Abstract
Diatomaceous earth (DE), a kind of natural and environmentally friendly concrete admixture with good pozzolanic and water absorption properties, is a potential high-quality internal curing material. DE internal curing agent was prepared by calcining excavated diatomaceous earth and applied in cement-based materials with a low water–binder ratio of 0.35 to study its effects on the autogenous shrinkage and early crack resistance of the materials. The results showed that DE was a kind of microfine powder with a unique and orderly microporous structure, and its main component was SiO2, which had good water-holding and -releasing properties. Pre-absorbed DE could effectively improve the internal relative humidity of cement-based materials with a low water–binder ratio. Under the standard of not reducing the 28 d compressive strength of mortar, compared to the benchmark group without DE, when 1% of cement is replaced by DE of equal mass, DE could effectively reduce the 7 d autogenous shrinkage of mortar by about 36.7% and delay the initial cracking time of mortar under the restraint condition of the ring by 10.7%. Therefore, it is an internal curing material with excellent performance.
1. Introduction
Currently, concrete is extensively used in civil engineering projects [1]. Against the backdrop of China’s active promotion of the western development strategy, many construction projects are facing severe challenges in terms of working environments. These projects have imposed stricter standards and requirements on the mechanical and durability properties of concrete, leading to the development of high-strength concrete with superior mechanical and durability properties.
The enhancement of concrete is typically achieved by reducing the water–binder ratio [2]. To maintain the workability of high-strength concrete, it is necessary to add high-efficiency water-reducing agents to keep its good fluidity and meet the pumping requirements on construction sites [3]. However, with the widespread application of high-strength concrete in the construction field, we also need to be aware of the adverse issues it may cause. High-strength concrete has a low content of free water available for hydration reactions, and the rapid decrease in free water content during cement hydration due to the large amount of cement used can lead to increased capillary stress, thus exacerbating material shrinkage issues caused by humidity gradients [4,5]. If high-strength concrete is not adequately moisturized during the early curing stage and hydration is incomplete, it can lead to autogenous shrinkage and drying shrinkage, resulting in cracks in the concrete structure at an early stage. These cracks not only provide a pathway for the intrusion of harmful substances from the outside but also weaken the load-bearing capacity of the concrete structure [6], leading to durability issues and failure to achieve the desired good durability of high-strength concrete [7,8]. This not only shortens the service life of the concrete structure but also significantly affects its early-stage strength development due to insufficient internal moisture, which slows down the hydration reaction rate of cement paste [9]. Additionally, repairing concrete cracks is not only labor-intensive and costly but also economically inefficient [10]. Therefore, compared to ordinary concrete, high-strength concrete has a higher demand for early-stage curing. To effectively mitigate its early-stage autogenous and drying shrinkage issues, it is necessary to select appropriate curing methods for high-strength concrete to ensure its durability.
Concrete curing can be divided into two basic types: external curing and internal curing. Ordinary concrete is typically cured through external methods such as water storage, watering, spraying, or covering with wet sacks [11]. However, these methods have long curing cycles, require significant manpower and resources, and may vary in effectiveness depending on the individual performing the curing. For cement-based materials with a low water–binder ratio, their dense structure and the difficulty of external curing water penetrating into the interior of the concrete make it challenging to provide timely moisture to promote the hydration reaction of the paste. Therefore, this curing method is not effective in mitigating the autogenous drying caused by internal water scarcity in concrete [12], thus limiting its ability to reduce paste shrinkage and ensure good durability.
As a result, there is a need for an alternative and more effective curing technique that can pre-store curing moisture inside high-strength concrete, replenish moisture when needed, and participate in the entire hydration process of the concrete until the relative humidity difference between the interior and exterior of the paste reaches zero. This internal curing approach can effectively mitigate autogenous and drying shrinkage.
Common internal curing methods include internal curing with saturated lightweight aggregate (LWA) [13,14] and super absorbent polymer (SAP) [15,16,17,18,19,20,21]. These materials used as internal curing carriers can continuously release water loaded inside the concrete, maintaining a relatively stable internal humidity environment. Although the use of the above internal curing agents can reduce the shrinkage development of cementitious materials, it may lead to negative effects such as reduced strength of cement-based materials [22,23,24,25], unstable volume development, and poor economy. Therefore, it has been a major challenge in the field of engineering materials to achieve a balance between reducing the shrinkage development of cementitious materials and ensuring that the incorporation of internal curing agents does not reduce the mechanical properties of cement-based materials.
Diatomaceous earth (DE), as a low-cost and easily accessible mineral resource, has a unique natural macropore/mesopore structure, which enables it to have good water absorption and release properties, making it a potential high-quality internal curing agent. The main component of diatomite is silicon dioxide [26], which has a certain pozzolanic activity and can participate in hydration reactions. Common lime mortar can also be used to restore ancient buildings through pozzolanic reactions, which can improve the surface strength of buildings. Therefore, to a certain extent, it can offset the decrease in mortar strength caused by the introduction of DE internal curing agent [27,28,29].
This article mainly uses local materials such as ordinary Portland cement (OPC), diatomaceous earth (DE), and river sand (RS) to design low water–binder ratio cement mortar. Part of the cement is replaced with DE, which can achieve internal curing effects with simple processing and low cost and is conducive to popularization and application. Additionally, the use of DE as a 1% cement substitute in the production of cement mortar reduces carbon dioxide emissions by 1.962 tons per 1000 m3 of brick masonry project, thus mitigating the environmental impact caused by partial cement replacement with DE [30]. Therefore, the purpose of this study is to determine the effects of replacing OPC with DE as an internal curing agent on the mechanical properties, shrinkage properties, crack resistance, and microstructure of low water–binder ratio cement mortar.
2. Materials and Methods
2.1. Materials
The materials used include OPC, DE, and RS. OPC is used as a binder, while DE is used to partially replace cement and serves as an internal curing agent. RS is used as aggregate. The DE is industrial-grade diatomite produced by Henan Boxu Environmental Protection, and its main component is silica, with a SiO2 content of 71.56% and a loss on ignition of 0.303%. Meanwhile, the OPC used is produced by Tianshan Cement Factory in Urumqi City, with a loss on ignition of 1.75%. DE, made of industrial-grade diatomite from Henan Boxu Environmental Protection, is crushed and sieved to ensure it passes through a #200 sieve, with a loss on ignition of 0.303%. The microscopic morphology of DE was captured by scanning electron microscopy, as shown in Figure 1. The chemical composition of OPC and DE, determined using X-ray fluorescence testing, is presented in Table 1. Tap water from Urumqi City is used for mixing, and polycarboxylate water-reducing agent is added to adjust the fluidity of the cement mortar.

Figure 1.
Microstructural image of DE.

Table 1.
Chemical composition of OPC and DE.
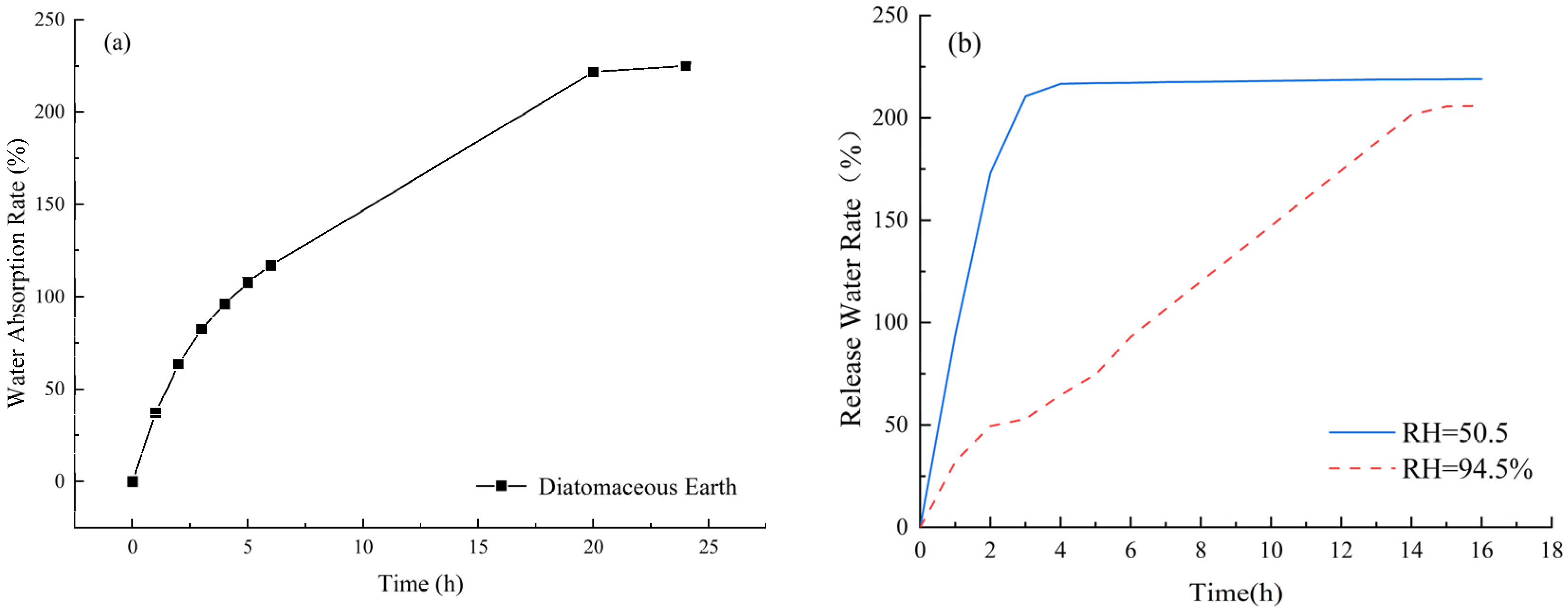
The water absorption and release performance of internal curing agents are crucial indicators for determining their efficiency. Water absorption determines the amount of internal curing water available for practical use, and a higher water absorption rate leads to more internal curing water being introduced. This, in turn, provides more water during hydration and evaporation, resulting in better shrinkage reduction in the slurry. The water release rate has a significant impact on the internal curing effect. If an internal curing agent has a high water absorption rate but a low water release rate, introducing it into concrete with a low water release rate or no water release would not only waste water resources and increase the self-weight of the concrete but also fail to achieve a good internal curing effect. Therefore, it is necessary to measure the water absorption and release characteristics of diatomite. Since DE is in powder form, with pores on its particle surface, its water absorption rate, measured using the tea bag method [31], is 230% of its mass, as shown in Figure 2a. By simulating the internal solution environment of cement mortar using potassium nitrate and a supersaturated solution of sodium bromide, and controlling the relative humidity at 94.5% and 50.5%, respectively, the water release performance of DE under different relative humidity conditions was measured, as shown in Figure 2b.
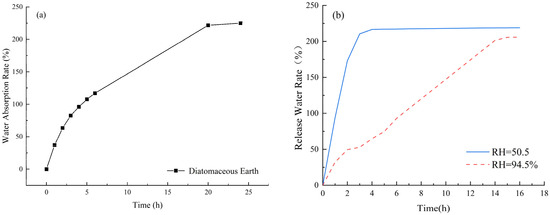
Figure 2.
The water absorption and release performance of DE. (a) Water absorption; (b) water release.
As shown in Figure 2b, under a relatively low humidity of 50.5%, the water release rate (the percentage of water loss to total water absorption) of DE tends to be stable at a 4-day age, releasing approximately 95% of water. Under a relatively high humidity of 94.5%, the water release rate of DE tends to be stable at a 16-day age, releasing approximately 89% of water. The excellent water absorption and release properties of DE lay a foundation for its application as an internal curing agent.
2.2. Methods
This article investigates the effects of replacing cement with DE at different replacement levels on the compressive strength, flexural strength, internal relative humidity, autogenous shrinkage, and early crack resistance of cement mortar with a water–binder ratio of 0.35.
The mix proportion of cement mortar refers to Table 2. Test specimens are prepared according to GB/T 17671-2021 “Test Method for Strength of Cement Paste and Sand (ISO Method)” [32]. Water and water-reducing agent are first added to the mixing pot and fully dissolved. Then, pre-wetted DE and OPC are added. After stirring at low speed for 30 s, RS is evenly added at the beginning of the second 30 s, and then stirred at high speed for 30 s. The mixing is stopped for 90 s, a scraper is used to scrape the cement paste on the blades, pot walls, and pot bottoms into the pot, and then the mixture is stirred at high speed for 60 s.

Table 2.
Mortar mix ratio.
2.2.1. Mechanical Properties Test
After mixing, the mixture is poured into a mold of 40 mm × 40 mm × 160 mm, then it is shaped through vibration and moved to a standard curing chamber (temperature: (20 ± 5) °C, relative humidity: (90 ± 5)%). It is demolded after 1 day, and then it continued to be cured under standard curing conditions for 3 d, 7 d, and 28 d, respectively, to test its compressive strength and flexural strength (three samples for each group).
2.2.2. Internal Relative Humidity Test
The mortar is placed into a 100 mm × 100 mm × 100 mm mold. After vibration, a 20 mm-diameter PE tube is inserted into the sample. A solid steel rod is placed inside the PE tube to prevent the cement mortar from floating to the top. The depth of insertion of both the PE tube and steel rod should be 50 mm. After 24 h of standard curing, the steel rod is pulled out and a temperature and humidity sensor probe is placed inside the PE tube. The specimen is immediately sealed. A TH20R-EX-H temperature and humidity recorder (HUAHANWEI, Wenzhou, China) is used for data measurement, accurate to ±2%. The seal is maintained during curing and tests are conducted for 168 h with a frequency of 1 h/time (three parallel samples for each group).
2.2.3. Autogenous Shrinkage Experiment
The self-shrinkage test specimen has a size of 100 mm × 100 mm × 515 mm. A NELD-ES730 (6-channel) non-contact concrete shrinkage deformation tester (Beijing Yichuang Times Technology Co., Beijing, China) is used. Before pouring the mortar, two layers of sealing film are laid at the bottom of the test mold, and lubricating oil is applied between the test mold and the film, as well as between the two layers of film. The targets are fixed at both ends of the test mold, with a spacing greater than 400 mm, and then the mortar is poured. The test mold and the test specimen are immediately sealed after pouring, and the displacement sensors are zeroed at both ends. The test is started before the initial setting of the mortar. The test temperature is maintained at a constant 20 ± 2 °C, with a test duration of 168 h and a data recording frequency of 1 h/time (three parallel samples for each group).
2.2.4. Ring Anti-Cracking Experiment
The early crack resistance performance is tested using the ring method, referring to the Guidelines for Durability Design and Construction of Concrete Structures (CCES p01-2018) [33]. In this test, the inner ring is a steel ring with a wall thickness of 25.4 mm, an outer diameter of 41.3 mm, and a height of 25.4 mm. The inner diameter of the outer ring is 66.7 mm. During the test, the mortar is poured into the ring and immediately transferred to a standard curing room for curing for 24 h after vibration molding. After that, the outer steel ring and the bottom plate are removed. The specimen is then moved to a plastic plate in a drying shrinkage chamber (temperature of 20 °C ± 2 °C, relative humidity of 50 ± 4%). The cracking location and crack development trend of the mortar ring are observed, and the initial cracking age is recorded. The crack width is also measured (three parallel samples for each group).
2.2.5. Micro-Experiment Methods
The chemically bound water content of cement mortar is determined using the high-temperature ignition method, the pore structure of the paste is measured by mercury intrusion porosimetry, the phase composition of the paste is determined by X-ray diffraction, and the micromorphology of the paste is observed by scanning electron microscopy.
3. Results
3.1. Mortar Mix Ratio and Compressive and Flexural Strength
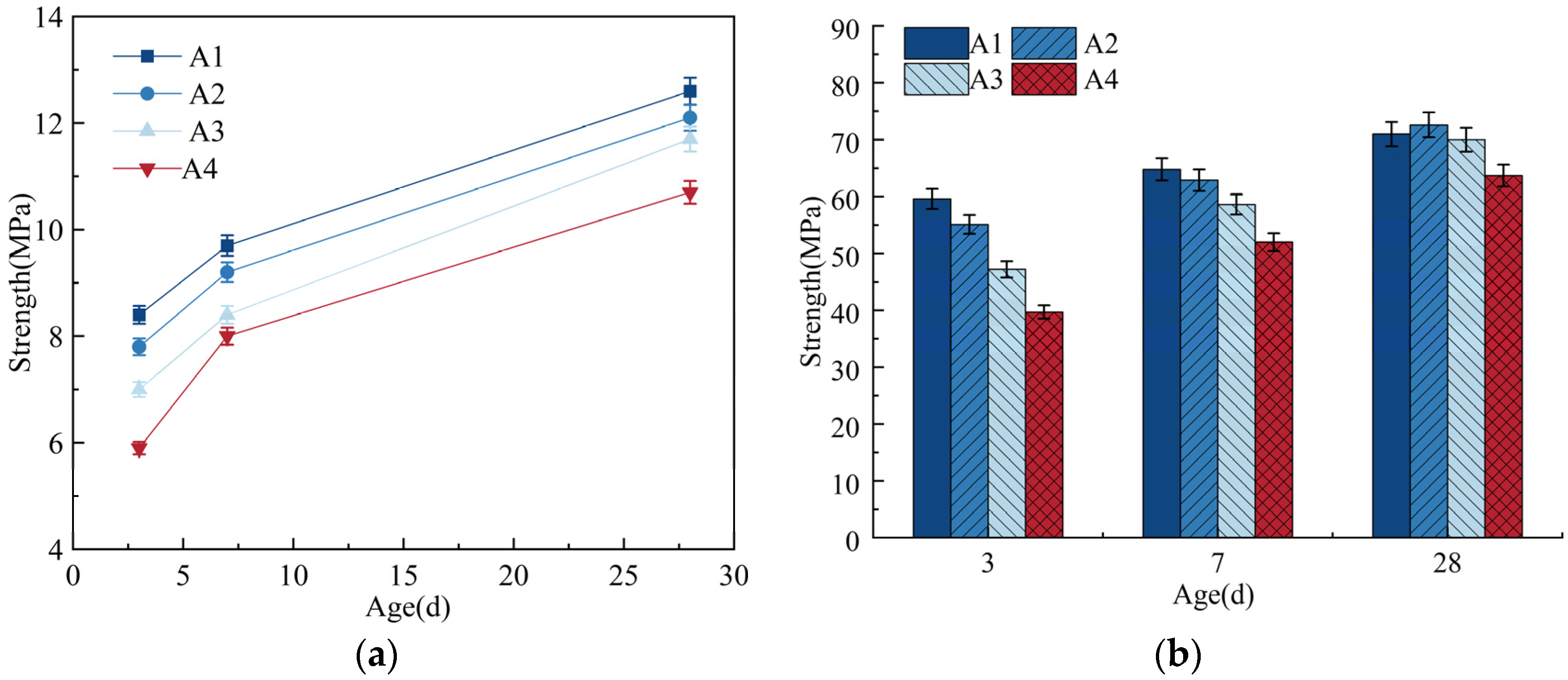
A total of 1%, 3%, and 5% of the cement were replaced with equal masses of DE. With a fixed cement–sand ratio of 1:1.3, the amount of water reducer was adjusted to make the flowability of the cement paste be 180 ± 5 mm. See Table 2 for the mix proportions of mortar. The compressive strength and flexural strength are shown in Figure 3.
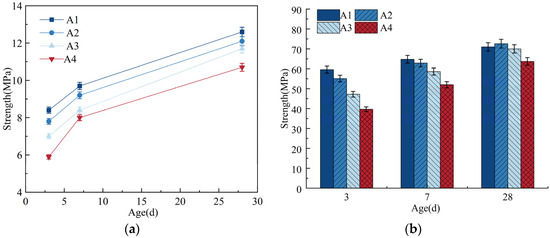
Figure 3.
Flexural strength and compressive strength. (a) Flexural strength; (b) compressive strength.
As can be seen from Table 2, when wet DE is used to replace cement to achieve the same flowability, it increases the internal curing water by 2% to 10% but also requires an increase in the dosage of water reducer by 0.01% to 0.04%. For concrete, strength is its most basic performance indicator and the primary consideration in structural design and quality inspection of construction work [34]. Therefore, even though the introduction of internal curing materials can effectively suppress shrinkage and cracking in cement mortar, it cannot be done at the cost of compromising the strength of the paste. As shown in Figure 3b, the early compressive strength of the mortar significantly decreases with the incorporation of DE. As the age increases, the compressive strength of the mortar continues to develop and gradually stabilizes, gradually approaching or even exceeding the compressive strength of the reference mortar during the same period. When the age is 28 days, the compressive strengths of groups A2, A3, and A4 increase to 102%, 98.6%, and 89.7% of the 28-day compressive strength of the reference mortar, respectively. This also indicates that the incorporation of DE slightly reduces the 3-day and 7-day compressive strengths of the mortar compared to the reference group but has a smaller impact on the 28-day compressive strength.
In summary, replacing 1% of cement with an equal mass of DE has a certain impact on the workability of cement mortar, but this can be addressed by appropriately adjusting the dosage of water reducer. Incorporating DE reduces the 3-day and 7-day strengths of the mortar but has a smaller effect on the 28-day compressive strength.
3.2. Variation in Relative Humidity Inside Mortar
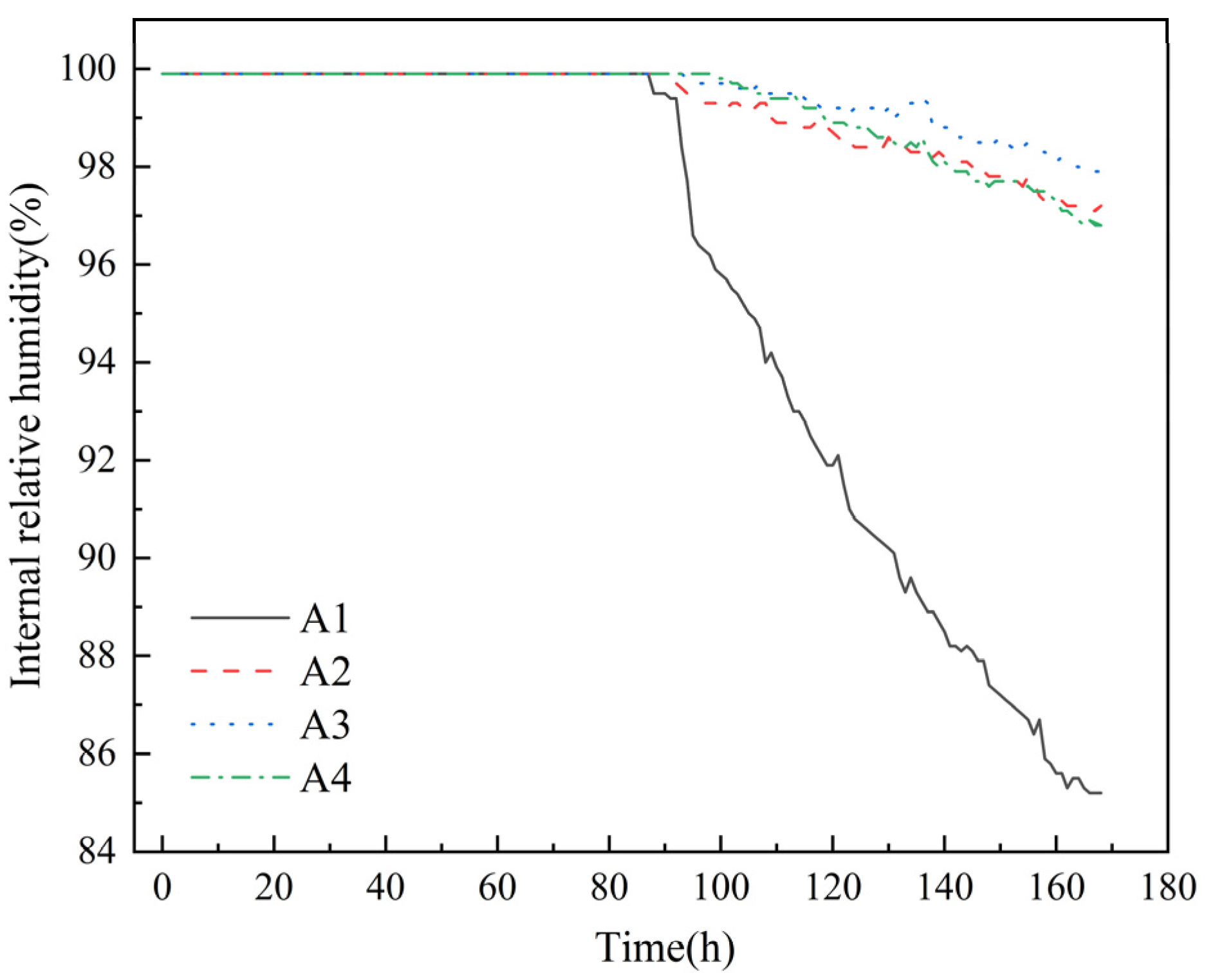
The experimental results of measuring the changes in relative humidity inside concrete over time with a high-precision hygrometer are shown in Figure 4.
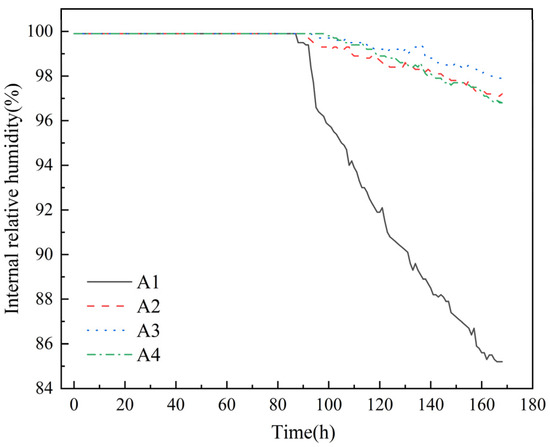
Figure 4.
Variation in relative humidity inside mortar.
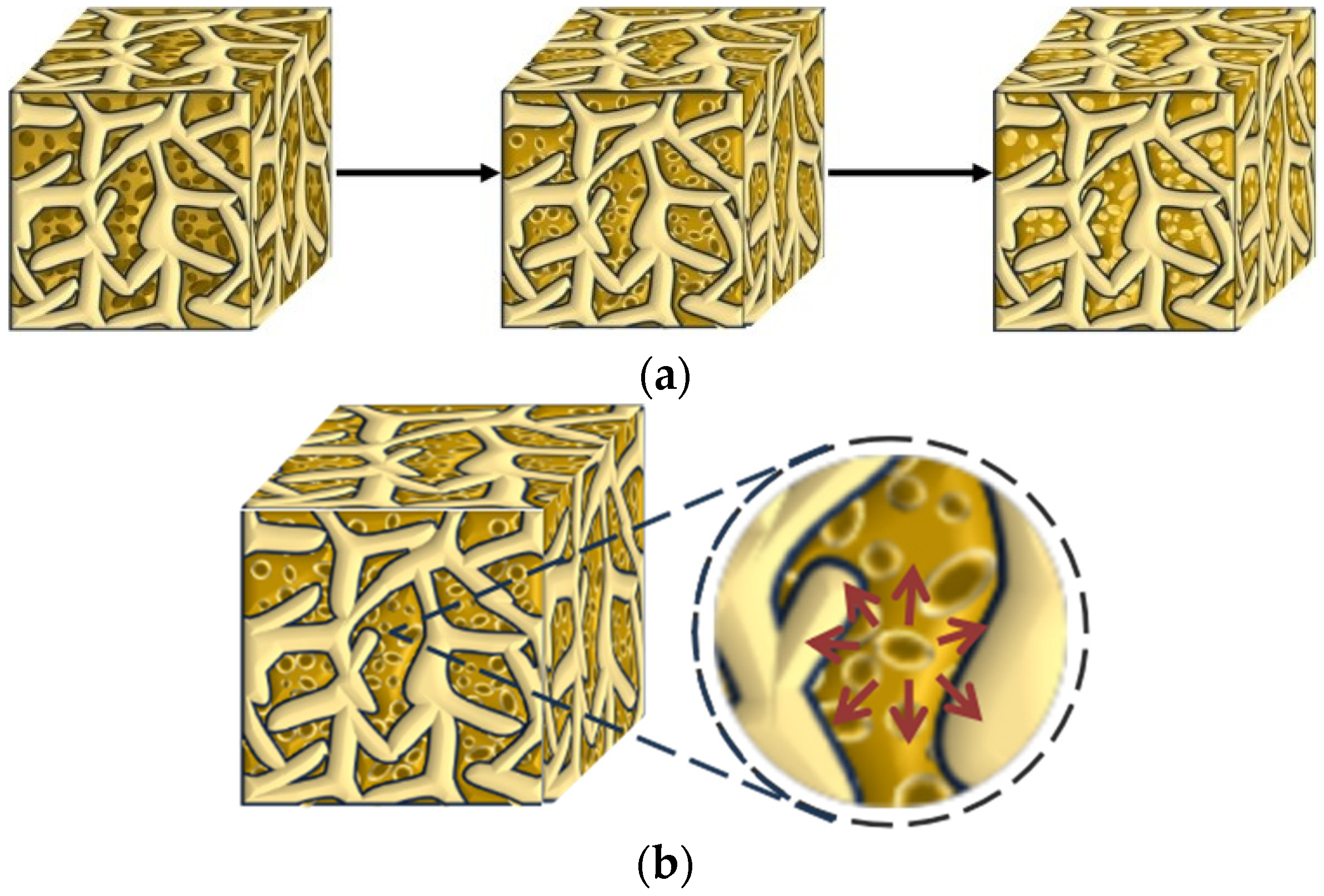
As shown in Figure 4, the incorporation of DE can delay the saturation time of mortar humidity. Compared with control group A1, the saturation times for groups with DE incorporation are prolonged by 2 h, 6 h, and 11 h, respectively. Meanwhile, the internal relative humidity decline rate of control group A1 is significantly the fastest, showing a steep decline. This is because although diatomite has certain water retention properties, it cannot eliminate the rapid water release caused by the initial water gradient drive [21]. After water release begins, it can significantly reduce the decline rate of internal relative humidity. At the same time, with the increase in diatomite content, the decline rate of internal relative humidity slows down significantly, indicating that the water released by diatomite can effectively compensate for the water reduction caused by cement hydration or diffusive evaporation [35]. The internal curing effect of diatomite is well exerted. The incorporation of DE can slightly increase the relative humidity of mortar, and with the increase in DE content, the decay rate of internal relative humidity of mortar decreases significantly. When the test age is 168 h, compared with the control group (A1), the internal relative humidity of the mortar in the pre-water absorption diatomite groups (A2, A3, A4) increases by 14.1%, 14.9%, and 13.6%, respectively. The schematic diagram of water release from DE inside mortar is shown in Figure 5.
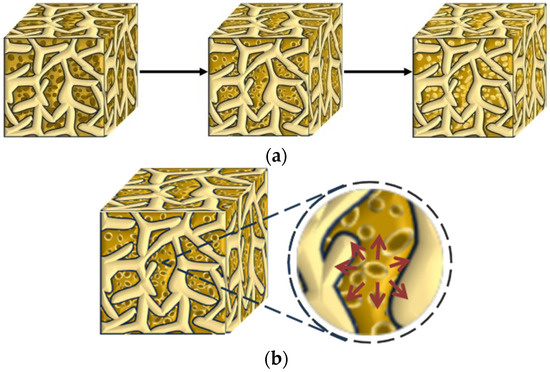
Figure 5.
Water release diagram of DE in mortar. (a) Schematic diagram of the change in saturated DE in mortar; (b) DE water release diagram. Legend: Saturated DE:  Semi-wet DE:
Semi-wet DE:  Dry DE:
Dry DE:  , Direction of Water Release:
, Direction of Water Release:  .
.
 Semi-wet DE:
Semi-wet DE:  Dry DE:
Dry DE:  , Direction of Water Release:
, Direction of Water Release:  .
.
3.3. Autogenous Shrinkage Performance of Mortar
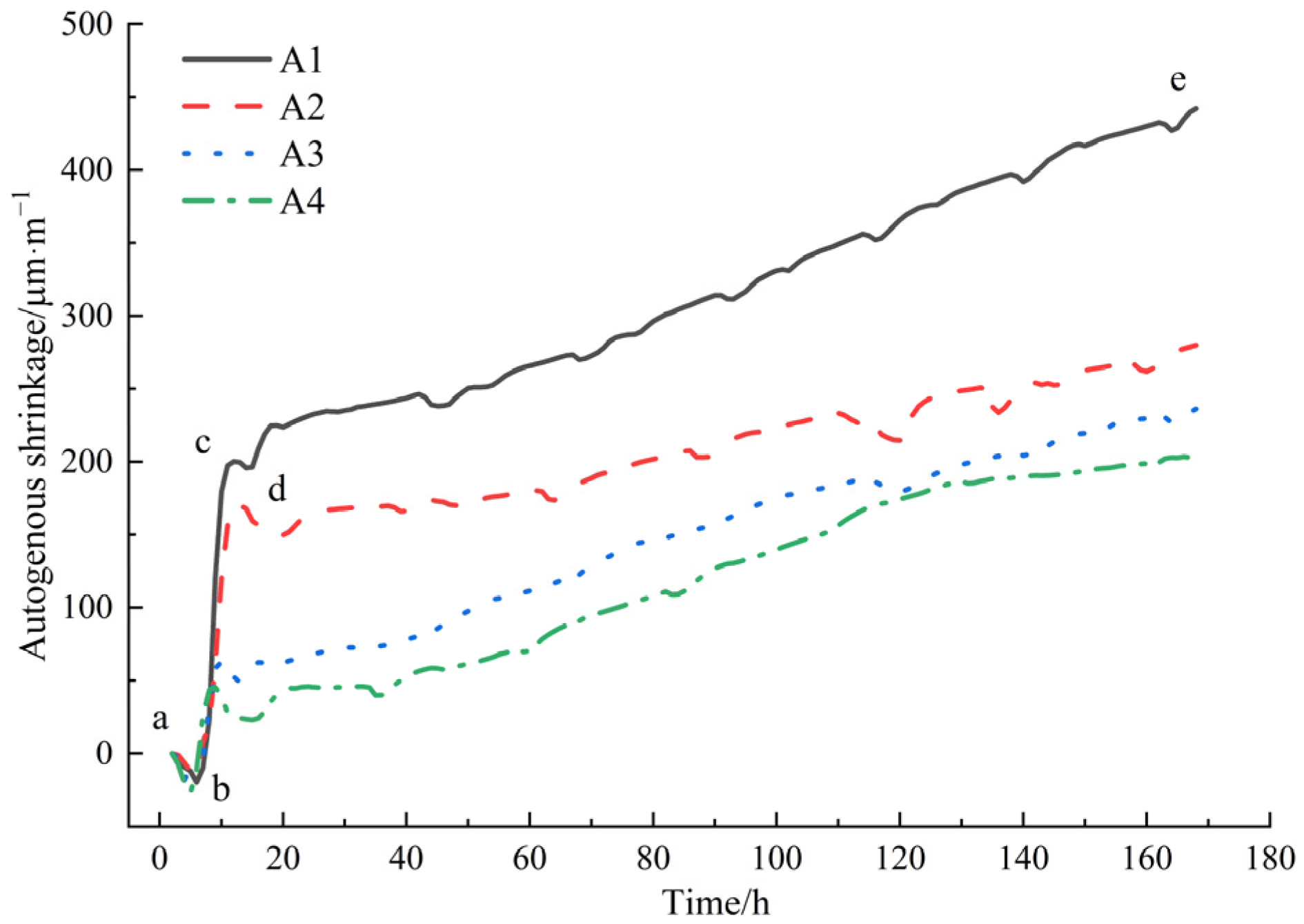
The results of the autogenous shrinkage tests of mortar are shown in Figure 6.
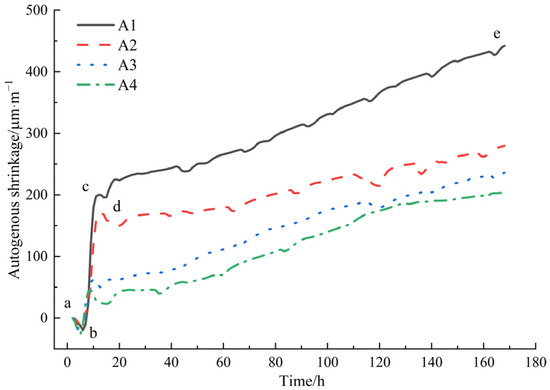
Figure 6.
Autogenous shrinkage performance of mortar.
As can be seen from Figure 6, the mortar without DE exhibited the fastest early autogenous shrinkage and the largest final autogenous shrinkage value. As the amount of DE incorporation increased, the autogenous shrinkage value of the mortar gradually decreased, consistent with the variation in the internal relative humidity of the mortar in Figure 4, that is, the lower the internal relative humidity of the mortar, the greater the autogenous shrinkage value of the mortar.
The autogenous shrinkage development of the control group (A1) could be divided into four stages: expansion-shrinkage-expansion-shrinkage, namely ab (brief expansion stage), bc (rapid shrinkage stage), cd (second expansion stage), and de (continuous shrinkage stage). The analysis of the deformation characteristics mechanism of the reference mortar at early ages is as follows. During the ab period, the control group mortar exhibits volume expansion. This is due to the mortar still being in a plastic flow state after pouring, with plastic settlement leading to vertical shrinkage and lateral expansion of the mortar. Moreover, the volume expansion rate caused by the hydration reaction of the cementing materials exceeds the shrinkage rate of the low water–binder ratio cement mortar, resulting in macroscopic volume expansion, with a peak expansion value of 20 μm/m. After the expansion deformation ends, rapid autogenous shrinkage occurs in the bc stage, with an increasing slope of the autogenous shrinkage curve. This is mainly because, during this period, rapid hydration reactions occur inside the cement mortar paste, releasing hydration heat. However, chemical shrinkage dominates over thermal expansion effects at this time, resulting in rapid shrinkage of the paste, with a peak shrinkage value of 200 μm/m. After this, the shrinkage curve exhibits a brief decline stage, where the cd stage shows a transient expansion process. This is related to the formation of ettringite, large-sized calcium hydroxide, and internal C-S-H during the hydration process [36]. The expansion-type hydration products offset some of the shrinkage, resulting in transient expansion. The reason for this brief duration is that the cement mortar with a low water–binder ratio has a dense structure without coarse aggregates, limiting the occurrence of expansion deformation. After the end of expansion deformation, the autogenous shrinkage of the mortar enters a gradual growth stage in the de period. The slope of the autogenous shrinkage deformation curve gradually decreases, becoming flatter, and the shrinkage increases slowly and steadily. The initial shrinkage value in this stage is 196 μm/m, and the autogenous shrinkage value at 168 h is 442 μm/m.
After adding DE, the self-shrinkage development curve changes significantly, as follows: (1) At the beginning of data collection, i.e., 3 h after adding water to the mortar, the mortar is in a slightly expansive state, with expansion values of 1.1 μm/m, 3.143 μm/m, and 7.416 μm/m (A2, A3, A4), respectively. (2) Compared to the reference group, the shrinkage time of the bc segment in the diatomite groups (A2, A3, A4) decreases, with shrinkage peaks of 170 μm/m, 63 μm/m, and 46 μm/m, respectively. Subsequently, they enter the cd segment, with extended duration of expansion and expansion peaks of 149 μm/m, 48 μm/m, and 23 μm/m, respectively. (3) The self-shrinkage values in the continuous shrinkage stage (de segment) decrease with the increase in the amount of diatomite added. Compared to the reference group, the final effective reduction rates after 7 days are 36.7%, 46.6%, and 54%, respectively. As can be seen in Figure 4, after the internal humidity saturation state of the mortar ends, approximately at around 80 h, the internal humidity of the mortar rapidly decreases, and the self-shrinkage caused by water loss also rapidly increases. The slope of the self-shrinkage development curve of the mortar becomes significantly steeper. In mortar mixed with diatomite, as the hydration reaction inside the paste continues, the free water content inside the paste gradually decreases, the internal relative humidity also decreases, and the capillary pressure decreases. Under negative pressure conditions, the water pre-stored in the pores of diatomite is gradually released, effectively reducing the rate and degree of internal self-shrinkage development in cement mortar.
When the admixture contents of diatomite are 1%, 3%, and 5%, theoretically, as the cement dosage changes to 99%, 97%, and 95% of the reference group, the total self-shrinkage of the paste should also be 99%, 97%, and 95% of the total self-shrinkage of the reference group, i.e., reductions in self-shrinkage of 1%, 3%, and 5%, respectively. However, in actuality, the reductions were 36.7%, 46.6%, and 54%, respectively. This demonstrates that the incorporation of pre-water-absorbed diatomite can effectively reduce the self-shrinkage development of mortar. Based on the selection criterion of minimal impact on the 28-day compressive strength of mortar, when the admixture content of DE is 1%, the reduction in self-shrinkage is significant without causing a decline in mechanical properties and with minimal impact on fluidity.
3.4. Early Cracking Resistance of Mortar
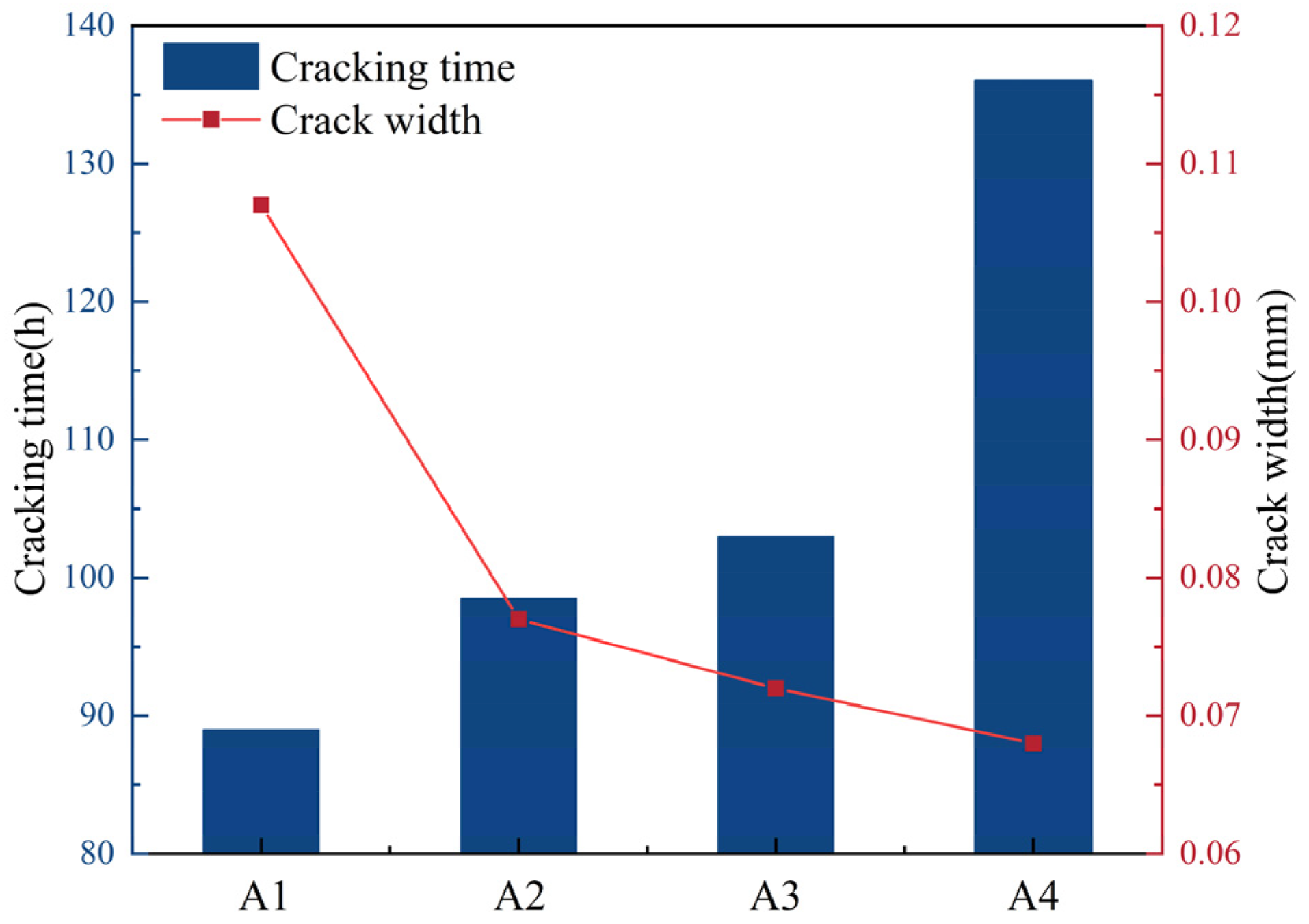
The results measured by the ring method are shown in Figure 7.
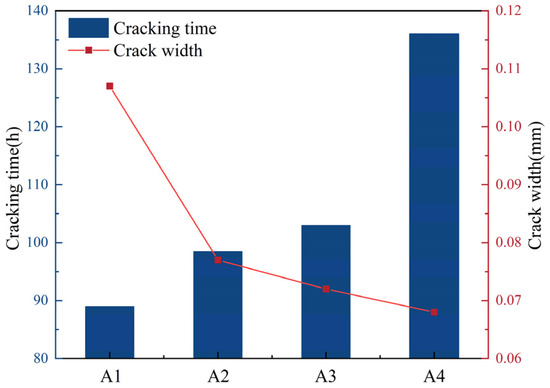
Figure 7.
Mortar initial cracking time and crack width diagram.
As can be seen from Figure 7, the addition of DE extends the initial cracking time of mortar and significantly reduces the crack width. When the amounts of DE mixed are 1% and 3%, the cracking times of mortar are delayed by 10.7% and 15.7%, respectively. At the same time, with the increase in DE content, the crack width is significantly reduced. The reason why diatomite can delay the initial cracking time of mortar is that, during the mixing process of mortar, the pre-water-absorbed DE is evenly distributed inside the slurry. As the cement hydration reaction proceeds, the moisture inside the cementitious material slurry gradually decreases, resulting in a humidity gradient difference and capillary pore negative pressure, which cause the diatomite to release the pre-stored water. This water compensates in a timely manner for water loss in the mortar, reducing the internal relative humidity gradient, promoting the hydration of cement, inhibiting the early self-shrinkage and drying shrinkage of the mortar, extending the initial cracking time of the mortar, and reducing the crack width of the mortar.
In summary, the rational use of DE can improve the internal relative humidity, significantly reduce its self-shrinkage, and enhance the early crack resistance of high-strength low water–binder ratio cement mortar without reducing its 28 d compressive strength. DE is an excellent internal curing material for low water–binder ratio cement mortar, and its introduction of internal curing water achieves good results, showing a good curing effect on low water–binder ratio cement mortar.
3.5. The Effect of DE on the Hydration Process of Cement Mortar
According to the mixing ratio in Table 2, samples were prepared and the chemically bound water content, XRD patterns, microscopic morphology via electron microscope images, and pore structure were measured at the ages of 3 d, 7 d, and 28 d.
3.5.1. The Influence of DE on the Chemically Bound Water Content of Cement Mortar
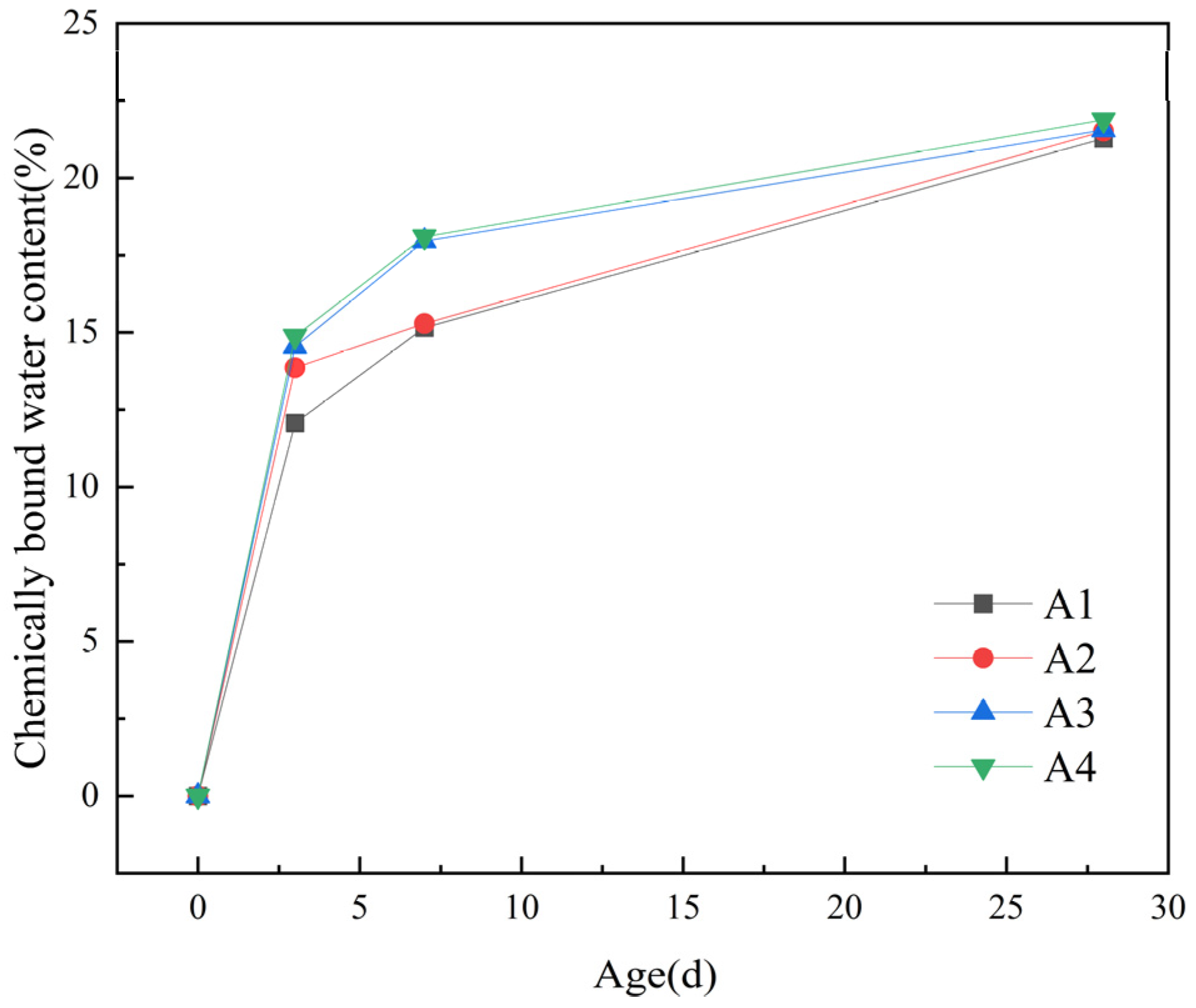
The curves of chemically bound water are shown in Figure 8.
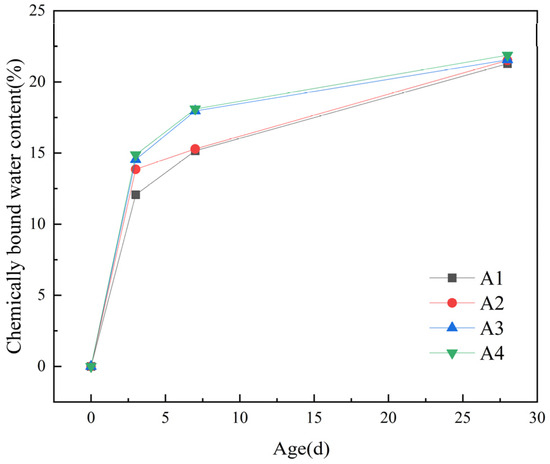
Figure 8.
Chemically bound water content.
From Figure 8, it can be observed that the chemically bound water content rapidly increases in the first 3 days. Compared to the baseline group (A1), the chemically bound water content in the groups doped with DE increased by 1.79%, 2.47%, and 2.81%, respectively. However, the growth becomes slower from 7 d to 28 d, with growth rates of 0.23%, 0.27%, and 0.6%, respectively. It can be seen that as the dosage of DE increases, the chemically bound water content of the cementitious material also increases. This can be attributed to two main reasons. Firstly, under the same water–binder ratio, the incorporation of DE releases water gradually when the internal relative humidity of the slurry decreases, which promotes the hydration of the slurry and thus increases the chemically bound water content of the hardened slurry. Secondly, in the middle and late stages of hydration (7–28 d), the pozzolanic activity of DE gradually takes effect. Under alkaline conditions, some of the reactive SiO2 and Al2O3 in DE react with the hydration product Ca(OH)2 of cement to produce secondary hydration products such as hydrated calcium silicate and hydrated calcium silicoaluminate, which increases the amount of hydration products and subsequently increases the chemically bound water content.
3.5.2. The Impact of DE on the Phase Composition of Hydration Products in Cement Mortar
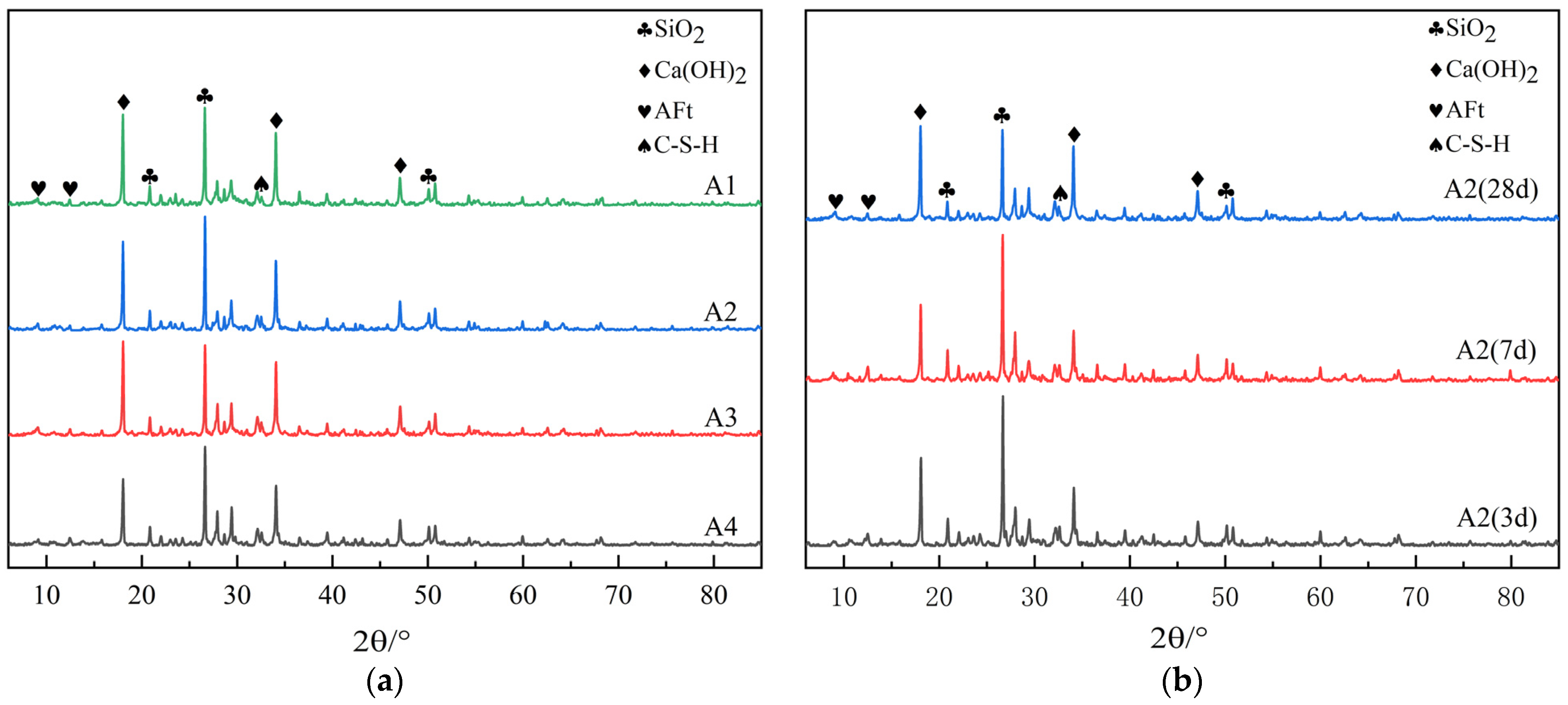
The hydration products of cement mortar mainly consist of Ca(OH)2 crystals and C-S-H gels. Among them, there are many types of C-S-H gels, most of which exist in an amorphous state in cement paste. It is difficult to detect C-S-H gels through XRD, but the changes in CH crystals are very obvious in the diffraction patterns. Therefore, to further analyze the impact of internal curing with diatomite earth on the hydration products of cement mortar, see Figure 9 for the XRD patterns of mortars with different dosages of DE and the XRD patterns of mortars with different ages after the addition of DE.
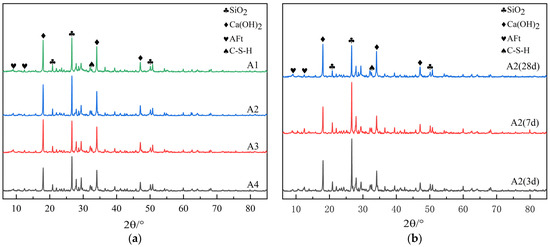
Figure 9.
XRD patterns. (a) XRD patterns of mortar with different DE contents; (b) XRD patterns of mortar at different ages after incorporation of DE.
As can be seen from Figure 9a, the development law of the diffraction peaks of crystalline hydration products in each group with different dosages of DE is basically similar, while the height of the diffraction peak mainly varies according to the dosage of DE. The diffraction peak of SiO2 is the highest in the spectra, but the difference among samples is not significant. This is mainly because a large amount of RS is contained in the cement paste, which cannot be avoided during sampling and testing. Therefore, the change in the SiO2 peak is not significant in each sample. C-S-H belongs to gels and exists in cement paste in an amorphous state with low crystallinity, so its diffraction peak is not obvious and difficult to distinguish in the XRD pattern. Therefore, the diffraction peak is not quantified, but the existence of C-S-H is observed in the pattern, which proves that C-S-H is generated during the hydration process of mortar. In Figure 9a, the difference in the Ca(OH)2 peak is the most obvious, and the peak decreases with the increase in DE dosage. This is mainly because the incorporated internal curing material DE has volcanic ash activity. Under alkaline conditions, SiO2 and Al2O3 in DE can react with Ca(OH)2 to produce secondary hydration products such as C-S-H gel and AFt. In the spectra, the existence of the AFt peak can be observed. Although the change is not significant, it can be observed that the peak increases with the increase in DE dosage. This phenomenon can explain why the incorporation of DE can delay the “second expansion stage” of autogenous shrinkage during the hydration period of cement mortar, which is caused by the production of a large amount of expansive AFt crystals during the hydration period of cement mortar.
As shown in the spectra of Figure 9b, the peak value of SiO2 gradually decreases while the peak value of Ca(OH)2 gradually increases as the age increases. This is mainly due to the hydration reaction, during which the internal relative humidity of mortar decreases. The difference in the relative humidity gradient stimulates diatomite to release stored water, which results in the secondary hydration of cementing materials, generating more Ca(OH)2. This enhances the alkalinity inside the cement paste, stimulating the potential activity of diatomite. As a result, its structure is destroyed, leading to reactions between SiO2, Al2O3 and Ca(OH)2 inside it, producing a large amount of C-S-H gel. This reduces the content of Ca(OH)2 and improves the degree of hydration of the mortar, which is consistent with the results of the chemically bound water content tests.
3.5.3. Effect of DE on the Micromorphology of Hydration Products of Cement Mortar
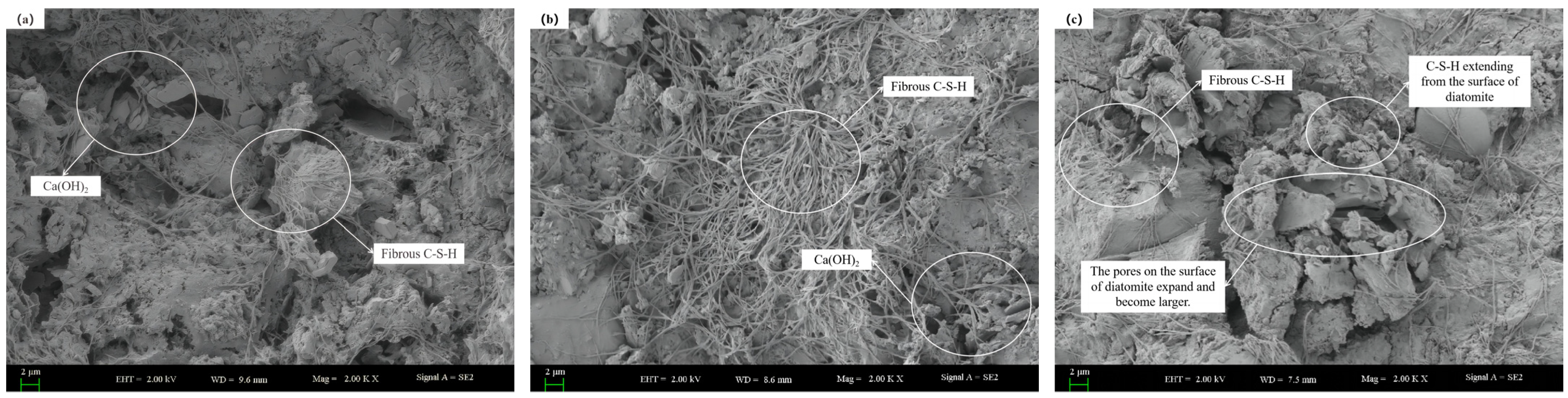
The micromorphologies of cement mortar at different ages after the addition of DE is shown in Figure 10. The micromorphologies of cement mortar without DE and with DE at 28 d age is shown in Figure 11.

Figure 10.
Microscopic morphologies of diatomite hardened paste at different ages. (a) 3 d microstructure diagram; (b) 7 d microstructure diagram; (c) 28 d microstructure diagram.
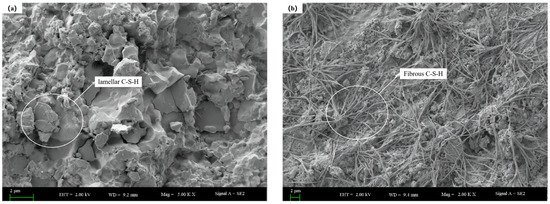
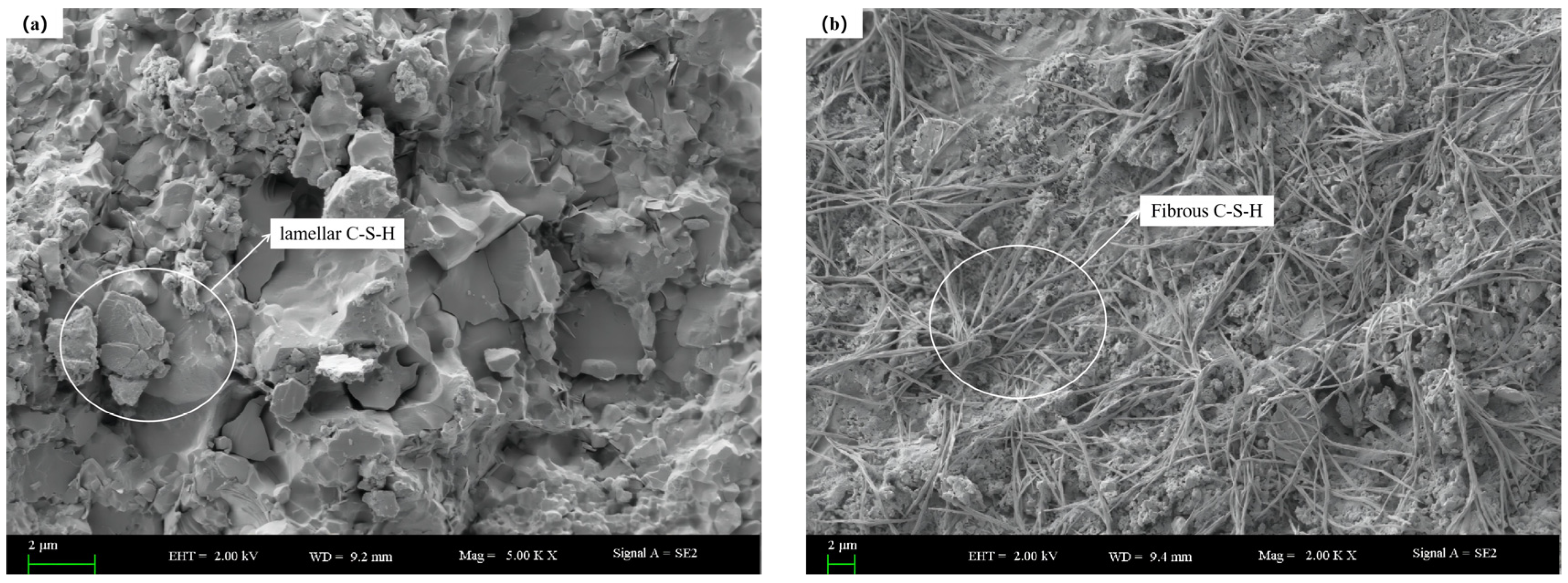
Figure 11.
The microstructures of 28 d age hardened slurry without DE and with DE: (a) Microscopic morphology of undoped DE; (b) micromorphology diagram with DE incorporation.
As shown in Figure 10, during the 3–7 d age period, a significant increase in C-S-H gel can be observed. At the 28 d age, a small amount of C-S-H gel was found on the surface of DE, which confirmed that DE participates in secondary hydration. Compared to the original scanning electron microscope image of DE, it can be found that the pores on the surface of DE transform into pore channels, leading to an increase in paste porosity, consistent with the mercury intrusion test results.
Figure 11 demonstrates a significant difference in the morphologies of C-S-H gel formed with and without the addition of DE. Without DE, the C-S-H gel is mainly in the form of flakes. However, with the addition of DE, the C-S-H gel appears as fibrous structures that intertwine and overlap. This difference is primarily due to the change in the calcium-to-silicon ratio of the paste after the addition of DE. As the calcium-to-silicon ratio increases, the particles transform from dense clumps on the surface to elongated fibrous structures [37]. Additionally, it can be observed that the microstructure without DE is denser with fewer pores visible, whereas the microstructure with DE exhibits a significant number of small pores and channels.
3.5.4. Effect of DE on the Pore Structure of Cement Mortar
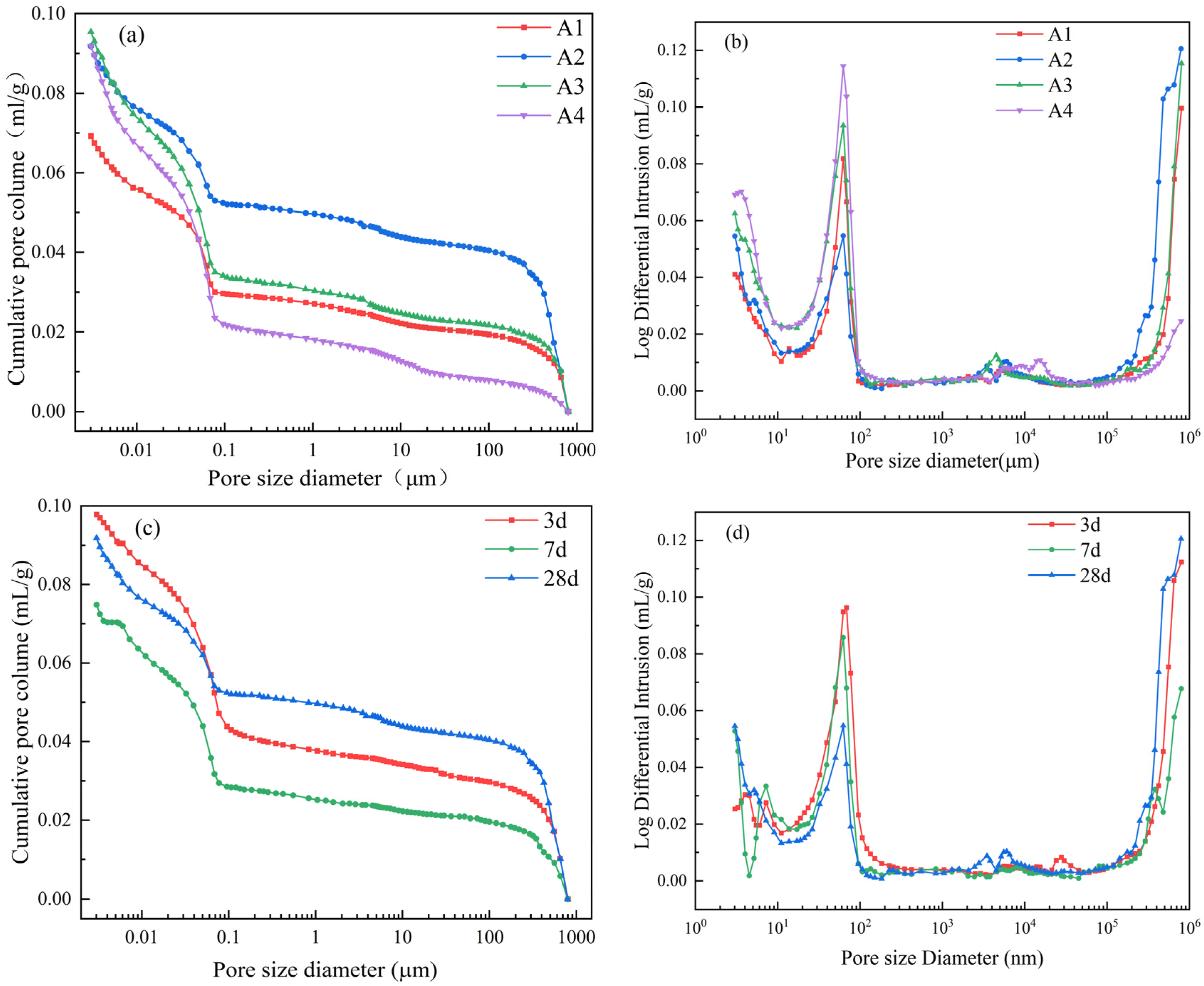
Figure 12 and Table 3 present the pore structure test results of internally cured mortar with DE. As can be seen from Figure 12a, when the water–binder ratio is 0.35, the pores of each sample are mainly distributed in gel pores below 0.1 µm and macropores above 100 µm. The presence of macropores may be caused by air pores introduced during the mixing process of the mortar or residual DE pores after water release.
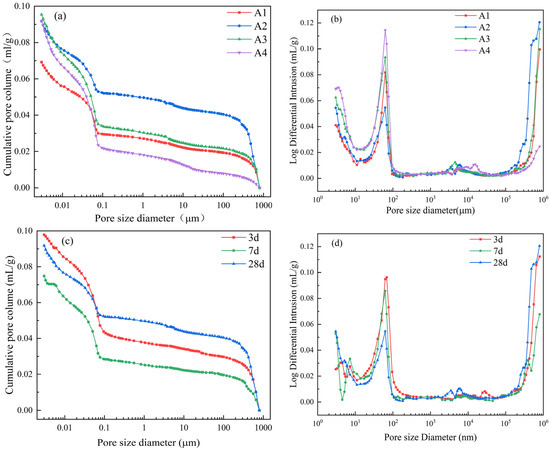
Figure 12.
Effect of diatomite internal curing on pore structure of mortar. (a) Pore size distribution integral curves; (b) pore size distribution differential curves; (c) integral curves of pore size distribution at different ages; (d) differential curves of pore size distribution at different ages.

Table 3.
The pore structure parameters and distribution of mortar with different DE doping amounts at 28 d and mortar with 1% DE doping amount at different ages.
As can be seen from Table 3, with the increase in DE content, the porosity of cement paste at 28 d age increases, but when the amount of DE is relatively large, the average pore size is relatively low. This indicates that the introduction of DE into cement mortar can increase the porosity within the cement paste, but it can also refine the pore structure. Combining Figure 12 with Table 3, it can be seen that as age increases, there is a decreasing trend in both the porosity and average pore size of the slurry. As shown in Figure 12d, the pore structure within the range of 10–100 nm gradually decreases with age. The reason is that, during the hydration process of the mortar, the relative humidity inside the slurry decreases, and the water pre-stored in DE is slowly released, enabling the hydration reaction to continue. The produced hydration products gradually fill the capillaries, refining the pore structure [38].
3.6. Shrinkage Reduction Mechanism of DE
According to the above experiments, replacing part of the cement with the same mass of DE can significantly reduce the autogenous shrinkage of cement mortar while maintaining the 28 d compressive strength, thereby improving the early crack resistance of cement mortar. The mechanism analysis is as follows.
- (1)
- SiO2 and Al2O3 in DE can undergo secondary hydration reactions with Ca(OH)2 to produce hydration products such as C-S-H and expansion-type AFt, compensating for some of the shrinkage. SiO2 and Al2O3 in DE have high contents and can react with Ca(OH)2 to produce ettringite, which prolongs the secondary expansion period of mortar autogenous shrinkage.
- (2)
- Replacing part of the cement with DE reduces the activity of the cementing material, thus reducing autogenous shrinkage from the source. Autogenous shrinkage is caused by the hydration of cementing materials such as cement. Although the main component of DE is silicon dioxide, its limited activity allows it to participate in some hydration reactions.
- (3)
- The slow release of water in DE increases the relative humidity inside the cement mortar and reduces the driving force of autogenous shrinkage. DE contains a large number of tiny pores and has good water absorption and release properties, which enable it to slowly release water during the hardening process of cement mortar, thereby increasing the internal relative humidity of cement mortar (see Figure 4) and reducing the driving force of autogenous shrinkage.
4. Conclusions
- (1)
- The unique natural macroporous/mesoporous structure of DE endows it with excellent water absorption and release properties, which can improve the internal relative humidity of mortar and significantly slow down the decline rate of internal relative humidity. Compared to the benchmark group, when the content of DE is 1%, the saturation duration of the internal humidity of mortar is extended by 3.4%.
- (2)
- Under the standard of not reducing the 28 d compressive strength of mortar, compared to the reference group, the mortar mix proportion of DE replacing 1% cement mass reduces the 3 d autogenous shrinkage value by 30.9% and the 7 d autogenous shrinkage value by 36.7%. In the ring-constrained crack resistance test, the initial cracking time is delayed by 10.7%, and the crack width is reduced by 28%.
- (3)
- The mechanism of DE reducing autogenous shrinkage comprises in three aspects. Firstly, SiO2 and Al2O3 in DE react with Ca(OH)2 to produce expansive ettringite, which compensates for part of the shrinkage. Secondly, DE replaces part of the cement, reducing the activity of cementing materials and thus reducing autogenous shrinkage from the source. Thirdly, the slow release of water from its porous structure can increase the internal relative humidity of mortar and reduce the driving force of autogenous shrinkage.
Author Contributions
Conceptualization, S.L. (Shunyi Liu); methodology, S.L. (Shunyi Liu); software, S.L. (Shunyi Liu); validation, S.L. (Shunyi Liu); formal analysis, S.L. (Shunyi Liu); investigation, S.L. (Shunyi Liu); resources, S.L. (Shunyi Liu); data curation, S.L. (Shunyi Liu); writing—original draft preparation, S.L. (Shunyi Liu); writing—review and editing, S.L. (Shunyi Liu), S.L. (Shuangxi Li), and C.J.; visualization, C.J.; supervision, S.L. (Shuangxi Li); project administration, S.L. (Shuangxi Li); funding acquisition, S.L. (Shuangxi Li). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (NSFC) [grant number 52269028].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to the fact that dataset production is part of the innovation of this paper, and the dataset will not be open-sourced at this time. We will continue to explore new possibilities in subsequent experimental research, and then the dataset will be made public along with the related code.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Menna, C.; Mata-Falcón, J.; Bos, F.P.; Vantyghem, G.; Ferrara, L.; Asprone, D.; Salet, T.; Kaufmann, W. Opportunities and challenges for structural engineering of digitally fabricated concrete. Cem. Concrete Res. 2020, 133, 106079. [Google Scholar] [CrossRef]
- Rong, Z.; Sun, W.; Xiao, H.; Wang, W. Effect of silica fume and fly ash on hydration and microstructure evolution of cement based composites at low water-binder ratios. Constr. Build. Mater. 2014, 51, 446–450. [Google Scholar] [CrossRef]
- Maruyama, I.; Teramoto, A. Temperature dependence of autogenous shrinkage of silica fume cement pastes with a very low water-binder ratio. Cem. Concr. Res. 2013, 50, 41–50. [Google Scholar] [CrossRef]
- Liu, J.; Shi, C.; Ma, X.; Khayat, K.H.; Zhang, J.; Wang, D. An overview on the effect of internal curing on shrinkage of high performance cement-based materials. Constr. Build. Mater. 2017, 146, 702–712. [Google Scholar] [CrossRef]
- Miao, Y.; Lu, Z.; Wang, F.; Wang, H.; Li, Y.; Lin, J.; Jiang, J. Shrinkage cracking evolvement in concrete cured under low relative humidity and its relationship with mechanical development. J. Build. Eng. 2023, 72, 106670. [Google Scholar] [CrossRef]
- Ramaglia, G.; Lignola, G.P.; Fabbrocino, F.; Prota, A. Numerical modelling of masonry barrel vaults reinforced with textile reinforced mortars. Key Eng. Mater. 2017, 747, 11–19. [Google Scholar] [CrossRef]
- Kumar, S.V.; GangaRao, H.V.S. Fatigue Response of Concrete Decks Reinforced with FRP Rebars. J. Struct. Eng. 1998, 124, 11–16. [Google Scholar] [CrossRef]
- Schutter, G.D. Durability of Marine Concrete Structures Damaged by Early age Thermal Cracking. In International RILEM Workshop on Life Prediction and Aging Management of Concrete Structures; Naus, D., Ed.; RILEM: Paris, France, 2000; pp. 177–186. [Google Scholar]
- Huang, Y.; Chen, G.; Yang, R.; Yu, R.; Xiao, R.; Wang, Z.; Xie, G.; Cheng, J. Hydration kinetics and microstructure development of Ultra-High Performance Concrete (UHPC) by high volume of phosphorus slag powder. Cem. Concr. Compos. 2023, 138, 104978. [Google Scholar] [CrossRef]
- Mamo, G.; Mattiasson, B. Alkaliphiles: The Emerging Biological Tools Enhancing Concrete Durability. Adv. Biochem. Eng./Biotechnol. 2020, 172, 293–342. [Google Scholar]
- Zeng, Y.; Li, X.; Jiang, D.; Ran, J. Study on the Effects of Innovative Curing Combinations on the Early Temperature Field of Concrete Box Girders. Buildings 2022, 12, 1808. [Google Scholar] [CrossRef]
- Tran, N.P.; Gunasekara, C.; Law, D.W.; Houshyar, S.; Setunge, S.; Cwirzen, A. A critical review on drying shrinkage mitigation strategies in cement-based materials. J. Build. Eng. 2021, 38, 102210. [Google Scholar] [CrossRef]
- Meddah, M.S.; Sato, R. Effect of curing methods on autogenous shrinkage and self-induced stress of high-performance concrete. ACI Mater. J. 2010, 107, 65–74. [Google Scholar]
- Lura, P. Autogenous Deformation and Internal Curing of Concrete. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2003. [Google Scholar]
- Jensen, O.M.; Hansen, P.F. Water-entrained cement-based materials: II. Experimental observations. Cem. Concr. Res. 2002, 32, 973–978. [Google Scholar] [CrossRef]
- Igarashi, S.; Watanabe, A. Experimental study on prevention of autogenous deformation by internal curing using super-absorbent polymer particles. In Proceedings of the International RILEM Conference on Volume Changes of Hardening Concrete: Testing and Mitigation, Lyngby, Denmark, 20–23 August 2006; pp. 77–86. [Google Scholar]
- Lura, P.; Durand, F.; Jensen, O.M. Autogenous strain of cement pastes with superabsorbent polymers. In Proceedings of the International RILEM Conference on Volume Changes of Hardening Concrete: Testing and Mitigation, Lyngby, Denmark, 20–23 August 2006; RILEM Publications SARL: Paris, France; pp. 57–65. [Google Scholar]
- Esteves, L.P. Internal Curing in Cement-Based Materials. Ph.D. Thesis, The University of Aveiro (UA), Aveiro, Portugal, 2009. [Google Scholar]
- Larianovsky, P. Internal Curing of Concrete Using Super-Absorbent Polymers. Master’s Thesis, Technion–Israel Institute of Technology, Haifa, Israel, 2007. [Google Scholar]
- Craeye, B.; Geirnaert, M.; De Schutter, G. Super absorbing polymers as an internal curing agent for mitigation of early-age cracking of high-performance concrete bridge decks. Constr. Build. Mater. 2011, 25, 1–13. [Google Scholar] [CrossRef]
- Hasholt, M.T.; Jensen, O.M.; Kovler, K.; Zhutovsky, S. Can superabsorent polymers mitigate autogenous shrinkage of internally cured concrete without compromising the strength? Constr. Build. Mater. 2012, 31, 226–230. [Google Scholar] [CrossRef]
- Ma, X.; Liu, J.; Shi, C. A review on the use of LWA as an internal curing agent of high performance cement-based materials. Constr. Build. Mater. 2019, 218, 385–393. [Google Scholar] [CrossRef]
- Kang, S.; Hong, S.; Moon, J. Importance of drying to control internal curing effects on field casting ultra-high performance concrete. Cem. Concr. Res. 2018, 108, 20–30. [Google Scholar] [CrossRef]
- Kang, S.; Hong, S.; Moon, J. The effect of superabsorbent polymer on various scale of pore structure in ultra-high performance concrete. Constr. Build. Mater. 2018, 172, 29–40. [Google Scholar] [CrossRef]
- Kang, S.; Hong, S.; Moon, J. Shrinkage characteristics of heat-treated ultra-high performance concrete and its mitigation using superabsorbent polymer based internal curing method. Cem. Concr. Compos. 2018, 89, 130–138. [Google Scholar] [CrossRef]
- Reka, A.A.; Pavlovski, B.; Fazlija, E.; Berisha, A.; Pacarizi, M.; Daghmehchi, M.; Sacalis, C.; Jovanovski, G.; Makreski, P.; Oral, A. Diatomaceous Earth: Characterization, thermal modification, and application. Open Chem. 2021, 19, 451–461. [Google Scholar] [CrossRef]
- Kontić, A.; Vasconcelos, G.; Melendez, C.B.; Azenha, M.; Sokolović, N. Influence of air entrainers on the properties of hydrated lime mortars. Constr. Build. Mater. 2023, 403, 132968. [Google Scholar] [CrossRef]
- Diaz, J.; Ševčík, R.; Mácová, P.; Menéndez, B.; Frankeová, D.; Slížková, Z. Impact of nanosilica on lime restoration mortars properties. J. Cult. Herit. 2022, 55, 210–220. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, H.; Xu, L.; Ge, W. A review of mechanical properties and carbonation behavior evolution of lime mortar for architectural heritages restoration. Miner. Miner. Mater. 2024, 3, 3. [Google Scholar] [CrossRef]
- Saidi, T.; Hasan, M. The effect of partial replacement of cement with diatomaceous earth (DE) on the compressive strength and absorption of mortar. J. King Saud Univ. Eng. Sci. 2022, 34, 250–259. [Google Scholar] [CrossRef]
- Schröfl, C.; Mechtcherine, V.; Gorges, M. Relation between the molecular structure and the efficiency of superabsorbent polymers (SAP) as concrete admixture to mitigate autogenous shrinkage. Cem. Concr. Res. 2012, 42, 865–873. [Google Scholar] [CrossRef]
- GB/T 17671-2021; Test Method for Strength of Cementitious Sand (ISO Method). National Technical Committee for Cement Standardisation: Beijing, China, 2021.
- CCES 01-2004; Guidelines for the Design and Construction of Concrete Structures for Durability. China Civil Engineering Society Standard: Beijing, China, 2008.
- Lin, H. Practice Analysis of the Development Trend of Concrete Strength. Concr. World 2021, 8, 52–55. [Google Scholar]
- Han, Y.; Zhang, J.; Luosun, Y.; Hao, T. Effect of internal curing on internal relative humidity and shrinkage of high strength concrete slabs. Constr. Build. Mater. 2014, 61, 41–49. [Google Scholar] [CrossRef]
- Mounanga, P.; Baroghel-Bouny, V.; Loukili, A.; Khelidj, A. Autogenous deformations of cement pastes: Part I. Temperature effects at early age and micro–macro correlations. Cem. Concr. Res. 2006, 36, 110–122. [Google Scholar] [CrossRef]
- Ma, Y.; Li, W.; Jin, M.; Liu, J.; Zhang, J.; Huang, J.; Lu, C.; Zeng, H.; Wang, J.; Zhao, H.; et al. Influences of leaching on the composition, structure and morphology of calcium silicate hydrate (C–S–H) with different Ca/Si ratios. J. Build. Eng. 2022, 58, 105017. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, K.; Gao, S.; Wang, F. Use of Zeolite as Both Internal Curing Agent and Supplementary Binder in Mortar. J. Mater. Civ. Eng. 2023, 35, 04023299. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).