Mechanism of Unfrozen Water Content Evolution during Melting of Cryogenic Frozen Coal Body Based on 2D NMR
Abstract
:1. Introduction
2. Coal Sample and Experiment
2.1. Coal Sample Preparation
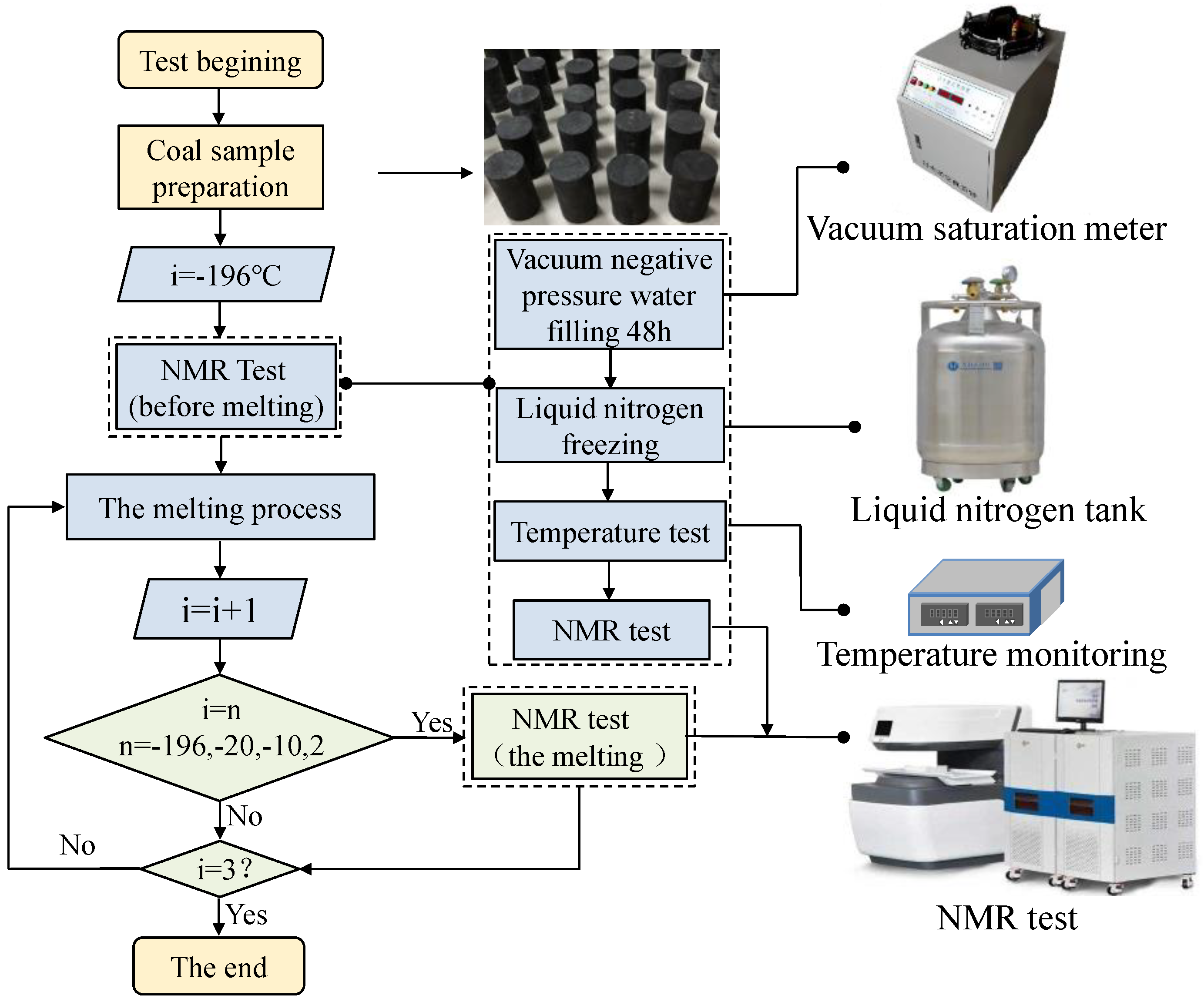
2.2. Experiment Method
- (1)
- Vacuum full of water machine: BSJ intelligent vacuum full of water machine.
- (2)
- Nuclear magnetic resonance core analyzer: Niumag MR-60 nuclear magnetic resonance core analyzer, the number of single sampling points (TD) was 1024, the cumulative sampling times (NS) was 32 times, the echo time (TE) was 0.233 ms, and the number of echoes (NECH) was 6000, the hydrogen proton resonance frequency was 21.3 MHz and the diameter of the probe coil was 60 mm.
3. Experimental Results and Analysis
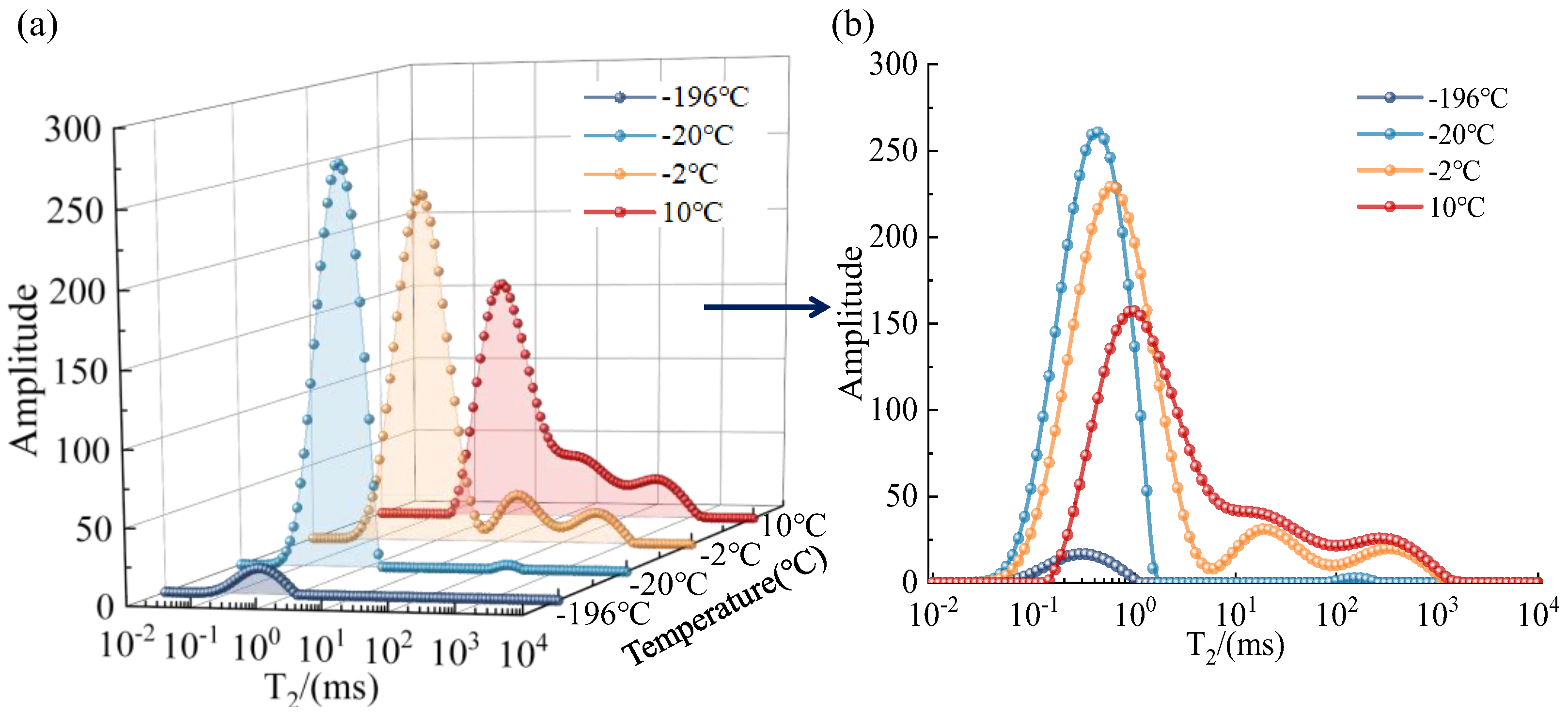
3.1. T2 Spectrum Test Results
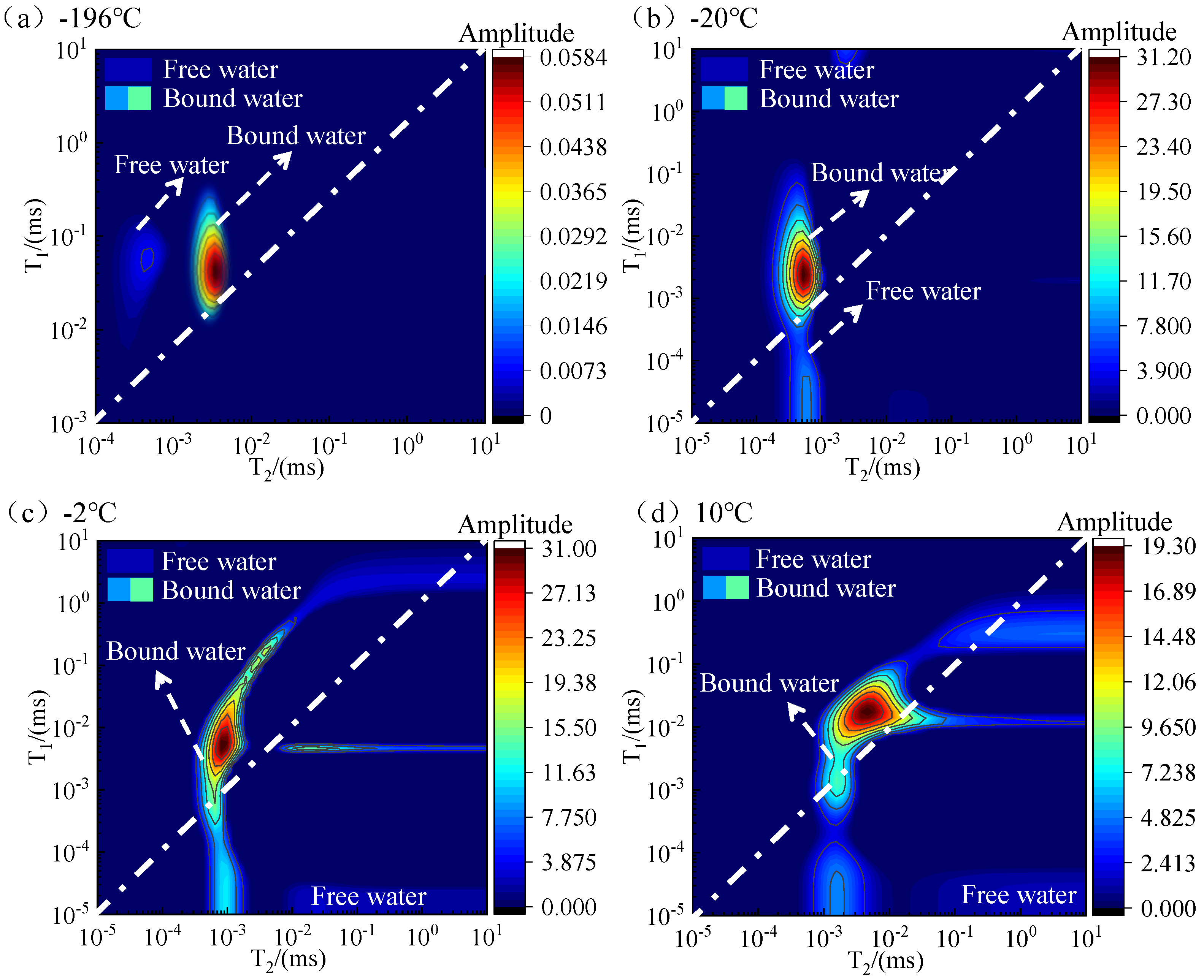
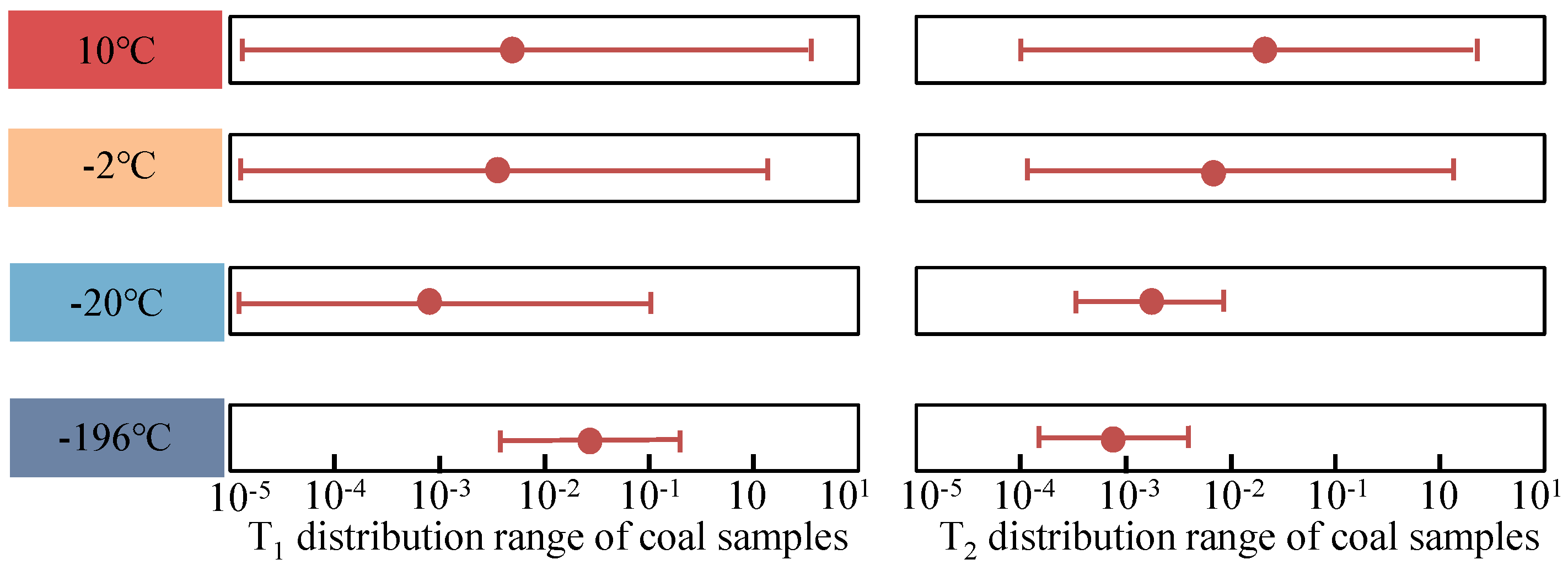
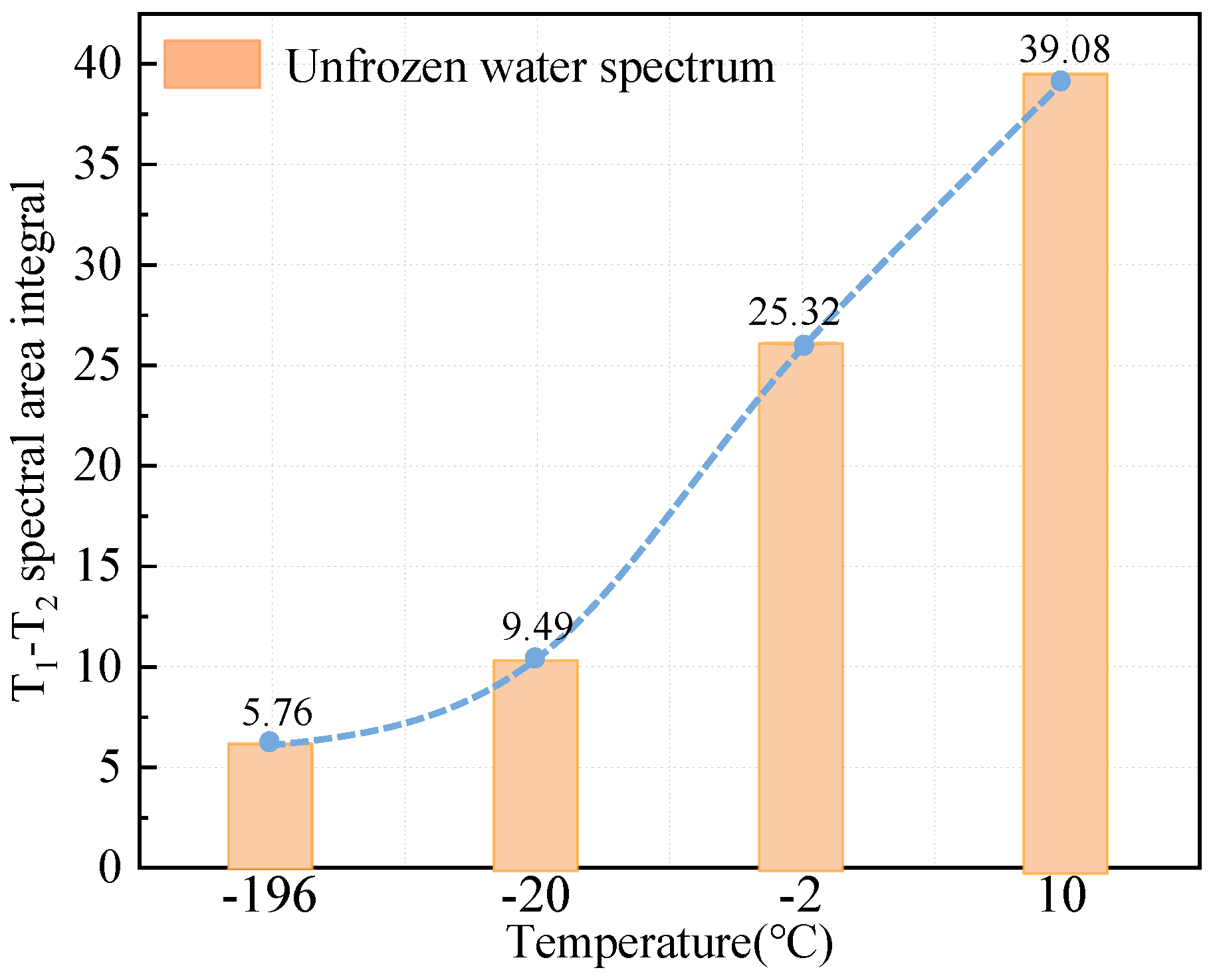
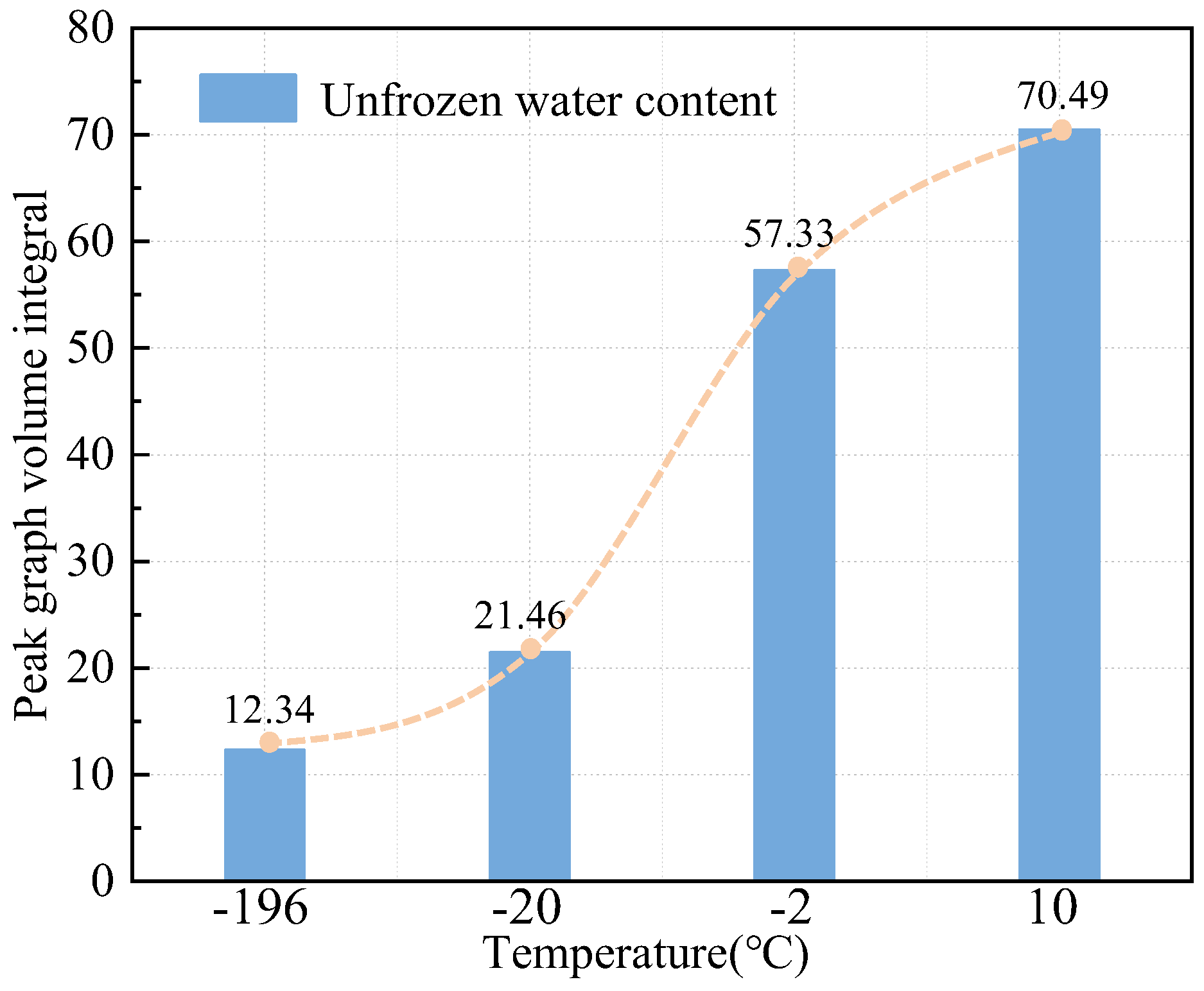
3.2. T1-T2 Spectrum Test Results
4. Evolution Mechanism of Unfrozen Water Content in Pore
4.1. Governing Equation of Water Ice Transition in Pore
4.2. Effect of Pore Temperature on Unfrozen Water Content
4.3. Effect of Potential Energy between Pore Wall and Unfrozen Water on Unfrozen Water Content
5. Conclusions
- (1)
- The two-dimensional NMR T1-T2 spectra reflected the unfrozen water space within the pore space. According to the T1-T2 spectra at different temperatures, the unfrozen water content could be obtained in ascending order with increasing temperature.
- (2)
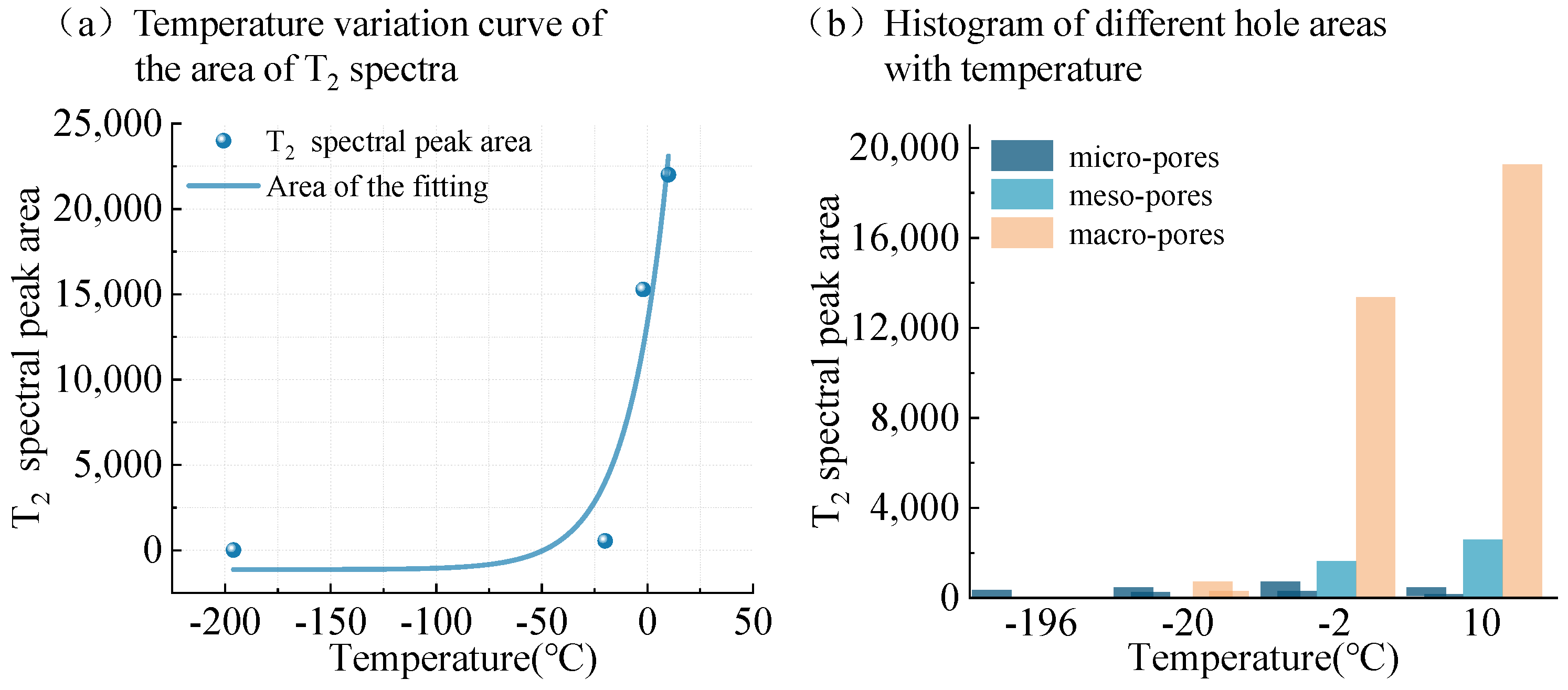
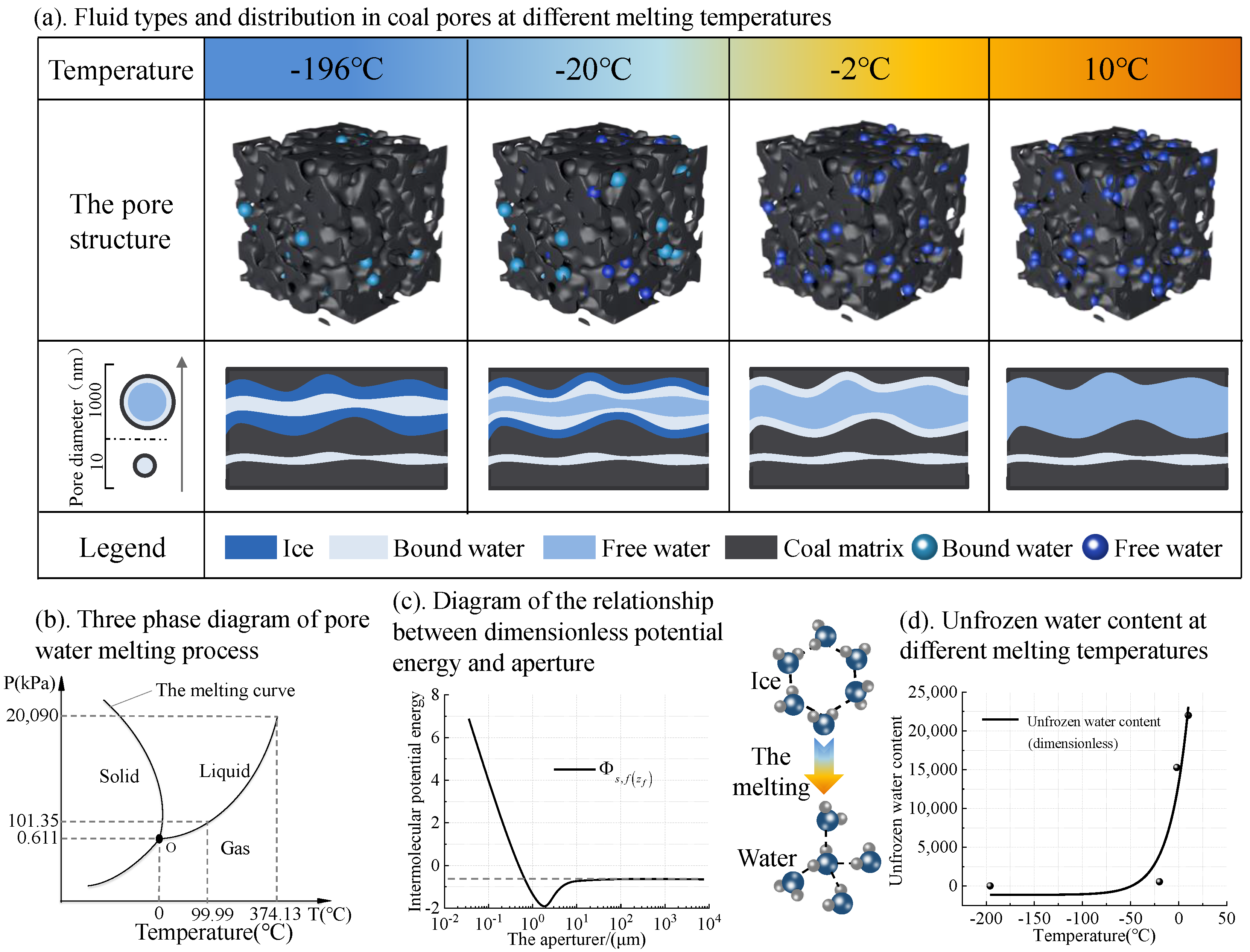
- In the melting process of frozen coal sample, the pore structure was composed of coal pore and ice pore. As the melting progresses, the small pores formed by the ice gradually disappeared, the number of small pores decreased and the number of intermediate pores increased.
- (3)
- The unfrozen water content was affected by temperature and pore size. The change of pore pressure with temperature directly affected the melting point of ice. The melting of coal samples started from the small pores until the temperature rose to a certain degree and the large pores began to melt.
- (4)
- Under the influence of intermolecular potential energy between water molecules and coal wall in the pore, the ice melting point decreased in small pore. Since the smaller the pore is, the larger the intermolecular potential energy is, a more significant effect occurs on the ice melting point reduction. This is the key reason why the ice in the small pores melted first during the melting process of frozen coal. In this paper, we only theoretically analyzed the effect of pore pressure on pore ice melting point and unfrozen water content under a single pore size condition. Due to the uneven pore size distribution of real coals, the control mechanism of pore size and distribution on unfrozen water content in coals with different degrees of metamorphism will be discussed in our future studies.
- (5)
- In this paper, it was found that the order of pore ice melting was small pores first and then medium-large pores, and the slow ice melting in medium-large pores will undoubtedly increase fracturing time and reduce the permeability-enhancement effect in the coal seam. At the same time, previous tests found that the freezing sequence was medium-large pores first and then small pores. Therefore, we believe that when applying low-temperature freeze-thaw fracturing technology, rapid freeze-thawing should be adopted to improve the freeze-thawing efficiency of medium-large pores, avoiding the waste of energy and time by freezing and thawing of small pores, so as to achieve the purpose of high-efficiency permeability enhancement and coalbed methane production increase. For example, rapid cyclic freezing and thawing or rapid hot and cold alternation can be adopted to fully reduce the consumption of liquid nitrogen, energy and time, as well as to promote the low-cost and high-efficiency application of this technology.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kleinhans, U.; Wieland, C.; Frandsen, F.J.; Spliethoff, H. Ash Formation and Deposition in Coal and Biomass Fired Combustion Systems: Progress and Challenges in the Field of Ash Particle Sticking and Rebound Behavior. Prog. Energy Combust. Sci. 2018, 68, 65–168. [Google Scholar] [CrossRef]
- Hasse, C.; Debiagi, P.; Wen, X.; Hildebrandt, K.; Vascellari, M.; Faravelli, T. Advanced Modeling Approaches for CFD Simulations of Coal Combustion and Gasification. Prog. Energy Combust. Sci. 2021, 86, 100938. [Google Scholar] [CrossRef]
- Ju, W.; Dong, J.; Chang, C. Evolution of Methane Permeability and Fracture Structure of Coal: Implications for Methane Extraction in Deep Coal Seams. RSC Adv. 2022, 12, 30227–30235. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Zhang, T.; Ma, T.; Tian, S.; Jia, X.; Jiang, Z. Effect of Water-Based SiO2 Nanofluid on Surface Wettability of Raw Coal. Energy 2022, 254, 124228. [Google Scholar] [CrossRef]
- Yan, F.; Lin, B.; Zhu, C.; Shen, C.; Zou, Q.; Guo, C.; Liu, T. A Novel ECBM Extraction Technology Based on the Integration of Hydraulic Slotting and Hydraulic Fracturing. J. Nat. Gas Sci. Eng. 2015, 22, 571–579. [Google Scholar] [CrossRef]
- Li, B.; Zhang, J.; Ding, Z.; Wang, B.; Li, P. A Dynamic Evolution Model of Coal Permeability during Enhanced Coalbed Methane Recovery by N 2 Injection: Experimental Observations and Numerical Simulation. RSC Adv. 2021, 11, 17249–17258. [Google Scholar] [CrossRef]
- Yan, H.; Wang, W.; Zhang, J.; Ma, D.; Zhou, N.; Wan, Z. Experimental Study on the Influence of Coal-Rock Interface Strength on Crack Propagation Law of Supercritical Carbon Dioxide Fracturing. Gas Sci. Eng. 2023, 112, 204943. [Google Scholar] [CrossRef]
- Sun, Y.; Zhai, C.; Qin, L.; Xu, J.; Yu, G. Coal Pore Characteristics at Different Freezing Temperatures under Conditions of Freezing–Thawing Cycles. Environ. Earth Sci 2018, 77, 525. [Google Scholar] [CrossRef]
- Akhondzadeh, H.; Keshavarz, A.; Al-Yaseri, A.Z.; Ali, M.; Awan, F.U.R.; Wang, X.; Yang, Y.; Iglauer, S.; Lebedev, M. Pore-Scale Analysis of Coal Cleat Network Evolution through Liquid Nitrogen Treatment: A Micro-Computed Tomography Investigation. Int. J. Coal Geol. 2020, 219, 103370. [Google Scholar] [CrossRef]
- Zou, Q.; Chen, Z.; Cheng, Z.; Liang, Y.; Xu, W.; Wen, P.; Zhang, B.; Liu, H.; Kong, F. Evaluation and Intelligent Deployment of Coal and Coalbed Methane Coupling Coordinated Exploitation Based on Bayesian Network and Cuckoo Search. Int. J. Min. Sci. Technol. 2022, 32, 1315–1328. [Google Scholar] [CrossRef]
- Sheta, S.; Afgan, M.S.; Hou, Z.; Yao, S.-C.; Zhang, L.; Li, Z.; Wang, Z. Coal Analysis by Laser-Induced Breakdown Spectroscopy: A Tutorial Review. J. Anal. At. Spectrom. 2019, 34, 1047–1082. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, H.; Lei, J.; Xie, J.; Li, L.; Gan, Y.; Deng, J.; Qi, R.; Liu, Y. Study on the Surface Wetting Mechanism of Bituminous Coal Based on the Microscopic Molecular Structure. RSC Adv. 2023, 13, 5933–5945. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Tan, Y.; Wang, F.; Luo, Y.; Zhao, Z. Improving Permeability of Coal Seams by Freeze-Fracturing Method: The Characterization of Pore Structure Changes under Low-Field NMR. Energy Rep. 2020, 6, 550–561. [Google Scholar] [CrossRef]
- Wan, X.; Pei, W.; Lu, J.; Zhang, X.; Yan, Z.; Pirhadi, N. Prediction of the Unfrozen Water Content in Soils Based on Premelting Theory. J. Hydrol. 2022, 608, 127505. [Google Scholar] [CrossRef]
- Huang, S.; Liu, F.; Liu, G.; Yu, S. Estimation of the Unfrozen Water Content of Saturated Sandstones by Ultrasonic Velocity. Int. J. Min. Sci. Technol. 2023, 33, 733–746. [Google Scholar] [CrossRef]
- Bi, J.; Wang, G.; Wu, Z.; Wen, H.; Zhang, Y.; Lin, G.; Sun, T. Investigation on Unfrozen Water Content Models of Freezing Soils. Front. Earth Sci. 2023, 10, 1039330. [Google Scholar] [CrossRef]
- Kruse, A.M.; Darrow, M.M. Adsorbed Cation Effects on Unfrozen Water in Fine-Grained Frozen Soil Measured Using Pulsed Nuclear Magnetic Resonance. Cold Reg. Sci. Technol. 2017, 142, 42–54. [Google Scholar] [CrossRef]
- Lu, J.; Pei, W.; Zhang, X.; Bi, J.; Zhao, T. Evaluation of Calculation Models for the Unfrozen Water Content of Freezing Soils. J. Hydrol. 2019, 575, 976–985. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, H.; Lv, J.; Dyck, M.; Wu, Q.; He, H. A Scientometric Review of Research Status on Unfrozen Soil Water. Water 2021, 13, 708. [Google Scholar] [CrossRef]
- Wan, X.; Pei, W.; Lu, J.; Qiu, E.; Yan, Z.; Pirhadi, N.; Zhu, J. Analytical Model to Predict Unfrozen Water Content Based on the Probability of Ice Formation in Soils. Permafr. Periglac. Process. 2022, 33, 436–451. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X.; Lu, J.; Pei, W.; Wang, C. Analysis of Volumetric Unfrozen Water Contents in Freezing Soils. Exp. Heat Transf. 2019, 32, 426–438. [Google Scholar] [CrossRef]
- Wen, H.; Bi, J.; Guo, D. Evaluation of the Calculated Unfrozen Water Contents Determined by Different Measured Subzero Temperature Ranges. Cold Reg. Sci. Technol. 2020, 170, 102927. [Google Scholar] [CrossRef]
- Huang, S.; Liu, Q.; Liu, Y.; Ye, Z.; Cheng, A. Freezing Strain Model for Estimating the Unfrozen Water Content of Saturated Rock under Low Temperature. Int. J. Geomech. 2018, 18, 04017137. [Google Scholar] [CrossRef]
- An, R.; Kong, L.; Li, C. Pore Distribution Characteristics of Thawed Residual Soils in Artificial Frozen-Wall Using NMRI and MIP Measurements. Appl. Sci. 2020, 10, 544. [Google Scholar] [CrossRef]
- Ahamed, M.A.A.; Perera, M.S.A.; Matthai, S.K.; Ranjith, P.G.; Li, D. Coal Composition and Structural Variation with Rank and Its Influence on the Coal-Moisture Interactions under Coal Seam Temperature Conditions—A Review Article. J. Pet. Sci. Eng. 2019, 180, 901–917. [Google Scholar] [CrossRef]
- Jin, X.; Yang, W.; Gao, X.; Zhao, J.-Q.; Li, Z.; Jiang, J. Modeling the Unfrozen Water Content of Frozen Soil Based on the Absorption Effects of Clay Surfaces. Water Resour. Res. 2020, 56, e2020WR027482. [Google Scholar] [CrossRef]
- Li, Z.; Chen, J.; Sugimoto, M. Pulsed NMR Measurements of Unfrozen Water Content in Partially Frozen Soil. J. Cold Reg. Eng. 2020, 34, 04020013. [Google Scholar] [CrossRef]
- Weng, L.; Wu, Z.; Liu, Q.; Chu, Z.; Zhang, S. Evolutions of the Unfrozen Water Content of Saturated Sandstones during Freezing Process and the Freeze-Induced Damage Characteristics. Int. J. Rock Mech. Min. Sci. 2021, 142, 104757. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Z.; Wang, J.; Zhao, Y.; Dou, Z. Quantification and Division of Unfrozen Water Content during the Freezing Process and the Influence of Soil Properties by Low-Field Nuclear Magnetic Resonance. J. Hydrol. 2021, 602, 126719. [Google Scholar] [CrossRef]
- Su, Z.; Tan, X.; Chen, W.; Jia, H.; Xu, F. A Model of Unfrozen Water Content in Rock during Freezing and Thawing with Experimental Validation by Nuclear Magnetic Resonance. J. Rock Mech. Geotech. Eng. 2022, 14, 1545–1555. [Google Scholar] [CrossRef]
- Li, H.; Yang, Z.; Wang, J. Unfrozen Water Content of Permafrost during Thawing by the Capacitance Technique. Cold Reg. Sci. Technol. 2018, 152, 15–22. [Google Scholar] [CrossRef]
- Song, Y.; Huang, F.; Li, X.; Han, D.; Zhao, L.; Liang, H.; Rui, M.; Wang, J.; Zhang, C. Water Status Evolution of Pork Blocks at Different Cooking Procedures: A Two-Dimensional LF-NMR T1-T2 Relaxation Study. Food Res. Int. 2021, 148, 110614. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-Q.; Venkataramanan, L.; Hürlimann, M.D.; Flaum, M.; Frulla, P.; Straley, C. T1–T2 Correlation Spectra Obtained Using a Fast Two-Dimensional Laplace Inversion. J. Magn. Reson. 2002, 154, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Huang, L.; Lv, X.; Ren, Y. Variation Features of Unfrozen Water Content of Water-Saturated Coal under Low Freezing Temperature. Sci Rep 2021, 11, 15398. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, L.; Yang, C. Discussion of the Applicability of the Generalized Clausius–Clapeyron Equation and the Frozen Fringe Process. Earth-Sci. Rev. 2015, 142, 47–59. [Google Scholar] [CrossRef]


| Coal Specimens | Proximate (wt %) | Ro,max (%) | Maceral Composition (vol %) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mad | Aad | Vad | FCad | V | I | E | M | ||
| Lignite | 6.16 | 6.94 | 28.97 | 57.93 | 0.62 | 45.76 | 48.27 | 1.03 | 4.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Zhang, X.; Qin, L.; Lin, B.; Mu, M.; Yang, W.; Lv, S.; Li, J. Mechanism of Unfrozen Water Content Evolution during Melting of Cryogenic Frozen Coal Body Based on 2D NMR. Appl. Sci. 2024, 14, 5182. https://doi.org/10.3390/app14125182
Liu T, Zhang X, Qin L, Lin B, Mu M, Yang W, Lv S, Li J. Mechanism of Unfrozen Water Content Evolution during Melting of Cryogenic Frozen Coal Body Based on 2D NMR. Applied Sciences. 2024; 14(12):5182. https://doi.org/10.3390/app14125182
Chicago/Turabian StyleLiu, Tong, Xian Zhang, Lei Qin, Baiquan Lin, Miao Mu, Wei Yang, Shiyin Lv, and Jiawei Li. 2024. "Mechanism of Unfrozen Water Content Evolution during Melting of Cryogenic Frozen Coal Body Based on 2D NMR" Applied Sciences 14, no. 12: 5182. https://doi.org/10.3390/app14125182







