The Interaction of Cytokines in Orthodontics: A Systematic Review
Abstract
:1. Introduction
- (1)
- (2)
- Immunoregulatory cytokines: IL2, IL3, IL4, IL5, IL7, IL10, IL12. These ILs, in fact, control the immune system response by stimulating differentiation into natural killer cells and B lymphocytes for antibody production [11];
- (3)
- Effector cytokines: interferons, chemokines, and stimulating factors (CSFs) that modulate defense processes toward infectious agents and neoplastic processes [9].
2. Materials and Methods
2.1. Protocol and Registration
2.2. Search Processing
2.3. Inclusion Criteria
- Criteria: Application in the present study;
- Population: Human subjects;
- Intervention: Fixed OT;
- Comparison: Groups with differing fixed OT;
- Outcome: Evaluation of cytokines levels variations before during and after fixed OT;
- Study design: Clinical trials.
2.4. Exclusion Criteria
2.5. Data Processing
2.6. Article Identification Procedure
2.7. Study Evaluation
2.8. Quality Assessment
3. Results
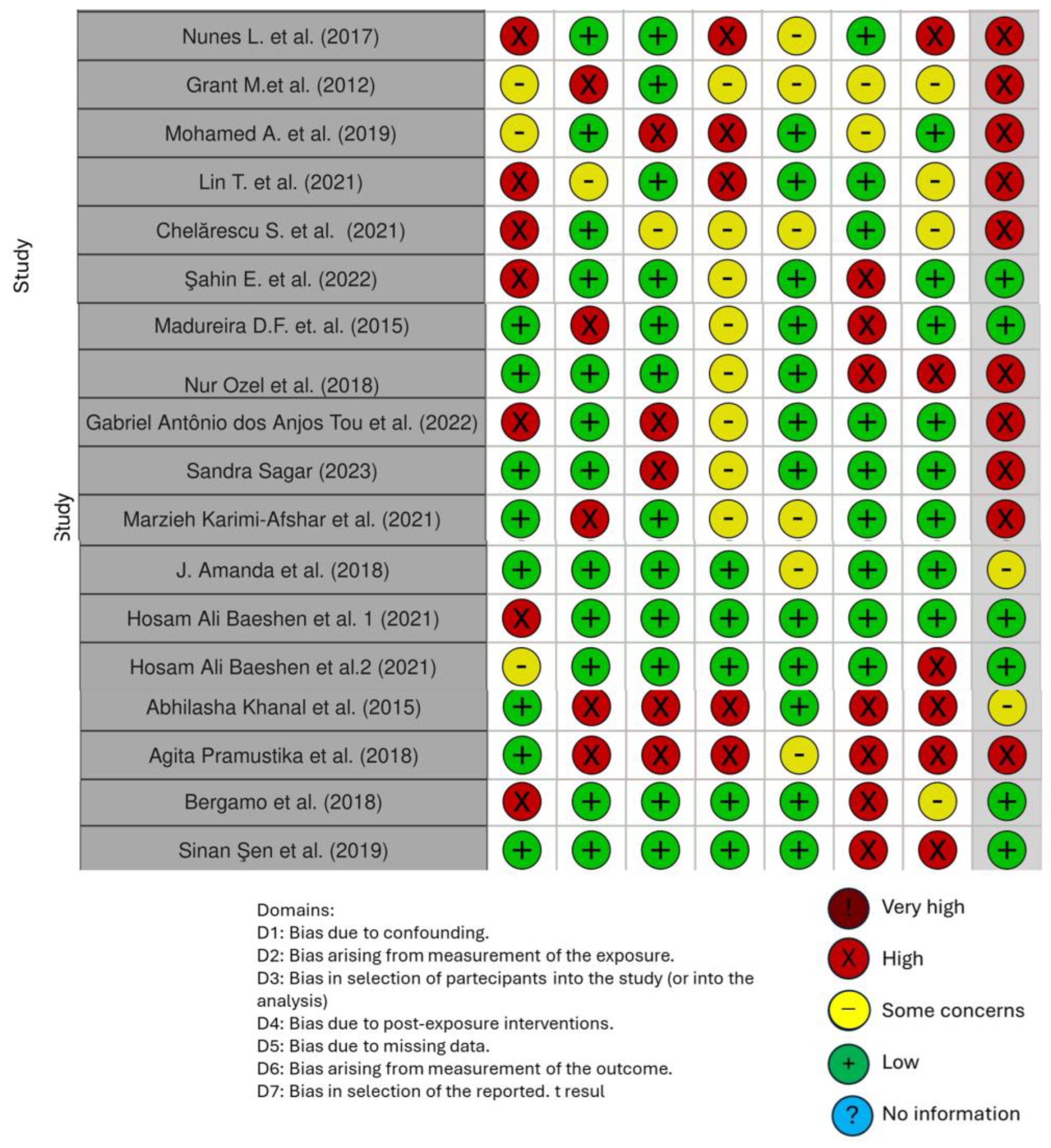
Quality Assessment and Risk of Bias of Included Articles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGEs | advanced glycation end products |
| ATP | adenosine triphosphate |
| BMI | body mass index |
| cAMP | cyclic adenosine monophosphate |
| CD326 | marker for epithelial cells |
| CD45+ | marker for leukocytes |
| DMT2 | type 2 diabetes mellitus |
| ELISA | enzyme-linked immuno-sorbent assay |
| ESP | elastomeric separator placement |
| GCF | gingival crevicular fluid |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| GS | glucosamine sulfate |
| CXCL8 | motif chemokine ligand 8 |
| CXCL9 | motif chemokine ligand 9 |
| HA | hyaluronic acid |
| IL | interleukin |
| LLLT | low-level laser therapy |
| MIs | mini-implants |
| MMPs | matrix metalloproteinases |
| NiTi | nickel titanium |
| NO | nitric oxide |
| OSI | oxidative stress index |
| OT | orthodontic treatment |
| OTM | orthodontic tooth movement |
| OA | osteoarthritis |
| OPG | osteopotegrin |
| OPN | osteopontin |
| OSI | oxidative stress index |
| Passive SL | passive self-ligating |
| PDL | periodontal ligament |
| PDT | photodynamic therapy |
| PEA | preadjusted edgewise appliance |
| PMICF | peri-miniscrew implant crevicular fluid |
| RME | rapid maxillary expansion |
| RANKL | receptor activator of nuclear factor κ-B ligand |
| SNPs | single-nucleotide polymorphism |
| SP | substance P |
| TAC | total antioxidant capacity |
| TGF | transforming growth factor |
| TGK- β1 | transforming growth factor-beta one |
| TIMPs | tissue inhibitors of MMPs |
| TMJ | temporomandibular joint |
| TNF | tumor necrosis factor |
| TOS | total oxidant state |
| VAS | visual analog scale |
| WSL | white spot lesions |
References
- Cytokine Storm and Sepsis Disease Pathogenesis. Available online: https://pubmed.ncbi.nlm.nih.gov/28555385/ (accessed on 28 January 2024).
- Kennedy, R.H.; Silver, R. Neuroimmune Signaling: Cytokines and the Central Nervous System. In Neuroscience in the 21st Century; Pfaff, D.W., Volkow, N.D., Rubenstein, J.L., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 883–922. [Google Scholar] [CrossRef]
- Striz, I.; Brabcova, E.; Kolesar, L.; Sekerkova, A. Cytokine Networking of Innate Immunity Cells: A Potential Target of Therapy. Clin. Sci. 2014, 126, 593–612. [Google Scholar] [CrossRef]
- Tamayo, E.; Fernández, A.; Almansa, R.; Carrasco, E.; Heredia, M.; Lajo, C.; Goncalves, L.; Gómez-Herreras, J.I.; de Lejarazu, R.O.; Bermejo-Martin, J.F. Pro- and Anti-Inflammatory Responses Are Regulated Simultaneously from the First Moments of Septic Shock. Eur. Cytokine Netw. 2011, 22, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G. Training Innate Immunity: The Changing Concept of Immunological Memory in Innate Host Defence. Eur. J. Clin. Investig. 2013, 43, 881–884. [Google Scholar] [CrossRef]
- Silk, A.W.; Margolin, K. Cytokine Therapy. Hematol. Oncol. Clin. N. Am. 2019, 33, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Huang, T.; Li, S.; Ma, A.; Pérez, J.L.; Sander, C.; Keskin, D.B.; Wu, C.J.; Fraenkel, E.; Hacohen, N. Dictionary of Immune Responses to Cytokines at Single-Cell Resolution. Nature 2024, 625, 377–384. [Google Scholar] [CrossRef]
- Jarczak, D.; Nierhaus, A. Cytokine Storm-Definition, Causes, and Implications. Int. J. Mol. Sci. 2022, 23, 11740. [Google Scholar] [CrossRef] [PubMed]
- Structural Biology of Shared Cytokine Receptors. Available online: https://pubmed.ncbi.nlm.nih.gov/18817510/ (accessed on 28 January 2024).
- Thomson, A.W.; Lotze, M.T.; Thomson, A.W.; Lotze, M.T. The Cytokine Handbook, Two-Volume Set, 4th ed.; Academic Press: Cambridge, MA, USA, 2003; ISBN 978-0-08-051879-4. [Google Scholar]
- Balzanelli, M.G.; Distratis, P.; Aityan, S.K.; Amatulli, F.; Catucci, O.; Cefalo, A.; De Michele, A.; Dipalma, G.; Inchingolo, F.; Lazzaro, R.; et al. An Alternative “Trojan Horse” Hypothesis for COVID-19: Immune Deficiency of IL-10 and SARS-CoV-2 Biology. Endocr. Metab. Immune Disord. Drug Targets 2022, 22, 1–5. [Google Scholar] [CrossRef]
- Liao, W.; Lin, J.-X.; Leonard, W.J. Interleukin-2 at the Crossroads of Effector Responses, Tolerance, and Immunotherapy. Immunity 2013, 38, 13–25. [Google Scholar] [CrossRef]
- Ergoren, M.C.; Paolacci, S.; Manara, E.; Dautaj, A.; Dhuli, K.; Anpilogov, K.; Camilleri, G.; Suer, H.K.; Sayan, M.; Tuncel, G.; et al. A Pilot Study on the Preventative Potential of Alpha-Cyclodextrin and Hydroxytyrosol against SARS-CoV-2 Transmission. Acta Bio Medica Atenei Parm. 2020, 91, e2020022. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Dipalma, G.; Inchingolo, A.M.; Malcangi, G.; Santacroce, L.; D’Oria, M.T.; Isacco, C.G.; Bordea, I.R.; Candrea, S.; Scarano, A.; et al. The 15-Months Clinical Experience of SARS-CoV-2: A Literature Review of Therapies and Adjuvants. Antioxidants 2021, 10, 881. [Google Scholar] [CrossRef]
- Ballini, A.; Cantore, S.; Farronato, D.; Cirulli, N.; Inchingolo, F.; Papa, F.; Malcangi, G.; Inchingolo, A.D.; Dipalma, G.; Sardaro, N.; et al. Periodontal Disease and Bone Pathogenesis: The Crosstalk between Cytokines and Porphyromonas Gingivalis. J. Biol. Regul. Homeost. Agents 2015, 29, 273–281. [Google Scholar]
- Ballini, A.; Gnoni, A.; Vito, D.D.; Dipalma, G.; Cantore, S.; Isacco, C.G.; Saini, R.; Santacroce, L.; Topi, S.; Scarano, A.; et al. Effect of Probiotics on the Occurrence of Nutrition Absorption Capacities in Healthy Children: A Randomized Double-Blinded Placebo-Controlled Pilot Study. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8645–8657. [Google Scholar]
- Inchingolo, A.D.; Carpentiere, V.; Piras, F.; Netti, A.; Ferrara, I.; Campanelli, M.; Latini, G.; Viapiano, F.; Costa, S.; Malcangi, G.; et al. Orthodontic Surgical Treatment of Impacted Mandibular Canines: Systematic Review and Case Report. Appl. Sci. 2022, 12, 8008. [Google Scholar] [CrossRef]
- Grant, M.; Wilson, J.; Rock, P.; Chapple, I. Induction of Cytokines, MMP9, TIMPs, RANKL and OPG during Orthodontic Tooth Movement. Eur. J. Orthod. 2013, 35, 644–651. [Google Scholar] [CrossRef]
- Madureira, D.F.; da Silva, J.M.; Teixeira, A.L.; Abreu, M.H.N.G.; Pretti, H.; Lages, E.M.B.; da Silva, T.A. Cytokine Measurements in Gingival Crevicular Fluid and Periodontal Ligament: Are They Correlated? Am. J. Orthod. Dentofac. Orthop. 2015, 148, 293–301. [Google Scholar] [CrossRef]
- Vidmar Šimic, M.; Maver, A.; Zimani, A.N.; Hočevar, K.; Peterlin, B.; Kovanda, A.; Premru-Sršen, T. Oral Microbiome and Preterm Birth. Front. Med. 2023, 10, 1177990. [Google Scholar] [CrossRef]
- Inchingolo, F.; Inchingolo, A.D.; Palumbo, I.; Trilli, I.; Guglielmo, M.; Mancini, A.; Palermo, A.; Inchingolo, A.M.; Dipalma, G. The Impact of Cesarean Section Delivery on Intestinal Microbiota: Mechanisms, Consequences, and Perspectives-A Systematic Review. Int. J. Mol. Sci. 2024, 25, 1055. [Google Scholar] [CrossRef]
- Inchingolo, F.; Martelli, F.S.; Gargiulo Isacco, C.; Borsani, E.; Cantore, S.; Corcioli, F.; Boddi, A.; Nguyễn, K.C.D.; De Vito, D.; Aityan, S.K.; et al. Chronic Periodontitis and Immunity, Towards the Implementation of a Personalized Medicine: A Translational Research on Gene Single Nucleotide Polymorphisms (SNPs) Linked to Chronic Oral Dysbiosis in 96 Caucasian Patients. Biomedicines 2020, 8, 115. [Google Scholar] [CrossRef]
- Di Francesco, F.; Lanza, A.; Di Blasio, M.; Vaienti, B.; Cafferata, E.A.; Cervino, G.; Cicciù, M.; Minervini, G. Application of Botulinum Toxin in Temporomandibular Disorders: A Systematic Review of Randomized Controlled Trials (RCTs). Appl. Sci. 2022, 12, 12409. [Google Scholar] [CrossRef]
- Crescente, G.; Minervini, G.; Spagnuolo, C.; Moccia, S. Cannabis Bioactive Compound-Based Formulations: New Perspectives for the Management of Orofacial Pain. Molecules 2022, 28, 106. [Google Scholar] [CrossRef]
- Pramstraller, M.; Simonelli, A.; Farina, R.; Trombelli, L. Biologically-Oriented Alveolar Ridge Preservation. Minerva Dent. Oral Sci. 2023, 72, 176–184. [Google Scholar] [CrossRef]
- Hexiang, Z.; Ziyan, C.; Jing, W.; Zhenlin, G. Biomechanical Characteristics of Orthodontic Tooth Movement before and after Increasing Alveolar Bone Mass with Periodontally Accelerated Osteogenic Orthodontics. Chin. J. Tissue Eng. Res. 2024, 28, 2133–2139. [Google Scholar] [CrossRef]
- Ramadanov, N.; Marinova-Kichikova, P.; Hable, R.; Dimitrov, D.; Becker, R. Comparison of Postoperative Serum Biomarkers after Total Hip Arthroplasty through Minimally Invasive versus Conventional Approaches: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Prosthesis 2023, 5, 694–710. [Google Scholar] [CrossRef]
- Osteoclast Activating Factor Is Now Interleukin-1 Beta: Historical Perspective and Biological Implications. Available online: https://pubmed.ncbi.nlm.nih.gov/3139850/ (accessed on 28 January 2024).
- Effects of Aging on RANKL and OPG Levels in Gingival Crevicular Fluid during Orthodontic Tooth Movement. Available online: https://pubmed.ncbi.nlm.nih.gov/16918678/ (accessed on 28 January 2024).
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast Differentiation and Activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Lin, T.; Yang, L.; Zheng, W.; Zhang, B. Matrix Metalloproteinases and Th17 Cytokines in the Gingival Crevicular Fluid during Orthodontic Tooth Movement. Eur. J. Paediatr. Dent. 2021, 22, 135–138. [Google Scholar] [CrossRef]
- Cantarella, G.; Cantarella, R.; Caltabiano, M.; Risuglia, N.; Bernardini, R.; Leonardi, R. Levels of Matrix Metalloproteinases 1 and 2 in Human Gingival Crevicular Fluid during Initial Tooth Movement. Am. J. Orthod. Dentofac. Orthop. 2006, 130, 568.e11–568.e16. [Google Scholar] [CrossRef]
- Expression of Genes for Gelatinases and Tissue Inhibitors of Metalloproteinases in Periodontal Tissues during Orthodontic Tooth Movement. Available online: https://pubmed.ncbi.nlm.nih.gov/17043917/ (accessed on 28 January 2024).
- Uematsu, S.; Mogi, M.; Deguchi, T. Interleukin (IL)-1 Beta, IL-6, Tumor Necrosis Factor-Alpha, Epidermal Growth Factor, and Beta 2-Microglobulin Levels Are Elevated in Gingival Crevicular Fluid during Human Orthodontic Tooth Movement. J. Dent. Res. 1996, 75, 562–567. [Google Scholar] [CrossRef]
- Gentile, C.; Gruppioni, E. A Perspective on Prosthetic Hands Control: From the Brain to the Hand. Prosthesis 2023, 5, 1184–1205. [Google Scholar] [CrossRef]
- Toti, Ç.; Kaςani, G.; Meto, A.; Droboniku, E.; Gurakuqi, A.; Tanellari, O.; Hysi, D.; Fiorillo, L. Early Treatment of Class II Division 1 Malocclusions with Prefabricated Myofunctional Appliances: A Case Report. Prosthesis 2023, 5, 1049–1059. [Google Scholar] [CrossRef]
- Sammartino, G.; Gasparro, R.; Marenzi, G.; Trosino, O.; Mariniello, M.; Riccitiello, F. Extraction of Mandibular Third Molars: Proposal of a New Scale of Difficulty. Br. J. Oral Maxillofac. Surg. 2017, 55, 952–957. [Google Scholar] [CrossRef]
- Lombardo, G.; Signoriello, A.; Marincola, M.; Bonfante, E.A.; Díaz-Caballero, A.; Tomizioli, N.; Pardo, A.; Zangani, A. Five-Year Follow-Up of 8 and 6 Mm Locking-Taper Implants Treated with a Reconstructive Surgical Protocol for Peri-Implantitis: A Retrospective Evaluation. Prosthesis 2023, 5, 1322–1342. [Google Scholar] [CrossRef]
- Vale, F.; Nunes, C.; Reis, J.; Travassos, R.; Ribeiro, M.; Marques, F.; Pedroso, A.; Marto, C.M.; Paula, A.B.; Francisco, I. Pharyngeal Obturator Prosthesis Ideal for Orthodontic Appliances: A Case Series. Prosthesis 2023, 5, 1129–1138. [Google Scholar] [CrossRef]
- Romeo, E.; Scaringi, R.; Lops, D.; Palazzolo, A. Single Crown Restorations Supported by One-Piece Zirconia Dental Implants: Case Series with a Mean Follow-Up of 58 Months. Prosthesis 2023, 5, 1060–1074. [Google Scholar] [CrossRef]
- Scandurra, C.; Gasparro, R.; Dolce, P.; Bochicchio, V.; Muzii, B.; Sammartino, G.; Marenzi, G.; Maldonato, N.M. The Role of Cognitive and Non-cognitive Factors in Dental Anxiety: A Mediation Model. Eur. J. Oral Sci. 2021, 129, e12793. [Google Scholar] [CrossRef] [PubMed]
- Soares Bonato, R.C.; Abel Mapengo, M.A.; de Azevedo-Silva, L.J.; Janson, G.; de Carvalho Sales-Peres, S.H. Tooth Movement, Orofacial Pain, and Leptin, Interleukin-1β, and Tumor Necrosis Factor-α Levels in Obese Adolescents. Angle Orthod. 2022, 92, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Cantore, S.; Mirgaldi, R.; Ballini, A.; Coscia, M.F.; Scacco, S.; Papa, F.; Inchingolo, F.; Dipalma, G.; De Vito, D. Cytokine Gene Polymorphisms Associate with Microbiogical Agents in Periodontal Disease: Our Experience. Int. J. Med. Sci. 2014, 11, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Balzanelli, M.G.; Distratis, P.; Lazzaro, R.; Pham, V.H.; Tran, T.C.; Dipalma, G.; Bianco, A.; Serlenga, E.M.; Aityan, S.K.; Pierangeli, V.; et al. Analysis of Gene Single Nucleotide Polymorphisms in COVID-19 Disease Highlighting the Susceptibility and the Severity towards the Infection. Diagnostics 2022, 12, 2824. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, N.; Perrini, P.; Marani, W.; Chaurasia, B.; Corsalini, M.; Scarano, A.; Rapone, B. Multiple Brain Abscesses of Odontogenic Origin. May Oral Microbiota Affect Their Development? A Review of the Current Literature. Appl. Sci. 2021, 11, 3316. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Inchingolo, A.M.; Bordea, I.R.; Malcangi, G.; Xhajanka, E.; Scarano, A.; Lorusso, F.; Farronato, M.; Tartaglia, G.M.; Isacco, C.G.; et al. SARS-CoV-2 Disease through Viral Genomic and Receptor Implications: An Overview of Diagnostic and Immunology Breakthroughs. Microorganisms 2021, 9, 793. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Gargiulo, C.I.; Malcangi, G.; Ciocia, A.M.; Patano, A.; Azzollini, D.; Piras, F.; Barile, G.; Settanni, V.; Mancini, A.; et al. Diagnosis of SARS-CoV-2 during the Pandemic by Multiplex RT-rPCR hCoV Test: Future Perspectives. Pathogens 2022, 11, 1378. [Google Scholar] [CrossRef]
- SARS-CoV-2 Disease Adjuvant Therapies and Supplements Breakthrough for the Infection Prevention. Available online: https://pubmed.ncbi.nlm.nih.gov/33806624/ (accessed on 1 February 2024).
- Marrapodi, M.M.; Mascolo, A.; di Mauro, G.; Mondillo, G.; Pota, E.; Rossi, F. The Safety of Blinatumomab in Pediatric Patients with Acute Lymphoblastic Leukemia: A Systematic Review and Meta-Analysis. Front. Pediatr. 2022, 10, 929122. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, A.; Tortora, C.; Argenziano, M.; Marrapodi, M.M.; Rossi, F. Emerging Roles of the Iron Chelators in Inflammation. Int. J. Mol. Sci. 2022, 23, 7977. [Google Scholar] [CrossRef] [PubMed]
- Crincoli, V.; Ballini, A.; Fatone, L.; Di Bisceglie, M.B.; Nardi, G.M.; Grassi, F.R. Cytokine Genotype Distribution in Patients with Periodontal Disease and Rheumatoid Arthritis or Diabetes Mellitus. J. Biol. Regul. Homeost. Agents 2016, 30, 863–866. [Google Scholar] [PubMed]
- Saloom, H.F.; Papageorgiou, S.N.; Carpenter, G.H.; Cobourne, M.T. The Effect of Obesity on Orofacial Pain during Early Orthodontic Treatment with Fixed Appliances: A Prospective Cohort Study. Eur. J. Orthod. 2018, 40, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Do Prado, W.L.; Lofrano, M.C.; Oyama, L.M.; Dâmaso, A.R. Obesidade e adipocinas inflamatórias: Implicações práticas para a prescrição de exercício. Rev. Bras. Med. Esporte 2009, 15, 378–383. [Google Scholar] [CrossRef]
- Malcangi, G.; Patano, A.; Ciocia, A.M.; Netti, A.; Viapiano, F.; Palumbo, I.; Trilli, I.; Guglielmo, M.; Inchingolo, A.D.; Dipalma, G.; et al. Benefits of Natural Antioxidants on Oral Health. Antioxidants 2023, 12, 1309. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Inchingolo, A.M.; Latini, G.; Ferrante, L.; Trilli, I.; Del Vecchio, G.; Palmieri, G.; Malcangi, G.; Inchingolo, A.D.; Dipalma, G. Oxidative Stress and Natural Products in Orthodontic Treatment: A Systematic Review. Nutrients 2023, 16, 113. [Google Scholar] [CrossRef] [PubMed]
- Profiles of Inflammation Factors and Inflammatory Pathways around the Peri-Miniscrew Implant. Available online: https://pubmed.ncbi.nlm.nih.gov/33834451/ (accessed on 27 January 2024).
- Interleukin-17, RANKL, and Osteoprotegerin Levels in Gingival Crevicular Fluid from Smoking and Non-Smoking Patients with Chronic Periodontitis during Initial Periodontal Treatment. Available online: https://pubmed.ncbi.nlm.nih.gov/19656027/ (accessed on 28 January 2024).
- LOX-1 Is Involved in IL-1β Production and Extracellular Matrix Breakdown in Dental Peri-Implantitis. Available online: https://pubmed.ncbi.nlm.nih.gov/28898769/ (accessed on 28 January 2024).
- Che, C.; Liu, J.; Yang, J.; Ma, L.; Bai, N.; Zhang, Q. Osteopontin Is Essential for IL-1β Production and Apoptosis in Peri-Implantitis. Clin. Implant Dent. Relat. Res. 2018, 20, 384–392. [Google Scholar] [CrossRef]
- Andrucioli, M.C.D.; Matsumoto, M.A.N.; Fukada, S.Y.; Saraiva, M.C.P.; Bergamo, A.Z.N.; Romano, F.L.; da Silva, R.A.B.; da Silva, L.A.B.; Nelson-Filho, P. Quantification of Pro-Inflammatory Cytokines and Osteoclastogenesis Markers in Successful and Failed Orthodontic Mini-Implants. J. Appl. Oral Sci. 2019, 27, e20180476. [Google Scholar] [CrossRef]
- Kaur, A.; Kharbanda, O.P.; Kapoor, P.; Kalyanasundaram, D. A Review of Biomarkers in Peri-Miniscrew Implant Crevicular Fluid (PMICF). Prog. Orthod. 2017, 18, 42. [Google Scholar] [CrossRef]
- Cytokine Profile Changes in Gingival Crevicular Fluid after Placement Different Brackets Types. Available online: https://pubmed.ncbi.nlm.nih.gov/29032048/ (accessed on 27 January 2024).
- Enhos, S.; Veli, I.; Cakmak, O.; Ucar, F.I.; Alkan, A.; Uysal, T. OPG and RANKL Levels around Miniscrew Implants during Orthodontic Tooth Movement. Am. J. Orthod. Dentofac. Orthop. 2013, 144, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, E.; Locarno, S.; Gallo, S.; Orsini, F.; Pini, C.; Farronato, M.; Thomaz, D.V.; Lenardi, C.; Piazzoni, M.; Tartaglia, G. Poloxamer-Based Hydrogel as Drug Delivery System: How Polymeric Excipients Influence the Chemical-Physical Properties. Polymers 2022, 14, 3624. [Google Scholar] [CrossRef] [PubMed]
- Gingival Crevicular Fluid Volume and Periodontal Parameters Alterations after Use of Conventional and Self-Ligating Brackets. Available online: https://pubmed.ncbi.nlm.nih.gov/27607519/ (accessed on 28 January 2024).
- Alterations in Plaque Accumulation and Gingival Inflammation Promoted by Treatment with Self-Ligating and Conventional Orthodontic Brackets. Available online: https://pubmed.ncbi.nlm.nih.gov/25992985/ (accessed on 28 January 2024).
- Gingival Crevicular Fluid Bone Turnover Biomarkers: How Postmenopausal Women Respond to Orthodontic Activation. Available online: https://pubmed.ncbi.nlm.nih.gov/28651765/ (accessed on 27 January 2024).
- Receptor Activator of NF(Kappa)B Ligand/Osteoprotegerin (RANKL/OPG) System and Osteopontin (OPN) Serum Levels in a Population of Apulian Postmenopausal Women. Available online: https://pubmed.ncbi.nlm.nih.gov/17215793/ (accessed on 28 January 2024).
- Khanal, A.; Hu, L.; Chen, L. Comparison of Expression Levels of RANKL and Interleukin-17A in Male and Female Orthodontic Patients with and without Appliances. Int. J. Periodontics Restor. Dent. 2015, 35, e28–e34. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, L.; Santacroce, L.; Dipalma, G.; Haxhirexha, K.; Topi, S.; Cantore, S.; Altini, V.; Pacifici, A.; De Vito, D.; Pettini, F.; et al. Gender Medicine: The Impact of Probiotics on Male Patients. Clin. Ter. 2021, 171, e8–e15. [Google Scholar] [CrossRef] [PubMed]
- Baeshen, H.A. Assessment of Salivary pro Inflammatory Cytokines Profile Level in Patients Treated with Labial and Lingual Fixed Orthodontic Appliances. PLoS ONE 2021, 16, e0249999. [Google Scholar] [CrossRef] [PubMed]
- Baeshen, H.A. Comparative Analysis of Growth Factors and Chemokine Secretions between Conventional Lingual and Labial Fixed Orthodontic Appliances. Niger. J. Clin. Pract. 2021, 24, 1438–1441. [Google Scholar] [CrossRef] [PubMed]
- Ata-Ali, F.; Plasencia, E.; Lanuza-Garcia, A.; Ferrer-Molina, M.; Melo, M.; Ata-Ali, J. Effectiveness of Lingual versus Labial Fixed Appliances in Adults According to the Peer Assessment Rating Index. Am. J. Orthod. Dentofac. Orthop. 2019, 155, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.B.; Singh, G. Cytokine Levels in Gingival Crevicular Fluid Samples of Patients Wearing Clear Aligners. J. Oral Biol. Craniofacial Res. 2020, 10, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, J.; Yang, K. Comparative Study of 660 and 830 Nm Photobiomodulation in Promoting Orthodontic Tooth Movement. Photobiomodul. Photomed. Laser Surg. 2019, 37, 349–355. [Google Scholar] [CrossRef]
- Photobiomodulation Impacts the Levels of Inflammatory Mediators during Orthodontic Tooth Movement? A Systematic Review with Meta-Analysis. Available online: https://pubmed.ncbi.nlm.nih.gov/34599400/ (accessed on 28 January 2024).
- Domínguez Camacho, A.; Montoya Guzmán, D.; Velásquez Cujar, S.A. Effective Wavelength Range in Photobiomodulation for Tooth Movement Acceleration in Orthodontics: A Systematic Review. Photobiomodul. Photomed. Laser Surg. 2020, 38, 581–590. [Google Scholar] [CrossRef]
- Fernandes, M.R.U.; Suzuki, S.S.; Suzuki, H.; Martinez, E.F.; Garcez, A.S. Photobiomodulation Increases Intrusion Tooth Movement and Modulates IL-6, IL-8 and IL-1β Expression during Orthodontically Bone Remodeling. J. Biophotonics 2019, 12, e201800311. [Google Scholar] [CrossRef] [PubMed]
- Cronshaw, M.; Parker, S.; Anagnostaki, E.; Lynch, E. Systematic Review of Orthodontic Treatment Management with Photobiomodulation Therapy. Photobiomodulation Photomed. Laser Surg. 2019, 37, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Current Protocol to Achieve Dental Movement Acceleration and Pain Control with Photo-Biomodulation. Available online: https://pubmed.ncbi.nlm.nih.gov/38229945/ (accessed on 28 January 2024).
- Inchingolo, A.M.; Malcangi, G.; Inchingolo, A.D.; Mancini, A.; Palmieri, G.; Di Pede, C.; Piras, F.; Inchingolo, F.; Dipalma, G.; Patano, A. Potential of Graphene-Functionalized Titanium Surfaces for Dental Implantology: Systematic Review. Coatings 2023, 13, 725. [Google Scholar] [CrossRef]
- Zhang, J.-M.; An, J. Cytokines, Inflammation and Pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Megha, K.B.; Joseph, X.; Akhil, V.; Mohanan, P.V. Cascade of Immune Mechanism and Consequences of Inflammatory Disorders. Phytomedicine 2021, 91, 153712. [Google Scholar] [CrossRef] [PubMed]
- Conlon, K.C.; Miljkovic, M.D.; Waldmann, T.A. Cytokines in the Treatment of Cancer. J. Interferon Cytokine Res. 2019, 39, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Nunes, L.; Quintanilha, L.; Perinetti, G.; Capelli, J. Effect of Orthodontic Force on Expression Levels of Ten Cyto kines in Gingival Crevicular Fluid. Arch. Oral Biol. 2017, 76, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Ozel, N.; Aksoy, A.; Kırzıoglu, F.Y.; Doguc, D.K.; Aksoy, T.A. Evaluation of Interleukin-1β Level and Oxidative Status in Gingival Crevicular Fluid during Rapid Maxillary Expansion. Arch. Oral Biol. 2018, 90, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Tou, G.A.D.A.; Diniz, I.M.A.; Ferreira, M.V.L.; de Mesquita, R.A.; Yamauti, M.; Silva, T.A.; Macari, S. Evaluation of Periodontal Parameters and Gingival Crevicular Fluid Cytokines in Children with Anterior Open Bite Receiving Passive Orthodontic Treatment with a Spur. Korean J. Orthod. 2022, 52, 142–149. [Google Scholar] [CrossRef]
- Correlation of Salivary Cytokine Il-17a and 1,25 Dihydroxycholecalciferol in Patients Undergoing Orthodontic Treatment. Available online: https://www.researchgate.net/publication/371320269_Correlation_of_Salivary_Cytokine_Il-17a_and_125_Dihydroxycholecalciferol_in_Patients_Undergoing_Orthodontic_Treatment (accessed on 26 January 2024).
- Karimi-Afshar, M.; Torabi, M.; Abdollahi, S.; Safarian, M.S.; Farsinejad, A. A Comparative Study on the IL-8 Expression in Gingival Crevicular Fluid during Early Alignment Stage of Orthodontic Treatment in Adults and Adolescents. J. Kerman Univ. Med. Sci. 2021, 28, 367–373. [Google Scholar] [CrossRef]
- Amanda, J.; Widayati, R.; Soedarsono, N.; Purwanegara, M.K. RANKL Concentrations in Early Orthodontic Treatment Using Passive Self-Ligating and Preadjusted Edgewise Appliance Bracket Systems. J. Phys. Conf. Ser. 2018, 1073, 042002. [Google Scholar] [CrossRef]
- Gujar, A.N.; Baeshen, H.A.; Alhazmi, A.; Bhandi, S.; Raj, A.T.; Patil, S.; Birkhed, D. Cytokine Levels in Gingival Crevicular Fluid during Orthodontic Treatment with Aligners Compared to Conventional Labial Fixed Appliances: A 3-Week Clinical Study. Acta Odontol. Scand. 2019, 77, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Comparison of Tumor Necrosis Factor-α Concentrations in Gingival Crevicular Fluid between Self-Ligating and Preadjusted Edgewise Appliances in the Early Leveling Stage of Orthodontic Treatment. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5863418/ (accessed on 26 January 2024).
- Liu, Q.; Guo, T.; Dang, W.; Song, Z.; Wen, Y.; Luo, H.; Wang, A. Correlation between Salivary Cytokine Profiles and White Spot Lesions in Adolescent Patients Receiving Clear Aligner Orthodontic Treatment. BMC Oral Health 2023, 23, 857. [Google Scholar] [CrossRef] [PubMed]
- Cen, X.; Liu, Y.; Wang, S.; Yang, X.; Shi, Z.; Liang, X. Glucosamine Oral Administration as an Adjunct to Hyaluronic Acid Injection in Treating Temporomandibular Joint Osteoarthritis. Oral Dis. 2018, 24, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Şen, S.; Orhan, G.; Zingler, S.; Katsikogianni, E.; Lux, C.J.; Erber, R. Comparison of Crevicular Fluid Cytokine Levels after the Application of Surface Sealants: A Randomized Trial. J. Orofac. Orthop. Fortschritte Kieferorthopadie 2019, 80, 242–253. [Google Scholar] [CrossRef]
- Teixeira, C.C.; Khoo, E.; Tran, J.; Chartres, I.; Liu, Y.; Thant, L.M.; Khabensky, I.; Gart, L.P.; Cisneros, G.; Alikhani, M. Cytokine Expression and Accelerated Tooth Movement. J. Dent. Res. 2010, 89, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.; Saidath, K.; Mohindroo, A.; Shetty, A.; Shetty, V.; Rao, S.; Shetty, P. Assessment and Measurement of Interleukin 6 in Periodontal Ligament Tissues during Orthodontic Tooth Movement. World J. Dent. 2019, 10, 88–92. [Google Scholar] [CrossRef]
- Chelărescu, S.; Șurlin, P.; Decusară, M.; Oprică, M.; Bud, E.; Teodorescu, E.; Elsaafin, M.N.; Păcurar, M. Evaluation of IL1β and IL6 Gingival Crevicular Fluid Levels during the Early Phase of Orthodontic Tooth Movement in Adolescents and Young Adults. Appl. Sci. 2021, 11, 521. [Google Scholar] [CrossRef]
- Şahin, E.; Cetinkaya, B.O.; Avci, B.; Kurt-Bayrakdar, S.; Elekdag-Turk, S. Human Randomized Controlled Trail Clinical and Biochemical Evaluation Oral Irrigator Effectiveness in Patients Under Orthodontic Treatment. preprint, 2022. [Google Scholar] [CrossRef]
- Santos, L.L.D.; Conti, A.C.C.F.; Fernandes, T.M.F.; Garlet, G.P.; Almeida, M.R.; Oltramari, P.V.P. Influence of Anxiety and Catastrophizing on Pain Perception in Orthodontic Treatment and Its Association with Inflammatory Cytokines. Braz. Oral Res. 2023, 37, e010. [Google Scholar] [CrossRef]
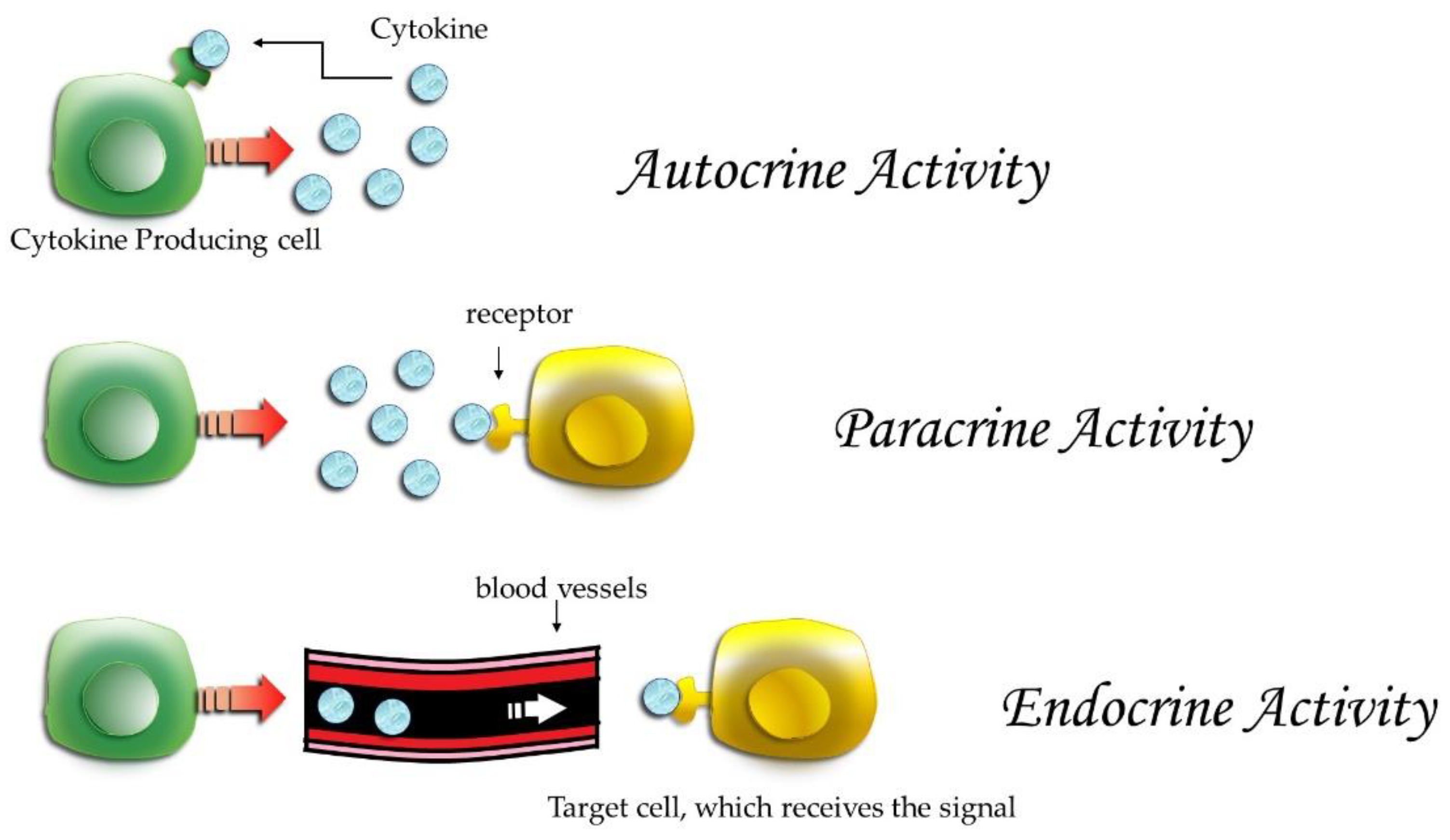
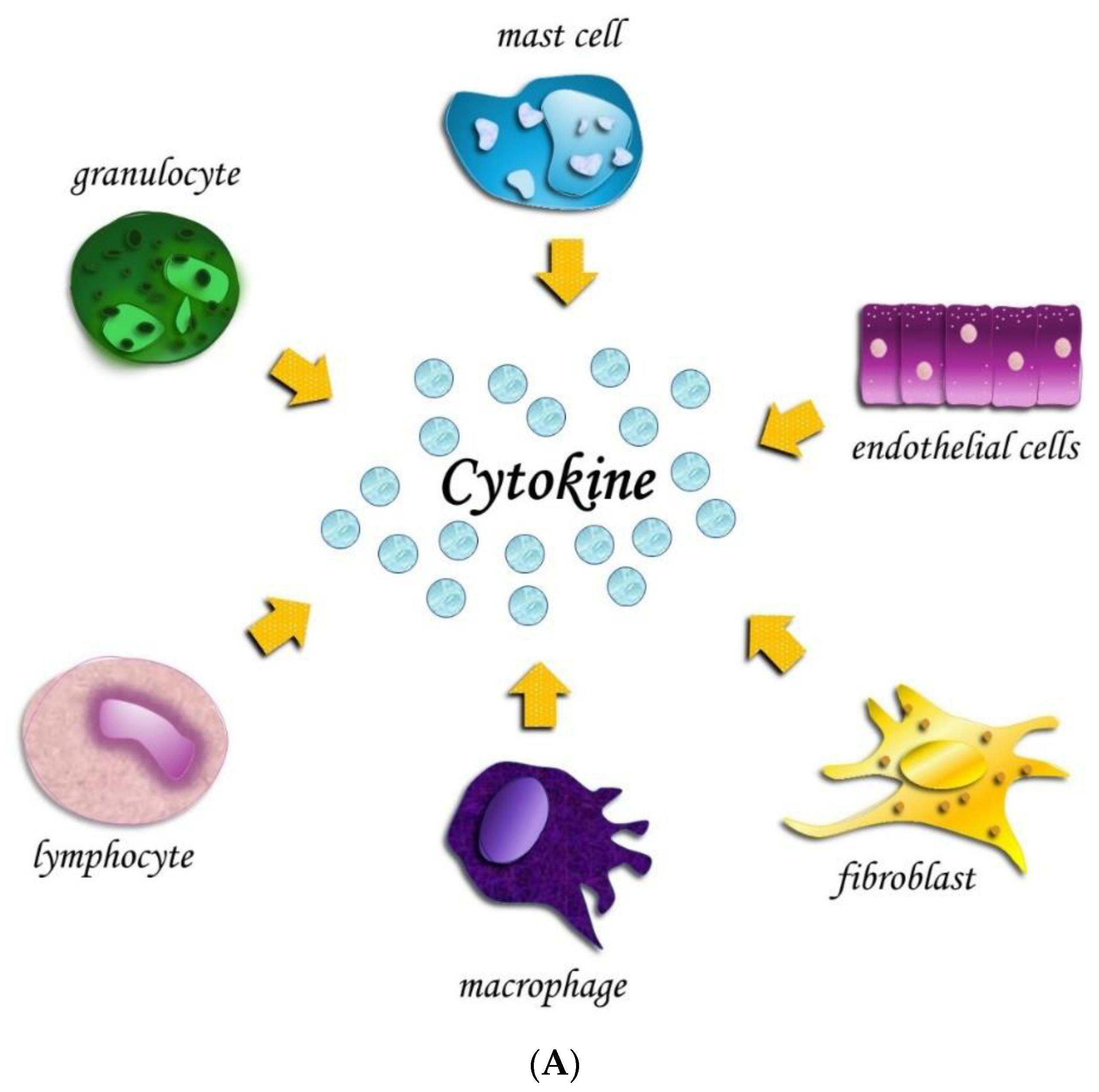
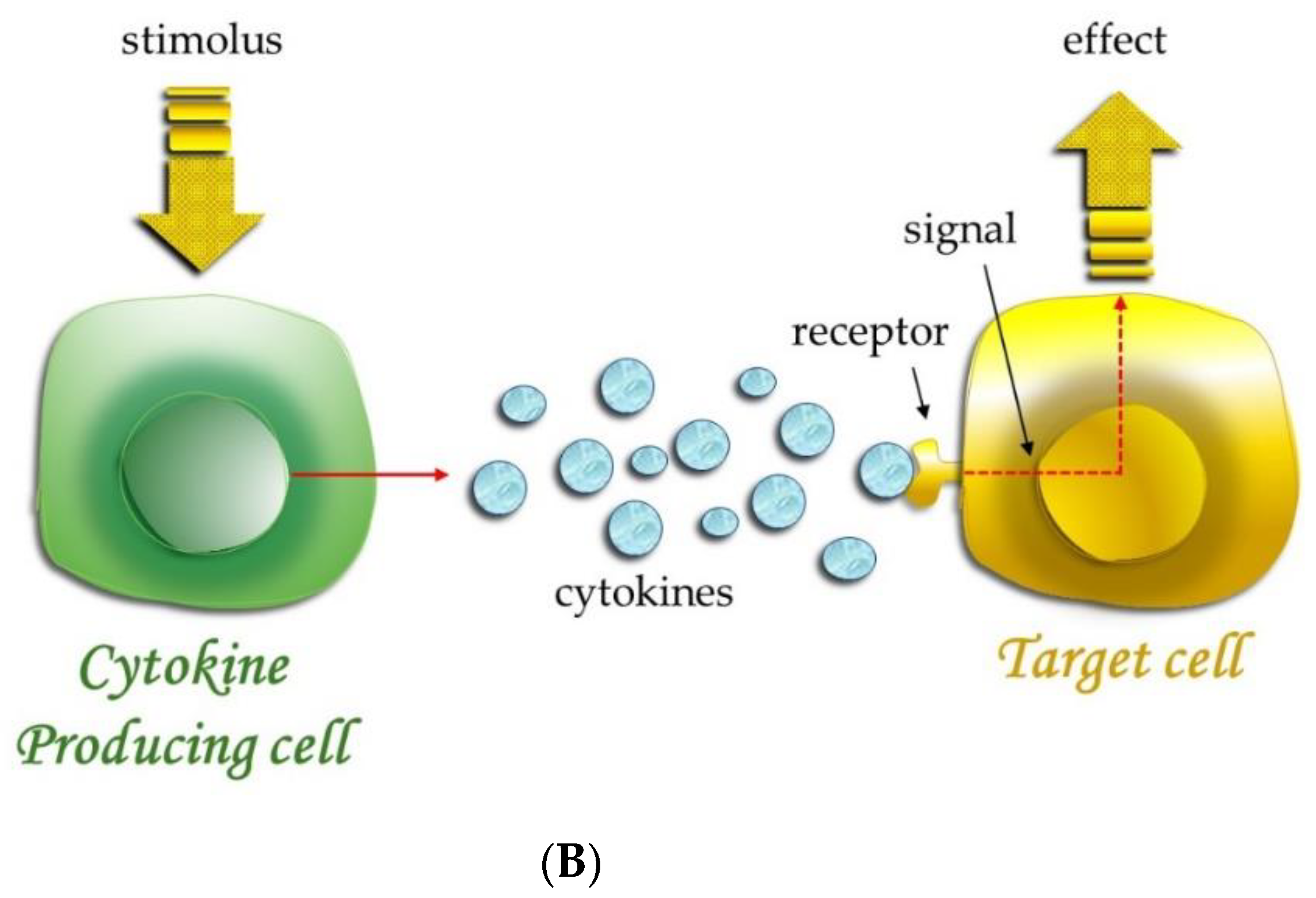
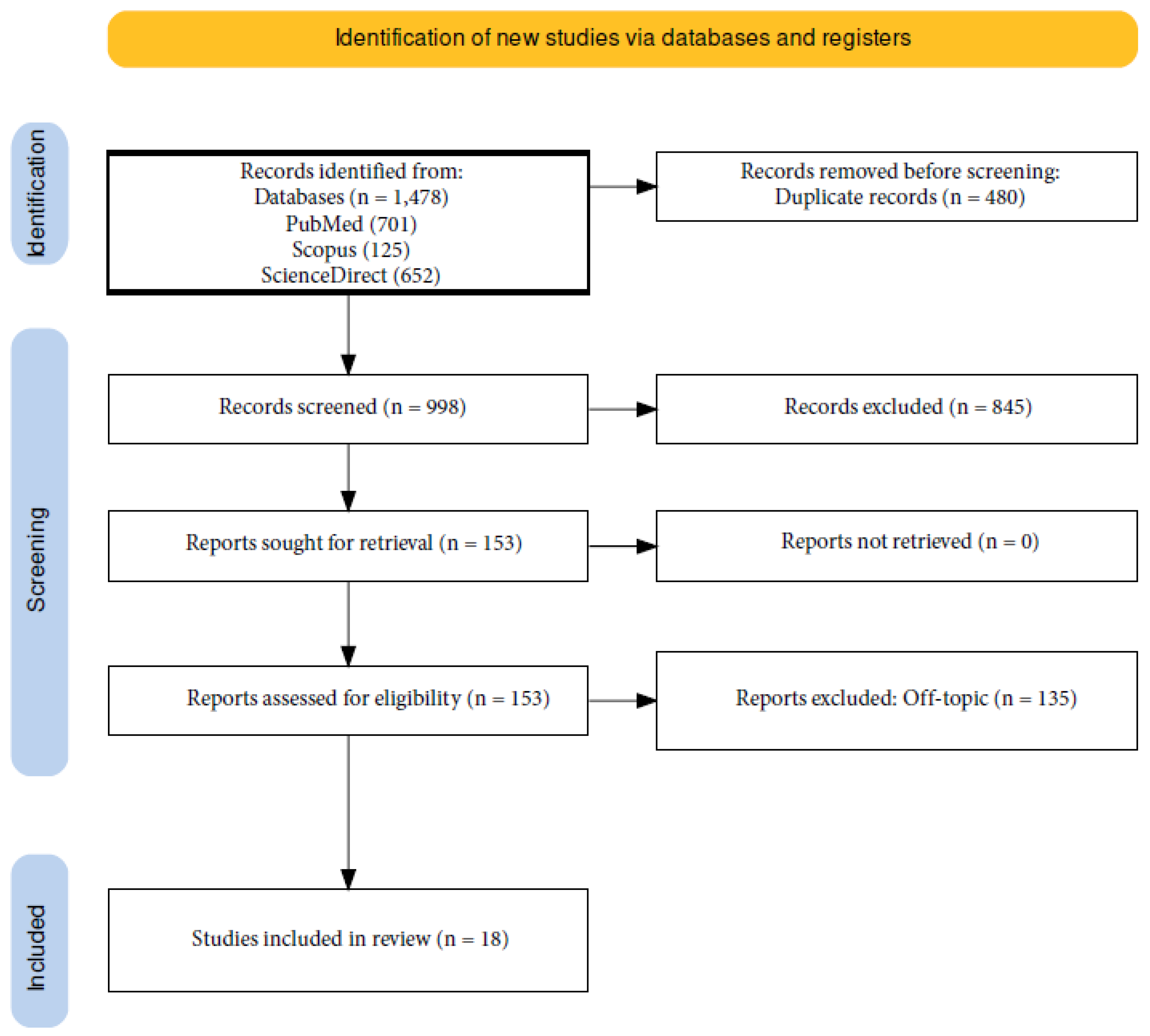
| Articles screening strategy | KEYWORDS: “A”: cytokines; “B”: OT; “C”: orthodontic therapy |
| Boolean Indicators: “A” AND “B”; “A” AND “C” | |
| Timespan: 1 January 2013 to 7 January 2024 | |
| Electronic databases: Pubmed; Scopus; ScienceDirect. |
| Authors (Year) | Type of the Study | Aim of the Study | Materials | Results |
|---|---|---|---|---|
| Nunes L. et al. (2017) [85] | Controlled Longitudinal study | To detect and quantify cytokine levels in GCF during initial OT | The study sample included 15 healthy patients, undergoing OT at the Orthodontic Clinic of the Faculty of Dentistry of the State University of Rio de Janeiro. Periodontal monitoring was performed at each stage of the OT, with assessments of visible plaque and of bleeding on palpation. Furthermore, GCF samples were collected from specific teeth at different stages of OT, using Luminex technology for cytokine quantification. | The results showed that total cytokine levels in the GCF remained constant in teeth subjected to orthodontic forces, suggesting a constant response to the light mechanical stimulus exerted by the NiTi brace inserted for alignment and leveling. In the control group, the levels of many cytokines in the GCF decreased significantly over time. |
| Grant M.et al. (2012) [18] | Controlled longitudinal intervention study | This study investigates on changes in cytokines and biomarkers of bone and tissue metabolism within GCF from patients undergoing OT. | GCF was collected from 20 volunteers during different stages of OT using Periopaper™ strips. The samples were obtained at baseline, before appliance placement, and 10 weeks after the first appliance placement, with additional collection at specific intervals following the application of distalizing forces. Analysis included assessing various cytokines (GM-CSF, interferon-gamma, IL-1beta, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, and TNFalpha), tissue biomarkers (MMP-9, TIMP-1 and 2), and bone metabolism indicators (RANKL and OPG). | Orthodontic force application increased pro-inflammatory markers in GCF, including IL-1beta, IL-8, TNFalpha, MMP-9, TIMPs 1 and 2 at tension sites near canines. Compression sites also exhibited elevated levels of IL-1beta, IL-8 (after 4 h), MMP-9 (after 7 and 42 days), and RANKL (after 42 days). |
| Mohamed A. et al. (2019) [86] | Prospective study | The text addresses orthodontic tooth movement as a result of cellular elements and periodontal ligament fluid, which plays a crucial role in the normal functioning of the periodontal ligament that surrounds all teeth in the oral cavity. | The article reports the results of a study conducted on 30 patients undergoing premolar extractions, analyzing changes in IL-6 levels in the periodontal ligament during OT. | This study suggests that IL-6 could play a key role in bone remodeling during orthodontic movement and could be considered as a potential biomarker to improve efficacy and management. |
| Lin T. et al. (2021) [31] | Prospective study | This study aimed to investigate the regulation of Th17 on MMPs expression during OTM. | Eighteen children in OT provided GCF samples at different time points: application day (T0), one hour (T1), 24 h (T2), one week (T3), 4 weeks (T4), and 12 weeks (T5) post-orthodontic force application. Cytokines and MMPs in GCF were measured using a Multiplex Luminex analyzer, and PDL tissues were stimulated by IL-17. | It was found that the expression of IL-17 was correlated with MMPs. After rhIL-17 treatment, the expression of MMP-1, MMP-2, and MMP-9 were up-regulated significantly. |
| Chelărescu S. et al. (2021) [87] | Randomized clinical trial | This study aims to analyze inflammatory mediators, particularly cytokines such as IL1β and IL6, in healthy adolescents and young adults during the acute phase of OT. | The research involved 20 patients, aged between 11 and 16 and between 17 and 28 undergoing OT. Crevicular fluid was collected before and 24 h after orthodontic activation. Fluid volume was measured with Periotron 8000, and cytokines were analyzed by immunoenzymatic methods. | The study uses bone remodeling biomarker analysis in crevicular fluid to monitor orthodontic treatment efficiency. Results show increased crevicular fluid volume and IL1β levels 24 h after treatment, with adolescents experiencing faster tooth movement compared to young adults. |
| Şahin E. et al. (2022) [88] | Controlled clinical trial | The aim of this study was to compare the effectiveness of oral irrigators with interdental brushes in patients with fixed OT. Furthermore, biochemical data, particularly on IL-1β concentrations in GCF, are presented, showing results favorable to the oral irrigator group. | There were two groups of patients with fixed OT. One of these used oral irrigator for oral hygiene, and the other interdental brushes. | The text suggests that the use of oral irrigators can have a positive impact on gum health, offering an effective alternative to interdental brushes, especially during fixed OT. |
| Madureira D.F. et al. (2015) [19] | Retrospective study | To analyze the expression of cytokines in GCF and PDL after mechanical stress. | Twenty-three healthy patients were included. The experimental group underwent a 0.980 N force for 1–28 days, while contralateral teeth served as controls. GCF and PDL samples were collected concurrently to analyze cytokines using cytometric bead array. | IL-6 production in the PDL significantly increased on day 1 after force application. Strong positive correlations between GCF and PDL in the experimental group were observed on days 3 (interferon-gamma), 7 (IL-10), 14 (IL-17A), and 28 (IL-17A, tumor necrosis factor-alpha), with strong negative correlations on days 14 (interferon-gamma) and 21 (IL-2, IL-10). |
| Nur Ozel et al. (2018) [89] | Prospective clinical study | To evaluate the impact of Rapid Maxillary Expansion (RME) on periodontal health, focusing on markers of oxidative stress and IL-1β levels in GCF. | GCF samples collected and analyzed for oxidative stress markers (TAC, TOS, OSI), IL-1β levels, and NO activity. | IL-1β levels insignificantly increased. NO levels increased during RME, associated with force loading and sutural relapse. TAC and TOS levels varied between buccal and palatal sides, with higher palatal levels. |
| Gabriel Antônio dos Anjos Tou et al. (2022) [90] | Prospective clinical study | To explore the molecular and clinical complexities of passive OT in 20 patients with indications for interceptive OT, anterior open bite, and good oral hygiene. | Measurement of cytokine levels (IL-8, IL-1β, IL-6, IL-10, TNF-α, and IL-12p70) in GCF. GCF samples collected at baseline, 24 h, and 7 days after bonding. | Increase in GCF cytokines observed 1 min to 1 h after bonding, peaking at 24 h, with a secondary peak at day 7. Insight into molecular dynamics of ILs during passive OT in children. Influence of lip pressure and perioral musculature on tooth movement and open-bite correction. |
| Sandra Sagar (2023) [91] | Prospective longitudinal study | To assess salivary levels of vitamin D3 and the pro-inflammatory cytokine IL-17A in OT patients. | Saliva samples collected from 97 patients aged 13 to 18 years with Class I malocclusion, analyzed by ELISA. | Significant decrease in mean salivary vitamin D3 levels from early to late phase, followed by stabilization in the log phase. Salivary levels of IL-17A decreased from early to late phase and further in the log phase. No significant gender-based differences in vitamin D3 levels. |
| Marzieh Karimi-Afshar et al. (2021) [92] | Descriptive–analytical study | To investigate the dynamics of IL-8 levels in GCF during OT. | GCF samples collected at different times: before OT, 24 h, 7 days, and 28 days after treatment. IL-8 levels measured using ELISA. | IL-8 concentration significantly decreased one day after treatment, followed by an increase at one and four weeks but did not reach baseline. Age groups (adolescents and adults) showed a significant difference in IL-8 concentration on the first day of treatment, with adults having lower IL-8 levels. No significant difference between sexes during the treatment period. |
| J. Amanda et al. (2018) [93] | Prospective clinical study | To investigate the dynamics of RANKL concentrations in GCF during early OT. | Patients aged 15 to 35 years divided into two experimental groups and a control group. RANKL concentrations analyzed by ELISA. | Passive slot bracket system showed higher RANKL concentrations at 168 h than the straight wire system. |
| Hosam Ali Baeshen et al. (2021) [72] | Cross-sectional comparative study | Investigate chemokines in saliva samples from patients treated with conventional lingual and labial fixed orthodontic appliances. | Forty saliva samples from subjects with lingual and labial braces. | CXCL8, CCL11, CCL2, and CCL5 showed higher secretion in subjects with labial braces. CXCL9 showed slightly higher secretion in subjects with lingual braces. |
| Hosam Ali Baeshen et al. (2021) [71] | Prospective comparative study | To analyze the impact of OT with lingual and labial fixed appliances. | Saliva samples from 20 patients with lingual appliances and 20 with labial fixed appliances. | Higher levels of CCL2, IL-17A, and IL-6 in patients with lingual braces compared to fixed labial braces. ELISA tests demonstrated lower levels of defensins (HNP-1, HNP-2, HBD-1, and HBD-2) in saliva of patients with lingual braces. |
| Abhilasha Khanal et al. (2015) [69] | Prospective comparative study | To analyze levels of IL-17A and RANKL in the GCF during OT in adolescents and young adults. | GCF samples collected from mesial, medial, and distal sides of the right maxillary canine. IL-17A and RANKL levels measured by ELISA. | Significant increase in IL-17A and RANKL levels in the treatment group compared with controls. Gender-specific differences observed, with males showing higher cytokine expression than females. Correlation between IL-17A and RANKL suggested a synergistic effect. |
| Agita Pramustika et al. (2018) [94] | Prospective experimental study | To examine the GCF of 18 patients before and after OT with passive self-ligating brackets and pre-ligated brackets. | Measure tumor necrosis factor-α (TNF-α) concentrations using ELISA kits. | TNF-α levels increased at 24 h in both experimental groups, decreasing significantly after 168 h in the passive elastomeric ligature group. |
| Bergamo et al. (2018) [62] | Randomized clinical trial | To investigate the correlation between bracket design and the ratio of five proinflammatory cytokines in GCF while exploring bacterial adhesion in the absence of tooth movement influence. | The study enrolled 20 participants aged 11 to 15 years. Conventional metallic brackets and two self-ligating brackets were affixed to maxillary incisors and canines. GCF samples were collected before and 60 days after bonding using standard filter paper strips. Cytokine levels (IL-12, IL-1α, IL-1β, IL-6, and TNF-α) were assessed through the LUMINEX assay. Checkerboard DNA–DNA hybridization analyzed the levels of red and orange bacterial complexes. Non-parametric tests were employed for data analysis at a 5% significance level. | Bracket design demonstrated an impact on cytokine levels and bacterial adhesion. This underscores the importance of considering bracket design in patients at risk of periodontal disease and root resorption. |
| Sinan Şen et al. (2019) [95] | Randomized clinical trial | To assess potential adverse biological effects of three commonly used surface sealants and a bonding primer on gingival tissues by analyzing cytokines in crevicular fluid following their application in orthodontic patients. | A single-center parallel trial with a split-mouth design was executed. Quadrants of 15 patients requiring OT with fixed appliances were randomly assigned to one of three surface sealants or a bonding primer. IL-8 and IL-10 levels in crevicular fluid were evaluated at four time points (before application, and at 30, 60, and 90 min after application). | The commonly used pre-bonding surface sealants did not exhibit a significant impact on inflammatory cytokine levels in crevicular fluid. Moreover, they did not seem to contribute to sensitization or hypersensitivity events, indicating their relatively benign biocompatibility in this study. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inchingolo, F.; Inchingolo, A.M.; Malcangi, G.; Ferrante, L.; Trilli, I.; Di Noia, A.; Piras, F.; Mancini, A.; Palermo, A.; Inchingolo, A.D.; et al. The Interaction of Cytokines in Orthodontics: A Systematic Review. Appl. Sci. 2024, 14, 5133. https://doi.org/10.3390/app14125133
Inchingolo F, Inchingolo AM, Malcangi G, Ferrante L, Trilli I, Di Noia A, Piras F, Mancini A, Palermo A, Inchingolo AD, et al. The Interaction of Cytokines in Orthodontics: A Systematic Review. Applied Sciences. 2024; 14(12):5133. https://doi.org/10.3390/app14125133
Chicago/Turabian StyleInchingolo, Francesco, Angelo Michele Inchingolo, Giuseppina Malcangi, Laura Ferrante, Irma Trilli, Angela Di Noia, Fabio Piras, Antonio Mancini, Andrea Palermo, Alessio Danilo Inchingolo, and et al. 2024. "The Interaction of Cytokines in Orthodontics: A Systematic Review" Applied Sciences 14, no. 12: 5133. https://doi.org/10.3390/app14125133








