Kaolin–Fly Ash Composite for Pb2+ and AsO43− Adsorption from Aqueous System
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Source Materials
2.2. Preparation of Composite Adsorbents
2.3. Model Solutions
2.4. Adsorption Experiments
2.5. Data Processing
2.6. Analytical Methods
3. Results
3.1. Characterisation of Source Material and Composite Adsorbents
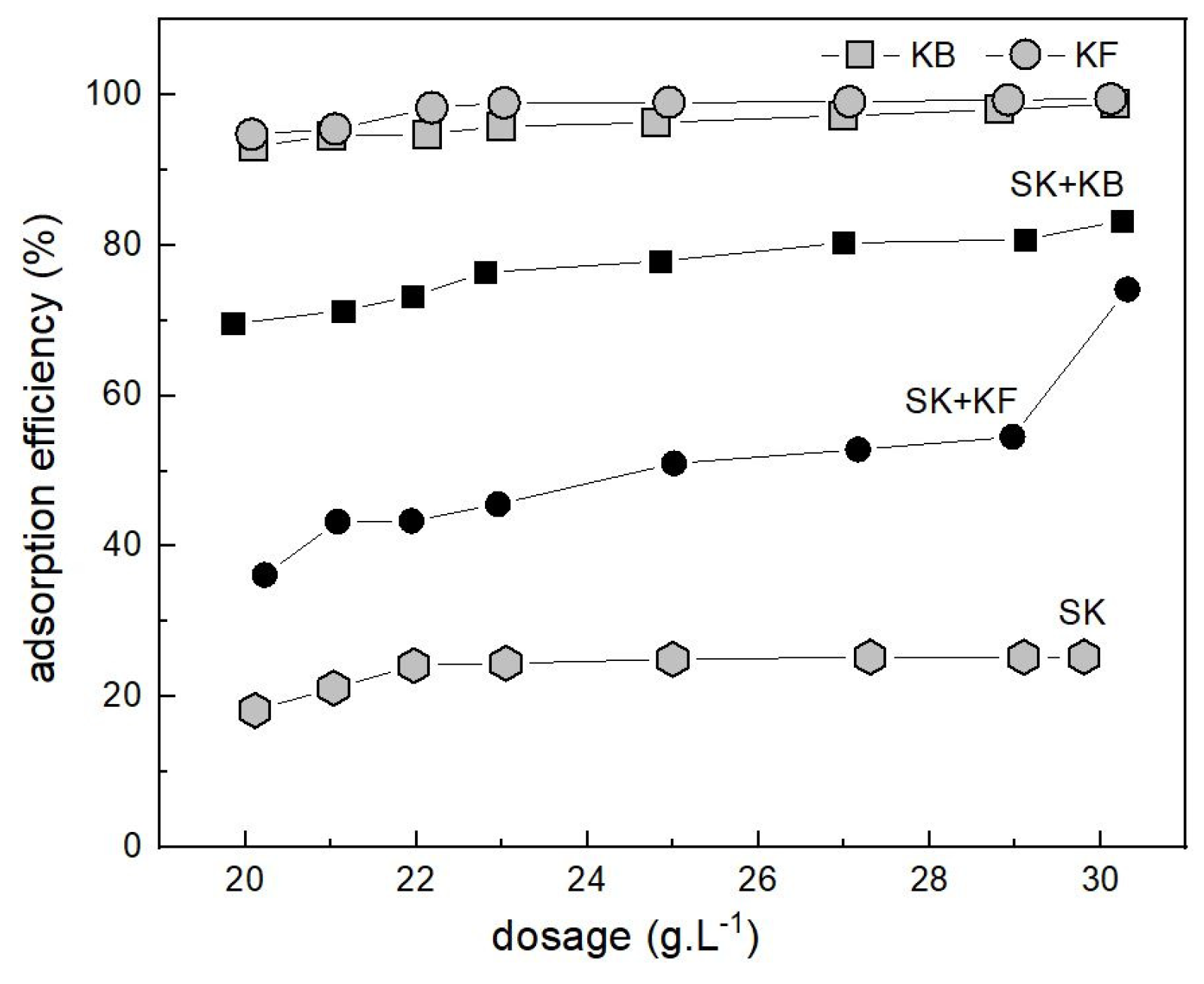
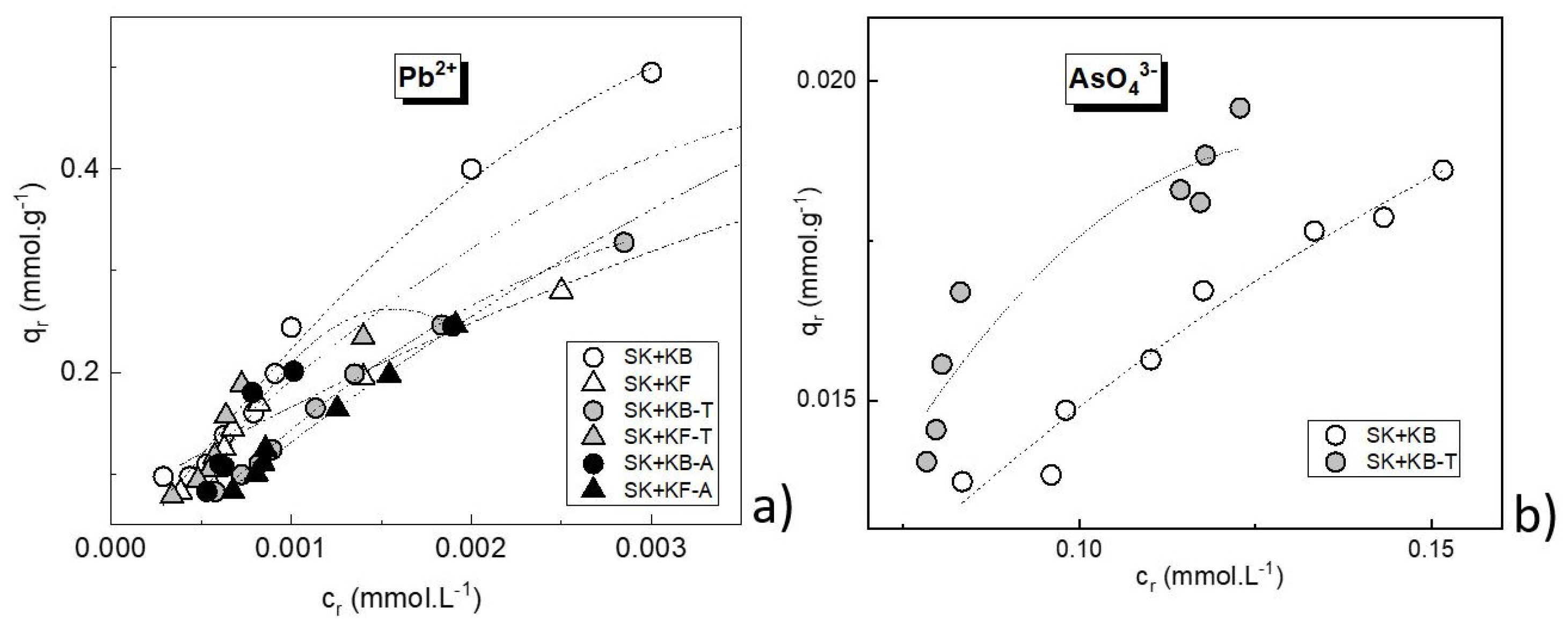
3.2. Adsorption of Pb2+ on Source Materials and Composite Adsorbents
3.3. Adsorption of AsO43− on Source Materials and Composite Adsorbents
3.4. Effect of Preparation Temperature on Sorbability of Pb2+ and AsO43− on Composite Adsorbents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roy, P.; Saha, A. Metabolism and toxicity of arsenic: A human carcinogen. Curr. Sci. 2002, 82, 38–45. Available online: https://www.jstor.org/stable/24105925 (accessed on 13 May 2024).
- Jiang, M.; Wang, Q.; Jin, X.; Chen, Z. Removal of Pb(II) from aqueous solution using modified and unmodified kaolinite clay. J. Hazard. Mater. 2009, 170, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Anjum, M.; Miandad, R.; Waqas, M.; Gehany, F.; Barakat, M. Remediation of wastewater using various nano-materials. Arab. J. Chem. 2019, 12, 4897–4919. [Google Scholar] [CrossRef]
- Khairnar, M.; Jain, V.; Wadgave, U.; Dhole, R.; Patil, S.; Chopade, S. Effect of different reverse osmosis water filters on fluoride content of drinking water. J. Ind. Assoc. Public Health Dent. 2018, 16, 165–168. [Google Scholar] [CrossRef]
- Mekonen, A.; Kumar, P.; Kumar, A. Integrated biological and physiochemical treatment process for nitrate and fluoride removal. Water Res. 2001, 35, 3127–3136. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.; Fucio, S.; Pascon, F.; Kantovitz, K.; Correr Sobrinho, L.; Puppin-Rontani, R. Effect of gamma irradiation on fluoride release and antibacterial activity of resin dental materials. Braz. Dent. J. 2009, 20, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, X.; Liu, S.; Li, Y.; Fan, J.; Lv, K. Effects of fluorine on photocatalysis. Chin. J. Catal. 2020, 41, 1451–1467. [Google Scholar] [CrossRef]
- Abou-El-Sherbini, K.S.; Hassanien, M.M. Study of organically-modified montmorillonite clay for the removal of copper(II). J. Hazard. Mater. 2010, 184, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Yang, Z.; Ren, K.; Tian, Z.; Dong, C.; Ma, R.; Yu, G.; Yang, W. Removal of antibiotics from water in the coexistence of suspended particles and natural or ganic matters using amino-acid-modified-chitosan flocculants: A combined ex perimental and theoretical study. J. Hazard. Mater. 2016, 317, 593–601. [Google Scholar] [CrossRef]
- Abbou, B.; Lebkiri, I.; Ouaddari, H.; Kadiri, L.; Ouass, A.; Habsaoui, A.; Lebkiri, A.; Rifi, E.H. Removal of Cd(II), Cu(II), and Pb(II) by adsorption onto natural clay: A kinetic and thermodynamic study. Turk. J. Chem. 2021, 45, 362–376. [Google Scholar] [CrossRef]
- Chen, L.; Chen, X.L.; Zhou, C.H.; Yang, H.M.; Ji, S.F.; Tong, D.S.; Zhong, Z.K.; Yu, W.H.; Chu, M.Q. Environmental-friendly montmorillonite-biochar composites: Facile production and tunable adsorption-release of ammonium and phosphate. J. Clean. Prod. 2017, 156, 648–659. [Google Scholar] [CrossRef]
- Doušová, B.; Fuitová, L.; Grygar, T.; Machovič, V.; Koloušek, D.; Herzogová, L.; Lhotka, M. Modified aluminosilicates as low-cost sorbents of As(III) from anoxic groundwater. J. Hazard. Mater. 2009, 165, 134–140. [Google Scholar] [CrossRef]
- González, A.; Navia, R.; Moreno, N. Fly ashes from coal and petroleum coke combustion: Current and innovative potential applications. Waste Manag. Res. 2009, 27, 976–987. [Google Scholar] [CrossRef]
- Alterary, S.S.; Marei, N.H. Fly ash properties, characterization, and applications: A review. J. King Saud. Univ. Sci. 2021, 33, 101536. [Google Scholar] [CrossRef]
- Mushtaq, F.; Zahida, M.; Bhattia, I.A.; Nasirb, S.; Hussain, T. Possible applications of coal fly ash in wastewater treatment. J. Environ. Manag. 2019, 240, 27–46. [Google Scholar] [CrossRef]
- Aigbe, U.O.; Ukhurebor, K.E.; Onyancha, R.B.; Osibote, O.A.; Darmokoesoemo, H.; Kusuma, H.S. Fly ash-based adsorbent for adsorption of heavy metals and dyes from aqueous solution: A review. J. Mater. Res. Technol. 2021, 14, 2751–2774. [Google Scholar] [CrossRef]
- Itskos, G.; Koukouzas, N.; Vasilatos, C.; Megremi, I.; Moutsatsou, A. Comparative uptake study of toxic elements from aqueous media by the different particle-size-fractions of fly ash. J. Hazard. Mater. 2010, 183, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.O.; Tran, T.; Park, Y.Y.; Kim, S.J.; Kim, M.J. Study on the kinetics of iron oxide leaching by oxalic acid. Int. J. Miner. Process. 2006, 80, 144–152. [Google Scholar] [CrossRef]
- Murray, H.H. Kaolin Applications. In Applied Clay Mineralogy. Occurrences, Processing and Application of Kaolins, Bentonites, Palygorskite, Sepiolite, and Common Clays, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2007; Chapter 5; pp. 85–109. [Google Scholar]
- Han, H.; Rafiq, M.K.; Zhou, T.; Xu, R.; Mašek, O.; Li, X. A critical review of clay-based composites with enhanced adsorption performance for metal and organic pollutants. J. Hazard. Mater. 2019, 369, 780–796. [Google Scholar] [CrossRef]
- Steiger, B.G.K.; Zhou, Z.; Anisimov, Y.A.; Evitts, R.W.; Wilson, L.D. Valorization of agro-waste biomass as composite adsorbents for sustainable wastewater treatment. Ind. Crop. Prod. 2023, 191, 115913. [Google Scholar] [CrossRef]
- Diagboyaa, P.N.; Olu-Owolabi, B.I.; Mtunzi, F.M.; Adebowale, K.O. Clay-carbonaceous material composites: Towards a new class of functional adsorbents for water treatment. Surf. Interfaces 2020, 19, 100506. [Google Scholar] [CrossRef]
- Diagboyaa, P.N.; Mtunzi, F.M.; Adebowale, K.O.; Düring, R.A.; Olu-Owolabi, B.I. Comparative empirical evaluation of the aqueous adsorptive sequestration potential of low-cost feldspar-biochar composites for ivermectin. Coll. Surf. A Physicochem. Eng. Asp. 2022, 634, 127930. [Google Scholar] [CrossRef]
- Bandura, L.; Panek, R.; Madej, J.; Franus, W. Synthesis of zeolite-carbon composites using high-carbon fly ash and their adsorption abilities towards petroleum substances. Fuel 2021, 283, 119173. [Google Scholar] [CrossRef]
- Gupt, C.B.; Bordoloi, S.; Sekharan, S.; Sarmah, A.K. A feasibility of Indian fy ash-bentonite as an alternative adsorbent composite to sand-bentonite mixes in landfill liner. Environ. Pollut. 2020, 265, 114811. [Google Scholar] [CrossRef] [PubMed]
- Doušová, B.; Koloušek, D.; Lhotka, M.; Keppert, M.; Urbanová, M.; Kobera, L.; Brus, J. Waste Brick Dust as Potential Sorbent of Lead and Cesium from Contaminated Water. Materials 2019, 12, 1647–1660. [Google Scholar] [CrossRef]
- Doušová, B.; Bedrnová, E.; Reiterman, P.; Keppert, M.; Koloušek, D.; Lhotka, M.; Mastný, L. Adsorption Properties of Waste Building Sludge for Environmental Protection. Minerals 2021, 11, 309. [Google Scholar] [CrossRef]
- Misak, N.Z. Langmuir isotherm and its application in ion-exchange reactions. React. Polym. 1993, 21, 53–64. [Google Scholar] [CrossRef]
- Wang, C.; Boithias, L.; Ning, Z.; Han, Y.; Sauvage, S.; Sánchez-Pérez, J.M.; Kuramochi, K.; Hatano, R. Comparison of Langmuir and Freundlich adsorption equations within the SWAT-K model for assessing potassium environmental losses at basin scale. Agric. Water Manag. 2017, 180, 205–211. [Google Scholar] [CrossRef]
- Guilhen, S.N.; Watanabe, T.; Tieko Silva, T.; Rovani, S.; Marumo, J.T.; Tenório, J.A.S.; Mašek, O.; de Araujo, L.G. Role of Point of Zero Charge in the Adsorption of Cationic Textile Dye on Standard Biochars from Aqueous Solutions: Selection Criteria and Performance Assessment. Recent Prog. Mater. 2022, 4, 010. [Google Scholar] [CrossRef]
- Carrales-Alvaradoa, D.H.; Rodríguez-Ramos, I.; Leyva-Ramosa, R.; Mendoza-Mendoza, E.; Villela-Martínez, D.E. Effect of surface area and physical–chemical properties of graphite and graphene-based materials on their adsorption capacity towards metronidazole and trimethoprim antibiotics in aqueous solution. Chem. Eng. J. 2020, 402, 126155. [Google Scholar] [CrossRef]
- Müller, B. Effect of particle size and surface area on the adsorption of albumin-bonded bilirubin on activated carbon. Carbon 2010, 48, 3607–3615. [Google Scholar] [CrossRef]
- Niu, Z.; Zhang, S.; Ma, M.; Wang, Z.; Zhao, H.; Wang, Y. Synthesis of novel waste batteries-sawdust-based adsorbent via a two-stage activation method for Pb2+ removal. Environ. Sci. Pollut. Res. 2019, 26, 4730–4745. [Google Scholar] [CrossRef] [PubMed]
- Doušová, B.; Grygar, T.; Martaus, A.; Fuitová, L.; Koloušek, D.; Machovič, V. Sorption of AsV on aluminosilicates treated with FeII nanoparticles. J. Colloid Interface Sci. 2006, 302, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Elizade-González, M.P.; Mattusch, J.; Einicke, W.D.; Wennrich, R. Sorption on natural solids for arsenic removal. Chem. Eng. J. 2001, 81, 187–195. [Google Scholar] [CrossRef]
- Huang, X.; Yang, G. Charge reversal and anion effects during adsorption of metal ions at clay surfaces: Mechanistic aspects and influence factors. Chem. Phys. 2020, 529, 110575. [Google Scholar] [CrossRef]
- Kosmulski, M. The pH dependent surface charging and points of zero charge. X. Update. Adv. Colloid Interface Sci. 2023, 319, 102973. [Google Scholar] [CrossRef]

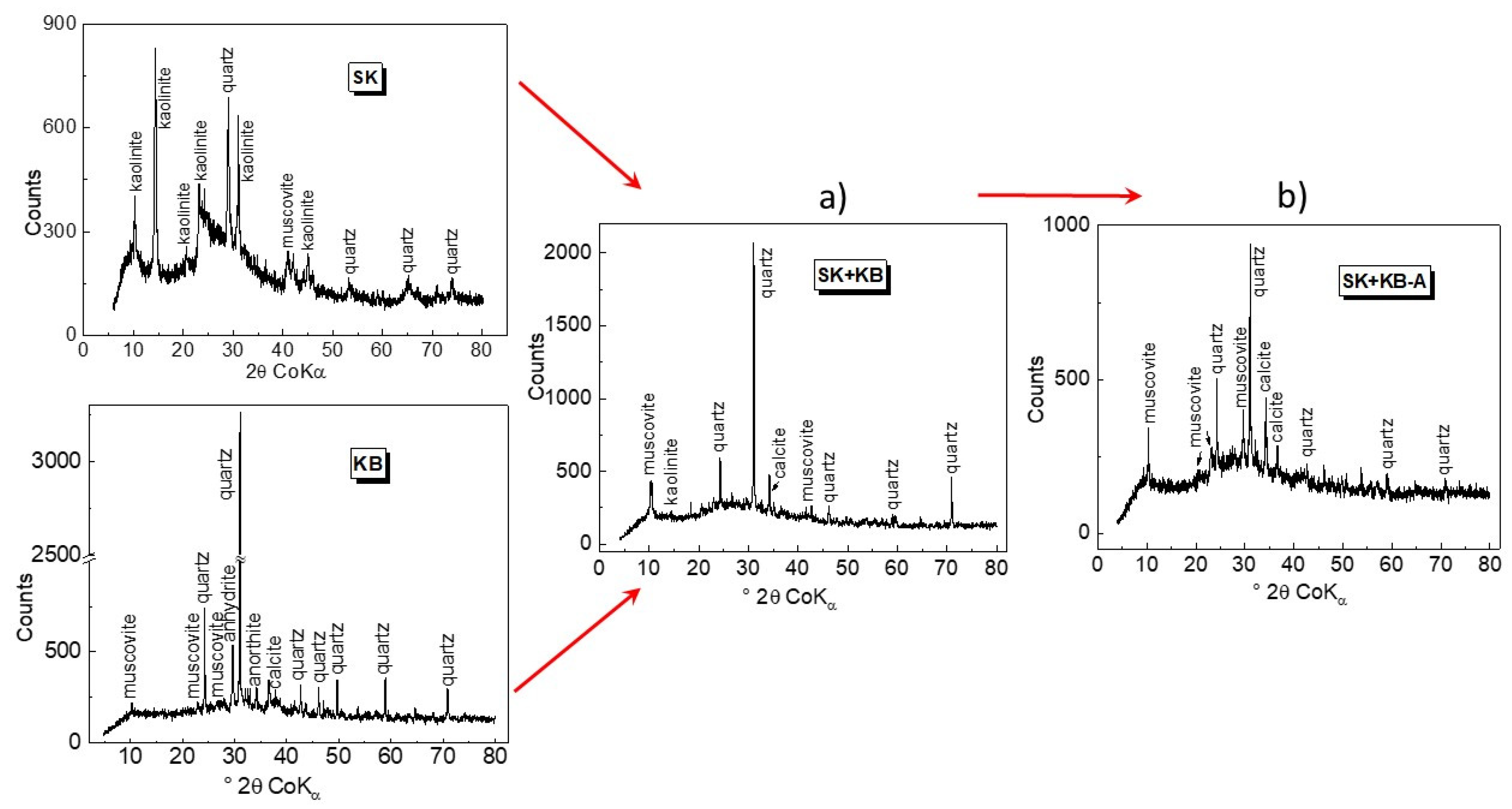
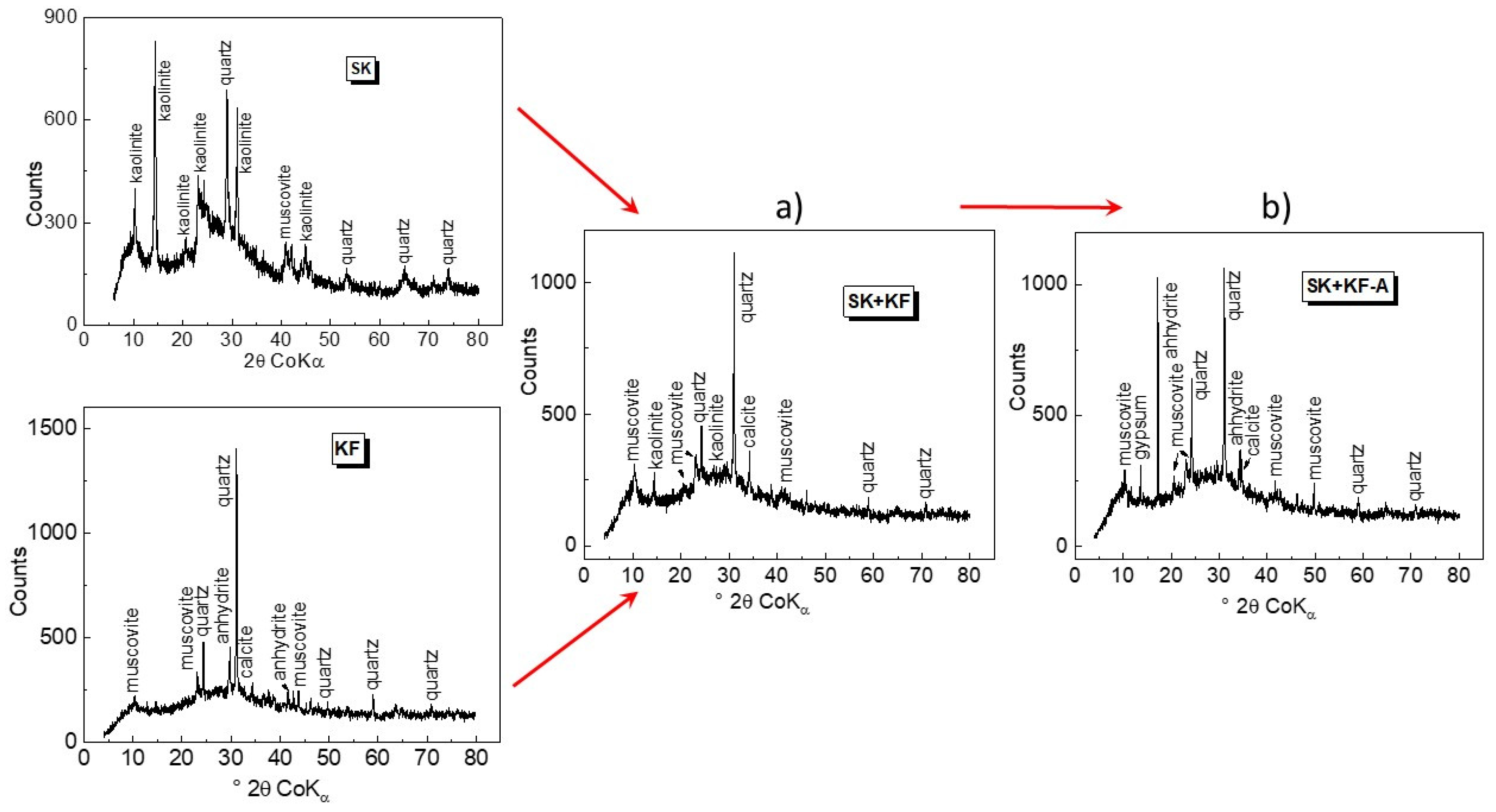
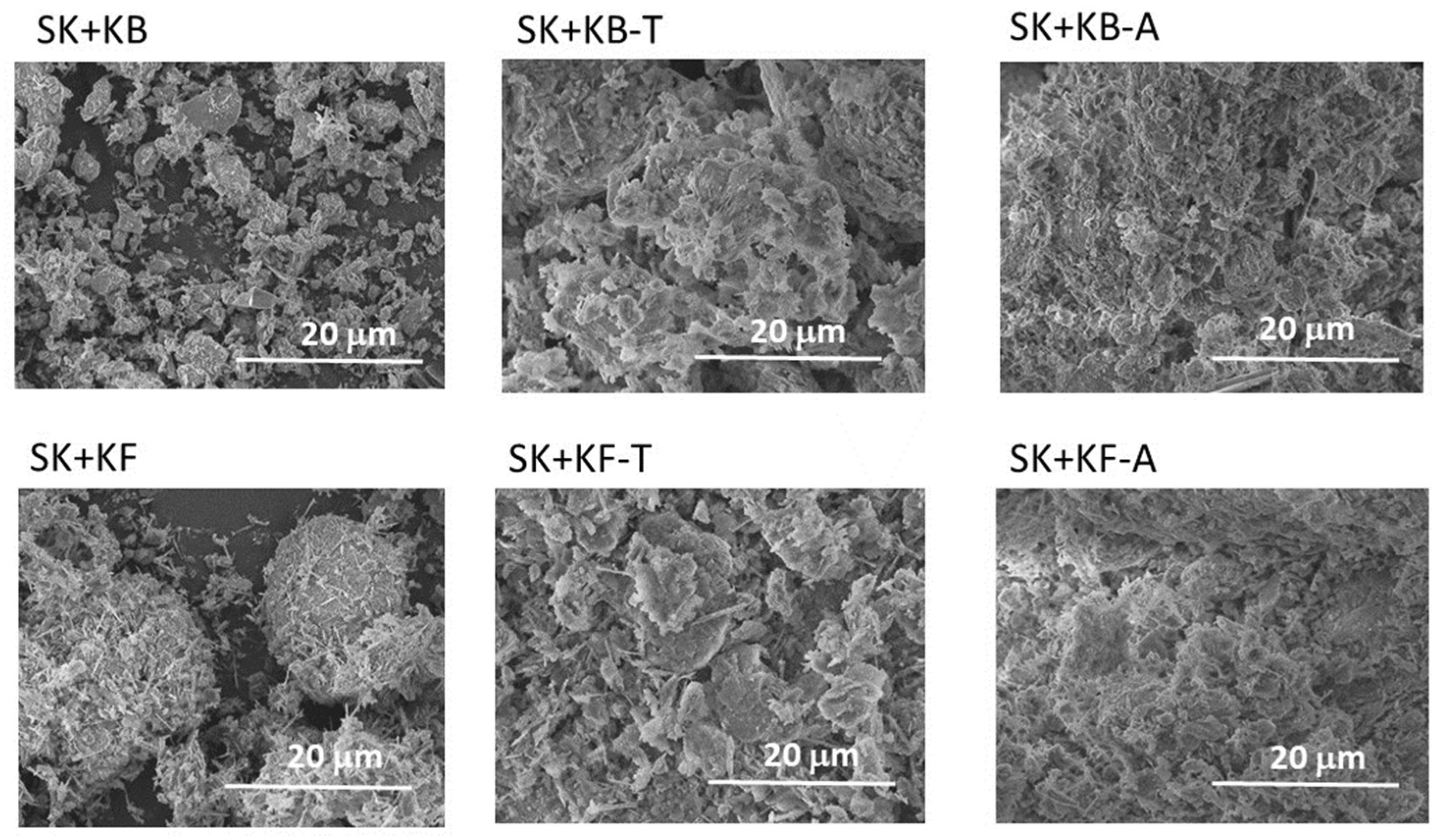


| Material | Chemical Composition (wt%) | SBET (m2.g−1) | pHZPC | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | TiO2 | CaO | MgO | SO3 | K2O | |||
| SK | 53.9 | 35.7 | 0.5 | 0.0 | 1.6 | 1.6 | 0.0 | 3.2 | 17.6 | 2.2 |
| KB | 42.1 | 17.0 | 5.0 | 1.5 | 24.0 | 2.5 | 0.7 | 4.3 | 48.4 | 11.1 |
| KF | 41.8 | 28.9 | 3.9 | 1.1 | 11.9 | 1.2 | 3.4 | 2.6 | 26.1 | 11.3 |
| Material | Mineralogical Composition |
|---|---|
| SK | kaolinite, muscovite, quartz |
| KB | muscovite, quartz, calcite, anhydrite, anorthite |
| KF | muscovite, quartz, calcite, anhydrite |
| SBET (m2.g−1) | pHZPC | |||||
|---|---|---|---|---|---|---|
| 20 °C | 65 °C-T | 110 °C-A | 20 °C | 65 °C-T | 110 °C-A | |
| SK+KB | 22.5 | 20.9 | 23.8 | 7.8 | 6.6 | 6.6 |
| SK+KF | 21.8 | 23.7 | 23.1 | 7.6 | 7.6 | 7.2 |
| Total Pore Volume (cm3.g−1) | ||||
|---|---|---|---|---|
| 20 °C | 65 °C-T | 110 °C-A | ||
| Source | SK | 0.053 | ||
| materials | KB | 0.047 | ||
| KF | 0.079 | |||
| Composites | SK+KB | 0.049 | 0.051 | 0.049 |
| SK+KF | 0.078 | 0.081 | 0.076 | |
| Langmuir Model | Freundlich Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| qmax (mmol g−1) | Qt (mmol g−1) | KL (L.mmol−1) | R2 | qmax (mmol g−1) | n | KF (mmol g−1) | R2 | ΔG (kJ.mol−1) | |
| SK | 0.04 | - | - | - | 0.04 | - | - | - | - |
| KB | 0.42 | 0.90 | 76.6 | 0.986 | 0.42 | 0.87 | 26.92 | 0.986 | −10.7 |
| KF | 0.43 | 0.23 | 140.6 | 0.667 | 0.43 | 0.66 | 2.59 | 0.736 | −12.3 |
| SK+KB | 0.50 | 0.58 | 512.1 | 0.933 | 0.50 | 0.80 | 50.90 | 0.980 | −15.5 |
| SK+KF | 0.47 | 1.07 | 213.1 | 0.938 | 0.47 | 0.61 | 11.91 | 0.911 | −13.3 |
| Langmuir Model | Freundlich Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| qmax (mmol g−1) | Qt (mmol g−1) | KL (L.mmol−1) | R2 | qmax (mmol g−1) | n | KF (mmol g−1) | R2 | ΔG (kJ.mol−1) | |
| SK | 0.005 | - | - | - | 0.005 | - | - | - | - |
| KB | 0.02 | 0.02 | 331.4 | 0.819 | 0.02 | 0.21 | 0.05 | 0.940 | −14.4 |
| KF | 0.02 | 0.02 | 720.8 | 0.920 | 0.02 | 0.14 | 0.04 | 0.788 | −16.3 |
| SK+KB | 0.03 | 0.04 | 7.37 | 0.948 | 0.02 | 0.55 | 0.05 | 0.957 | −5.0 |
| SK+KF | 0.01 | - | - | - | 0.01 | - | - | - | - |
| Langmuir Model | Freundlich Model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Preparation Temperature | qmax (mmol g−1) | Qt (mmol g−1) | KL (L.mmol−1) | R2 | qmax (mmol g−1) | n | KF (mmol g−1) | R2 | ΔG (kJ.mol−1) | |
| SK+KB SK+KF | 20 °C | 0.50 | 0.57 | 512.1 | 0.933 | 0.50 | 0.80 | 50.90 | 0.980 | −15.5 |
| 65 °C | 0.30 | 1.23 | 74.8 | 0.992 | 0.30 | 0.91 | 71.74 | 0.985 | −11.4 | |
| 110 °C (autoclave) | 0.48 | 1.80 | 68.65 | 0.824 | 0.49 | 0.90 | 88.55 | 0.885 | −10.5 | |
| 20 °C 65 °C | 0.47 0.30 | 1.07 0.54 | 213.1 202.9 | 0.938 0.902 | 0.47 0.30 | 0.61 0.43 | 11.90 15.92 | 0.911 0.773 | −13.3 −15.4 | |
| 110 °C (autoclave) | 0.50 | 0.58 | 281.7 | 0.923 | 0.30 | 0.71 | 17.66 | 0.918 | −14.0 | |
| Langmuir Model | Freundlich Model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Preparation Temperature | qmax (mmol g−1) | Qt (mmol g−1) | KL (L.mmol−1) | R2 | qmax (mmol g−1) | n | KF (mmol g−1) | R2 | ΔG (kJ.mol−1) | |
| SK+KB SK+KF | 20 °C | 0.02 | 0.04 | 7.4 | 0.948 | 0.02 | 0.55 | 0.05 | 0.957 | −5.0 |
| 65 °C | 0.02 | 0.03 | 9.5 | 0.790 | 0.02 | 0.52 | 0.06 | 0.794 | −5.6 | |
| 110 °C (autoclave) | 0.01 | - | - | - | 0.01 | - | - | - | - | |
| 20 °C 65 °C | 0.01 0.009 | - - | - - | - - | 0.01 0.007 | - - | - - | - - | - - | |
| 110 °C (autoclave) | 0.003 | - | - | - | 0.003 | - | - | 0.918 | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doušová, B.; Bedrnová, E.; Maxová, K.; Koloušek, D.; Lhotka, M.; Pilař, L.; Angelis, M. Kaolin–Fly Ash Composite for Pb2+ and AsO43− Adsorption from Aqueous System. Appl. Sci. 2024, 14, 5358. https://doi.org/10.3390/app14125358
Doušová B, Bedrnová E, Maxová K, Koloušek D, Lhotka M, Pilař L, Angelis M. Kaolin–Fly Ash Composite for Pb2+ and AsO43− Adsorption from Aqueous System. Applied Sciences. 2024; 14(12):5358. https://doi.org/10.3390/app14125358
Chicago/Turabian StyleDoušová, Barbora, Eva Bedrnová, Kateřina Maxová, David Koloušek, Miloslav Lhotka, Lukáš Pilař, and Milan Angelis. 2024. "Kaolin–Fly Ash Composite for Pb2+ and AsO43− Adsorption from Aqueous System" Applied Sciences 14, no. 12: 5358. https://doi.org/10.3390/app14125358
APA StyleDoušová, B., Bedrnová, E., Maxová, K., Koloušek, D., Lhotka, M., Pilař, L., & Angelis, M. (2024). Kaolin–Fly Ash Composite for Pb2+ and AsO43− Adsorption from Aqueous System. Applied Sciences, 14(12), 5358. https://doi.org/10.3390/app14125358






