A. Alleviation of nickel toxicity in finger millet (Eleusine coracana L.) germinating seedlings by exogenous application of salicylic acid and nitric oxide. Crop J. 2017, 5, 240–250. [Google Scholar] [CrossRef]
- Ghazijahani, T.G.; Jiao, H.; Holloway, S. Influence of a cutout on circular steel hollow sections under cyclic loading. J. Constr. Steel Res. 2014, 100, 12–20. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- IUNG. Analytical Methods in Agricultural Chemistry Stations Part II. In Plant Analyses; Institute of Soil Science and Plant Cultivation: Puławy, Poland, 1972; pp. 25–83. [Google Scholar]
- Doke, N. Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and the hyphal wall components. Physiol. Plant Pathol. 1983, 23, 345–357. [Google Scholar] [CrossRef]
- Messner, B.; Boll, M. Cell suspension cultures of spruce (P. abies): Inactivation of extracellular enzymes by fungal elicitor-induced transient release of hydrogen peroxide (oxidative burst). Plant Cell Tissue Organ Cult. 1994, 39, 69–78. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Effect of light on lipid peroxidation in chloroplasts. Biochem. Biophys. Res. Commun. 1968, 19, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Law, M.Y.; Charles, S.A.; Halliwell, B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. The effect of hydrogen peroxide and paraquat. Biochem. J. 1983, 210, 899–903. [Google Scholar] [CrossRef]
- Costa, H.; Gallego, S.M.; Tomaro, M.L. Effect of UV-B radiation on antioxidant defense system in sunflower cotyledons. Plant Sci. 2002, 162, 939–945. [Google Scholar] [CrossRef]
- Bates, I.S.; Waldern, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Bandurska, H. Does proline accumulated in leaves of water deficit stressed barley plants confine cell membrane injury? I. Free proline accumulation and membrane injury index in drought and osmotically stressed plants. Acta Physiol. Plant. 2000, 22, 409–415. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Day, T.A. Relating UV-B radiation screening effectiveness of foliale to absorbing-compound concentration andanatomical characteristis in a diverse group of plants. Oecologia 1993, 95, 542–550. [Google Scholar] [CrossRef]
- Stefova, M.; Kulevanova, S.; Stafilov, T. Assay of flavonols andquantification of quercetin in medicinal plants by HPLC with UV-diode array detection. J. Liq. Chromatogr. Relat. 2001, 24, 2283–2292. [Google Scholar] [CrossRef]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Scudeletti, D.; Crusciol, C.A.C.; Bossolani, J.W.; Moretti, L.G.; Momesso, L.; Servaz Tubana, B.; De Castro, S.G.Q.; De Oliveira, E.F.; Hungria, M. Trichoderma asperellum inoculation as a tool for attenuating drought stress in sugarcane. Front. Plant Sci. 2021, 12, 570. [Google Scholar] [CrossRef]
- Waqas, M.; Yaning, C.; Iqbal, H.; Shareef, M.; ur Rehman, H.; Bilal, H.M. Synergistic consequences of salinity and potassium deficiency in quinoa: Linking with stomatal patterning, ionic relations and oxidative metabolism. Plant Physiol. Biochem. 2021, 159, 17–27. [Google Scholar] [CrossRef]
- Rady, M.M.; Boriek, S.H.; El-Mageed, A.; Taia, A.; Seif El-Yazal, M.A.; Ali, E.F.; Hassan, F.A.; Abdelkhalik, A. Exogenous gibberellic acid or dilute bee honey boosts drought stress tolerance in Vicia faba by rebalancing osmoprotectants, antioxidants, nutrients, and phytohormones. Plants 2021, 10, 748. [Google Scholar] [CrossRef]
- Tahmasebi, A.; Niazi, A. Comparison of Transcriptional Response of C3 and C4 plants to drought stress using meta-analysis and systems biology approach. Front. Plant Sci. 2021, 12, 668736. [Google Scholar] [CrossRef]
- Khalvandi, M.; Siosemardeh, A.; Roohi, E.; Keramati, S. Salicylic acid alleviated the effect of drought stress on photosynthetic characteristics and leaf protein patterns in winter wheat. Heliyon 2021, 7, e05908. [Google Scholar] [CrossRef] [PubMed]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Naz, S.; Altaf, M.M.; Khan, L.U.; Tiwari, R.K.; Lal, M.K.; Shahid, M.A.; Kumar, R.; et al. Melatonin improves drought stress tolerance of tomatoes by modulating plant growth, root architecture, photosynthesis, and antioxidant defense system. Antioxidants 2022, 11, 309. [Google Scholar] [CrossRef] [PubMed]
- Franzoni, G.; Cocetta, G.; Ferrante, A. Effect of glutamic acid foliar applications on lettuce under water stress. Physiol. Mol. Biol. Plants 2021, 27, 1059–1072. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.; Tudela, J.; Martınez-Sanchez, A.; Luna, M. Harvest maturity indicators of leafy vegetables. Stewart Postharvest Rev. 2012, 8, 1–9. [Google Scholar]
- Waskiewicz, A.; Gładysz, O.; Beszterda, M.; Golinski, P. Water stress and crop plants. In Water Stress and Crop Plants: A Sustainable Approach; Ahmad, P., Ed.; Wiley: Chichester, UK, 2006; Volume 2, pp. 393–411. [Google Scholar]
- Sayyari, M.; Ghavami, M.; Ghanbari, F.; Kordi, S. Assessment of salicylic acid impacts on growth rate and some physiological parameters of lettuce plants under drought stress conditions. Int. J Agric. Crop Sci. 2013, 5, 1951–1957. [Google Scholar]
- Mou, B. Genetic Variation of Beta-carotene and Lutein Contents in Lettuce. J. Am. Soc. Hortic. Sci. 2009, 130, 870–876. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Rahman, M.M.; Ansary, M.M.U.; Keya, S.S.; Abdelrahman, M.; Miah, M.G.; Phan Tran, L.S. Silicon in mitigation of abiotic stress-induced oxidative damage in plants. Crit. Rev. Biotechnol. 2021, 41, 918–934. [Google Scholar] [CrossRef] [PubMed]
- Ghanbary, E.; Fathizadeh, O.; Pazhouhan, I.; Zarafshar, M.; Tabari, M.; Jafarnia, S.; Parad, G.A.; Bader, M.K.F. Drought and pathogen effects on survival, leaf physiology, oxidative damage, and defense in two Middle Eastern oak species. Forests 2021, 12, 247. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Kapoor, D. Fascinating regulatory mechanism of silicon for alleviating drought stress in plants. Plant Physiol. Biochem. 2021, 166, 1044–1053. [Google Scholar] [CrossRef]
- Bankole, A.E.; Umebese, C.E.; Feyisola, R.T.; Bamise, T. Influence of Salicylic Acid on the Growth of Lettuce (Lactuca sativa var longifolia) During Reduced Leaf Water Potential. J. Appl. Sci. Environ. Manag. 2018, 22, 543. [Google Scholar] [CrossRef]
- Nazar, R.; Umar, S.; Khan, N.A.; Sareer, O. Salicylic acid supplementation improves photosynthesis and growth in mustard through changes in proline accumulation and ethylene formation under drought stress. S. Afr. J. Bot. 2015, 98, 84–94. [Google Scholar] [CrossRef]
- Iqbal, M.; Hussain, I.; Liaqat, H.; Ashraf, M.A.; Rasheed, R.; Rehman, A. Exogenously applied selenium reduces oxidative stress and induces heat tolerance in spring wheat. Plant Physiol. Biochem. 2015, 94, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Christophe, S.; Jean-Christophe, A.; Annabelle, L.; Alain, O.; Marion, P.; Anne-Sophie, V. Plant N fluxes and modulation by nitrogen, heat and water stresses: A review based on comparison of legumes and non legume plants. In Abiotic Stress in Plants–Mechanisms and Adaptations; Shanker, A., Venkateswarlu, B., Eds.; Intech Open Access Publisher: Rijeka, Croatia, 2011; pp. 79–118. [Google Scholar]
- Robredo, A.; Pérez-López, U.; Miranda-Apodaca, J.; Lacuesta, M.; Mena-Petite, A.; Muñoz-Rueda, A. Elevated CO2 reduces the drought effect on nitrogen metabolism in barley plants during drought and subsequent recovery. Environ. Exp. Bot. 2011, 71, 399–408. [Google Scholar] [CrossRef]
- Giri, A.; Heckathorn, S.; Mishra, S.; Krause, C. Heat stress decreases levels of nutrient-uptake and -assimilation proteins in tomato roots. Plants 2017, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; Hadavi, E.; Hekmati, J. Effect of salicylic acid, malic acid, citric acid and sucrose on antioxidant activity, membrane stability and ACC-Oxidase activity in relation to vase life of carnation cut flowers. J. Agric. Technol. 2012, 8, 2053–2063. [Google Scholar] [CrossRef]
- He, M.; Dijkstra, F.A. Drought effect on plant nitrogen and phosphorus: A meta-analysis. New Phytol. 2014, 204, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Waqas, M.; Wang, L.; Jones, J.J.; Josef Turetschek, R.; Engelmeier, D.; Geilfus, C.; Koch, M. Short-term phosphorus deficiency induces flavonoid accumulation in the lamina of pak choi: A finishing treatment that influences inner quality. Sci. Hortic. 2023, 314, 111953. [Google Scholar] [CrossRef]
- Biswas, D.K.; Ma, B.L.; Morrison, M.J. Changes in leaf nitrogen and phosphorus content, photosynthesis, respiration, growth, and resource use efficiency of a rapeseed cultivar as affected by drought and high temperatures. Can. J. Plant Sci. 2019, 99, 488–498. [Google Scholar] [CrossRef]
- Uchida, R. Essential nutrients for plant growth-nutrient functions and deficiency symptoms. In Plant Nutrient Management in Hawaii’s Soils, Approaches for Tropical and Subtropical Agriculture; : Silva, J.A., Uchida, R., Eds.; College of Tropical Agriculture and Human Resources, University of Hawaii: Honolulu, Hawaii, 2000; pp. 31–55. [Google Scholar]
- Maathuis, F.J. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef]
- Munsif, F.; Farooq, U.; Arif, M.; Shah, T.; Jehangir, M.; Zaheer, S.; Akhtar, K.; Khan, M.S.; Ahmad, I.; Ahmad, W.; et al. Potassium and salicylic acid function synergistically to promote the drought resilience through upregulation of antioxidant profile for enhancing potassium use efficiency and wheat yield. Ann. Appl. Biol. 2022, 180, 273–282. [Google Scholar] [CrossRef]
- Cockburn, W. Stomatal Mechanism as the Basis of the Evolution of CAM and C4 Photosynthesis. Plant Cell Environ. 1983, 6, 275–279. [Google Scholar] [CrossRef]
- Outlaw, W.H.; Lowry, O.H. Organic acid and potassium accumulation in guard cells during stomatal opening. Proc. Nat. Acad. Sci. USA 1977, 74, 4434–4438. [Google Scholar] [CrossRef] [PubMed]
- McAinsh, M.R.; Pittman, J.K. Shaping the calcium signature. New Phytol. 2009, 181, 275–294. [Google Scholar] [CrossRef]
- Mengel, K.; Kirkby, E.A. (Eds.) Principles of Plant Nutrition; International Potash Institute: Worblaufen-Bern, Switzerland, 2006. [Google Scholar]
- Fiedor, L.; Kania, A.; Myśliwa-Kurdziel, B.; Orzeł, Ł.; Stochel, G. Understanding chlorophylls: Central magnesium ion and phytyl as structural determinants. Biochim. Biophys. Acta 2008, 1777, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Wanli, G.; Nazimc, H.; Lianga, Z.; Dongfeng, Y.D. Magnesium deficiency in plants: An urgent problem. Crop J. 2016, 4, 83–91. [Google Scholar]
- Marschner, H. Marschner’s mineral nutrition of plants. J. Plant Physiol. 2012, 134, 308–315. [Google Scholar] [CrossRef]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef]
- Martinez, J.P.; Ledent, J.F.; Bajji, M.; Kinet, J.M.; Lutts, S. Effect of water stress on growth, Na+ and K+ accumulation and water use efficiency in relation to osmotic adjustment in two populations of Atriplex halimus L. Plant Growth Regul. 2003, 41, 63–73. [Google Scholar] [CrossRef]
- Xiao, F.; Zhou, H. Plant salt response: Perception, signaling, and tolerance. Front. Plant Sci. 2023, 13, 1053699. [Google Scholar] [CrossRef]
- Abubakar, M.; Kadiec, P.; Melante, W. Impact of drought on nutrient composition of vegetables. J. Plant Nutr. 2022, 44, 365–422. [Google Scholar]
- Bai, X.; Zhu, J.J.; Zhang, P. Severe salt stress in Vaccinium myrtillus (L.) in response to Na+ ion toxicity. Environ. Exp. Bot. 2012, 76, 49–53. [Google Scholar]
- Erel, R.; Ben-Gal, A.; Dag, A.; Schwartz, A.; Yermiyahu, U. Sodium replacement of potassium in physiological processes of olive trees (var. Barnea) as affected by drought. Tree Physiol. 2014, 34, 1102–1117. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Wang, Y.F.; Zhao, C.F.; Yang, M.; Wang, G.X.; Zhang, R.H. The quantitative proteomic analysis provides insight into the effects of drought stress in maize. Photosynthetica 2021, 59, 1–11. [Google Scholar] [CrossRef]
- Babaei, K.; Moghaddam, M.; Farhadi, N.; Pirbalouti, A.G. Morphological, physiological and phytochemical responses of Mexican marigold (Tagetes minuta L.) to drought stress. Sci. Hortic. 2021, 284, 110–116. [Google Scholar] [CrossRef]
- Babaei, L.; Sharifani, M.M.; Darvishzadeh, R.; Abbaspour, N.; Henareh, M. Impact of drought stress on photosynthetic response of some pear species. Int. J. Hortic. Sci. Technol. 2021, 8, 353–369. [Google Scholar]
- Song, X.; Zhou, G.; He, Q. Critical leaf water content for maize photosynthesis under drought stress and its response to re-watering. Sustainability 2021, 13, 7218. [Google Scholar] [CrossRef]
- Ors, S.; Ekinci, M.; Yildirim, E.; Sahin, U.; Turan, M.; Dursun, A. Interactive effects of salinity and drought stress on photosynthetic characteristics and physiology of tomato (Lycopersicon esculentum L.) seedlings. S. Afr. J. Bot. 2021, 137, 335–339. [Google Scholar] [CrossRef]
- Kaur, H.; Manna, M.; Thakur, T.; Gautam, V.; Salvi, P. Imperative role of sugar signaling and transport during drought stress responses in plants. Physiol. Plant. 2021, 171, 833–848. [Google Scholar] [CrossRef]
- Ahmad, H.; Li, J. Impact of water deficit on the development and senescence of tomato roots grown under various soil textures of Shaanxi, China. BMC Plant Biol. 2021, 21, 241. [Google Scholar] [CrossRef]
- Ullah, A.; Farooq, M. The challenge of drought stress for grain legumes and options for improvement. Arch. Agron. Soil Sci. 2021, 68, 1601–1618. [Google Scholar] [CrossRef]
- Ullah, A.; Al-Rajhi, R.S.; Al-Sadi, A.M.; Farooq, M. Wheat genotypes with higher intercellular CO2 concentration, rate of photosynthesis, and antioxidant potential can better tolerate drought stress. J. Soil Sci. Plant Nutr. 2021, 21, 2378–2391. [Google Scholar] [CrossRef]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll fluorescence, photoinhibition and abiotic stress: Does it make any difference the fact to be a C3 or C4 species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Faraloni, C.; Cutino, I.; Petruccelli, R.; Leva, A.R.; Lazzeri, S.; Torzillo, G. Chlorophyll fluorescence technique as a rapid tool for in vitro screening of olive cultivars (Olea europaea L.) tolerant to drought stress. Environ. Exp. Bot. 2011, 73, 49–56. [Google Scholar] [CrossRef]
- Fracheboud, Y.; Jompuk, C.; Ribaut, J.M.; Stamp, P.; Leipner, J. Genetic analysis of cold-tolerance of photosynthesis in maize. Plant Mol. Biol. 2004, 56, 241–253. [Google Scholar] [CrossRef]
- Gorbe, E.; Calatayud, A. Applications of chlorophyll fluorescence imaging technique in horticultural research: A review. Sci. Hortic. 2012, 138, 24–35. [Google Scholar] [CrossRef]
- Calatayud, A.; Roca, D.; Martínez, P.F. Spatial-temporal variations in rose leaves under water stress conditions studied by chlorophyll fluorescence imaging. Plant Physiol. Biochem. 2006, 44, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Łoboda, T. Fluorescencja Chlorofilu w Badaniach Stanu Fizjologicznego Roślin; Wydawnictwo SGGW: Warszawa, Poland, 2010. [Google Scholar]
- Ruban, A.V.; Horton, P. Regulation of non-photochemical quenching of chlorophyll fluorescence in plants. Funct. Plant Biol. 1995, 22, 221–230. [Google Scholar] [CrossRef]
- Umar, M.; Siddiqui, Z.S. Physiological performance of sunflower genotypes under combined salt and drought stress environment. Acta Bot. Croat. 2020, 77, 36–44. [Google Scholar] [CrossRef]
- Buonocore, G.; Perrone, S.; Tataranno, M.L. Oxygen toxicity: Chemistry and biology of reactive oxygen species. In Seminars in Fetal and Neonatal Medicine; WB Saunders: Philadelphia, PA, USA, 2010; Volume 15, pp. 186–190. [Google Scholar] [CrossRef]
- Inupakutika, M.A.; Sengupta, S.; Devireddy, A.R.; Azad, R.K.; Mittler, R. The evolution of reactive oxygen species metabolism. J. Exp. Bot. 2016, 67, erw382. [Google Scholar] [CrossRef]
- Ruban, A.V. Nonphotochemical chlorophyll fluorescence quenching: Mechanism and effectiveness in protecting plants from photodamage. Plant Physiol. 2016, 170, 1903–1916. [Google Scholar] [CrossRef]
- Mallhi, Z.I.; Rizwan, M.; Mansha, A.; Ali, Q.; Asim, S.; Ali, S.; Ahmad, P. Citric acid enhances plant growth, photosynthesis, and phytoextraction of lead by alleviating the oxidative stress in castor beans. Plants 2019, 8, 525. [Google Scholar] [CrossRef]
- Krause, G.H.; Weis, E. Chlorophyll fluorescence as a tool in plant physiology. Photosynth. Res. 1984, 5, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Zhou, P.; Xiao, Q.; Shi, D. Effects of foliar application of organic acids on alleviation of aluminum toxicity in Lettuce. J. Plant. Nutr. Soil Sci. 2014, 177, 421–430. [Google Scholar] [CrossRef]
- Ceusters, N.; Valcke, R.; Frans, M.; Claes, J.E.; Van den Ende, W.; Ceusters, J. Performance index and PSII connectivity under drought and contrasting light regimes in the CAM orchid Phalaenopsis. Front. Plant Sci. 2019, 10, 1012. [Google Scholar] [CrossRef] [PubMed]
- Bacarin, M.A.; Deuner, S.; Silva, F.S.P.D.; Cassol, D.; Silva, D.M. Chlorophyll a fluorescence as indicative of the salt stress on Brassica napus L. Braz. J. Plant Physiol. 2011, 23, 245–253. [Google Scholar] [CrossRef]
- Kumar, D. Salicylic acid signaling in disease resistance. Plant Sci. 2014, 228, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Bukhat, S.; Manzoor, H.; Athar, H.U.R.; Zafar, Z.U.; Azeem, F.; Rasul, S. Salicylic acid induced photosynthetic adaptability of Raphanus sativus to salt stress is associated with antioxidant capacity. J. Plant Growth Regul. 2020, 39, 809–822. [Google Scholar] [CrossRef]
- Bandurska, H.; Stroinski, A. The effect of salicylic acid on barley response to water deficit. Acta Physiol. Plant. 2005, 27, 379–386. [Google Scholar] [CrossRef]
- El Tayeb, M.A.; Ahmed, N.L. Response of wheat cultivars to drought and salicylic acid. Am.-Eurasian J. Agric. Environ. Sci. 2010, 3, 1–7. [Google Scholar]
- Parveen, A.; Arslan Ashraf, M.; Hussain, I.; Perveen, S.; Rasheed, R.; Mahmood, Q.; Hussain, S.; Ditta, A.; Hashem, A.; Al-Arjani, A.-B.F.; et al. Promotion of growth and physiological characteristics in water-stressed Triticum aestivum in relation to foliar-application of salicylic acid. Water 2021, 13, 1316. [Google Scholar] [CrossRef]
- Hussain, M.; Malik, M.A.; Farooq, M.; Ashraf, M.Y.; Cheema, M.A. Improving drought tolerance by exogenous application of glycinebetaine and salicylic acid in sunflower. J. Agron. Crop Sci. 2008, 194, 193–199. [Google Scholar] [CrossRef]
- Hayat, S.; Hasan, S.A.; Fariduddin, Q.; Ahmad, A. Growth of tomato (Lycopersicon esculentum) in response to salicylic acid under water stress. J. Plant Interact. 2008, 3, 297–304. [Google Scholar] [CrossRef]
- Umebese, C.E.; Olatimilehin, T.O.; Ogunsusi, T.A. Salicylic acid protects nitrate reductase activity, growth and proline in amaranth and tomato plants during water deficit. Am. J. Agric. Biol. Sci. 2009, 4, 224–229. [Google Scholar] [CrossRef]
- Sadeghipour, O.; Aghaei, P. Biochemical changes of common bean (Phaseolus vulgaris L.) to pretreatment with salicylic acid (SA) under water stress conditions. Int. J. Biosci. 2012, 2, 14–22. [Google Scholar]
- Bakry, B.A.; El-Hariri, D.M.; Sadak, M.S.; El-Bassiouny, H.M.S. Drought stress mitigation by foliar application of salicylic acid in two linseed varieties grown under newly reclaimed sandy soil. J. Appl. Sci. Res. 2012, 8, 3503–3514. [Google Scholar]
- De Carvalho, M.H.C.; Brunet, J.; Bazin, J.; Kranner, I.; d’Arcy-Lameta, A.; Zuily-Fodil, Y.; Contour-Ansel, D. Homoglutathione synthetase and glutathione synthetase in drought-stressed cowpea leaves: Ex-pression patterns and accumulation of lowmolecular-weight thiols. J. Plant Physiol. 2010, 167, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Camaille, M.; Fabre, N.; Clément, C.; Ait Barka, E. Advances in wheat physiology in response to drought and the role of plant growth promoting rhizobacteria to trigger drought tolerance. Microorganisms 2021, 9, 687. [Google Scholar] [CrossRef] [PubMed]
- Franzisky, B.L.; Solter, J.; Xue, C.; Harter, K.; Stahl, M.; Geilfus, C.-M. In planta exploitation of leaf apoplastic compounds: A window of opportunity for spatiotemporal studies of apoplastic metabolites, hormones and physiology. Biorxiv 2023. [Google Scholar]
- Ali, Q.; Javed, M.T.; Noman, A.; Haider, M.Z.; Waseem, M.; Iqbal, N.; Waseem, M.; Shah, M.S.; Shahzad, F.; Perveen, R. Assessment of drought tolerance in mung bean cultivars/lines as depicted by the activities of germination enzymes, seedling’s antioxidative potential and nutrient acquisition. Arch. Agron. Soil Sci. 2018, 64, 84–102. [Google Scholar] [CrossRef]
- Demidchik, V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Raza, G.; Ali, K.; Ashraf, M.Y.; Mansoor, S.; Javid, M.; Asad, S. Overexpression of an H+−PPase gene from Arabidopsis in sugarcane improves drought tolerance, plant growth, and photosynthetic responses. Turk. J. Biol. 2016, 40, 109–119. [Google Scholar] [CrossRef]
- Rosalie, R.; Léchaudel, M.; Dhuique-Mayer, C.; Dufossé, L.; Joas, J. Antioxidant and enzymatic responses to oxidative stress induced by cold temperature storage and ripening in mango (Mangifera indica L. cv. ‘Cogshall’) in relations to carotenoid content. J. Plant Physiol. 2018, 224, 75–85. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Senaratna, T.; Touchell, D.; Bunn, E.; Dixon, K. Acetyl salicylic acid (Aspirin) and salicylic acid induce multiple stress tolerance in bean and tomato plants. Plant Growth Regul. 2000, 30, 157–161. [Google Scholar] [CrossRef]
- Kadioglu, A.; Saruhan, N.; Saglam, A.; Terzi, R.; Acet, T. Exogenous salicylic acid alleviates effects of long term drought stress and delays leaf rolling by inducing antioxidant system. Plant Growth Regul. 2011, 64, 27–37. [Google Scholar] [CrossRef]
- Saruhan, N.; Saglam, A.; Kadioglu, A. Salicylic acid pretreatment induces drought tolerance and delays leaf rolling by inducing antioxidant systems in maise genotypes. Acta Physiol. Plant. 2011, 34, 97–106. [Google Scholar] [CrossRef]
- Biareh, V.; Shekari, F.; Sayfzadeh, S.; Zakerin, H.; Hadidi, E.; Beltrão, J.G.T.; Mastinu, A. Physiological and qualitative response of Cucurbita pepo L. to salicylic acid under controlled water stress conditions. Horticulturae 2022, 8, 79. [Google Scholar] [CrossRef]
- Gebaly, S.G.; Ahmed, F.M.; Namich, A.A. Effect of spraying some organic, amino acids and potassium citrate on alleviation of drought stress in cotton plant. J. Plant Prod. 2013, 4, 1369–1381. [Google Scholar] [CrossRef]
- Levi, A.; Paterson, A.H.; Cakmak, I.; Saranga, Y. Metabolite and mineral analyses of cotton near-isogenic lines introgressed with QTLs for productivity and drought-related traits. Physiol. Plant. 2011, 141, 265–275. [Google Scholar] [CrossRef]
- Miyazawa, K. Drought stress alleviation of cabbage seedlings by citric acid application. Acta Hortic. 2016, 1112, 101–108. [Google Scholar] [CrossRef]
- Ababaf, M.; Omidi, H.; Bakhshandeh, A. Changes in antioxidant enzymes activities and alkaloid amount of Catharanthus roseus in response to plant growth regulators under drought condition. Ind. Crops Prod. 2021, 167, 113505. [Google Scholar] [CrossRef]
- Aftab, T.; Khan, M.M.A.; Da Silva, J.A.T.; Idrees, M.; Naeem, M.; Moinuddin. Role of salicylic acid in promoting salt stress tolerance and enhanced artemisinin production in Artemisia annua L. J. Plant Growth Regul. 2011, 30, 425–435. [Google Scholar] [CrossRef]
- Després, C.; Fobert, P.R. In vivo biochemical characterisation of transcription factors regulating plant defense response to disease. Can. J. Plant Pathol. 2006, 28, 3–15. [Google Scholar] [CrossRef]
- Wani, A.B.; Chadar, H.; Wani, A.H.; Singh, S.; Upadhyay, N. Salicylic acid to decrease plant stress. Environ. Chem. Lett. 2017, 15, 101–123. [Google Scholar] [CrossRef]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef]
- Farooq, A.; Bukhari, S.A.; Akram, N.A.; Ashraf, M.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Exogenously applied ascorbic acid-mediated changes in osmoprotection and oxidative defense system enhanced water stress tolerance in different cultivars of safflower (Carthamus tinctorious L.). Plants 2020, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K.; et al. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef]
- Osama, S.; El Sherei, M.; Al-Mahdy, D.A.; Bishr, M.; Salama, O. Effect of salicylic acid foliar spraying on growth parameters, γ-pyrones, phenolic content and radical scavenging activity of drought stressed Ammi visnaga L. plant. Ind. Crops Prod. 2019, 134, 1–10. [Google Scholar] [CrossRef]
- Shafiq, S.; Akram, N.A.; Ashraf, M.; Arshad, A. Synergistic effects of drought and ascorbic acid on growth, mineral nutrients and oxidative defense system in canola (Brassica napus L.) plants. Acta Physiol. Plant. 2014, 36, 1539–1553. [Google Scholar] [CrossRef]
- Smirnoff, N. Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free Radic. Biol. Med. 2018, 122, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, A.F.G.; da Silva Gomes, J.W.; Avilez, A.A.; Sarria, S.D.; Broetto, F.; Vieites, R.L.; de Souza Guimarães, M.L.C. Foliar salicylic acid application to mitigate the effect of water deficiency on potato (Solanum tuberosum L.). Plant Stress 2023, 7, 100135. [Google Scholar] [CrossRef]
- Li, J.; Cang, Z.; Jiao, F.; Bai, X.; Zhang, D.; Zhai, R. Influence of drought stress on photosynthetic characteristics and protective enzymes of potato at seedling stage. J. Saudi Soc. Agric. Sci. 2017, 16, 82–88. [Google Scholar] [CrossRef]
- Tajik, S.; Zarinkamar, F.; Soltani, B.M.; Nazari, M. Induction of phenolic and flavonoid compounds in leaves of saffron (Crocus sativus L.) by salicylic acid. Sci. Hortic. 2019, 257, 108751. [Google Scholar] [CrossRef]
- Khalil, N.; Fekry, M.; Bishr, M.; El-Zalabani, S.; Salama, O. Foliar spraying of salicylic acid induced accumulation of phenolics, increased radical scavenging activity and modified the composition of the essential oil of water stressed Thymus vulgaris L. Plant Physiol. Biochem. 2018, 123, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Sharma, S.; Samir, P. Research, Reactive oxygen species, oxidative stress and ROS scavenging system in plants. J. Chem. Pharm. Res. 2016, 8, 595–604. [Google Scholar]
- Rana, V.; Ram, S.; Nehra, K. Review proline biosynthesis and its role in abiotic stress. Int. J. Agric. Innov. Res. 2017, 6, 2319–2473. [Google Scholar]
- Cappellari, L.D.R.; Chiappero, J.; Palermo, T.B.; Giordano, W.; Banchio, E. Volatile organic compounds from rhizobacteria increase the biosynthesis of secondary metabolites and improve the antioxidant status in Mentha piperita L. grown under salt stress. Agronomy 2020, 10, 1094. [Google Scholar] [CrossRef]
- Palmer, I.A.; Chen, H.; Chen, J.; Chang, M.; Li, M.; Liu, F.; Fu, Z.Q. Novel salicylic acid analogs induce a potent defense response in Arabidopsis. Int. J. Mol. Sci. 2019, 20, 3356. [Google Scholar] [CrossRef]
- Pál, M.; Janda, T.; Majláth, I.; Szalai, G. Involvement of salicylic acid and other phenolic compounds in light-dependent cold acclimation in maise. Int. J. Mol. Sci. 2020, 21, 1942. [Google Scholar] [CrossRef]
- Ali, B. Salicylic acid: An efficient elicitor of secondary metabolite production in plants. Biocatal. Agric. Biotechnol. 2020, 31, 101884. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Amirjani, M.R.; Mahdiyeh, M. Antioxidative and biochemical responses of wheat to drought stress. J. Agric. Biol. Sci. 2013, 8, 291–301. [Google Scholar]
- Habib, N.; Ali, Q.; Ali, S.; Javed, M.T.; Haider, M.Z.; Perveen, R.; Shahid, M.R.; Rizwan, M.; Abdel-Daim, M.M.; Elkelish, A.; et al. Use of nitric oxide and hydrogen peroxide for better yield of wheat (Triticum aestivum L.) under water deficit Conditions: Growth, Osmoregulation, and Antioxidative Defense Mechanism. Plants 2020, 9, 285. [Google Scholar] [CrossRef] [PubMed]
- Naz, H.; Akram, N.A.; Ashraf, M. Impact of ascorbic acid on growth and some physiological attributes of cucumber (Cucumis sativus) plants under water-deficit conditions. Pak. J. Bot. 2016, 48, 877–883. [Google Scholar]
- Askari, E.; Ehsanzadeh, P. Drought stress mitigation by foliar application of salicylic acid and their interactive effects on physiological characteristics of fennel (Foeniculum vulgare Mill.) genotypes. Acta Physiol. Plant. 2015, 37, 4. [Google Scholar] [CrossRef]
- Razmi, N.; Ebadi, A.; Daneshian, J.; Jahanbakhsh, S. Salicylic acid induced changes on antioxidant capacity, pigments and grain yield of soybean genotypes in water deficit condition. J. Plant Interact. 2017, 12, 457–464. [Google Scholar] [CrossRef]
- Khapte, P.S.; Kumar, P.; Burman, U.; Kumar, P. Deficit irrigation in tomato: Agronomical and physio-biochemical implications. Sci. Hortic. 2019, 248, 256–264. [Google Scholar] [CrossRef]
- Li, Q.; Wang, G.; Wang, Y.; Yang, D.; Guan, C.; Ji, J. Foliar application of salicylic acid alleviate the cadmium toxicity by modulation the reactive oxygen species in potato. Ecotoxicol. Environ. Saf. 2019, 172, 317–325. [Google Scholar] [CrossRef]
- Lobato, A.K.D.S.; Barbosa, M.A.M.; Alsahli, A.A.; Lima, E.J.A.; Silva, B.R.S.D. Exogenous salicylic acid alleviates the negative impacts on production components, biomass and gas exchange in tomato plants under water deficit improving redox status and anatomical responses. Physiol. Plant. 2021, 172, 869–884. [Google Scholar] [CrossRef] [PubMed]
| PEG Level | Foliar Spray | ||||
|---|---|---|---|---|---|
| Non Treated | H2O | SA | MA | CA | |
| Plant Yielding (g·Plant−1) | |||||
| Control | 17.45 ± 1.77 a | 16.32 ± 3.02 a | 18.09 ± 2.36 a | 19.46 ± 0.74 a | 17.24 ± 1.61 a |
| PEG 5% | 12.67 ± 3.81 a | 10.95 ± 4.11 a | 12.05 ± 5.57 a | 10.67 ± 0.13 a | 12 ± 2.44 a |
| PEG 7.5% | 11.31 ± 0.56 a | 10.65 ± 0.7 a | 11.76 ± 1.96 a | 12.8 ± 1.32 a | 11.78 ± 0.63 a |
| Dry matter plant yielding (g·plant−1) | |||||
| Control | 1.25 ± 0.13 ab | 1 ± 0.01 ab | 1.15 ± 0.14 ab | 1.29 ± 0.06 b | 1.08 ± 0.09 ab |
| PEG 5% | 0.92 ± 0.28 ab | 0.81 ± 0.23 ab | 0.86 ± 0.11 ab | 0.96 ± 0.06 ab | 0.87 ± 0.17 ab |
| PEG 7.5% | 0.89 ± 0.04 ab | 0.75 ± 0.05 a | 1.06 ± 0.08 ab | 1.01 ± 0.12 ab | 0.8 ± 0.04 ab |
| PEG Level | Foliar Spray | ||||
|---|---|---|---|---|---|
| Non Treated | H2O | SA | MA | CA | |
| N | |||||
| Control | 0.039 ± 0.01 b | 0.034 ± 0.01 ab | 0.031 ± 0.01 ab | 0.033 ± 0.01 ab | 0.031 ± 0.01 ab |
| PEG 5% | 0.018 ± 0.0 a | 0.016 ± 0.0 a | 0.018 ± 0.0 a | 0.021 ± 0.0 ab | 0.016 ± 0.0 a |
| PEG 7.5% | 0.016 ± 0.0 a | 0.015 ± 0.0 a | 0.019 ± 0.0 ab | 0.02 ± 0.0 ab | 0.015 ± 0.0 ab |
| P | |||||
| Control | 0.004 ± 0.001 a | 0.003 ± 0.001 a | 0.003 ± 0.001 a | 0.004 ± 0.001 a | 0.003 ± 0.001 a |
| PEG 5% | 0.002 ± 0.0 a | 0.002 ± 0.0 a | 0.003 ± 0.001 a | 0.003 ± 0.0 a | 0.002 ± 0.0 a |
| PEG 7.5% | 0.002 ± 0.0 a | 0.002 ± 0.001 a | 0.003 ± 0.001 a | 0.003 ± 0.001 a | 0.002 ± 0.001 a |
| K | |||||
| Control | 0.072 ± 0.006 c | 0.057 ± 0.013 ab | 0.059 ± 0.018 ab | 0.069 ± 0.016 c | 0.058 ± 0.011 ab |
| PEG 5% | 0.039 ± 0.009 ab | 0.04 ± 0.001 ab | 0.044 ± 0.004 ab | 0.051 ± 0.002 ab | 0.04 ± 0.004 ab |
| PEG 7.5% | 0.041 ± 0.003 ab | 0.034 ± 0.006 a | 0.047 ± 0.006 ab | 0.047 ± 0.003 ab | 0.032 ± 0.007 a |
| Ca | |||||
| Control | 0.013 ± 0.001 b | 0.011 ± 0.001 ab | 0.01 ± 0.003 ab | 0.013 ± 0.002 b | 0.01 ± 0.001 ab |
| PEG 5% | 0.008 ± 0.002 ab | 0.007 ± 0.001 a | 0.008 ± 0.0 ab | 0.008 ± 0.001 ab | 0.006 ± 0.001 a |
| PEG 7.5% | 0.007 ± 0.001 a | 0.006 ± 0.001 a | 0.007 ± 0.001 a | 0.007 ± 0.0 ab | 0.006 ± 0.0 a |
| Mg | |||||
| Control | 0.005 ± 0.0 c | 0.004 ± 0.0 bc | 0.004 ± 0.001 bc | 0.005 ± 0.0 c | 0.004 ± 0.0 bc |
| PEG 5% | 0.003 ± 0.0 ab | 0.002 ± 0.0 a | 0.003 ± 0.0 ab | 0.003 ± 0.0 ab | 0.002 ± 0.0 a |
| PEG 7.5% | 0.002 ± 0.0 a | 0.002 ± 0.0 a | 0.002 ± 0.0 a | 0.002 ± 0.0 a | 0.002 ± 0.0 a |
| Na | |||||
| Control | 0.004 ± 0.0 b | 0.003 ± 0.0 ab | 0.003 ± 0.001 ab | 0.004 ± 0.0 ab | 0.003 ± 0.0 ab |
| PEG 5% | 0.003 ± 0.0 ab | 0.002 ± 0.0 a | 0.002 ± 0.0 ab | 0.003 ± 0.0 ab | 0.002 ± 0.0 a |
| PEG 7.5% | 0.002 ± 0.0 a | 0.002 ± 0.0 a | 0.003 ± 0.0 ab | 0.002 ± 0.0 ab | 0.002 ± 0.0 ab |
| PEG Level | Foliar Spray | ||||
|---|---|---|---|---|---|
| Non Treated | H2O | SA | MA | CA | |
| F0 | |||||
| Control | 7780 ± 309 a–c | 8300 ± 710 a–c | 7965 ± 253 a–c | 7813 ± 246 a–c | 8713 ± 196 c |
| PEG 5% | 8523 ± 862 bc | 8181 ± 508 a–c | 8496 ± 375 bc | 7943 ± 131 a–c | 7119 ± 607 ab |
| PEG 7.5% | 7061 ± 362 ab | 6935 ± 854 a | 7911 ± 94 a–c | 7976 ± 350 a–c | 8214 ± 835 a–c |
| Fv | |||||
| Control | 46,978 ± 1462 b | 47,249 ± 1326 b | 45,049 ± 987 ab | 45,418 ± 1577 ab | 48,766 ± 927 b |
| PEG 5% | 45,917 ± 3213 ab | 46,632 ± 2094 ab | 47,683 ± 2668 b | 45,797 ± 800 ab | 41,809 ± 3076 ab |
| PEG 7.5% | 39,681 ± 1983 a | 41,636 ± 3567 ab | 43,651 ± 558 ab | 44,106 ± 1548 ab | 46,642 ± 5167 ab |
| FM | |||||
| Control | 54,759 ± 1531 a–c | 55,550 ± 1766 bc | 53,014 ± 754 a–c | 53,232 ± 1593 a–c | 57,479 ± 735 c |
| PEG 5% | 54,445 ± 4019 a–c | 54,813 ± 2566 a–c | 56,179 ± 3030 bc | 53,740 ± 847 a–c | 48,929 ± 3659 ab |
| PEG 7.5% | 46,743 ± 2169 a | 48,572 ± 4377 ab | 51,563 ± 635 a–c | 52,082 ± 1898 a–c | 54,857 ± 5997 a–c |
| Fv/F0 | |||||
| Control | 6.04 ± 0.16 a | 5.72 ± 0.42 a | 5.66 ± 0.29 a | 5.82 ± 0.27 a | 5.6 ± 0.23 a |
| PEG 5% | 5.4 ± 0.24 a | 5.7 ± 0.15 a | 5.61 ± 0.1 a | 5.77 ± 0.12 a | 5.88 ± 0.17 a |
| PEG 7.5% | 5.62 ± 0.29 a | 6.02 ± 0.32 a | 5.52 ± 0.04 a | 5.53 ± 0.05 a | 5.67 ± 0.09 a |
| Fv/FM | |||||
| Control | 0.86 ± 0.0 a | 0.85 ± 0.01 a | 0.85 ± 0.01 a | 0.85 ± 0.01 a | 0.85 ± 0.01 a |
| PEG 5% | 0.84 ± 0.01 a | 0.85 ± 0.0 a | 0.85 ± 0.0 a | 0.85 ± 0.0 a | 0.85 ± 0.0 a |
| PEG 7.5% | 0.85 ± 0.01 a | 0.86 ± 0.01 a | 0.85 ± 0.0 a | 0.85 ± 0.0 a | 0.85 ± 0.0 a |
| PiABS | |||||
| Control | 5.11 ± 0.93 a–c | 4.44 ± 1.57 a–c | 4.41 ± 0.47 a–c | 4.88 ± 0.51 a–c | 3.77 ± 0.68 ab |
| PEG 5% | 3.74 ± 0.67 ab | 4.69 ± 0.56 a–c | 3.83 ± 0.42 ab | 4.26 ± 0.38 ab | 6.36 ± 0.85 c |
| PEG 7.5% | 5.73 ± 0.22 bc | 4.54 ± 0.24 a–c | 3.88 ± 0.39 ab | 3.58 ± 0.22 a | 3.98 ± 0.6 ab |
| ABS/RC | |||||
| Control | 1.91 ± 0.09 ab | 2.1 ± 0.19 b | 2.05 ± 0.24 ab | 1.97 ± 0.13 ab | 2.24 ± 0.06 b |
| PEG 5% | 2.19 ± 0.21 b | 1.99 ± 0.19 ab | 2.12 ± 0.13 b | 1.99 ± 0.13 ab | 1.66 ± 0.18 a |
| PEG 7.5% | 1.82 ± 0.03 ab | 1.95 ± 0.13 ab | 2.1 ± 0.11 b | 2.09 ± 0.01 ab | 2.06 ± 0.14 ab |
| TR0/RC | |||||
| Control | 1.64 ± 0.07 ab | 1.79 ± 0.14 bc | 1.74 ± 0.19 a–c | 1.68 ± 0.1 a–c | 1.9 ± 0.04 c |
| PEG 5% | 1.85 ± 0.17 bc | 1.7 ± 0.16 a–c | 1.79 ± 0.1 bc | 1.7 ± 0.11 a–c | 1.42 ± 0.15 a |
| PEG 7.5% | 1.55 ± 0.04 ab | 1.66 ± 0.11 a–c | 1.78 ± 0.09 bc | 1.77 ± 0.01 bc | 1.75 ± 0.12 a–c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleiber, T.; Chadzinikolau, T.; Formela-Luboińska, M.; Lartey, J.L.; Kosiada, T. Enhancing Lettuce Drought Tolerance: The Role of Organic Acids in Photosynthesis and Oxidative Defense. Appl. Sci. 2024, 14, 5119. https://doi.org/10.3390/app14125119
Kleiber T, Chadzinikolau T, Formela-Luboińska M, Lartey JL, Kosiada T. Enhancing Lettuce Drought Tolerance: The Role of Organic Acids in Photosynthesis and Oxidative Defense. Applied Sciences. 2024; 14(12):5119. https://doi.org/10.3390/app14125119
Chicago/Turabian StyleKleiber, Tomasz, Tamara Chadzinikolau, Magda Formela-Luboińska, Jeffrey Larte Lartey, and Tomasz Kosiada. 2024. "Enhancing Lettuce Drought Tolerance: The Role of Organic Acids in Photosynthesis and Oxidative Defense" Applied Sciences 14, no. 12: 5119. https://doi.org/10.3390/app14125119







