Pathogens in the Food Chain: Escherichia coli Strains in Raw Milk Originating from Ewes Treated for Mastitis with Various Therapeutic Protocols
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of Sampled Animal Groups
2.2. Methodology: Isolation and Phenotypic Characterization of Escherichia coli
Bio-Typing: By Conventional Biochemical Tests and Platform VITEK 2 System
2.3. Sero-Typing: Serological Identification of E. coli Isolates
2.4. Vero-Typing: Detection of Verocytotoxins Producing E. coli Serotypes
2.5. Phenotypic Evaluation of Virulence Markers and Exoenzymes Production
2.5.1. Hemolysin Production
2.5.2. Serum Resistance
2.5.3. Gelatinase Production Test
2.5.4. Phenotypic Assessment Detection of Lipase, Caseinase, Lecithinase, and Amylase
- -
- The lipolytic activity (lipase production) of E. coli isolates was assessed using Tween 80-agar media (composed of 15 mL/L Tween 80, 5 g/L tryptone, 2.5 g/L yeast extract, 5 g/L NaCl, and 20 g/L agar; pH 7.2 ± 0.2). The plates were spot inoculated with the isolates and incubated aerobically for 24–48 h at 37 °C. The presence of clear zones (halos) around the colonies, following staining with methyl red solution (0.2 g/L methyl red in 95% ethanol), indicated the presence of lipolytic activity (lipase production) [46].
- -
- The production of caseinase from the isolates was detected using Skimmed Milk Agar (SMA, HiMedia Laboratories GmbH, Modautal, Germany). The isolates were streaked onto SMA plates and then incubated aerobically for 24 h at 37 °C. The presence of transparent zones around the colonies indicated caseinase production [42].
- -
- To assess lecithinase activity, isolates were cultured on an Egg Yolk Agar base (HiMedia Laboratories GmbH, Modautal, Germany) supplemented with 2.5% yolk. Incubation was conducted for 18–48 h initially, with further incubation for up to 7 days to account for potential delayed lipase activity. The plates were then examined for the presence of opaque zones around the colonies to confirm lecithinase production [42].
- -
- For amylolytic enzymes, the E. coli isolates were streaked onto Starch Agar media plates containing 10 g/L soluble starch, 5 g/L tryptone, 3 g/L yeast extract, and 20 g/L agar at pH 7.2 ± 0.2. The plates were then incubated aerobically for 24–48 h at 37 °C. Following incubation, the plates were flooded with potassium iodide solution and allowed to react for 10 min. The presence of transparent zones surrounding the colonies indicated amylase production [47,48].
2.6. Biofilm Formation Assay
2.7. Antimicrobial Susceptibility Testing
2.7.1. Determination of Minimum Inhibitory Concentrations (MICs) for E. coli Isolates
2.7.2. Detection of Extended-Spectrum Beta-Lactamase (ESBL) Producers Phenotype in E. coli Isolates
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
- Udders affected by clinical mastitis harbor a diverse range of pathogenic E. coli “O” serotypes, including O157, at a higher prevalence compared to healthy and treated udders.
- These isolates exhibit virulent traits, such as toxin production, hemolysin production, antibiotic resistance, ESBL activity, and biofilm formation.
- Antibiotic resistance is influenced by the therapeutic protocol employed, particularly the choice of antibiotic.
- Even after the end of the withdrawal period, some strains of the E. coli “O” serotype remain in the milk, thus posing a serious threat to public health if this milk is consumed raw or if it enters the dairy process without pasteurization.
- The aforementioned threat is exacerbated by the relatively high prevalence of antibiotic-resistant isolates.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Available online: https://ec.europa.eu/eurostat/statistics (accessed on 25 March 2024).
- van den Brom, R.; de Jong, A.; van Engelen, E.; Heuvelink, A.; Vellema, P. Zoonotic risks of pathogens from sheep and their milk borne transmission. Small Rumin. Res. 2020, 189, 106123. [Google Scholar] [CrossRef] [PubMed]
- Baylis, C. Raw milk and raw milk cheeses as vehicles for infection by Verocytotoxin-producing Escherichia coli. Int. J. Dairy Technol. 2009, 62, 293–307. [Google Scholar] [CrossRef]
- Nespolo, C.; Brandelli, A. Characterization of cheeses produced with ovine and caprine milk and microbiological evaluation of processing areas in the dairy plant in Brazil. Int. Food Res. J. 2012, 19, 1713–1721. [Google Scholar]
- Colonna, A.; Durham, C.; Meunier-Goddik, L. Factors affecting consumers’ preferences for and purchasing decisions regarding pasteurized and raw milk specialty cheeses. J. Dairy Sci. 2011, 94, 5217–5226. [Google Scholar] [CrossRef] [PubMed]
- Lahne, J.; Trubek, A. “A little information excites us”. Consumer sensory experience of Vermont artisan cheese as active practice. Appetite 2014, 78, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Milpa, M.; Arriaga-Jordán, C.M.; Cesín-Vargas, A.; Espinoza-Ortega, A. Characterisation of consumers of traditional foods: The case of Mexican fresh cheeses. Br. Food J. 2016, 118, 915–930. [Google Scholar] [CrossRef]
- Quigley, L.; O’Sullivan, O.; Stanton, C.; Beresford, T.P.; Ross, R.P.; Fitzgerald, G.F.; Cotter, P.D. The complex microbiota of raw milk. FEMS Microbiol. Rev. 2013, 37, 664–698. [Google Scholar] [CrossRef]
- Lamas, A.; Sanjulián, L.; Cepeda, A.; Fente, C.; Regal, P. Milk Microbiota: A Source of Antimicrobial-Producing Bacteria with Potential Application in Food Safety. Proceedings 2021, 70, 11. [Google Scholar] [CrossRef]
- Carloni, E.; Petruzzelli, A.; Amagliani, G.; Brandi, G.; Caverni, F.; Mangili, P.; Tonucci, F. Effect of farm characteristics and practices on hygienic quality of ovine raw milk used for artisan cheese production in central Italy. Anim. Sci. J. 2015, 87, 591–599. [Google Scholar] [CrossRef]
- Baines, J. The role of machine milking in quality milk production and ensuring dairy cow welfare. Cattle Pract. 2010, 18, 48–52. [Google Scholar]
- Stefan, G.; Baraitareanu, S. Approaches of Milking Biosecurity and Milking Parlour Hygiene in Dairy Farms [Internet]. In Recent Developments on Mastitis—Treatment and Control [Working Title]; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Gonzalo, C. Milk hygiene in small ruminants: A review. Span. J. Agric. Res. 2018, 15, e05R02. [Google Scholar] [CrossRef]
- Tonamo, A.; Komlósi, I.; Varga, L.; Czeglédi, L.; Peles, F. Bacteriological Quality of Raw Ovine Milk from Different Sheep Farms. Animals 2020, 10, 1163. [Google Scholar] [CrossRef]
- Toghdory, A.; Ghoorchi, T.; Asadi, M.; Bokharaeian, M.; Najafi, M.; Ghassemi Nejad, J. Effects of Environmental Temperature and Humidity on Milk Composition, Microbial Load, and Somatic Cells in Milk of Holstein Dairy Cows in the Northeast Regions of Iran. Animals 2022, 12, 2484. [Google Scholar] [CrossRef]
- López-Carlos, M.A.; Hernández-Briano, P.; Aguilera-Soto, J.I.; Carrillo-Muro, O.; Medina-Flores, C.A.; Méndez-Llorente, F.; Aréchiga-Flores, C.F. Effect of milking hygiene, herd size, water hardness and temperature-humidity index on milk quality of dairy farms. Rev. Bras. Zootec. 2023, 52, e20210189. [Google Scholar] [CrossRef]
- Marcone, G.; Carnovale, F.; Arney, D.; De Rosa, G.; Napolitano, F. A simple method for on-farm evaluation of sheep welfare using animal-based indicators. Small Rumin. Res. 2022, 208, 106636. [Google Scholar] [CrossRef]
- Jiménez, L.; Perea, J.M.; Caballero-Villalobos, J.; Angón, E.; Cecchinato, A.; Amalfitano, N.; Oliete, B.; Arias, R. A Stochastic Frontier Approach to Study the Relationship between the Hygienic Quality of Bulk Tank Sheep Milk and Technical Efficiency of the Coagulation Process. Foods 2024, 13, 873. [Google Scholar] [CrossRef]
- Gonzalo, C.; Juárez, M.T.; García-Jimeno, M.C.; De La Fuente, L.F. Bulk tank somatic cell count and total bacterial count are affected by target practices and milking machine features in dairy sheep flocks in Castilla y León region, Spain. Small Rumin. Res. 2019, 178, 22–29. [Google Scholar] [CrossRef]
- Lianou, D.T.; Michael, C.K.; Vasileiou, N.G.C.; Petinaki, E.; Cripps, P.J.; Tsilipounidaki, K.; Katsafadou, A.I.; Politis, A.P.; Kordalis, N.G.; Ioannidi, K.S.; et al. Extensive Countrywide Field Investigation of Somatic Cell Counts and Total Bacterial Counts in Bulk-Tank Raw Milk in Sheep Flocks in Greece. Foods 2021, 10, 268. [Google Scholar] [CrossRef]
- Lianou, D.T.; Petinaki, E.; Cripps, P.J.; Gougoulis, D.A.; Michael, C.K.; Tsilipounidaki, K.; Skoulakis, A.; Katsafadou, A.I.; Vasileiou, N.G.C.; Giannoulis, T.; et al. Prevalence, Patterns, Association with Biofilm Formation, Effects on Milk Quality and Risk Factors for Antibiotic Resistance of Staphylococci from Bulk-Tank Milk of Goat Herds. Antibiotics 2021, 10, 1225. [Google Scholar] [CrossRef] [PubMed]
- Condoleo, R.; Giangolini, G.; Chiaverini, A.; Patriarca, D.; Scaramozzino, P.; Mezher, Z. Occurrence of Listeria monocytogenes and Escherichia coli in Raw Sheep’s Milk from Farm Bulk Tanks in Central Italy. J. Food Prot. 2020, 83, 1929–1933. [Google Scholar] [CrossRef]
- Bagel, A.; Sergentet, D. Shiga Toxin-Producing Escherichia coli and Milk Fat Globules. Microorganisms 2022, 10, 496. [Google Scholar] [CrossRef]
- Otero, V.; Sánchez, S.; Herrera-León, S.; Rodríguez-Calleja, J.-M.; Otero, A.; García-López, M.-L.; Santos, J.A. Detection and characterization of Shiga toxin-producing Escherichia coli (STEC) in bulk tank ewes’ milk and sheep farm environment. Small Rumin. Res. 2017, 154, 110–114. [Google Scholar] [CrossRef]
- Available online: https://eur-lex.europa.eu/eli/reg/2004/852/oj (accessed on 2 January 2024).
- Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32004R0853 (accessed on 24 March 2024).
- Valero, A.; Hernandez, M.; De Cesare, A.; Manfreda, G.; González-García, P.; Rodríguez-Lázaro, D. Survival kinetics of Listeria monocytogenes on raw sheep milk cured cheese under different storage temperatures. Int. J. Food Microbiol. 2014, 184, 39–44. [Google Scholar] [CrossRef]
- Yoon, Y.; Lee, S.; Choi, K.H. Microbial benefits and risks of raw milk cheese. Food Control 2016, 63, 201–215. [Google Scholar] [CrossRef]
- Condoleo, R.; Mezher, Z.; Marozzi, S.; Guzzon, A.; Fischetti, R.; Senese, M.; Sette, S.; Bucchini, L. Risk Assessment of Human Listeriosis from Semisoft Cheeses Made from Raw Sheep’s Milk in Lazio and Tuscany (Italy). Risk Anal. 2016, 37, 661–676. [Google Scholar] [CrossRef]
- Napoleoni, M.; Villa, L.; Barco, L.; Busani, L.; Cibin, V.; Lucarelli, C.; Tiengo, A.; Dionisi, A.M.; Conti, F.; Da Silva Nunes, F.R.; et al. A Strong Evidence Outbreak of Salmonella Enteritidis in Central Italy Linked to the Consumption of Contaminated Raw Sheep Milk Cheese. Microorganisms 2021, 9, 2464. [Google Scholar] [CrossRef]
- Cardozo, M.V.; Nespolo, N.; Delfino, T.C.; de Almeida, C.C.; Pizauro, L.J.L.; Valmorbida, M.K.; Pereira, N.; Avila, F.A.D. Raw milk cheese as a potential infection source of pathogenic and toxigenic food born pathogens. Food Sci. Technol. 2021, 41, 355–358. [Google Scholar] [CrossRef]
- Barandika, J.F.; Alvarez-Alonso, R.; Jado, I.; Hurtado, A.; García-Pérez, A.L. Viable Coxiella burnetii in hard cheeses made with unpasteurized milk. Int. J. Food Microbiol. 2019, 303, 42–45. [Google Scholar] [CrossRef]
- Basanisi, M.G.; La Bella, G.; Nobili, G.; Raele, D.A.; Cafiero, M.A.; Coppola, R.; Damato, A.M.; Fraccalvieri, R.; Sottili, R.; La Salandra, G. Detection of Coxiella burnetii DNA in sheep and goat milk and dairy products by droplet digital PCR in south Italy. Int. J. Food Microbiol. 2022, 366, 109583. [Google Scholar] [CrossRef]
- Condoleo, R.; Palumbo, R.; Mezher, Z.; Bucchini, L.; Taylor, R.A. Microbial risk assessment of Escherichia coli shiga-toxin producers (STEC) in raw sheep’s milk cheeses in Italy. Food Control. 2022, 137, 108951. [Google Scholar] [CrossRef]
- Giadinis, N.; Arsenos, G.; Tsakos, P.; Psychas, V.; Dovas, C.; Papadopoulos, E.; Karatzias, H.; Fthenakis, G. Milk-drop syndrome of ewes: Investigation of the causes in dairy sheep in Greece. Small Rumin. Res. 2012, 106, 33–35. [Google Scholar] [CrossRef]
- Gonzales-Barron, U.; Gonçalves-Tenório, A.; Rodrigues, V.; Cadavez, V. Foodborne pathogens in raw milk and cheese of sheep and goat origin: A meta-analysis approach. Curr. Opin. Food Sci. 2017, 18, 7–13. [Google Scholar] [CrossRef]
- Al-Gburi, N.M. Prevalence of Escherichia coli O157: H7 in camels fecal samples. J. Genet. Environ. Resour. Conserv. 2016, 4, 46–50. Available online: https://www.researchgate.net/publication/310477771_Prevalence_of_Escherichia_coli_O157H7_in_camels_fecal_samples (accessed on 24 March 2024).
- Bosilevac, J.M.; Gassem, M.A.; Al Sheddy, I.A.; Almaiman, S.A.; Al-Mohizea, I.S.; Alowaimer, A.; Koohmaraie, M. Prevalence of Escherichia coli O157:H7 and Salmonella in camels, cattle, goats, and sheep harvested for meat in Riyadh. J. Food Prot. 2015, 78, 89–96. [Google Scholar] [CrossRef]
- Tzschoppe, M.; Martin, A.; Beutin, L. A rapid procedure for the detection and isolation of enterohaemorrhagic Escherichia coli (EHEC) serogroup O26, O103, O111, O118, O121, O145 and O157 strains and the aggregative EHEC O104:H4 strain from ready-to-eat vegetables. Int. J. Food Microbiol. 2012, 152, 19–30. [Google Scholar] [CrossRef]
- Godbout-DeLasalle, F.; Higgins, R. Biotyping of clinical isolates of Escherichia coli of animal origin, using the Analytab API 20E system. Can. J. Vet. Res. 1986, 50, 418–421. [Google Scholar] [PubMed] [PubMed Central]
- Akinjogunla, O.J.; Akaka, B.C.; Okon, M.U.; Inyang, C.U.; Umoh, M.I.; Etukudo, I.U. Serological identification, virulence factors, antibiogram and plasmid profile of Escherichia coli serotypes in raw milk and pasteurized milk products. Sci. Afr. 2022, 17, e01314. [Google Scholar] [CrossRef]
- Akinjogunla, O.J.; Akaka, B.C.; Inyang, C.U. Epidemiological Investigation, Serotypes and Distribution of Verocytotoxigenic Escherichia coli (VTEC) in Raw Milk and Milk Products in Uyo, Nigeria. Niger. J. Biotechnol. 2020, 37, 10–20. [Google Scholar] [CrossRef]
- Vaish, R.; Pradeep, M.; Setty, C.R.; Kandi, V. Evaluation of Virulence Factors and Antibiotic Sensitivity Pattern of Escherichia coli Isolated from Extraintestinal Infections. Cureus 2016, 8, e604. [Google Scholar] [CrossRef]
- Orole, O.O.; Gambo, S.M.; Fadayomi, V.S. Characteristics of Virulence Factors and Prevalence of Virulence Markers in Resistant Escherichia coli from Patients with Gut and Urinary Infections in Lafia, Nigeria. Microbiol. Insights 2022, 15, 11786361221106993. [Google Scholar] [CrossRef]
- Sharma, S.; Bhat, G.K.; Shenoy, S. Virulence factors and drug resistance in Escherichia coli isolated from extraintestinal infections. Indian J. Med Microbiol. 2007, 25, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Samad, M.Y.A.; Razak, C.A.; Salleh, A.B.; Yunus, W.Z.W.; Ampon, K.; Basri, M. A plate assay for primary screening of lipase activity. J. Microbiol. Methods 1989, 9, 51–56. [Google Scholar] [CrossRef]
- Amoozegar, M.A.; Malekzadeh, F.; Malik, K.A. Production of amylase by newly isolated moderate halophile, Halobacillus sp. strain MA-2. J. Microbiol. Methods 2003, 52, 353–359. [Google Scholar] [CrossRef]
- Akinjogunla, O.J.; Adenugba, I.T.; Inyang, U.O. Extracellular Hydrolytic Enzymes and Location of Multidrug Resistance. World J. Biomed. Res. 2017, 4, 50–60. [Google Scholar]
- Tremblay, Y.D.; Vogeleer, P.; Jacques, M.; Harel, J. High-throughput microfluidic method to study biofilm formation and host-pathogen interactions in pathogenic Escherichia coli. Appl. Environ. Microbiol. 2015, 81, 2827–2840. [Google Scholar] [CrossRef] [PubMed]
- Novais, A.; Vuotto, C.; Pires, J.; Montenegro, C.; Donelli, G.; Coque, T.M. Diversity and biofilm-production ability among isolates of Escherichia coli phylogroup D belonging to ST69, ST393 and ST405 clonal groups. BMC Microbiol. 2013, 13, 144. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standard Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; CLSI Document M100-28; Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2018. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing. The Breakpoint Tables for Interpretations of MICs and Zone Diameters; Version 3.0, Valid from 2013-01-01; EUCAST: Basel, Switzerland, 2013. [Google Scholar]
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk Susceptibility Tests; M2-A6; NCCLS: Villanova, PA, USA, 2000. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing, EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters. 2016. Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_5.0_Breakpoint_Table_01.pdf (accessed on 2 January 2024).
- Halabi, M.K.; Lahlou, F.A.; Diawara, I.; El Adouzi, Y.; Marnaoui, R.; Benmessaoud, R.; Smyej, I. Antibiotic Resistance Pattern of Extended Spectrum Beta Lactamase Producing Escherichia coli Isolated From Patients with Urinary Tract Infection in Morocco. Front. Cell. Infect. Microbiol. 2021, 11, 720701. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing (M100), 30th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Sampah, J.; Owusu-Frimpong, I.; Aboagye, F.T.; Owusu-Ofori, A. Prevalence of carbapenem-resistant and extended-spectrum beta-lactamase-producing Enterobacteriaceae in a teaching hospital in Ghana. PLoS ONE 2023, 18, e0274156. [Google Scholar] [CrossRef]
- Owusu, F.A.; Obeng-Nkrumah, N.; Gyinae, E.; Kodom, S.; Tagoe, R.; Tabi, B.K.A.; Dayie, N.T.K.D.; Opintan, J.A.; Egyir, B. Occurrence of Carbapenemases, Extended-Spectrum Beta-Lactamases and AmpCs among Beta-Lactamase-Producing Gram-Negative Bacteria from Clinical Sources in Accra, Ghana. Antibiotics 2023, 12, 1016. [Google Scholar] [CrossRef]
- Friker, B.; Morach, M.; Püntener, S.; Cernela, N.; Horlbog, J.; Stephan, R. Assessing the microbiological quality of raw goats’ and ewes’ tank milk samples in Switzerland. Int. Dairy J. 2019, 102, 104609. [Google Scholar] [CrossRef]
- El Malt, L.M.; El Wanis, S.A.A.; El-Zamkan, M.A.; Hafoda, M.A. Molecular characterization of Escherichia coli isolated from raw sheep and goat milk. J. Agric. Sci. Vet. Med. 2017, 5, 127–138. Available online: http://www.ijasvm.com/currentissue.php (accessed on 2 January 2024).
- Ombarak, R.A.; Hinenoya, A.; Awasthi, S.P.; Iguchi, A.; Shima, A.; Elbagory, A.M.; Yamasaki, S. Prevalence and pathogenic potential of Escherichia coli isolates from raw milk and raw milk cheese in Egypt. Int. J. Food Microbiol. 2016, 221, 69–76. [Google Scholar] [CrossRef]
- Mørk, T.; Waage, S.; Tollersrud, T.; Kvitle, B.; Sviland, S. Clinical mastitis in ewes; bacteriology, epidemiology and clinical features. Acta Vet. Scand. 2007, 49, 23. [Google Scholar] [CrossRef]
- El-Seedy, F.R.; Raheel, I.; Hassan, W.H.; Ramadan, A.G. Antimicrobial and Virulence Characteristics of Escherichia coli Isolated from Mastitis and Endometritis Cases in Sheep and Goats. J. Vet. Med. Res. 2023, 30, 12–18. [Google Scholar] [CrossRef]
- Fotou, K.; Tzora, A.; Voidarou Ch Alexopoulos, A.; Plessas, S.; Avgeris, I.; Bezirtzoglou, E.; Akrida-Demertzi, K.; Demertzis, P.G. Isolation of microbial pathogens of subclinical mastitis from raw sheep’s milk of Epirus (Greece) and their role in its hygiene. Anaerobe 2011, 17, 315–319. [Google Scholar] [CrossRef]
- Sancak, Y.C.; Sancak, H.; Isleyici, O.; Durmaz, H. Presence of Escherichia coli O157 and O157:H7 in raw milk and van herby cheese. J. Vet. Res. 2015, 59, 511–514. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, X.; Wu, J.; Liu, Q.; Pang, X.; Yang, H. Metabolic characterisation of eight Escherichia coli strains including “Big Six” and acidic responses of selected strains revealed by NMR spectroscopy. Food Microbiol. 2020, 88, 103399. [Google Scholar] [CrossRef]
- Beier, R.C.; Franz, E.; Bono, J.L.; Mandrell, R.E.; Fratamico, P.M.; Callaway, T.R.; Andrews, K.; Poole, T.L.; Crippen, T.L.; Sheffield, C.L.; et al. Disinfectant and antimicrobial susceptibility profiles of the big six non-O157 Shiga toxin-producing Escherichia coli strains from food animals and humans. J. Food Prot. 2016, 79, 1355–1370. [Google Scholar] [CrossRef]
- Detert, K.; Schmidt, H. Sporadic Detection of Escherichia coli O104:H4 Strain C227/11Φcu in the Edible Parts of Lamb’s Lettuce Cultured in Contaminated Agricultural Soil Samples. Microorganisms 2023, 11, 2072. [Google Scholar] [CrossRef]
- Amemiya, K.; Rozak, D.A.; Dankmeyer, J.L.; Dorman, W.R.; Marchand, C.; Fetterer, D.P.; Worsham, P.L.; Purcell, B.K. Shiga-Toxin-Producing Strains of Escherichia coli O104:H4 and a Strain of O157:H7, Which Can Cause Human Hemolytic Uremic Syndrome, Differ in Biofilm Formation in the Presence of CO2 and in Their Ability to Grow in a Novel Cell Culture Medium. Microorganisms 2023, 11, 1744. [Google Scholar] [CrossRef]
- Perez-Corrales, C.; Peralta-Barquero, V.; Duarte-Martinez, F.; Oropeza-Barrios, G. A 6-year retrospective study of clinical features, microbiology, and genomics of Shiga toxin-producing Escherichia coli in children who presented to a tertiary referral hospital in Costa Rica. Microbiol. Spectr. 2024, 12, e0305623. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Furevi, A.; Perepelov, A.V.; Guo, X.; Cao, H.; Wang, Q.; Reeves, P.R.; Knirel, Y.A.; Wang, L.; Widmalm, G. Structure and genetics of Escherichia coli O antigens. FEMS Microbiol. Rev. 2019, 44, 655–683. [Google Scholar] [CrossRef]
- DebRoy, C.; Roberts, E.; Fratamico, P.M. Detection of O antigens in Escherichia coli. Anim. Heal. Res. Rev. 2011, 12, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Croxen, M.A.; Law, R.J.; Scholz, R.; Keeney, K.M.; Wlodarska, M.; Finlay, B.B. Recent advances in understanding enteric pathogenic Escherichia coli. Clin. Microbiol. Rev. 2013, 26, 822–880. [Google Scholar] [CrossRef] [PubMed]
- Sarowska, J.; Futoma-Koloch, B.; Jama-Kmiecik, A.; Frej-Madrzak, M.; Ksiazczyk, M.; Bugla-Ploskonska, G.; Choroszy-Krol, I. Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: Recent reports. Gut Pathog. 2019, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Pakbin, B.; Brück, W.M.; Rossen, J.W.A. Virulence Factors of Enteric Pathogenic Escherichia coli: A Review. Int. J. Mol. Sci. 2021, 22, 9922. [Google Scholar] [CrossRef] [PubMed]
- Doranga, S.; Conway, T. Nitrogen assimilation by E. coli in the mammalian intestine. mBio 2024, 15, e0002524. [Google Scholar] [CrossRef]
- Conway, T.; Cohen, P.S. Commensal and Pathogenic Escherichia coli Metabolism in the Gut. Microbiol. Spectr. 2015, 3, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Foster-Nyarko, E.; Pallen, M.J. The microbial ecology of Escherichia coli in the vertebrate gut. FEMS Microbiol. Rev. 2022, 46, fuac008. [Google Scholar] [CrossRef]
- Algburi, A.; Zehm, S.; Netrebov, V.; Bren, A.B.; Chistyakov, V.; Chikindas, M.L. Subtilosin prevents biofilm formation by inhibiting bacterial quorum sensing. Probiotics Antimicrob. Proteins 2017, 9, 81–90. [Google Scholar] [CrossRef]
- Ciofu, O.; Rojo-Molinero, E.; Macià, M.D.; Oliver, A. Antibiotic treatment of biofilm infections. APMIS 2017, 125, 304–319. [Google Scholar] [CrossRef] [PubMed]
- Shree, P.; Singh, C.K.; Sodhi, K.K.; Surya, J.N.; Singh, D.K. Biofilms: Understanding the structure and contribution towards bacterial resistance in antibiotics. Med. Microecol. 2023, 16, 100084. [Google Scholar] [CrossRef]
- Hathroubi, S.; Mekni, M.A.; Domenico, P.; Nguyen, D.; Jacques, M. Biofilms: Microbial Shelters Against Antibiotics. Microb. Drug Resist. 2017, 23, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Atlaw, N.A.; Keelara, S.; Correa, M.; Foster, D.; Gebreyes, W.; Aidara-Kane, A.; Harden, L.; Thakur, S.; Fedorka-Cray, P.J. Evidence of sheep and abattoir environment as important reservoirs of multidrug resistant Salmonella and extended-spectrum beta-lactamase Escherichia coli. Int. J. Food Microbiol. 2021, 363, 109516. [Google Scholar] [CrossRef] [PubMed]
- Bezabih, Y.M.; Sabiiti, W.; Alamneh, E.; Bezabih, A.; Peterson, G.M.; Bezabhe, W.M.; Roujeinikova, A. The global prevalence and trend of human intestinal carriage of ESBL-producing Escherichia coli in the community. J. Antimicrob. Chemother. 2020, 76, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Obaidat, M.M.; Gharaibeh, W.A. Sheep and goat milk in Jordan is a reservoir of multidrug resistant extended spectrum and AmpC beta-lactamases Escherichia coli. Int. J. Food Microbiol. 2022, 377, 109834. [Google Scholar] [CrossRef]
- Osińska, A.; Korzeniewska, E.; Korzeniowska-Kowal, A.; Wzorek, A.; Harnisz, M.; Jachimowicz, P.; Buta-Hubeny, M.; Zieliński, W. The challenges in the identification of Escherichia coli from environmental samples and their genetic characterization. Environ. Sci. Pollut. Res. 2022, 30, 11572–11583. [Google Scholar] [CrossRef]
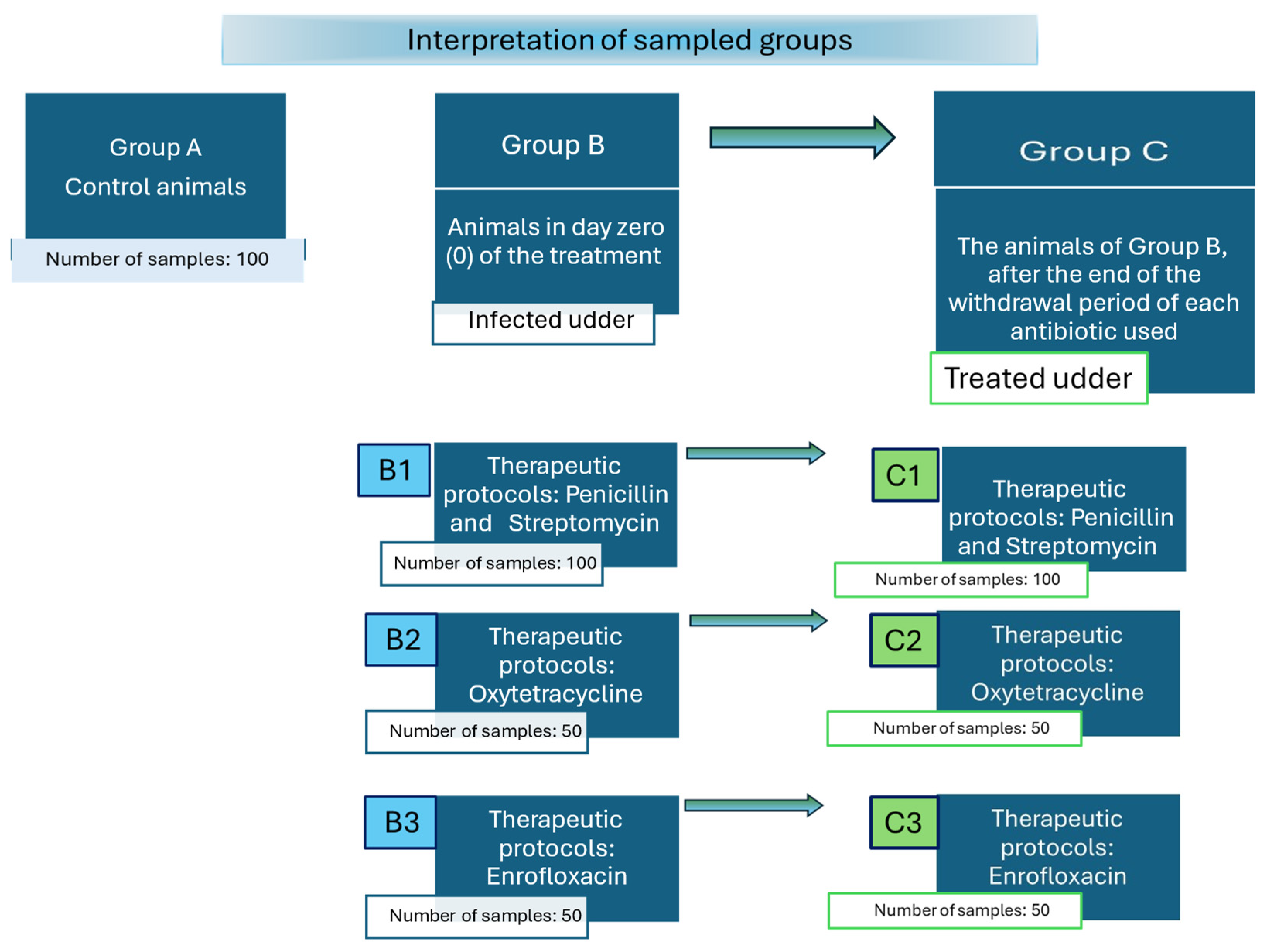
| Experimental Group | Substance | Therapeutic Protocol |
|---|---|---|
| Group 1 | Penicillin/streptomycin (Commercial preparation containing per mL 200,000 IU of procain penicillin G and 250 mg dihydrostreptomycine | 1 mL/25 kg; BW a qd b IM c for 5 days |
| Group 2 | Oxytetracycline | 8 mg/kg BW; qd SC d or IM for 5 days |
| Group 3 | Enrofloxacin | 2.5–5 mg/kg qd SC for 5 days |
| Group A * Controls (Healthy Udders) (n = 100) ** | Group B (Mastitis, before Treatment) (n = 200) | Group C1 (Treatment Udders with Penicillin and Streptomycin, on the First Day after the End of the Withdrawal Period of Each Antibiotic Used) (n = 100) | Group C2 (Treatment Udders with Oxytetracycline, on the First Day after the End of the Withdrawal Period of each Antibiotic Used) (n = 50) | Group C3 (Treatment Udders with Enrofloxacin, on the First Day after the End of the Withdrawal Period of each Antibiotic Used) (n = 50) | Total (n) | |
|---|---|---|---|---|---|---|
| Non-E. coli O157:H7 | 3.77 ± 0.52 (n = 5) *** | 1.91 ± 0.71 (n = 55) | 0.87 ± 0.18 (n = 8) | 1.99 ± 0.83 (n = 7) | 1.19 ± 0.61 (n = 13) | 88 |
| E. coli O157:H7 Sorbitol (+) | 0 | 2.36 (n = 1) | 1.90 (n = 1) | 1.65 ± 0.35 (n = 2) | 0 | 4 |
| E. coli O157:H7 Sorbitol (−) | 0 | 2.29 ± 0.25 (n = 3) | 1.98 (n = 1) | 1.72 (n = 1) | 2.61 (n = 1) | 5 |
| Total | 5 | 59 | 10 | 10 | 14 | 98 |
| Metabolic/Virulent Traits * | Group A** (n = 5 ***) | Group B (n = 55) | Group C1 (n = 8) | Group C2 (n = 7) | Group C3 (n = 13) |
|---|---|---|---|---|---|
| Lipase | 3 (60.00%) | 19 (34.55%) | 3 (37.50%) | 2 (28.57%) | 1 (7.70%) |
| Caseinase | 2 (40.00%) | 18 (32.73%) | 3 (37.50%) | 4 (57.14%) | 9 (69.23%) |
| Amylase | 4 (80.00%) | 44 (80.00%) | 7 (87.50%) | 6 (85.71%) | 13 (100%) |
| Lecithinase | 2 (40.00%) | 10 (18.19%) | 3 (37.50%) | 2 (28.57%) | 2 (15.40%) |
| Gelatinase | 3 (60.00%) | 44 (80.00%) | 7 (87.50%) | 7 (100%) | 9 (69.23%) |
| Serum resistance | 1 (20.00%) | 31 (56.36%) | 8 (100%) | 7 (100%) | 12 (92.31%) |
| Hemolysin | 4 (80.00%) | 20 (36.37%) | 5 (62.50%) | 4 (57.14%) | 6 (46.15%) |
| Serotoxin 1 | 0 (0%) | 17 (30.91%) | 6 (75.00%) | 4 (57.14%) | 3 (23.07%) |
| Serotoxin 2 | 2 (40.00%) | 8 (14.55%) | 0 (0%) | 3 (42.85%) | 4 (30.77%) |
| Metabolic/ Virulent Traits * | Sorbitol (−) | Sorbitol (+) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Strain no 1 (Group B) ** | Strain no 2 (Group B) | Strain no 3 (Group B) | Strain no 4 (Group C1) | Strain no 5 (Group C2) | Strain no 6 (Group C3) | Strain no 7 (Group B) | Strain no 8 (Group C1) | Strain no 9 (Group C2) | Strain no 10 (Group C2) | |
| Lipase | No | No | No | No | No | No | No | No | No | No |
| Caseinase | No | No | No | Yes | No | No | Yes | No | Yes | No |
| Amylase | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Lecithinase | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes |
| Gelatinase | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Serum resistance | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Hemolysin | Yes | No | No | No | Yes | Yes | No | Yes | yes | Yes |
| Serotoxin 1 | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Serotoxin 2 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| ESBL phenotype | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Biofilm formation | strong | weak | strong | moderate | No | strong | weak | weak | strong | No |
| Antimicrobial Agents | Experimental Group | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B1 (n = 30) a | C1 (n = 10) | B2 (n = 13) | C2 (n = 9) | B3 (n = 13) | C3 (n = 14) | |||||||||||||
| S b | I | R | S | I | R | S | I | R | S | I | R | S | I | R | S | I | R | |
| Mechanism of action: Inhibition of cell wall synthesis | ||||||||||||||||||
| AMP c | 12 | 5 | 13 | 1 | 1 | 8 | 2 | - | 11 | 3 | - | 6 | 2 | - | 11 | 1 | - | 13 |
| CEP | 25 | 3 | 2 | 5 | 3 | 2 | 8 | 2 | 3 | 5 | - | 4 | 8 | 2 | 3 | 10 | 4 | - |
| CEF | 25 | - | 5 | 9 | - | 1 | 12 | - | 1 | 8 | - | 1 | 12 | - | 1 | 14 | - | - |
| Mechanism of action: Binding at the 30s ribosomal subunit | ||||||||||||||||||
| AM | 30 | - | - | 10 | - | - | 13 | - | - | 9 | - | - | 13 | - | - | 1 | - | 1 |
| TET | 22 | 1 | 7 | 5 | 1 | 4 | 6 | 2 | 5 | 6 | 1 | 2 | 6 | 2 | 5 | 3 | 2 | 9 |
| GEN | 27 | 3 | - | 9 | 1 | - | 9 | 4 | - | 3 | 4 | 2 | 9 | 4 | - | 8 | 4 | 2 |
| Mechanism of action: Binding at the 50s ribosomal subunit of the 70s ribosome | ||||||||||||||||||
| CHL | 23 | 1 | 6 | 6 | 2 | 2 | 10 | 3 | - | 6 | 3 | - | 10 | 3 | - | 10 | 3 | 1 |
| STR | 21 | 2 | 7 | 4 | 1 | 5 | 7 | 2 | 4 | 2 | 1 | 6 | 7 | 2 | 4 | 5 | 2 | 7 |
| Mechanism of action: Inhibition of the tetrahydrofolic acid synthesis | ||||||||||||||||||
| SUL | 30 | - | - | 10 | - | - | 13 | 1 | - | 9 | - | - | 13 | - | - | 14 | - | - |
| TRI/SU | 30 | - | - | 10 | - | - | 13 | - | - | 9 | - | - | 13 | - | - | 14 | - | - |
| Mechanism of action: Inhibition of DNA gyrase | ||||||||||||||||||
| NAL | 19 | - | 11 | 8 | - | 2 | 7 | - | 6 | 6 | - | 3 | 7 | - | 6 | 9 | - | 5 |
| CIP | 27 | 2 | 1 | 7 | 2 | 1 | 10 | 3 | - | 6 | 2 | 1 | 10 | 3 | - | 3 | 4 | 7 |
| Production of extended spectrumβ-lactamase-ESBLs phenotypes | ||||||||||||||||||
| Yes | 8 | 4 | 9 | 4 | 5 | 10 | ||||||||||||
| No | 22 | 6 | 4 | 5 | 8 | 4 | ||||||||||||
| Antimicrobial Agents | Tracking/Labeling of E. coli O157:H7 Isolates | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Strain no 1 B * (−) ** | Strain no 2 B (−) | Strain no 3 B (−) | Strain no 4 C1 (−) | Strain no 5 C3 (−) | Strain no 6 B (+) | Strain no 7 C1 (+) | Strain no 8 C2 (+) | Strain no 9 C2 (+) | |
| Mechanism of action: Inhibition of cell wall synthesis *** | |||||||||
| AMP a | R ^ | R | R | R | R | R | R | R | R |
| CEP | S | S | I | S | I | R | I | R | S |
| CEF | R | S | S | S | S | S | S | S | S |
| Mechanism of action: Binding at the 30s ribosomal subunit | |||||||||
| AM | S | S | R | S | R | S | S | S | S |
| TET | R | R | R | R | R | R | S | R | S |
| GEN | I | I | I | S | I | I | S | S | I |
| Mechanism of action: Binding at the 50s ribosomal subunit of the 70s ribosome | |||||||||
| CHL | R | S | S | S | S | S | S | S | I |
| STR | R | I | R | R | R | S | I | S | R |
| Mechanism of action: Inhibition of the tetrahydrofolic acid synthesis | |||||||||
| TRI/SU | S | S | S | S | S | S | S | S | S |
| SUL | S | S | S | S | S | S | S | S | S |
| Mechanism of action: Inhibition of DNA gyrase | |||||||||
| NAL | S | R | S | S | S | R | S | R | S |
| CIP | S | I | R | S | R | I | S | R | S |
| Number of classes in which the strains were found resistant | |||||||||
| 3 | 3 | 4 | 3 | 4 | 3 | 1 | 3 | 2 | |
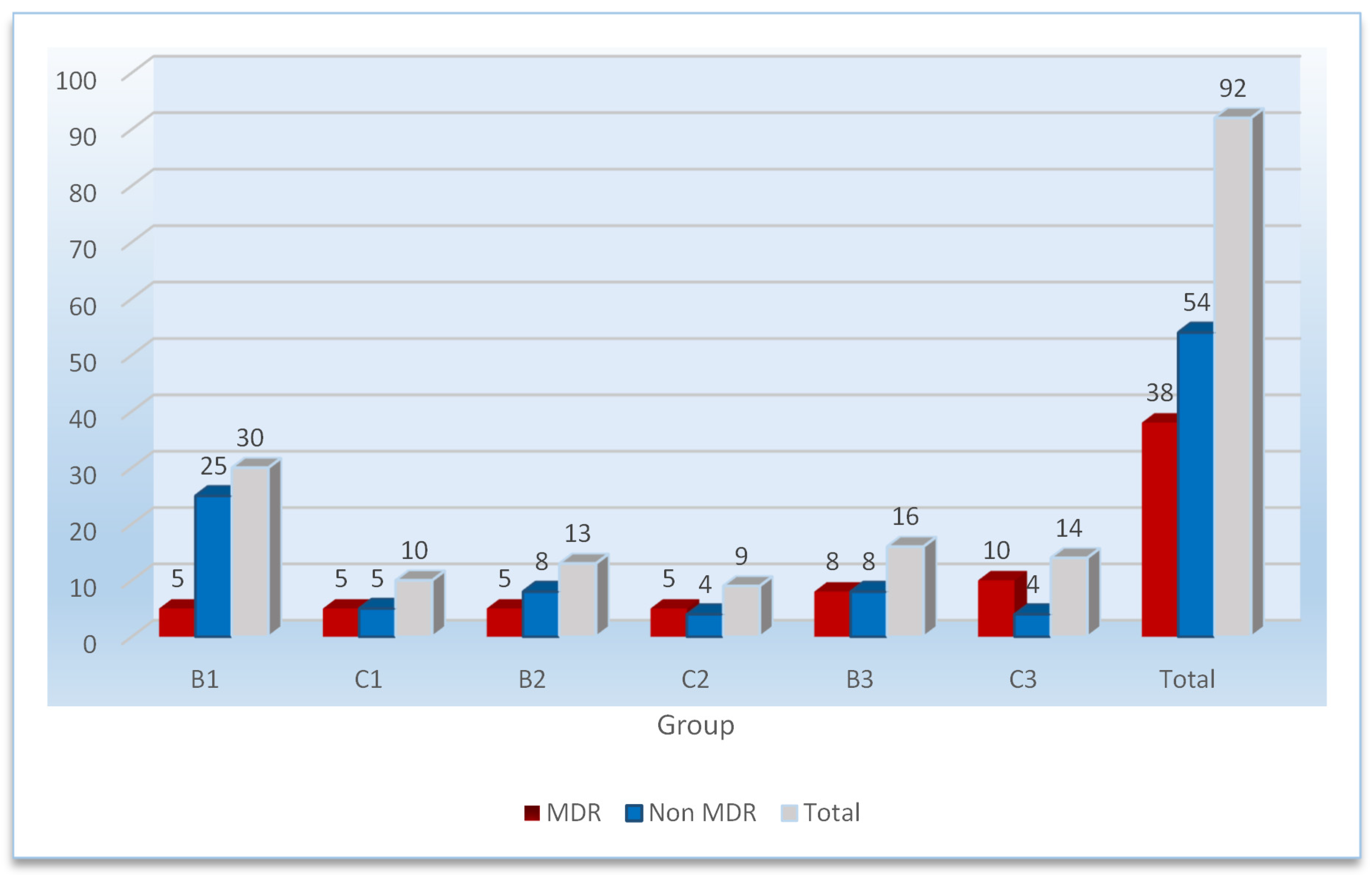
| Group | − * | + ** | ++ | +++ | Total |
|---|---|---|---|---|---|
| B1 *** | 14 | 2 | 4 | 10 | 30 |
| C1 | 2 | 1 | 2 | 5 | 10 |
| B2 | 8 | 2 | 1 | 2 | 13 |
| C2 | 3 | - | 3 | 3 | 9 |
| B3 | 9 | 1 | 4 | 2 | 16 |
| C3 | 8 | - | 1 | 5 | 14 |
| Total | 44 | 6 | 15 | 27 | 92 |
| Group | Non-O157:H7 E. coli “O” Serotypes | Total |
|---|---|---|
| A * | O5, O103 (n = 2) **, O45 | 4 |
| B1 | O103 (n = 6), O118 (n = 2), O128, O111 (n = 5), O145 (n = 3), O61, O78, O25, O121 (n = 2), O104, O45 (n = 2), O115, O130, O18, O88 | 29 |
| B2 | O28 (n = 3), O111 (n = 2), O125, O145 (n = 2), O104, O26 | 10 |
| B3 | O26 (n = 2), O111 (n = 3), O25, O145, O106, O104, O103, O125, O28, O152 | 13 |
| C1 | O103 (n = 3), O61, O121, O118, O104, O145 | 8 |
| C2 | O28 (n = 2), O111, O104, O26, O45 | 6 |
| C3 | O26 (n = 2), O111 (n = 4), O145 (n = 2), O25, O106, O104, O28, O152 | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotou, K.; Rozos, G.; Zaralis, K.; Dadamogia, A.; Stavropoulou, E.; Demertzis, P.; Akrida-Demertzi, K.; Tzora, A.; Voidarou, C. Pathogens in the Food Chain: Escherichia coli Strains in Raw Milk Originating from Ewes Treated for Mastitis with Various Therapeutic Protocols. Appl. Sci. 2024, 14, 5383. https://doi.org/10.3390/app14135383
Fotou K, Rozos G, Zaralis K, Dadamogia A, Stavropoulou E, Demertzis P, Akrida-Demertzi K, Tzora A, Voidarou C. Pathogens in the Food Chain: Escherichia coli Strains in Raw Milk Originating from Ewes Treated for Mastitis with Various Therapeutic Protocols. Applied Sciences. 2024; 14(13):5383. https://doi.org/10.3390/app14135383
Chicago/Turabian StyleFotou, Konstantina, Georgios Rozos, Konstantinos Zaralis, Aikaterini Dadamogia, Elisavet Stavropoulou, Panagiotis Demertzis, Konstantoula Akrida-Demertzi, Athina Tzora, and Chrysoula (Chrysa) Voidarou. 2024. "Pathogens in the Food Chain: Escherichia coli Strains in Raw Milk Originating from Ewes Treated for Mastitis with Various Therapeutic Protocols" Applied Sciences 14, no. 13: 5383. https://doi.org/10.3390/app14135383
APA StyleFotou, K., Rozos, G., Zaralis, K., Dadamogia, A., Stavropoulou, E., Demertzis, P., Akrida-Demertzi, K., Tzora, A., & Voidarou, C. (2024). Pathogens in the Food Chain: Escherichia coli Strains in Raw Milk Originating from Ewes Treated for Mastitis with Various Therapeutic Protocols. Applied Sciences, 14(13), 5383. https://doi.org/10.3390/app14135383








