Light and Nutrient Conditions Influence Fucoxanthin Production of the Microalgae Cyclotella meneghiniana
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Culture Conditions
2.2. Species Identification
2.3. Cell Densities and Determination of Photosystem II Photosynthetic Functions
2.4. Determination of Photosynthetic Pigment Contents
2.4.1. Determination of Fucoxanthin and β-Carotene
2.4.2. Determination of and Chlorophyll-a, Chlorophyll-c, and Carotenoids
2.5. Statistical Analysis
3. Results and Discussion
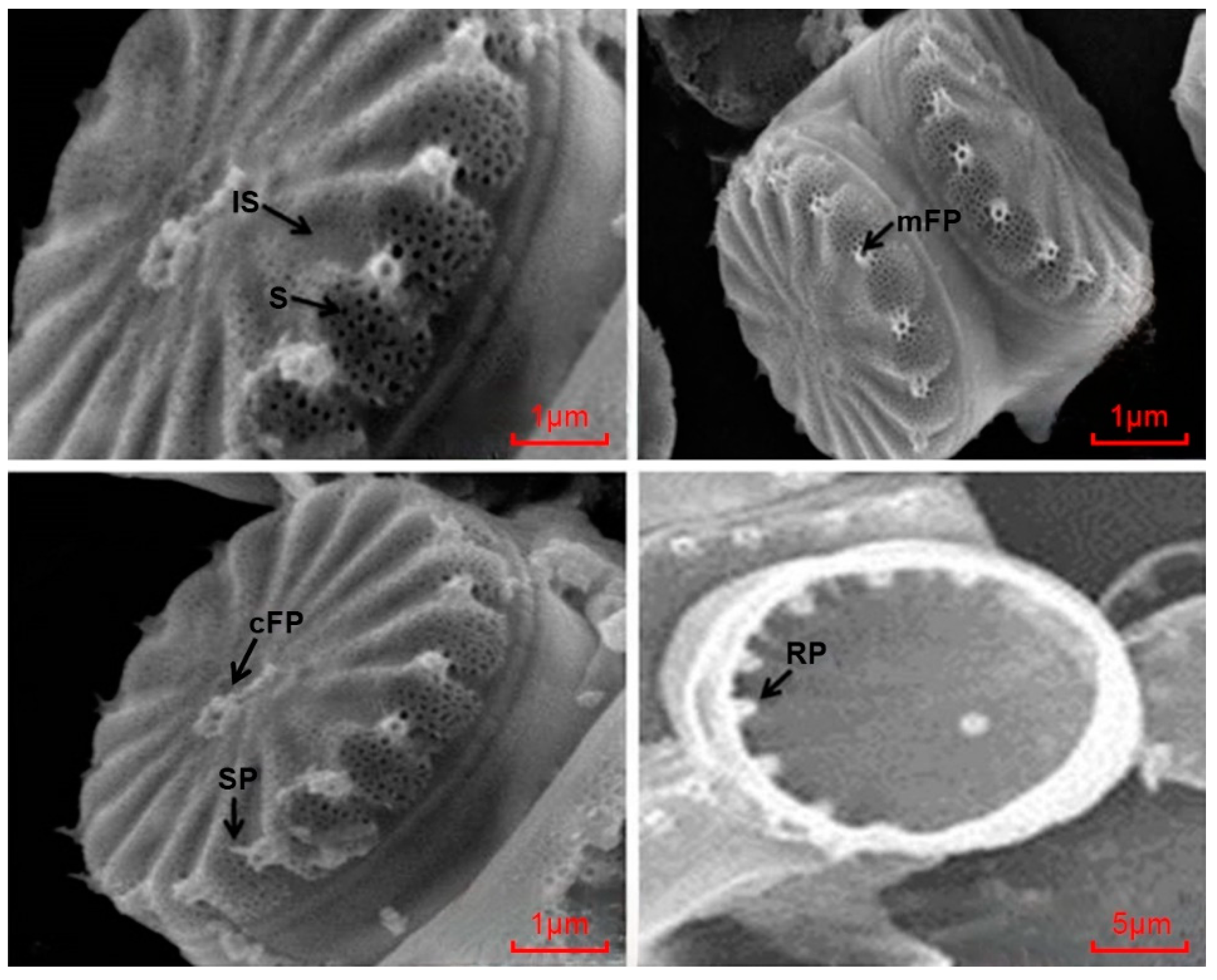
3.1. Molecular Identification of C. meneghiniana
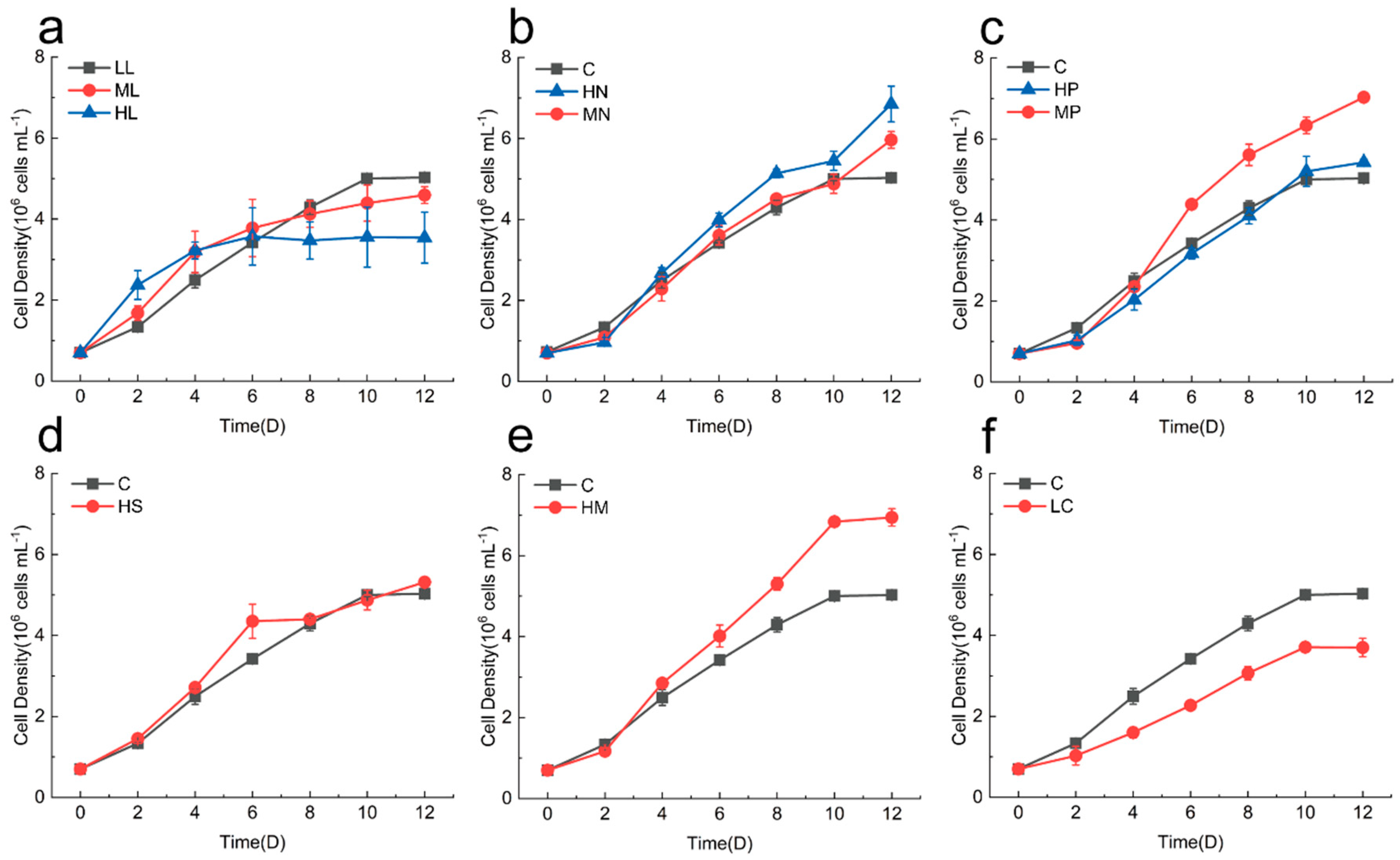
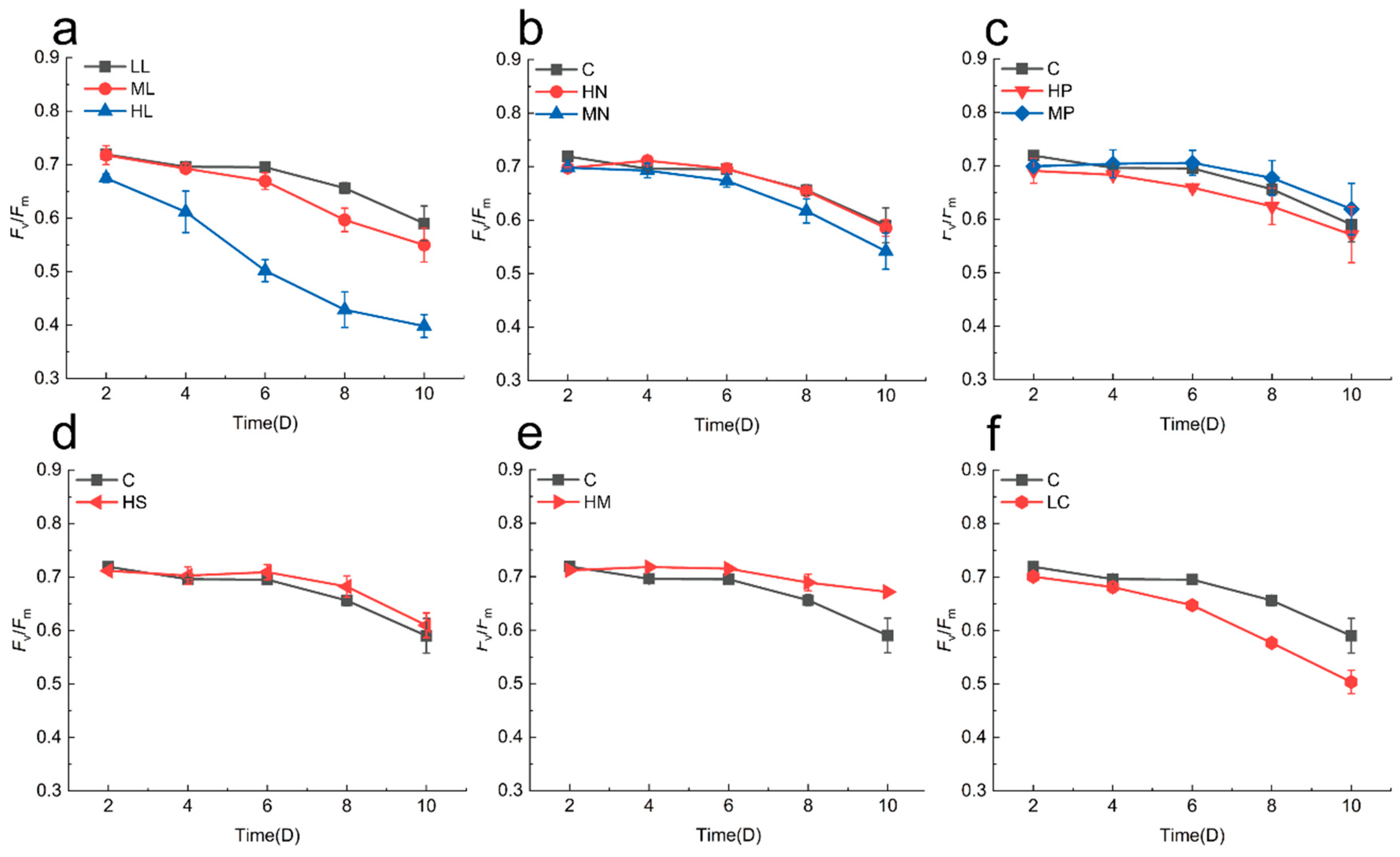
3.2. Physiological Effects on Cyclotella meneghiniana
3.3. Influence of Different Conditions on the Content of Photosynthetic Pigments
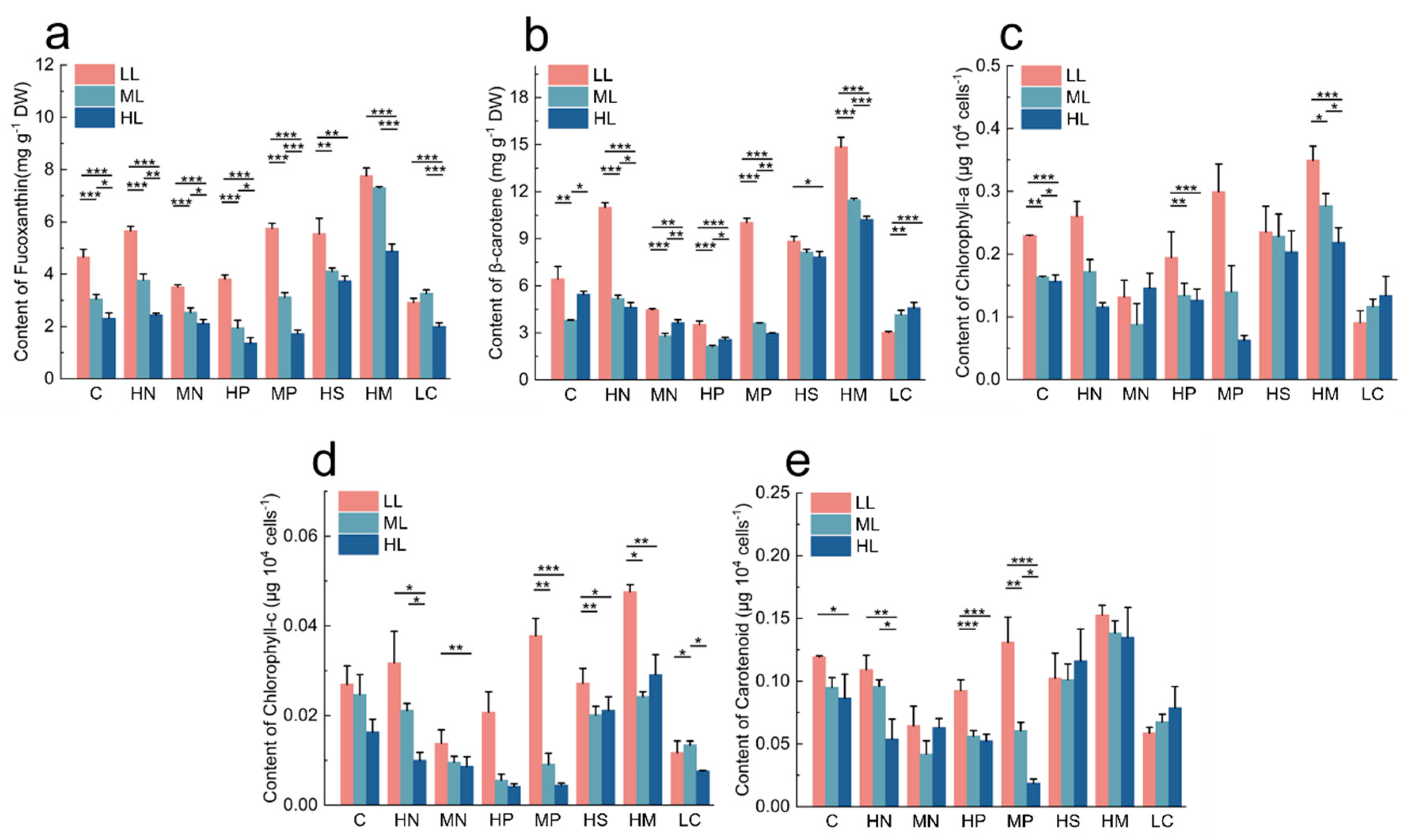
3.3.1. Effect of Light on Photosynthetic Pigment Production
3.3.2. Effect of Nutrient Conditions on the Content of Photosynthetic Pigments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Factories 2018, 17, 36. [Google Scholar] [CrossRef]
- Goshtasbi, H.; Okolodkov, Y.B.; Movafeghi, A.; Awale, S.; Safary, A.; Barar, J.; Omidi, Y. Harnessing microalgae as sustainable cellular factories for biopharmaceutical production. Algal Res. 2023, 74, 103237. [Google Scholar] [CrossRef]
- Udayan, A.; Pandey, A.K.; Sirohi, R.; Sreekumar, N.; Sang, B.-I.; Sim, S.J.; Kim, S.H.; Pandey, A. Production of microalgae with high lipid content and their potential as sources of nutraceuticals. Phytochem. Rev. 2023, 22, 833–860. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; De Morais, R.M.S.C.; De Morais, A.M.M.B. Health applications of bioactive compounds from marine microalgae. Life Sci. 2013, 93, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Mavrommatis, A.; Tsiplakou, E.; Zerva, A.; Pantiora, P.D.; Georgakis, N.D.; Tsintzou, G.P.; Madesis, P.; Labrou, N.E. Microalgae as a Sustainable Source of Antioxidants in Animal Nutrition, Health and Livestock Development. Antioxidants 2023, 12, 1882. [Google Scholar] [CrossRef]
- Khaw, Y.S.; Yusoff, F.M.; Tan, H.T.; Noor Mazli, N.A.I.; Nazarudin, M.F.; Shaharuddin, N.A.; Omar, A.R.; Takahashi, K. Fucoxanthin Production of Microalgae under Different Culture Factors: A Systematic Review. Mar. Drugs 2022, 20, 592. [Google Scholar] [CrossRef]
- Hou, H.Y.; Xiang, W.P.; Zhang, J.R.; Cheng, P.F.; Zhou, C.X. Strain and light selection improved fucoxanthin content in the diatom. Acta Hydrobiol. Sin. 2020, 44, 912–919. [Google Scholar] [CrossRef]
- Peng, J.; Yuan, J.-P.; Wu, C.-F.; Wang, J.-H. Fucoxanthin, a Marine Carotenoid Present in Brown Seaweeds and Diatoms: Metabolism and Bioactivities Relevant to Human Health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef]
- Pereira, H.; Sá, M.; Maia, I.; Rodrigues, A.; Teles, I.; Wijffels, R.H.; Navalho, J.; Barbosa, M. Fucoxanthin production from Tisochrysis lutea and Phaeodactylum tricornutum at industrial scale. Algal Res. 2021, 56, 102322. [Google Scholar] [CrossRef]
- Li, M.; Feng, H.; Ouyang, X.; Ling, J. Determination of Fucoxanthin in Bloom-Forming Macroalgae by HPLC-UV. J. Chromatogr. Sci. 2021, 59, 978–982. [Google Scholar] [CrossRef]
- Sarmiento-Padilla, A.L.; Moreira, S.; Oliveira Rocha, H.A.; Araujo, R.G.; Govea-Salas, M.; Pinales-Marquez, C.D.; Ruiz, H.A.; Rodriguez-Jasso, R.M. Circular bioeconomy in the production of fucoxanthin from aquatic biomass: Extraction and bioactivities. J. Chem. Technol. Biotechnol. 2022, 97, 1363–1378. [Google Scholar] [CrossRef]
- Heffernan, N.; Smyth, T.J.; FitzGerald, R.J.; Vila-Soler, A.; Mendiola, J.; Ibáñez, E.; Brunton, N.P. Comparison of extraction methods for selected carotenoids from macroalgae and the assessment of their seasonal/spatial variation. Innov. Food Sci. Emerg. Technol. 2016, 37, 221–228. [Google Scholar] [CrossRef]
- Wu, S.-J.; Liou, C.-J.; Chen, Y.-L.; Cheng, S.-C.; Huang, W.-C. Fucoxanthin Ameliorates Oxidative Stress and Airway Inflammation in Tracheal Epithelial Cells and Asthmatic Mice. Cells 2021, 10, 1311. [Google Scholar] [CrossRef]
- Zhao, D.; Kwon, S.-H.; Chun, Y.S.; Gu, M.-Y.; Yang, H.O. Anti-Neuroinflammatory Effects of Fucoxanthin via Inhibition of Akt/NF-κB and MAPKs/AP-1 Pathways and Activation of PKA/CREB Pathway in Lipopolysaccharide-Activated BV-2 Microglial Cells. Neurochem. Res. 2017, 42, 667–677. [Google Scholar] [CrossRef]
- Xiao, H.; Zhao, J.; Fang, C.; Cao, Q.; Xing, M.; Li, X.; Hou, J.; Ji, A.; Song, S. Advances in Studies on the Pharmacological Activities of Fucoxanthin. Mar. Drugs 2020, 18, 634. [Google Scholar] [CrossRef]
- Bae, M.; Kim, M.-B.; Park, Y.-K.; Lee, J.-Y. Health benefits of fucoxanthin in the prevention of chronic diseases. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2020, 1865, 158618. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chen, C.-Y.; Varjani, S.; Chang, J.-S. Producing fucoxanthin from algae-Recent advances in cultivation strategies and downstream processing. Bioresour. Technol. 2022, 344, 126170. [Google Scholar] [CrossRef]
- Zarekarizi, A.; Hoffmann, L.; Burritt, D. Approaches for the sustainable production of fucoxanthin, a xanthophyll with potential health benefits. J. Appl. Phycol. 2019, 31, 281–299. [Google Scholar] [CrossRef]
- Lu, X.; Sun, H.; Zhao, W.; Cheng, K.-W.; Chen, F.; Liu, B. A Hetero-Photoautotrophic Two-Stage Cultivation Process for Production of Fucoxanthin by the Marine Diatom Nitzschia laevis. Mar. Drugs 2018, 16, 219. [Google Scholar] [CrossRef]
- Pajot, A.; Huynh, G.H.; Picot, L.; Marchal, L.; Nicolau, E. Fucoxanthin from Algae to Human, an Extraordinary Bioresource: Insights and Advances in up and Downstream Processes. Mar. Drugs 2022, 20, 222. [Google Scholar] [CrossRef]
- Araújo, R.; Vázquez Calderón, F.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Garcia Tasende, M.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current Status of the Algae Production Industry in Europe: An Emerging Sector of the Blue Bioeconomy. Front. Mar. Sci. 2021, 7, 626389. [Google Scholar] [CrossRef]
- Terasaki, M.; Hirose, A.; Narayan, B.; Baba, Y.; Kawagoe, C.; Yasui, H.; Saga, N.; Hosokawa, M.; Miyashita, K. Evaluation of recoverable functional lipid components of several brown seaweeds (phaeophyta) from Japan with special reference to fucoxanthin and fucosterol contents. J. Phycol. 2009, 45, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Mohamadnia, S.; Tavakoli, O.; Faramarzi, M.A.; Shamsollahi, Z. Production of fucoxanthin by the microalga Tisochrysis lutea: A review of recent developments. Aquaculture 2020, 516, 734637. [Google Scholar] [CrossRef]
- Wang, S.; Wu, S.; Yang, G.; Pan, K.; Wang, L.; Hu, Z. A review on the progress, challenges and prospects in commercializing microalgal fucoxanthin. Biotechnol. Adv. 2021, 53, 107865. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wei, D.; Xie, J. Diatoms as cell factories for high-value products: Chrysolaminarin, eicosapentaenoic acid, and fucoxanthin. Crit. Rev. Biotechnol. 2020, 40, 993–1009. [Google Scholar] [CrossRef] [PubMed]
- Foo, S.C.; Yusoff, F.M.; Ismail, M.; Basri, M.; Yau, S.K.; Khong, N.M.H.; Chan, K.W.; Ebrahimi, M. Antioxidant capacities of fucoxanthin-producing algae as influenced by their carotenoid and phenolic contents. J. Biotechnol. 2017, 241, 175–183. [Google Scholar] [CrossRef]
- Kim, S.M.; Jung, Y.-J.; Kwon, O.-N.; Cha, K.H.; Um, B.-H.; Chung, D.; Pan, C.-H. A potential commercial source of fucoxanthin extracted from the microalga Phaeodactylum tricornutum. Appl. Biochem. Biotechnol. 2012, 166, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Guo, Y.; Li, Y.; Chen, B.; Ma, H.; Wang, H.; Wang, L.; Yuan, D. Effective fucoxanthin production in the flagellate alga Poterioochromonas malhamensis by coupling heterotrophic high-cell-density fermentation with illumination. Front. Bioeng. Biotechnol. 2022, 10, 1074850. [Google Scholar] [CrossRef] [PubMed]
- Tachihana, S.; Nagao, N.; Katayama, T.; Yusoff, F.M.; Banerjee, S.; Shariff, M.; Yamada, Y.; Imaizumi, Y.; Toda, T.; Furuya, K. High productivity of fucoxanthin and eicosapentaenoic acid in a marine diatom Chaetoceros gracilis by perfusion culture under high irradiance. Algal Res. 2023, 72, 103123. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, J.; Zhang, J.; Su, S.; Huang, L.; Ye, J. Kinetic modelling of microalgal growth and fucoxanthin synthesis in photobioreactor. Int. J. Chem. React. Eng. 2022, 20, 723–734. [Google Scholar] [CrossRef]
- Mousavi Nadushan, R.; Hosseinzade, I. Optimization of production and antioxidant activity of fucoxanthin from marine haptophyte algae, Isochrysis galbana. Iran. J. Fish. Sci. 2020, 19, 2901–2908. [Google Scholar] [CrossRef]
- Xia, S.; Gao, B.; Fu, J.; Xiong, J.; Zhang, C. Production of fucoxanthin, chrysolaminarin, and eicosapentaenoic acid by Odontella aurita under different nitrogen supply regimes. J. Biosci. Bioeng. 2018, 126, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Zittelli, G.C.; Lauceri, R.; Faraloni, C.; Benavides, A.M.S.; Torzillo, G. Valuable pigments from microalgae: Phycobiliproteins, primary carotenoids, and fucoxanthin. Photochem. Photobiol. Sci. 2023, 22, 1733–1789. [Google Scholar] [CrossRef] [PubMed]
- Novoveska, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.-F. Microalgal Carotenoids: A Review of Production, Current Markets, Regulations, and Future Direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gong, P.; Cai, Q.; Zhang, C.; Gao, B. Maximizing fucoxanthin production in Odontella aurita by optimizing the ratio of red and blue light-emitting diodes in an auto-controlled internally illuminated photobioreactor. Bioresour. Technol. 2022, 344, 126260. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Liu, P.-Y.; Chang, Y.-H.; Nagarajan, D.; Latagan, M.J.D.; de Luna, M.D.G.; Chen, J.-H.; Chang, J.-S. Optimizing cultivation strategies and scaling up for fucoxanthin production using Pavlova sp. Bioresour. Technol. 2024, 399, 130609. [Google Scholar] [CrossRef] [PubMed]
- Ishika, T.; Laird, D.W.; Bahri, P.A.; Moheimani, N.R. Co-cultivation and stepwise cultivation of Chaetoceros muelleri and Amphora sp. for fucoxanthin production under gradual salinity increase. J. Appl. Phycol. 2019, 31, 1535–1544. [Google Scholar] [CrossRef]
- Roeding, A.; Boekema, E.; Buechel, C. The structure of FCPb, a light-harvesting complex in the diatom Cyclotella meneghiniana. Photosynth. Res. 2018, 135, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Gardian, Z.; Litvin, R.; Bina, D.; Vacha, F. Supramolecular organization of fucoxanthin-chlorophyll proteins in centric and pennate diatoms. Photosynth. Res. 2014, 121, 79–86. [Google Scholar] [CrossRef]
- Truong, T.Q.; Park, Y.J.; Winarto, J.; Huynh, P.K.; Moon, J.; Choi, Y.B.; Song, D.-G.; Koo, S.Y.; Kim, S.M. Understanding the Impact of Nitrogen Availability: A Limiting Factor for Enhancing Fucoxanthin Productivity in Microalgae Cultivation. Mar. Drugs 2024, 22, 93. [Google Scholar] [CrossRef]
- Gelzinis, A.; Augulis, R.; Buechel, C.; Robert, B.; Valkunas, L. Confronting FCP structure with ultrafast spectroscopy data: Evidence for structural variations. Phys. Chem. Chem. Phys. 2021, 23, 806–821. [Google Scholar] [CrossRef] [PubMed]
- Guillard, R.R.; Ryther, J.H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, N.; Gogoi, A.; Kalita, R.D.; Ahmed, G.A.; Buragohain, A.K.; Choudhury, A. Luminescence studies of fresh water diatom frustules. Indian J. Phys. 2010, 84, 665–669. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001; Volume 1, pp. 131–142. ISBN 978-0-87969-577-4. [Google Scholar]
- Zhang, B.Y.; Wang, G.C.; Zhang, Y.; Han, X.T.; Lu, S.H.; Qi, Y.Z.; Zou, J.Z.; Zeng, C.K.; Tseng, C.K. Cloning and sequence analysis of 5.8s rDNA and its region from Prorocentrum donghaiense and P. micans APBM. Oceanol. Limnol. Sin. 2004, 35, 272–277. [Google Scholar]
- Senapin, S.; Phiwsaiya, K.; Withyachumnarnkul, K.B. Development of primers and a procedure for specific identification of the diatom Thalassiosira weissflogii. Aquac. Int. 2011, 19, 693–704. [Google Scholar] [CrossRef]
- Guo, B.; Liu, B.; Yang, B.; Sun, P.; Lu, X.; Liu, J.; Chen, F. Screening of Diatom Strains and Characterization of Cyclotella cryptica as A Potential Fucoxanthin Producer. Mar. Drugs 2016, 14, 125. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Cho, H.-K.; Shin, H.-S. Physicochemical properties and antioxidant activities of commercial vinegar drinks in Korea. Food Sci. Biotechnol. 2012, 21, 1729–1734. [Google Scholar] [CrossRef]
- Ritchie, R.J. Consistent Sets of Spectrophotometric Chlorophyll Equations for Acetone, Methanol and Ethanol Solvents. Photosynth. Res. 2006, 89, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Seawater Analysis; Fisheries Research Board of Canada: Ottawa, ON, Canada, 1968. [Google Scholar]
- Rzodkiewicz, M.; Gąbka, M.; Szpikowska, G.; Woszczyk, M. Diatom assemblages as indicators of salinity gradients: A case study from a coastal lake. Oceanol. Hydrobiol. Stud. 2017, 46, 325–339. [Google Scholar] [CrossRef]
- Beszteri, B.; John, U.; Medlin, L.K. An assessment of cryptic genetic diversity within the Cyclotella meneghiniana species complex (Bacillariophyta) based on nuclear and plastid genes, and amplified fragment length polymorphisms. Eur. J. Phycol. 2007, 42, 47–60. [Google Scholar] [CrossRef]
- Goswami, B.; Choudhury, A.; Buragohain, A.K. Luminescence properties of a nanoporous freshwater diatom. Luminescence 2012, 27, 16–19. [Google Scholar] [CrossRef]
- McLachlan, J. The growth of unicellular algae in artificial and enriched sea water media. Can. J. Microbiol. 1959, 5, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Hevia-Orube, J.; Orive, E.; David, H.; Díez, A.; Laza-Martínez, A.; Miguel, I.; Seoane, S. Molecular and morphological analyses of solitary forms of brackish Thalassiosiroid diatoms (Coscinodiscophyceae), with emphasis on their phenotypic plasticity. Eur. J. Phycol. 2016, 51, 11–30. [Google Scholar] [CrossRef]
- Xia, S.; Wang, K.; Wan, L.; Li, A.; Hu, Q.; Zhang, C. Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Mar. Drugs 2013, 11, 2667–2681. [Google Scholar] [CrossRef] [PubMed]
- Kanamoto, A.; Kato, Y.; Yoshida, E.; Hasunuma, T.; Kondo, A. Development of a Method for Fucoxanthin Production Using the Haptophyte Marine Microalga Pavlova sp. OPMS 30543. Mar. Biotechnol. 2021, 23, 331–341. [Google Scholar] [CrossRef]
- Ahn, C.-Y.; Lee, J.-Y.; Oh, H.-M. Control of Microalgal Growth and Competition by N: P Ratio Manipulation. Korean J. Environ. Biol. 2013, 31, 61–68. [Google Scholar] [CrossRef]
- Rashid, N.; Rehman, M.S.U.; Han, J. Enhanced growth rate and lipid production of freshwater microalgae by adopting two-stage cultivation system under diverse light and nutrients conditions. Water Environ. J. 2015, 29, 533–540. [Google Scholar] [CrossRef]
- Kraus, C.N.; Bonnet, M.-P.; de Souza Nogueira, I.; Pereira Souza Lobo, M.T.M.; da Motta Marques, D.; Garnier, J.; Galli Vieira, L.C. Unraveling Flooding Dynamics and Nutrients’ Controls upon Phytoplankton Functional Dynamics in Amazonian Floodplain Lakes. Water 2019, 11, 154. [Google Scholar] [CrossRef]
- Mikaelyan, A.S.; Pautova, L.A.; Chasovnikov, V.K.; Mosharov, S.A.; Silkin, V.A. Alternation of diatoms and coccolithophores in the north-eastern Black Sea: A response to nutrient changes. Hydrobiologia 2015, 755, 89–105. [Google Scholar] [CrossRef]
- Zhang, X.; Mei, X.; Gulati, R.D.; Liu, Z. Effects of N and P enrichment on competition between phytoplankton and benthic algae in shallow lakes: A mesocosm study. Environ. Sci. Pollut. Res. 2015, 22, 4418–4424. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Chen, L.; Cheng, W.; Liu, T. Combined production of fucoxanthin and EPA from two diatom strains Phaeodactylum tricornutum and Cylindrotheca fusiformis cultures. Bioprocess Biosyst. Eng. 2018, 41, 1061–1071. [Google Scholar] [CrossRef]
- Shakouri, A.; Balouch, G.M. The effects of nitrate and phosphate on growth of algae, Ulva rigida. Iran. J. Fish. Sci. 2020, 19, 59–66. [Google Scholar] [CrossRef]
- Lin, Q.; Liang, J.-R.; Huang, Q.-Q.; Luo, C.-S.; Anderson, D.M.; Bowler, C.; Chen, C.-P.; Li, X.-S.; Gao, Y.-H. Differential cellular responses associated with oxidative stress and cell fate decision under nitrate and phosphate limitations in Thalassiosira pseudonana: Comparative proteomics. PLoS ONE 2017, 12, e0184849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Kong, Z.; Chen, S.; Ran, Z.; Ye, M.; Xu, J.; Zhou, C.; Liao, K.; Cao, J.; Yan, X. The comparative study for physiological and biochemical mechanisms of Thalassiosira pseudonana and Chaetoceros calcitrans in response to different light intensities. Algal Res. 2017, 27, 89–98. [Google Scholar] [CrossRef]
- Shi, P.; Shen, H.; Wang, W.; Yang, Q.; Xie, P. Habitat-specific differences in adaptation to light in freshwater diatoms. J. Appl. Phycol. 2016, 28, 227–239. [Google Scholar] [CrossRef]
- Heiden, J.P.; Bischof, K.; Trimborn, S. Light Intensity Modulates the Response of Two Antarctic Diatom Species to Ocean Acidification. Front. Mar. Sci. 2016, 3, 260. [Google Scholar] [CrossRef]
- Raven, J.A. The cost of photoinhibition. Physiol. Plant. 2011, 142, 87–104. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Silva, S.O.; Baptista, J.M.; Malcata, F.X. Light requirements in microalgal photobioreactors: An overview of biophotonic aspects. Appl. Microbiol. Biotechnol. 2011, 89, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Parveen, A.; Bhatnagar, P.; Gautam, P.; Bisht, B.; Nanda, M.; Kumar, S.; Vlaskin, M.S.; Kumar, V. Enhancing the bio-prospective of microalgae by different light systems and photoperiods. Photochem. Photobiol. Sci. 2023, 22, 2687–2698. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Wu, T.; Fu, Y.; He, Y.; Mao, X.; Chen, F. Storage carbon metabolism of Isochrysis zhangjiangensis under different light intensities and its application for co-production of fucoxanthin and stearidonic acid. Bioresour. Technol. 2019, 282, 94–102. [Google Scholar] [CrossRef]
- Gao, F.; Teles Cabanelas Itd, I.; Wijffels, R.H.; Barbosa, M.J. Process optimization of fucoxanthin production with Tisochrysis lutea. Bioresour. Technol. 2020, 315, 123894. [Google Scholar] [CrossRef] [PubMed]
- McClure, D.D.; Luiz, A.; Gerber, B.; Barton, G.W.; Kavanagh, J.M. An investigation into the effect of culture conditions on fucoxanthin production using the marine microalgae Phaeodactylum tricornutum. Algal Res. 2018, 29, 41–48. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, X.; Liu, J. Screening of Isochrysis strains for simultaneous production of docosahexaenoic acid and fucoxanthin. Algal Res. 2019, 41, 101545. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, L.; Zhao, Y.; Lin, Q.; Huang, S.; Jin, Y.; Ma, Z.; Guan, W. Antagonistic and synergistic effects of warming and microplastics on microalgae: Case study of the red tide species Prorocentrum donghaiense. Environ. Pollut. 2022, 307, 119515. [Google Scholar] [CrossRef]
- Zarandi-Miandoab, L.; Hejazi, M.-A.; Bagherieh-Najjar, M.-B.; Chaparzadeh, N. Optimization of the Four Most Effective Factors on β-Carotene Production by Dunaliella salina Using Response Surface Methodology. Iran. J. Pharm. Res. 2019, 18, 1566–1579. [Google Scholar] [CrossRef] [PubMed]
- Papagiannakis, E.; Van Stokkum, I.H.M.; Fey, H.; Büchel, C.; Van Grondelle, R. Spectroscopic Characterization of the Excitation Energy Transfer in the Fucoxanthin–Chlorophyll Protein of Diatoms. Photosynth. Res. 2005, 86, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.M.; Bukhari, S.A.H.; Zeng, J.; QuAN, X.; Ali, E.; Muhammad, N.; Zhang, G. Nitrogen (N) metabolism related enzyme activities, cell ultrastructure and nutrient contents as affected by N level and barley genotype. J. Integr. Agric. 2017, 16, 190–198. [Google Scholar] [CrossRef]
- Marella, T.K.; Tiwari, A. Marine diatom Thalassiosira weissflogii based biorefinery for co-production of eicosapentaenoic acid and fucoxanthin. Bioresour. Technol. 2020, 307, 123245. [Google Scholar] [CrossRef]
- Mohamadnia, S.; Tavakoli, O.; Faramarzi, M.A. Production of fucoxanthin from the microalga Tisochrysis lutea in the bubble column photobioreactor applying mass transfer coefficient. J. Biotechnol. 2022, 348, 47–54. [Google Scholar] [CrossRef]
- Mohamadnia, S.; Tavakoli, O.; Faramarzi, M.A. Enhancing production of fucoxanthin by the optimization of culture media of the microalga Tisochrysis lutea. Aquaculture 2021, 533, 736074. [Google Scholar] [CrossRef]
- Kim, S.M.; Kang, S.-W.; Kwon, O.-N.; Chung, D.; Pan, C.-H. Fucoxanthin as a major carotenoid in Isochrysis aff. galbana: Characterization of extraction for commercial application. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 477–483. [Google Scholar] [CrossRef]
- Jiang, Y.; Laverty, K.S.; Brown, J.; Brown, L.; Chagoya, J.; Burow, M.; Quigg, A. Effect of silicate limitation on growth, cell composition, and lipid production of three native diatoms to Southwest Texas desert. J. Appl. Phycol. 2015, 27, 1433–1442. [Google Scholar] [CrossRef]
- Mao, X.; Chen, S.H.Y.; Lu, X.; Yu, J.; Liu, B. High silicate concentration facilitates fucoxanthin and eicosapentaenoic acid (EPA) production under heterotrophic condition in the marine diatom Nitzschia laevis. Algal Res. 2020, 52, 102086. [Google Scholar] [CrossRef]
- Twiss, M.R.; Gouvêa, S.P.; Bourbonniere, R.A.; McKay, R.M.L.; Wilhelm, S.W. Field Investigations of Trace Metal Effects on Lake Erie Phytoplankton Productivity. J. Great Lakes Res. 2005, 31, 168–179. [Google Scholar] [CrossRef]
- Sun, P.; Wong, C.-C.; Li, Y.; He, Y.; Mao, X.; Wu, T.; Ren, Y.; Chen, F. A novel strategy for isolation and purification of fucoxanthinol and fucoxanthin from the diatom Nitzschia laevis. Food Chem. 2019, 277, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Pasquet, V.; Chérouvrier, J.-R.; Farhat, F.; Thiéry, V.; Piot, J.-M.; Bérard, J.-B.; Kaas, R.; Serive, B.; Patrice, T.; Cadoret, J.-P.; et al. Study on the microalgal pigments extraction process: Performance of microwave assisted extraction. Process Biochem. 2011, 46, 59–67. [Google Scholar] [CrossRef]

| Microalgae | Fucoxanthin Contents (mg g−1 DW) | Sample Condition | References |
|---|---|---|---|
| Cyclotella meneghiniana | 7.76 | Dried | In this study |
| Saccharina japonica | 0.03 | Dried | Foo et al., 2017 [26] |
| Skeletonema costatum | 0.36 | Dried | Foo et al., 2017 [26] |
| Odontella sinensis | 1.18 | Dried | Foo et al., 2017 [26] |
| Nitzschia laevis | 1.68 | Dried | Sun et al., 2019 [87] |
| Isochrysis galbana | 2.19 | Dried | Foo et al., 2017 [26] |
| Chaetoceros gracilis | 2.24 | Dried | Kim et al., 2012 [83] |
| Chaetoceros calcitrans | 2.33 | Dried | Goiris et al., 2012 [88] |
| Nitzschia sp. | 4.92 | Dried | Kim et al., 2012 [83] |
| Cylindrotheca closterium | 5.23 | Dried | Pasquet et al., 2011 [89] |
| Isochrysis galbana | 6.04 | Dried | Kim et al., 2012 [83] |
| Phaeodactylum tricornutum | 8.55 | Dried | Kim et al., 2012 [83] |
| Isochrysis aff. galbana | 18.23 | Dried | Kim et al., 2012 [83] |
| Odontella aurita | 21.67 | Dried | Song et al., 2013 [56] |
| Isochrysis zhangjiangensis | 23.29 | Dried | Li et al., 2019 [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chinnappan, S.; Cai, J.; Li, Y.; Yang, Z.; Sheng, Y.; Cheng, K.; Du, H.; Liu, W.; Li, P. Light and Nutrient Conditions Influence Fucoxanthin Production of the Microalgae Cyclotella meneghiniana. Appl. Sci. 2024, 14, 5504. https://doi.org/10.3390/app14135504
Chinnappan S, Cai J, Li Y, Yang Z, Sheng Y, Cheng K, Du H, Liu W, Li P. Light and Nutrient Conditions Influence Fucoxanthin Production of the Microalgae Cyclotella meneghiniana. Applied Sciences. 2024; 14(13):5504. https://doi.org/10.3390/app14135504
Chicago/Turabian StyleChinnappan, Santhoshkumar, Jingting Cai, Yanfei Li, Zhenxiong Yang, Yangjie Sheng, Keying Cheng, Hong Du, Wenhua Liu, and Ping Li. 2024. "Light and Nutrient Conditions Influence Fucoxanthin Production of the Microalgae Cyclotella meneghiniana" Applied Sciences 14, no. 13: 5504. https://doi.org/10.3390/app14135504






