Advances and Applications of Three-Dimensional-Printed Patient-Specific Chest Phantoms in Radiology: A Systematic Review
Abstract
1. Introduction
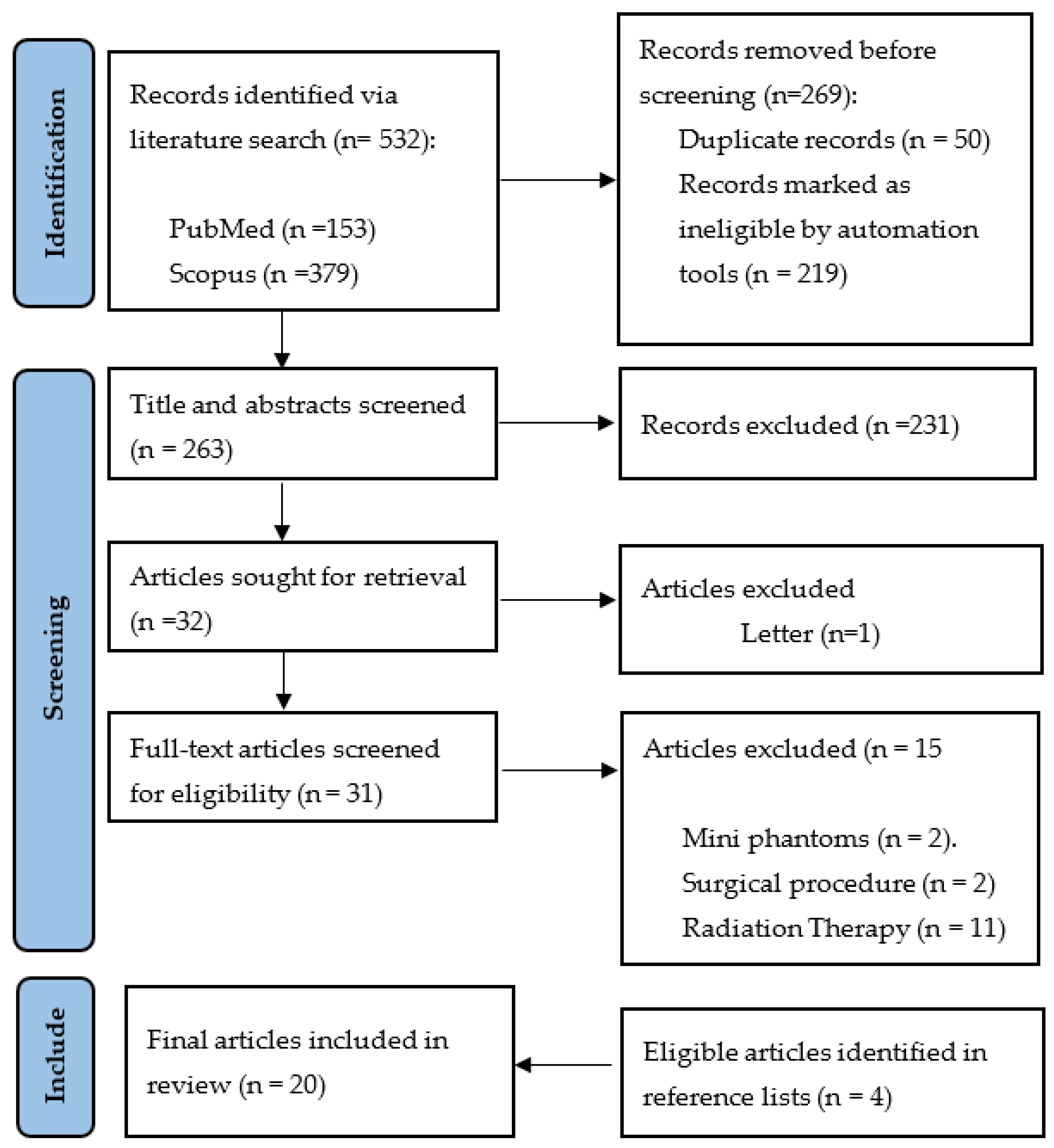
2. Methodology
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Article Selection and Quality Assessment
2.4. Data Extraction and Synthesis
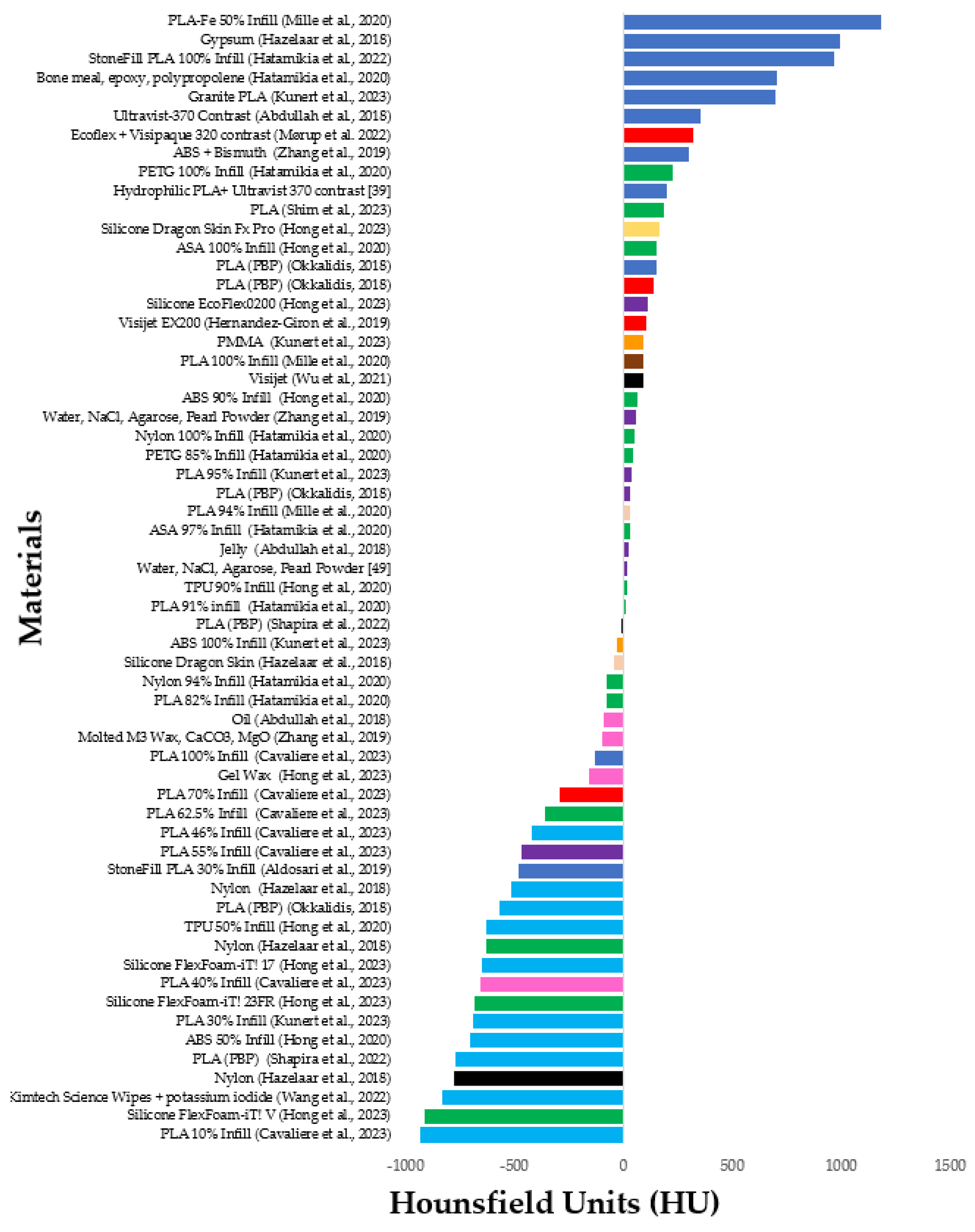
| Article | Year | Study Purpose | Country of Origin | Organs | 3DPM/Modelling Segmentation Software/Printer Costs and Time | 3D-Printing Materials | Lesions | Key Findings and Limitations |
|---|---|---|---|---|---|---|---|---|
| [30] | 2018 | Low-cost cardiac phantom for optimising cardiac CT protocols. | Australia | Heart | FDM 3D Slicer https://www.slicer.org/, accessed on 1 May 2017 Creatbot DM Plus USD 70 12.1 h | ABS Contrast (aorta) Oil (fat) Jelly (muscle) | 0 | A low-cost radiation equivalent, commercial phantom derived with filling materials having similar CT attenuation value to those of the real patient’s images. Aorta, fat, and muscle had HU differences of 8%, −3%, and 5% relative to patient, respectively, representing a maximum error up to 27 HU. The phantom lacks haemodynamic flow and was not developed from real patients’ images. Testing scanning protocols were not investigated. |
| [31] | 2019 | Pulmonary artery phantom with simulated embolism for optimising CTPA protocols. | Australia | Pulmonary trunk and arteries | SLS AnalyzeDirect V 12.0 (AnalyzeDirect, Inc., Lexana, KS, USA) Printer N/P Costs N/P Time N/P | Elastoplastic | 2 pulmonary emboli | Geometrically accurate, optimised protocols for PE detection with dose reduction by up to 80%, lacked HU equivalence test, static rather than dynamic representing blood flow. |
| [32] | 2023 | Feasibility of low-cost thoracic phantom for CT reproducibility assessments. Proposed application for CT quality assurance and dose optimisation. | USA | Lung, Fat, Muscle, Bones, vessels, nodules | FDM inPrint, Materialise NV, Leuven Ultimaker 5S EUR 270 (AUD 450) 3 days | PLA at varied infills | 1 | Comprehensive thoracic model, not radiation and geometrically equivalent. Bone, fat, muscle, lung, vessels, and lesions had HU differences of −69%, −903%, −1772%, −7%, −319%, and −75% relative to the patient, respectively. Representing a maximum HU error of up to 505 HU. Although PLA is a widely popular material, there was a lack of systematic assessment of recent materials with mixed metallic additives for better HU replication. |
| [33] | 2023 | Low-cost patient-specific lung tumour phantoms for imaging algorithm validation. | Austria | Lung tumours | FDM Materialize Mimics Research 23.0 (Materialize, Leuven, Belgium) Original Prusa i3 MK3S Costs N/P Time N/P | PLA, ASA, PETG, Nylon at varied infills. | 12 (6 different samples of 2 tumours) | Homogenous and heterogeneous tumours created with varied infills between central and peripheral aspects. Good radiation equivalence, achieving average attenuations between −100 and 100 HU, consistent with the 17 patient samples. Adequate geometrical agreement of 97% for the 6 lesion samples and 78% for the smaller 6 lesion samples. Smaller lesions were less geometrically accurate due to spatial resolution limitations of the printer. |
| [34] | 2022 | Feasibility of CT-derived skeletal thorax phantom with realistic heterogeneous cortical and spongy bone attenuation. Proposed application for validation of CT procedures. | Austria | Ribs, vertebral column, soft tissue | FDM Materialize Mimics Research 21.0 software (Materialize, Leuven, Belgium) Original Prusa i3 MK3S Costs N/P Time N/P | StoneFill PLA at varied infills and perimeters. | 0 | Radiation equivalence of heterogeneous bone was achieved (−482 to 968 HU) with a single print material, facilitating a simple fabrication process. HU differences of −9.8%, −150%, −7.5%, and −9.4% for the cancellous bone of the dorsal vertebral column, vertebral body, ribs, and soft tissue, respectively, representing a maximum error up to 30 HU by varying infill. Cortical bone matched patient attenuations (230–910 HU) by varying number of perimeters. |
| [35] | 2020 | Feasibility of CT-derived skeletal thorax phantom with morphological and radiological accuracy. Proposed applications include exposure optimisation, medical education, skills practice, and surgical guidance. | Austria | Ribs, vertebral column | PolyJet Materialize Mimics USL 21.0, Materialize, Leuven, Belgium). Connex3 Objet500 Costs: N/P 120 h printing, ≥12 days production | Bone meal powder, epoxy and polypropylene amalgamate injected into rigid Vero pure white mould, flexible Agilus30 Clear (FLX935) for encapsulating the skeletal integument. SUP706B supporting material | 0 | Reproduced average HU accurately. Dorsal vertebral column, vertebral bodies and ribs had a 1.6%, −8.8%, and −3% HU difference between that of the patient, respectively, with a maximum HU error of 19 HU. Lacked heterogeneous bone composition, unable to achieve above 705 HU, 85% geometrical overlap—physical discrepancy between structures due to printing in separate parts. |
| [36] | 2018 | Feasibility of creating a thorax phantom based on a patient with lung cancer for X-ray quality analysis. Proposed for protocol optimisation and software validation. | The Netherlands | Ribs, vertebral column, scapulae, soft tissue, lung surface, airways, lung blood vessels, nodules | Binder Jetting and SLS Materialize Mimics (18.0.0.524, Materialise, Leuven, Belgium) Zcorp 650 and EOS GmbH USD 3500 Time N/P | Gypsum (bone) Nylon (tumours, lung structures), Silicon Dragon Skin (cast for soft tissue). | 3 | HUs varied from patient, with lower lung and higher bone/soft tissue values. HU differences between patient and phantom were 124 %, 49%, −26%, and −28.6% for soft tissue, bone, lung structures and lesions, respectively, giving an HU error up to 221 HU. Accurate geometrical comparison to patient image with mean differences < 1 mm for all tissues. Multiple printed parts assembled, posed challenge to accuracy of spatial relationships. Lacked aerated lung density. |
| [37] | 2019 | Lung phantom with modelled vessels, used for CT image quality assessment and validating reconstruction methods. | The Netherlands | Lung vessels, soft-tissue, vertebral column | MJM ProJet HD 3000 3D Slicer https://www.slicer.org/, accessed on 1 May 2018 $few hundred Time N/P | Visijet EX200 (vessels), PMMA (soft tissue), Teflon (vertebra) | 0 | Shape and HUs varied from patients. Lower lung (air representation, lack of parenchyma) and higher vessels, bone, and soft tissue attenuations. Marked HU differences of 2000%, 11.43%, 271.88%, and 352.38% compared to the patient for vessels, lung interstitium, soft tissue, and vertebra, respectively, giving an error up to 99 HU. MultiJet printing is expensive, despite allowing high level of detail and smooth surfaces. |
| [38] | 2020 | Patient-specific chest phantoms with lesions. Proposed for validating quantitative CT software, calibrating CT intensity (quality assurance), education. | South Korea | Right lung lobe, airway, lesions | FDM Materialize Mimics (Inc., Leuven, Belgium) DP200, Shindoh Co and Ultimaker 3 Cost N/P Time N/P | ABS, TPU (different infills) | N/P | Lung parenchyma of ABS (−705 ± 108 HU) and TPU phantoms (−630 ± 62 HU) were within range of patient attenuations (−600 to −900 HU). Solid nodules differed between patients by 31% and 86% for ABS and TPU phantoms, respectively, with an error up to 85 HU. Added artificial lesions. Bone was ignored due to higher HU requirements. Tissue texture was unnatural due to laminae from successively layering material. |
| [39] | 2023 | Patient-specific chest phantom with lesions of realistic HU proposed for validating quantitative CT software, CT intensity calibration, educational purposes and patient communication. | South Korea | Lung lobes, lesions, spine, ribs, heart, muscle, skin, fat | FDM Materialize Mimics (Inc., Louvain, Belgium) Stratasys Fortus 900MC and Ultimaker S5 Cost N/P Time N/P | Flexible TPU (heart), hydrophilic PLA + contrast (bone), Cast: Silicone (FlexFoam-iT! Series, Lesions), Gel wax (fat), Ecoflex0020 silicone (muscle), Silicon Dragon (Skin) | 6 | Comprehensive thoracic model, HU was within range of normal values for all structures except bone (200 HU instead of >1000 HU) as the contrast was not well absorbed. Attenuation differences between patient and phantom for muscle, fat, skin, and solid nodules were 0%, −39% 36%, and 19%, respectively. Accurate dimensions within 0.2 ± 0.18 mm. Lesions fabricated and randomly placed, rather than based on real patient data. Axial slice rather than entire torso. |
| [40] | 2023 | Reproduce an axial slice of a commercial thorax phantom, proposed for optimising radiation exposures for specific patient groups that are not adequately represented by commercial phantoms (pregnant women, overweight individuals). | Germany | Lung, Muscle, Breast tissue, bone and cartilage | FDM 3D Slicer https://www.slicer.org/, accessed on 1 May 2023 industrial MEX printer (3ntr A2 V4; 3ntr, Oleggio, Italy-multi-material) EUR 39 (AUD 64—exclude printer) 58 h | PLA (infill: 95% muscle, 30% lung), Granite-PLA (bone), PETG (Cartilage), ABS (breast adipose), PMMA (glandular breast) | 0 | Commercial phantom derived rather than based on real patients. Similar HU achieved to commercial phantom, except bone was 160 HU lower, and lung 110 HU higher. All tissues in range of human norms. Does not differentiate between muscle and fat layers. Slight geometrical differences: post-polymerisation shrinkage of ABS and lengthening due to segmentation errors. Multi-material printer allowing 3 different materials to be printed in one step is expensive and not widely available. Phantom fails to distinguish between cortical bone, cancellous bone, and bone marrow. |
| [41] | 2020 | Patient-derived low-cost paediatric torso phantom from only 2 materials, for CT imaging assessment and dosimetry purposes. | USA | Lung, Soft tissue, heart, oesophagus, ribs, clavicles, scapula, vertebral column | FDM 3D Slicer https://slic3r.org/ accessed on 1 May 2019 Ultimaker 3 (dual extrusion) USD 160 1 week/~120 h | PLA (soft tissues and others), PLA-Fe (bones) at different infills | 0 | Very similar HU to patient with an error of 100–200 HU for soft tissue and bone, respectively. Strong linear correlation between infill density and CT number. Automated process printed in one build without the need for post-processing and backfilling. Only a 10 cm axial cross-section was reproduced. Does not differentiate between muscle, fat, and skin soft tissue layers. |
| [18] | 2022 | Patient-specific 3D-printed coronary artery model for CTPA optimisation. | Denmark | Coronary arteries | FDM Invesalius 3 (Invesalius, Brazil) Dimension Elite EUR 43 7 h | Platinum curved silicone rubber (Ecoflex 00-35) + Visipaque contrast + gelatine + NaCl | 0 | Coronary artery model demonstrated accurate radiation equivalence, within 15% of patient HUs. Protocols with ASiR-V above 60% were non-diagnostic. Embedded in an expensive commercial phantom and with a porcine heart, not true to patient. |
| [42] | 2018 | Propose a new method of 3D-printing patient chest using PBP variation of filament extrusion amount per unit distance. | Malta | N/P | FDM N/A T-Rex 2 (Formbot) Cost N/P Time N/P | PLA | 0 | PBP produced a significantly wider HU range compared to VID method and more closely resembled patient HU’s, however, with longer printing times. Morphologically more similar by visual inspection. Converts CT image directly into printer instructions to control extrusion rate per voxel, without intermediate step of segmentation. Phantom dimensions and tissues included are undescribed. High enough bone attenuation was not achieved. Different scanners and parameters used for patients and phantoms may explain different HUs. |
| [43] | 2023 | PixelPrint method to print COVID-19 lung phantoms by modifying printhead speed, with constant filament extrusion rate. Proposed for validation of algorithms and protocol optimisation. | USA | N/P | FDM N/A Lulzbot TAZ 6 Cost N/P Time N/P | PLA | 0 | Converts CT image directly into printer instructions to control the printhead speed per voxel, without the intermediate step of segmentation. Subjective radiologist assessment determined that there were non-clinically significant differences (mean score difference: 0.03–0.29) between real patient and phantom slices in terms of diagnostic confidence, image contrast and image noise (p < 0.0005, effect size = 0.03–0.31), as well as resolution (p > 0.05) on a scoring scale of 1–5. |
| [44] | 2022 | Evaluation of PixelPrint method to print COVID-19 lung phantoms of different severity with accurate geometry, texture, and attenuation profiles. Proposed for protocol optimisation, CT research and ground-truths for radiomics. | USA | Lung (parenchyma and vessels). | FDM N/A Lulzbot TAZ 6 Cost N/P 24 h | PLA | 0 | Phantom attenuations were achieved by different volumes of filament per voxel. Mean HU differences between patient and phantom for lung parenchyma and vessels were within 15 HU. Geometrically equivalent within printer resolution error. Strong radiomics correlation of contrast and texture between patient and phantom images (r >0.95). |
| [45] | 2023 | Compare the detection sensitivity of paediatric lung nodules using different image reconstruction methods. | South Korea | Lung Nodules | SLA TeraRecon 3D program (USA) RS pro-800 Cost N/P Time N/P | PLA | 3 | Determined that the fast non-local means filter is better than iterative reconstruction at reducing image noise whilst preserving contrast and sharpness for better lung nodule detection. Printed the irregular shape of the nodules extracted from real patient data, however, lacked formal morphological and geometrical analysis. Nodules did not reflect the various attenuations of the patients’ nodules (−37 to 665 HU), however, were within range (145–185 HU). Lacked vessels and parenchyma. Embedded into an expensive commercial phantom. |
| [46] | 2022 | Feasibility of using low-density paper and inkjet printing to simulate diseased lung parenchyma and lung nodules as ground truths for radiomics. Proposed for application of CT protocol optimisation and software validation. | USA | Lung parenchyma and nodules | Inkjet Printing ITK-SNAP (ITKSNAP.org. accessed on 1 May 2021) HP Deskjet 6940 Cost N/P Time N/P | Kimtech Science Wipes with potassium iodide solution | 1 | Phantom slices achieved good Pearson correlation of attenuations compared to patient slices (r = 0.83–0.92). Lung parenchyma (−830 to 200 HU) was unable to re-create near air densities <−1000 HU due to limitations of paper substrate. Radiomic comparisons showed a median absolute difference of 6.1% and good morphological consensus with shaped features demonstrating <25% difference. |
| [47] | 2021 | Aortic dissection phantom with TEVAR stent in situ for optimising routine follow-up CTA protocols. | Switzerland | Aorta | PolyJet 3D Slicer (version 4.9.0, www.slicer.org accessed on 26 July 2021; MA, USA) Printer: N/P Cost N/P Time N/P | Visijet CE-NT, Agilus, | 0 | A patient-specific aortic dissection 3D-printed model with a TEVAR stent was developed, having similar material and radiological properties to humans. Dose reduction of at least 20% enabled by reducing kVp from 120 to 80, whilst maintaining diagnostic image quality. Lacked haemodynamic flow and realistic surrounding tissue environment. |
| [48] | 2019 | Development of a cost-effective personalised chest phantom, proposed for dose optimisation. | China | Skin, fat, muscle, lung, lesion, ribs, scapula, sternal angle | Method: N/P Mimics Research 17.0 image analysis software (Materialize, Belgium) Printer: N/P, photosensitive printer Cost: N/P Time: N/P | ABS (skin shell), Molted M3 wax + CaCO3 + MgO (Fat), ABS-Bismuth (bones), water, agarose, NaCl + pearl powder (Muscle and lesions), foamed silica gel (lung). | 1 | A patient-specific chest phantom consisting of a 3D-printed skin and fat shell with filling materials, similar in morphology and radiation attenuation properties to the real CT. HU differences of 25%, 30%, 20%, and 35% between patient and phantom for fat, muscle, bone, and tumour, respectively. This represents a 20 HU difference for fat, muscle, and lesion and a 55 HU difference on average for bone. Lacked geometrical analysis as well as HU analysis for lung tissue and skin. |
| Article | Scanner | Parameters | Skin | Fat | Muscle | Soft Tissue Combined | Vessels | Bone | Lung Parenchyma | Lung Nodules | Airways | Heart | Breast |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [30] | Alexion, Toshiba Medical Systems Co Ltd., Otowara, Japan) | 120 kVp, 200 mA | - | Oil −92.4 HU | Jelly 25.9 HU | - | Contrast 354.3 HU | - | - | - | - | - | - |
| [32] | Siemens Somatom Force (Siemens Healthineers, Erlangen, Germany) | 120 kVp, 50 mAs | - | PLA (40% infill) −657 ± 55.46 HU | PLA (55% infill) −469 ± 79.16 HU | - | PLA (70% infill) −295 ± 43.93 HU | PLA (100% infill) −132.16 ± 103.66 HU | PLA (10% infill) −933.17 ± 63.89 HU | PLA (62.5% infill) −357 ± 56.12 HU | - | - | - |
| [33] | SOMATOM Definition AS, Siemens Healthineers, Germany | 120 kVp, 200 mAs | - | - | - | - | - | - | - | ASA (100%) 155 HU, 30 HU (97%), PLA: −75 HU (82% infill), 10 HU (91% Infill), Nylon: 54 HU (100%), −75 HU (94%), PETG: 227 (100%), 47 (85%) | - | - | - |
| [34] | SOMATOM Definition AS, Siemens Healthineers. Erlangen Germany | 120 kVp, 315 mAs | - | - | - | - | - | StoneFill PLA (30–100% Infill) = −482 to 968 HU | - | - | - | - | - |
| [35] | SOMATOM Definition AS, Siemens Healthineers. Erlangen Germany | 120 kVp, 315 mAs | - | - | - | - | - | Bone meal powder, epoxy, polypropylene = 42–705 HU | - | - | - | - | - |
| [36] | GE Discovery CT590 | 120 kVp | - | - | - | Silicone Dragon Skin −168 to 95 HU (μ = −43 HU) | Nylon = −779 to −229 (μ = −512 HU) | Gypsum= 372–995 HU (μ = 731) | Nylon = −779 to −229 (μ = −512 HU) | Nylon = −632 to 50 HU (μ = −130 HU) | Nylon = −779 to −229 (μ = −512 HU) | - | - |
| [37] | Toshiba Aquilion Genesis | 120 kVp, Sure Exposure | - | - | - | PMMA 119 ± 10 HU | Visijet Ex200 104 ± 22 HU | Teflon 119 ± 8 HU | Air −985 ± 18 HU | - | - | - | - |
| [38] | dual-source CT SOMATOM Definition Flash, Siemens | 120 kVp | - | - | - | - | - | - | 50% infill: ABS −705 ± 108 HU, TPU −630 ± 62 HU | 90% Infill: ABS 68 ± 16 HU TPU 15 ± 18 HU | - | - | - |
| [39] | dual-source CT SOMATOM Definition Flash, Siemens | 120 kVp | Silicone Dragon Skin Fx Pro 165 ± 29 HU | Gel wax −160 ± 21 HU | Silicone ExoFlex0200 111 ± 23 HU | - | - | Hydrophilic PLA + contrast 200 ± 24 HU | Silicone FlexFoam-iT! 17 −651 ± 16 HU | FlexFoam-iT! V:−909 ±18 HU, FlexFoam-iT! 23FR: −683 ± 23 HU | - | Flexible TPU N/A | - |
| [40] | GE Bright Speed; General Electrics, Boston, MA, USA | 120 kVp, 200 mA, 0.8 s | - | - | PLA (95% Infill): 35 ± 25 HU | - | - | Granite PLA composite filament 700 ± 50 HU | PLA (30%) −690 ± 80 HU | - | - | - | ABS (adipose) −30 ± 10 HU PMMA (glandular) 95 ± 15 HU |
| [41] | Siemens Biograph mCT | 120 kVp, 250 mAs | - | - | - | PLA (94%) 31 ± 79 HU | - | PLA-Fe (50%) 1180 ± 1107 HU | PLA (46%) −417 ± 434 HU | - | - | PLA, 94 ± 46 HU | - |
| [18] | GE Revolution GE Healthcare Waukesha, WI, USA | 100 kVp, 50–570 mA | - | - | - | - | Ecoflex, contrast, 318 ± 4 HU | - | - | - | - | - | - |
| [42] | Phillips, Brilliance 64 | 120 kVp, 339 mA | - | - | PLA 32 HU | - | PLA 139 HU | PLA 153 HU | PLA −570 HU | - | - | - | - |
| [44] | GE Revolution, Siemens Sensation-64 | Not mentioned | - | - | - | - | PLA −3.9 ± 18.6 HU | - | PLA −771 ± 34 HU | - | - | - | - |
| [45] | SOMATOM Definition AS, Siemens Healthineers. | 80 and 100 kVp | - | - | - | - | - | - | - | PLA 145–185 HU | - | - | - |
| [46] | Siemens Somatom Force | 120 kVp, 200 mAs | - | - | - | - | - | - | Kimtech Science Wipes + KI −830 to 200 HU | Kimtech Science Wipes + KI N/A | - | - | - |
| [47] | Siemens Somatom Force | 120 kVp 150 mAs | - | - | - | - | Visijet CE-NT 90.6 HU | - | - | - | - | - | - |
| [48] | Phillips, Brilliance 256 | 120 kVp, 260 mAs | ABS N/A | Molted M3 wax, CaCO3, MgO, −100 to −60 HU | Water, NaCl, Agarose, pearl powder, 20–60 HU | - | - | ABS + Bismuth 120–300 HU | Foamed silica gel N/A | Water, NaCl, Agarose, pearl powder 17–49 HU | - | - | - |
3. Results
3.1. Three-Dimensional-Printing Thoracic Organs
3.2. Three-Dimensional-Printing Methods
3.3. Purposes of 3D-Printed Chest Phantoms
3.4. Quality of Studies
4. Discussion
4.1. Quality of Studies
4.2. Three-Dimensional-Printing Methods and Materials
4.3. Three-Dimensional-Printing Thoracic Organs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, Z.; Wong, Y.H.; Yeong, C.H. Patient-specific 3D-printed low-cost models in medical education and clinical practice. Micromachines 2023, 14, 464. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, K.M. Rapid prototyping technologies. In Rapid Prototyping in Cardiac Disease; Borrello, J., Backeris, P., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 41–49. [Google Scholar]
- Rossi, T.; Williams, A.; Sun, Z. Three-Dimensional Printed Liver Models for Surgical Planning and Intraoperative Guidance of Liver Cancer Resection: A Systematic Review. Appl. Sci. 2023, 13, 10757. [Google Scholar] [CrossRef]
- Ghantous, Y.; Nashef, A.; Mohanna, A.; Abu-El-naaj, I. Three-dimensional technology applications in maxillofacial reconstructive surgery: Current surgical implications. Nanomaterials 2020, 10, 2523. [Google Scholar] [CrossRef] [PubMed]
- Haleem, A.; Javaid, M.; Suman, R.; Singh, R.P. 3D printing applications for radiology: An overview. Indian J. Radiol. Imaging 2021, 31, 10–17. [Google Scholar] [PubMed]
- Filippou, V.; Tsoumpas, C. Recent advances on the development of phantoms using 3D printing for imaging with CT, MRI, PET, SPECT, and ultrasound. J. Med. Phys. 2018, 45, e740–e760. [Google Scholar] [CrossRef] [PubMed]
- Scalzetti, E.M.; Huda, W.; Bhatt, S.; Ogden, K.M. A method to obtain mean organ doses in a Rando phantom. Health Phys. 2008, 95, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Okkalidis, N. 3D printing methods for radiological anthropomorphic phantoms. Phys. Med. Biol. 2022, 67, 15TR04. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Figl, M.; Unger, E.; Buschmann, M.; Homolka, P. X-ray attenuation of bone, soft and adipose tissue in CT from 70 to 140 kV and comparison with 3D printable additive manufacturing materials. Sci. Rep. 2022, 12, 14580. [Google Scholar] [CrossRef] [PubMed]
- Kunert, P.; Trinkl, S.; Giussani, A.; Reichert, D.; Brix, G. Tissue equivalence of 3D printing materials with respect to attenuation and absorption of X-rays used for diagnostic and interventional imaging. J. Med. Phys. 2022, 49, 7766–7778. [Google Scholar] [CrossRef]
- Tino, R.; Yeo, A.; Leary, M.; Brandt, M.; Kron, T. A Systematic Review on 3D-Printed Imaging and Dosimetry Phantoms in Radiation Therapy. Technol. Cancer Res. Treat. 2019, 18, 1533033819870208. [Google Scholar] [CrossRef]
- Germann, M.; Shim, S.; Angst, F.; Saltybaeva, N.; Boss, A. Spiral breast computed tomography (CT): Signal-to-noise and dose optimization using 3D-printed phantoms. Eur. J. Radiol. 2021, 31, 3693–3702. [Google Scholar] [CrossRef] [PubMed]
- Jahnke, P.; Schwarz, S.; Ziegert, M.; Schwarz, F.B.; Hamm, B.; Scheel, M. Paper-based 3D printing of anthropomorphic CT phantoms: Feasibility of two construction techniques. Eur. J. Radiol. 2019, 29, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- Irnstorfer, N.; Unger, E.; Hojreh, A.; Homolka, P. An anthropomorphic phantom representing a prematurely born neonate for digital x-ray imaging using 3D printing: Proof of concept and comparison of image quality from different systems. Sci. Rep. 2019, 9, 14357. [Google Scholar] [CrossRef] [PubMed]
- Homolka, P.; Figl, M.; Wartak, A.; Glanzer, M.; Dünkelmeyer, M.; Hojreh, A.; Hummel, J. Design of a head phantom produced on a 3D rapid prototyping printer and comparison with a RANDO and 3M lucite head phantom in eye dosimetry applications. Phys. Med. Biol. 2017, 62, 3158–3174. [Google Scholar] [CrossRef] [PubMed]
- Rossman, A.H.; Catenacci, M.; Zhao, C.; Sikaria, D.; Knudsen, J.E.; Dawes, D.; Gehm, M.E.; Samei, E.; Wiley, B.J.; Lo, J.Y. Three-dimensionally-printed anthropomorphic physical phantom for mammography and digital breast tomosynthesis with custom materials, lesions, and uniform quality control region. J. Med. Imaging 2019, 6, 021604. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Pegues, H.; Samei, E.; Lo, J.Y.; Wiley, B.J. Controlling the attenuation of 3D-printed physical phantoms for computed tomography with a single material. Med. Phys. 2022, 49, 2582–2589. [Google Scholar] [CrossRef] [PubMed]
- Mørup, S.D.; Stowe, J.; Precht, H.; Gervig, M.H.; Foley, S. Design of a 3D printed coronary artery model for CT optimization. Radiography 2022, 28, 426–432. [Google Scholar] [CrossRef]
- Sindi, R.; Wong, Y.H.; Yeong, C.H.; Sun, Z. Development of patient-specific 3D-printed breast phantom using silicone and peanut oils for magnetic resonance imaging. Quant. Imaging Med. Surg. 2020, 10, 1237–1248. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-H.; Park, M.; Yoon, M.S.; Lee, Y. Quantitative evaluation of total variation noise reduction algorithm in CT images using 3D-printed customized phantom for femur diagnosis. J. Korean Phys. Soc. 2022, 81, 450–459. [Google Scholar] [CrossRef]
- Li, X.; Wu, B.; Zou, Y.; Zhang, G.; Liu, S.; Zhao, L.; Zhang, Z.; Wu, W.; Liu, C.; Ai, S. Development of a 3D-printed pelvic CT phantom combined with fresh pathological tissues of bone tumor. Quant. Imaging Med. Surg. 2022, 12, 4647–4657. [Google Scholar] [CrossRef]
- Leitão, C.A.; Salvador, G.L.d.O.; Tazoniero, P.; Warszawiak, D.; Saievicz, C.; Jakubiak, R.R.; Escuissato, D.L. Dosimetry and comparison between different CT protocols (low dose, ultralow dose, and conventional CT) for lung nodules’ detection in a phantom. Radiol. Res. Pract. 2021, 2021, 6667779. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.; Landau, J.; Ebner, L.; Bütikofer, Y.; Leidolt, L.; Brela, B.; May, M.; Heverhagen, J.; Christe, A. Performance of ultralow-dose CT with iterative reconstruction in lung cancer screening: Limiting radiation exposure to the equivalent of conventional chest X-ray imaging. Eur. Radiol. 2016, 26, 3643–3652. [Google Scholar] [CrossRef] [PubMed]
- Mascalchi, M.; Picozzi, G.; Puliti, D.; Diciotti, S.; Deliperi, A.; Romei, C.; Falaschi, F.; Pistelli, F.; Grazzini, M.; Vannucchi, L.; et al. Lung Cancer Screening with Low-Dose CT: What We Have Learned in Two Decades of ITALUNG and What Is Yet to Be Addressed. Diagnostics 2023, 13, 2197. [Google Scholar] [CrossRef] [PubMed]
- McCollough, C.H.; Leng, S. Use of artificial intelligence in computed tomography dose optimisation. Ann. ICRP 2020, 49, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.; Flohr, T. Computed tomography recent history and future perspectives. J. Med. Imaging 2021, 8, 052109. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, e1–e34. [Google Scholar] [CrossRef] [PubMed]
- Cano-Vicent, A.; Tambuwala, M.M.; Hassan, S.S.; Barh, D.; Aljabali, A.A.A.; Birkett, M.; Arjunan, A.; Serrano-Aroca, Á. Fused deposition modelling: Current status, methodology, applications and future prospects. Addit. Manuf. 2021, 47, 102378. [Google Scholar] [CrossRef]
- Crowe, M.; Sheppard, L.; Campbell, A. Reliability analysis for a proposed critical appraisal tool demonstrated value for diverse research designs. J. Clin. Epidemiol. 2012, 65, 375–383. [Google Scholar] [CrossRef]
- Abdullah, K.A.; McEntee, M.F.; Reed, W.; Kench, P.L. Development of an organ-specific insert phantom generated using a 3D printer for investigations of cardiac computed tomography protocols. J. Med. Radiat. Sci. 2018, 65, 175–183. [Google Scholar] [CrossRef]
- Aldosari, S.; Jansen, S.; Sun, Z. Patient-specific 3D printed pulmonary artery model with simulation of peripheral pulmonary embolism for developing optimal computed tomography pulmonary angiography protocols. Quant. Imaging Med. Surg. 2019, 9, 75–85. [Google Scholar] [CrossRef]
- Cavaliere, C.; Baldi, D.; Brancato, V.; Aiello, M.; Salvatore, M. A customized anthropomorphic 3D-printed phantom to reproducibility assessment in computed tomography: An oncological case study. Front. Oncol. 2023, 13, 1123796. [Google Scholar] [CrossRef] [PubMed]
- Hatamikia, S.; Gulyas, I.; Birkfellner, W.; Kronreif, G.; Unger, A.; Oberoi, G.; Lorenz, A.; Unger, E.; Kettenbach, J.; Figl, M.; et al. Realistic 3D printed CT imaging tumor phantoms for validation of image processing algorithms. Phys. Medica 2023, 105, 102512. [Google Scholar] [CrossRef] [PubMed]
- Hatamikia, S.; Kronreif, G.; Unger, A.; Oberoi, G.; Jaksa, L.; Unger, E.; Koschitz, S.; Gulyas, I.; Irnstorfer, N.; Buschmann, M.; et al. 3D printed patient-specific thorax phantom with realistic heterogenous bone radiopacity using filament printer technology. J. Med. Phys. 2022, 32, 438–452. [Google Scholar] [CrossRef] [PubMed]
- Hatamikia, S.; Oberoi, G.; Unger, E.; Kronreif, G.; Kettenbach, J.; Buschmann, M.; Figl, M.; Knäusl, B.; Moscato, F.; Birkfellner, W. Additively Manufactured Patient-Specific Anthropomorphic Thorax Phantom With Realistic Radiation Attenuation Properties. Front. Bioeng. Biotechnol. 2020, 8, 385. [Google Scholar] [CrossRef] [PubMed]
- Hazelaar, C.; Eijnatten, M.; Dahele, M.; Wolff, J.; Forouzanfar, T.; Slotman, B.; Verbakel, W.F.A.R. Using 3D printing techniques to create an anthropomorphic thorax phantom for medical imaging purposes. J. Med. Phys. 2018, 45, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Giron, I.; den Harder, J.M.; Streekstra, G.J.; Geleijns, J.; Veldkamp, W.J.H. Development of a 3D printed anthropomorphic lung phantom for image quality assessment in CT. Phys. Medica 2019, 57, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Lee, S.; Kim, G.B.; Lee, S.M.; Kim, N.; Seo, J.B. Development of a CT imaging phantom of anthromorphic lung using fused deposition modeling 3D printing. Medcine 2020, 99, e18617. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Moon, S.; Seo, J.B.; Kim, N. Development of a patient-specific chest computed tomography imaging phantom with realistic lung lesions using silicone casting and three-dimensional printing. Sci. Rep. 2023, 13, 3941. [Google Scholar] [CrossRef]
- Kunert, P.; Schlattl, H.; Trinkl, S.; Giussani, A.; Klein, L.; Janich, M.; Reichert, D.; Brix, G. Reproduction of a conventional anthropomorphic female chest phantom by 3D-printing: Comparison of image contrasts and absorbed doses in CT. J. Med. Phys. 2023, 50, 4734–4743. [Google Scholar] [CrossRef]
- Mille, M.M.; Griffin, K.T.; Maass-Moreno, R.; Lee, C. Fabrication of a pediatric torso phantom with multiple tissues represented using a dual nozzle thermoplastic 3D printer. J. Appl. Clin. Med. Phys. 2020, 21, 226–236. [Google Scholar] [CrossRef]
- Okkalidis, N. A novel 3D printing method for accurate anatomy replication in patient-specific phantoms. J. Med. Phys. 2018, 45, 4600–4606. [Google Scholar] [CrossRef] [PubMed]
- Shapira, N.; Donovan, K.; Mei, K.; Geagan, M.; Roshkovan, L.; Gang, G.J.; Abed, M.; Linna, N.B.; Cranston, C.P.; O’Leary, C.N.; et al. Three-dimensional printing of patient-specific computed tomography lung phantoms: A reader study. PNAS Nexus 2023, 2, pgad026. [Google Scholar] [CrossRef] [PubMed]
- Shapira, N.; Donovan, K.; Mei, K.; Geagan, M.; Roshkovan, L.; Litt, H.I.; Gang, G.J.; Stayman, J.W.; Shinohara, R.T.; Noël, P.B. PixelPrint: Three-dimensional printing of realistic patient-specific lung phantoms for CT imaging. In Medical Imaging 2022: Physics of Medical Imaging; SPIE: Bellingham, DC, USA, 2022; Volume 12031. [Google Scholar] [CrossRef]
- Shim, J.; Yoon, M.; Lee, Y. Comparison of filtered back projection with fast non-local means denoising approach and iterative reconstruction in pediatric chest CT image using 3D printed lung nodules. J. Korean Phys. Soc. 2023, 82, 1114–1123. [Google Scholar] [CrossRef]
- Wang, J.; Falkson, S.R.; Guo, H.H. Radiopaque Recreations of Lung Pathologies from Clinical Computed Tomography Images Using Potassium Iodide Inkjet 3-dimensional Printing: Proof of Concept. J. Thorac. Imaging 2022, 37, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.A.; Squelch, A.; Jansen, S.; Sun, Z. Optimization of computed tomography angiography protocols for follow-up type b aortic dissection patients by using 3d printed model. Appl. Sci. 2021, 11, 6844. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, H.; Zhao, H.; He, Z.; Shi, L.; He, Y.; Ju, N.; Rong, Y.; Qiu, J. Design and fabrication of a personalized anthropomorphic phantom using 3D printing and tissue equivalent materials. Quant. Imaging Med. Surg. 2019, 9, 94–100. [Google Scholar] [CrossRef]
- George, E.; Liacouras, P.; Rybicki, F.J.; Mitsouras, D. Measuring and Establishing the Accuracy and Reproducibility of 3D Printed Medical Models. Radiographics 2017, 37, 1424–1450. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-A.; Squelch, A.; Sun, Z. Investigation of three-dimensional printing materials for printing aorta model replicating type B aortic dissection. Curr. Med. Imaging Rev. 2021, 17, 843–849. [Google Scholar] [CrossRef]
- Sorooshfard, E.; Tahmasbi, M.; Chegeni, N.; Birgani, M.J.T. Evaluating the effects of variation in CT scanning parameters on the image quality and Hounsfield units for optimization of dose in radiotherapy treatment planning: A semi-anthropomorphic thorax phantom study. J. Cancer Res. Ther. 2023, 19, 426–434. [Google Scholar] [CrossRef]
- Dowsett, D.; Kenny, P.; Johnston, E. Interactions of X- and gamma radiation with matter. In The Physics of Diagnostic Imaging, 2nd ed.; Dowsett, D., Kenny, P., Johnston, E., Eds.; CRC Press: London, UK, 2012; pp. 113–141. [Google Scholar]
- Koyotokagaku. Product Data: Multipurpose Chest Phantom N1 “LUNGMAN”. Available online: https://www.kyotokagaku.com/en/products_data/ph-1_01/ (accessed on 25 April 2024).
- Ma, X.; Buschmann, M.; Unger, E.; Homolka, P. Classification of X-ray attenuation properties of additive manufacturing and 3D printing materials using computed tomography from 70 to 140 kVp. Front. Bioeng. Biotechnol. 2021, 9, 763960. [Google Scholar] [CrossRef]
- Madison, K.; Weygand, J.; Andreozzi, J.M.; Hunt, D.; Perez, B.A.; Graham, J.A.; Gage, R. Methodology for computed tomography characterization of commercially available 3D printing materials for use in radiology/radiation oncology. J. Appl. Clin. Med. Phys. 2023, 24, e13999. [Google Scholar] [CrossRef]
- Savi, M.; Andrade, M.A.B.; Potiens, M.P.A. Commercial filament testing for use in 3D printed phantoms. Radiat. Phys. Chem. Oxf. Engl. 1993 2020, 174, 108906. [Google Scholar] [CrossRef]
- van Eijnatten, M.; Koivisto, J.; Karhu, K.; Forouzanfar, T.; Wolff, J. The impact of manual threshold selection in medical additive manufacturing. Int. J. Comput. Assist. Radiol. Surg. 2017, 12, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Mei, K.; Geagan, M.; Roshkovan, L.; Litt, H.I.; Gang, G.J.; Shapira, N.; Stayman, J.W.; Noël, P.B. Three-dimensional printing of patient-specific lung phantoms for CT imaging: Emulating lung tissue with accurate attenuation profiles and textures. J. Med. Phys. 2022, 49, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed]
- Gharleghi, R.; Dessalles, C.A.; Lal, R.; McCraith, S.; Sarathy, K.; Jepson, N.; Otton, J.; Barakat, A.I.; Beier, S. 3D Printing for Cardiovascular Applications: From End-to-End Processes to Emerging Developments. Ann. Biomed. Eng. 2021, 49, 1598–1618. [Google Scholar] [CrossRef] [PubMed]
- Iftekar, S.F.; Aabid, A.; Amir, A.; Baig, M. Advancements and Limitations in 3D Printing Materials and Technologies: A Critical Review. Polymers 2023, 15, 2519. [Google Scholar] [CrossRef] [PubMed]
- Formlabs. Guide to 3D Printing Materials: Types, Applications, and Properties. Available online: https://formlabs.com/asia/blog/3d-printing-materials/ (accessed on 16 June 2024).
- Simplify3D. Filament Properties Table. Available online: https://www.simplify3d.com/resources/materials-guide/properties-table/ (accessed on 16 June 2024).
- Barile, G.; Leoni, A.; Muttillo, M.; Paolucci, R.; Fazzini, G.; Pantoli, L. Fused-Deposition-Material 3D-Printing Procedure and Algorithm Avoiding Use of Any Supports. Sensors 2020, 20, 470. [Google Scholar] [CrossRef] [PubMed]
- Formlabs. Flexible 3D Printing Guide: Compare Processes, Materials, and Applications. Available online: https://formlabs.com/blog/flexible-3d-printing-materials-and-processes/ (accessed on 16 June 2024).
- Majca-Nowak, N.; Pyrzanowski, P. The Analysis of Mechanical Properties and Geometric Accuracy in Specimens Printed in Material Jetting Technology. J. Mater. 2023, 16, 3014. [Google Scholar] [CrossRef]
- 3D Systems. VisiJet® EX200 Plastic Material for 3-D Modeling. Available online: https://www.pdmodels.co.uk/datasheets/Visijet_EX200_Info_0509.pdf (accessed on 17 June 2024).
- 3D Systems. USP Class VI and ISO 10993-1 Information. Available online: https://support.3dsystems.com/s/article/materials-usp-class-vi-and-iso-10993-1-information?language=en_US (accessed on 17 June 2024).
- 3D Systems. Safety Data Sheet. Available online: https://printer-docs-public.s3.amazonaws.com/sites/default/files/sds-files/professional/VisiJet_EX200/SDS_24184_MTR_UGHS_EN_04-17-2024-VisiJet%20EX%20200%2C%20VisiJet%20M3%20Crystal.pdf (accessed on 17 June 2024).
- Huang, J.; Duan, B.; Cai, P.; Manuka, M.; Hu, H.; Hong, Z.; Cao, R.; Jian, S.; Ma, B. On-demand setting of extrusion-based 3D printing gypsum using a heat-induced accelerator. Constr. Build. Mater. 2021, 304, 124624. [Google Scholar] [CrossRef]
- Form Futura 3D Printing Materials. PMMA Filament. Available online: https://formfutura.com/c/filaments/pmma/ (accessed on 17 June 2024).
- Smooth-On. FlexFoam-iT!™ X. Available online: https://www.smooth-on.com/products/flexfoam-it-x/ (accessed on 18 June 2024).
- Seeram, E. Computed Tomography: Physical Principles, Clinical Applications, and Quality Control, 3rd ed.; Elsevier: Philadelphia, PA, USA, 2008; pp. 84–102. [Google Scholar]
- Ceh, J.; Youd, T.; Mastrovich, Z.; Peterson, C.; Khan, S.; Sasser, T.A.; Sander, I.M.; Doney, J.; Turner, C.; Leevy, W.M. Bismuth infusion of ABS enables additive manufacturing of complex radiological phantoms and shielding equipment. Sensors 2017, 17, 459. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Xie, K.; Wu, X.; Lu, Z.; Li, C.; Sun, J.; Lin, T.; Sui, J.; Ni, X. Generating synthetic CT from low-dose cone-beam CT by using generative adversarial networks for adaptive radiotherapy. J. Radiat. Oncol. 2021, 16, 202. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Wang, W.; Mao, Z.; He, Y.; Chen, S.; Liu, G.; Su, J.; Feng, P.; Shi, Y.; Yan, C.; et al. Multimaterial 3D and 4D Bioprinting of Heterogenous Constructs for Tissue Engineering. Adv. Mater. 2023, e2307686. [Google Scholar] [CrossRef] [PubMed]


| Search Strategy 1 | AND | Search Strategy 2 | AND | Search Strategy 3 |
| 3D printing OR 3D printed OR 3D-printed OR 3D-printing OR 3-D-printed OR 3-dimensional Printing OR Three-dimensional printing OR three-dimensional printing OR three-dimensional (3D) printing OR three-dimensional (3-D) printer OR 3D printable OR 3D printer OR Additively Manufactured OR Additively manufacturing OR fused deposition modelling OR FDM OR Selective laser sintering OR SLS OR MultiJet printing OR PolyJet Printing OR Resin-based Vat photopolymerization OR vat polymerisation OR Vat polymerization OR VPP | lung OR pulmonary OR chest OR thorax OR bronchial OR respiratory OR alveoli OR alveolar OR lungs OR pleura OR thoracic | phantom OR simulation OR Model OR Patient-replica OR construction OR design OR fabrication OR Patient-specific OR replica OR replication OR reproduction OR mould |
| Article | Preliminaries | Introduction | Design | Data Collection | Ethics/Conflicts of Interest | Results | Discussion | Total |
|---|---|---|---|---|---|---|---|---|
| [30] | 4 | 4 | 5 | 2 | 4 | 4 | 3 | 26/35 (74%) |
| [31] | 4 | 5 | 4 | 3 | 5 | 4 | 4 | 29/35 (83%) |
| [32] | 4 | 5 | 4 | 3 | 5 | 3 | 3 | 27/35 (77%) |
| [33] | 4 | 5 | 3 | 3 | 5 | 2 | 5 | 27/35 (77%) |
| [34] | 5 | 5 | 3 | 3 | 5 | 2 | 3 | 26/35 (74%) |
| [35] | 5 | 5 | 3 | 2 | 5 | 4 | 3 | 27/35 (77%) |
| [36] | 5 | 5 | 4 | 3 | 5 | 3 | 5 | 30/35 (86%) |
| [37] | 5 | 5 | 4 | 4 | 2 | 2 | 5 | 27/35 (77%) |
| [38] | 5 | 2 | 4 | 4 | 4 | 2 | 3 | 24/35 (69%) |
| [39] | 5 | 4 | 4 | 2 | 5 | 2 | 4 | 26/35 (74%) |
| [40] | 4 | 5 | 4 | 3 | 4 | 3 | 4 | 27/35 (77%) |
| [41] | 4 | 5 | 4 | 4 | 5 | 3 | 4 | 29/35 (83%) |
| [18] | 4 | 5 | 3 | 3 | 4 | 2 | 4 | 25/35 (71%) |
| [42] | 3 | 4 | 3 | 3 | 4 | 2 | 4 | 23/35 (66%) |
| [43] | 4 | 5 | 3 | 5 | 4 | 3 | 5 | 29/35 (83%) |
| [44] | 3 | 2 | 2 | 3 | 4 | 2 | 1 | 17/35 (49%) |
| [45] | 4 | 4 | 3 | 2 | 2 | 2 | 3 | 20/35 (57%) |
| [46] | 5 | 4 | 2 | 3 | 2 | 2 | 4 | 22/35 (63%) |
| [47] | 5 | 5 | 3 | 4 | 5 | 3 | 5 | 30/35 (86%) |
| [48] | 5 | 5 | 4 | 2 | 5 | 2 | 3 | 26/35 (74%) |
| Material (Printing Method) | Advantages | Disadvantages |
|---|---|---|
| PLA (FDM) | Low melting point [59] Simple print process [32] Non-toxic and biocompatible [59] Rigid and strong [60] Wide variety of colours [59] Inexpensive and highly available [59] Suitable for soft tissue and muscle replication as exhibits radiodensities between 32 and 185 HU at 100% infill [40,42,45] | Brittle [59] Rough surface finish [59] Surface texture is unnatural due to laminae or stair-step appearance [43] Low heat resistance—can warp and melt under sun exposure [61] Prone to oozing effect [61] FDM requires removal of supporting material for overhanging parts [62] |
| ABS (FDM) | Relatively low attenuations, making it suitable as a surrogate for adipose tissue [40] Tough, and impact resistant, makes for robust moulds to encase filler materials [39] | Prone to shrinkage and warping during cooling after the print [40] Requires removal of supporting material for overhanging parts [62] Toxic [28] Affected by humidity [28] |
| TPU (FDM and SLS) | Flexible polymer [63] Low radiodensities of around −200 HU, suitable for representing subsolid, minimally attenuating lesions [38] Higher resolution enabled with SLS as compared to FDM printing [63] | TPU used with FDM printers is not functionally strong as compared to SLS [63] |
| Nylon/Polyamide 12 (SLS) | High-detail resolution and strength. Suitable for small structures requiring low radiodensities (~−700 to −130 HU) [36] Does not require supporting material due to free powder acting as the supporting material [36] | Free unsintered powder may remain trapped in parts of the model [36] High-cost printers [36] Prone to thermal distortion [36] Rough and grainy surface finish [36] |
| PETG (FDM) | Suitable for cartilage tissue, exhibiting ~170 ± 20 HU [40] Simple to print, flexible and strong [60] Glossy and smooth surface finish [60] Negligible warping [60] Water resistant [60] | Easily scratched and absorbs moisture [59] Can produce thin hairs on the surface due to stringing (oozing material) [60] |
| Vero PureWhite (PolyJet) | Rigid radiopaque photopolymer [64] Fine resolution and accuracy [64] Durable [64] Suitable for moulds encapsulating materials [35] | Brittle [64] |
| Filaments doped with high-density additives—StoneFill PLA, PLA with iron, granite-PLA, ABS with added Bismuth (FDM), Bone meal powder amalgamate (casting) | Higher atomic numbers and densities enabled to better replicate radiodensities of cortical bone, which is not achievable with base polymer materials [10]. StoneFil PLA—density of 1.54 g/cm3 [10] | Long-term damage of the extrusion nozzle due to abrasion from high-density additives [8] Bone meal amalgamate casting—requires more than 24 h to cure, introduces air bubble artifacts and necessitates a sealed compartment to prevent leaking into neighbouring areas. These considerations are relevant to casting in general [35] |
| VisiJet EX200 (Multi-Jet) | Very tough and durable [65] Transparent—allows visualisation of internal structures [66] High resolution—enables smooth curves or sharp edges [67] Biocompatible [68] | May cause skin irritation [69] Slight odour [69] Requires supporting material for overhanging structures [64] Expensive printer [37] |
| Gypsum Powder (Binder Jetting) | Low cost and accessible [70] High Density of 1.57 g/cm3, gives radiodensities between 372 and 995 HU, similar to bone [10,36] | Low strength [70] Porous [70] |
| PMMA (FDM) | Transparent—allows visualisation of internal structures [71] Strong and durable [71] Resistance to UV and other weather exposures [71] Density of 1.12 g/cm3 [10]. Suitable radiodensity for glandular tissue at ~95 HU [35] | Shrinks and warps without a heated printing bed [71] Harmful gasses emitted during printing—requires good ventilation [71] |
| Silicone of the FlexFoam-IT series (casting) [72] | Expandable and durable, suitable densities for representing skin and lung parenchyma according to expansion factor [39] Silicone of the FlexFoam-IT series has short curing time of less than 2 h [34]. Silicone Dragon Skin has a long shelf-life and fast curing time (<16 h) [36] | Pot life of only 1 min after opening [39] Requires a silicone-releasing agent in order to remove the mould [39] Requires a completely sealed mould in order to avoid leaking into neighbouring areas [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silberstein, J.; Sun, Z. Advances and Applications of Three-Dimensional-Printed Patient-Specific Chest Phantoms in Radiology: A Systematic Review. Appl. Sci. 2024, 14, 5467. https://doi.org/10.3390/app14135467
Silberstein J, Sun Z. Advances and Applications of Three-Dimensional-Printed Patient-Specific Chest Phantoms in Radiology: A Systematic Review. Applied Sciences. 2024; 14(13):5467. https://doi.org/10.3390/app14135467
Chicago/Turabian StyleSilberstein, Jenna, and Zhonghua Sun. 2024. "Advances and Applications of Three-Dimensional-Printed Patient-Specific Chest Phantoms in Radiology: A Systematic Review" Applied Sciences 14, no. 13: 5467. https://doi.org/10.3390/app14135467
APA StyleSilberstein, J., & Sun, Z. (2024). Advances and Applications of Three-Dimensional-Printed Patient-Specific Chest Phantoms in Radiology: A Systematic Review. Applied Sciences, 14(13), 5467. https://doi.org/10.3390/app14135467