Abstract
Insect frass and vermicompost hold potential applications as fertilizers, with their abilities to improve plant resilience against unfavorable environmental conditions and increase their resistance to pests and diseases. In this study, we explored the effects of vermicompost fertilization, mealworm frass, and superworm frass as potential plant fertilizers for red beet cultivation. We analyzed the connections among chemical parameters, rhizobiome structure and function, and the biometrics of fertilizer-treated plants. In general, soils enriched with vermicompost and superworm frass exhibited the highest macroelement contents. Dry superworm frass fertilization was characterized by the increased availabilities of total nitrogen, NH4-N, and NO3-N. The use of vermicompost and mealworm frass resulted in significantly higher red beet biomass values. The presence of the highest N-fixation potential and key hormonal substances involved in plant development, such as auxins and gibberellins, was demonstrated using wet superworm frass. The results indicated that wet superworm frass, similar to vermicompost and dry superworm frass, exhibits high chemoheterotrophic potential. This suggests an r-type strategy and high adaptive flexibility of rhizobial bacteria. As a consequence, both life in the root zone and the microbiome itself may be better adapted to sudden, unfavorable environmental changes or attacks by plant pathogens.
1. Introduction
Despite the current challenges in the agricultural processing industry, ecological production remains essential. There is a noticeable trend among consumers toward goods produced in an environmentally friendly manner [1,2,3]. Although the concept of organic farming is many years old and is a dynamically developing field, its definition is still imprecise. According to universal terminology, organic production emphasizes sustainable practices, avoids the use of mineral fertilizers and pesticides, and promotes environmental protection [4,5,6]. This appeals to end consumers, who, along with the developing economy and increasing income, place more and more emphasis on conscious food choices [7,8].
Although growing plants organically is a challenge in large areas, it is easier to grow for one’s own needs. In Poland, this is due to the fact that the majority of soils are of classes IVa, IVb, V, and VI, i.e., of medium, poor, and very poor quality [9]. By growing plants in private gardens in accordance with organic practices, it is easier to maintain control over the soil and microclimate and to adjust crop rotation and fertilization [10].
Undoubtedly, red beet (Beta vulgaris L.) is one of the most frequently cultivated vegetables, as the beet has long been used in traditional cuisines in Europe. It hails from the Mediterranean Region and has seen a surge in cultivation across Europe, America, and Asia in recent times, thanks to its rising popularity [11]. Furthermore, beet has the capacity to both treat and prevent multiple diseases. Numerous studies have emphasized the bioactive properties of beet in the antioxidant, anti-inflammatory, antitumor, liver-protective, cognitive enhancement, and blood pressure management realms [12]. However, the expenditure on its cultivation is one of the highest among cultivated plants. It requires large financial, material, and energy expenditures. The soil should be deeply loosened. Fertilization itself should be moderate. Optimal beet fertilization should be (in mg/dm) 70–90 N (N-NH4 + N-NO3), 50–70 P, 175–250 K, 60–80 Mg, and 1500–2500 Ca. The optimal pH (H2O) for beet cultivation should be in the range of 6.0–7.5 [13]. A careful approach to nitrogen fertilization is particularly important, due to the plant’s excessive ability to intake nitrates in storage roots [14]. Too much nitrogen may contribute to excessive root growth, poorer color, and difficulty in storage. A low dose of nitrogen causes intense coloration of beet leaves, rapid drying, and low root yield. As in the case of growing other plants, the basis for fertilization should be determining the current content of available elements in the soil. In the case of beet cultivation, it is important to maintain a minimum rotation period, i.e., four years. This will reduce the occurrence of weeds, pests, and diseases in the plantation [15,16,17].
In the context of the dynamic development of ecology and the gradual displacement of conventional solutions in plant cultivation and animal breeding, alternative solutions should be carefully considered. One of these is organic fertilizer and feed produced through insect activity [18]. Insects partially solve the problem of the need for high-protein food in animal farms, poultry, and fish. Farm insects, such as the mealworm (Tenebrio molitor L.), the black fly (Hermetia illucens L.), and the superworm (Zophobas morio F.), due to their chemical compositions, can be very good components for feed mixtures [19,20]. This is primarily due to their high protein content, which ranges from 42% to 63%. This makes insects a good substitute for soybean or fish meal [21,22]. The nutritional value of these foods will increase in the coming years. This will translate into large amounts of obtained excrement [23]. Insect frass has many agricultural advantages. The application of black fly frass, which contains bioavailable macroelements, facilitates the partial recovery of phosphorus forms that are otherwise inaccessible to plants, thereby enhancing soil microbiota [24,25]. In turn, frass from the mealworm may increase the contents of nutrients such as nitrogen, phosphorus, potassium, magnesium, and sodium. Moreover, it may increase the abundance of microorganisms supporting the mineralization of organic matter [26,27].
To determine the future impacts of soil improvement on the agroecosystem and crops, rhizobium analysis can be used as a biomarker, along with the identification of functional characteristics of the microbiome [28]. The taxonomic structure of the bacterial rhizobium exhibits an r-type life strategy that is directed toward the dynamic decomposition of organic matter. If soil addition causes a rearrangement of the taxonomic structure toward a K-type life strategy (e.g., oligotrophy), it will reduce the adaptive flexibility of the rhizobiome and loosen the bacteria–root relationship [29]. As a result, biological activity in the root zone will decrease, the distribution of nutrients by plant growth-promoting rhizobacteria (PGPR) will decrease, and the gates of infection for soil phytopathogens will open. The combination of structural and functional analyses of the root zone with appropriate statistical tools can help determine the suitability of new organic and natural fertilizers, which are currently being dynamically introduced in accordance with the idea of "zero waste" and sustainable agriculture.
Our research objective was to establish causal relationships among chemical parameters, rhizobium structure and function, and plant biometrics altered by soil fertilization using frass and vermicompost. We assume that frass improves soil chemical parameters and plant growth as much as, or better than, vermicompost. Moreover, we assume that the fertilizers used have different effects on the soil, plant, and rhizosphere microbiota.
2. Materials and Methods
2.1. Origin and Preparation of Fertilizers
All types of frass were produced and collected from long-term cultures of insects and the California earthworm (Eisenia fetida Sav.), conducted at the Department of Entomology, Phytopathology and Molecular Diagnostics, Faculty of Agriculture and Forestry, University of Warmia and Mazury in Olsztyn. Frass was collected in batches after consumption of the provided insect food. Vermicompost was collected after composting plant waste for 12 months. The collected fertilizers were stored in tight bags for a month for initial hygienization and standardization. The chemical properties of vermicompost and insect frass are provided in Table S1.
2.2. Conducting a Greenhouse Experiment
Red beet was selected for the experiment due to its high flexibility in various forms of fertilization, as well as its use in small-scale or greenhouse cultivation. The experiment was carried out in 1 m × 1 m vegetable shelters. Each fertilizer was mixed with mineral soil with a low total nitrogen content (<0.2%) and with the addition of 5% peat (v/v). The fertilizer dose was standardized to provide the soil with 10 g N/m2. The mass of the fertilizer was calculated from the total nitrogen content (as determined by the Kjeldahl method) in the fresh mass of vermicompost or frass. During each plot, the seeds were planted in five rows of 20 seeds per row. The plants were watered with tap water, maintaining humidity at approximately 60% of the soil’s water volume. The ambient temperature and humidity were maintained (daily average from 16 to 24 °C). After 60 days, NDVI measurements of the crop were performed (PolyPen RP 410, Merazet, Poznań, Poland), and the soil and root zones were collected for further analysis. Ten plants from each row were used for biomass analysis. Dry mass measurements were performed using a moisture analyzer (Radwag, Radom, Poland). Each treatment was performed in four replicates.
2.3. Soil Chemical Analysis
The approach to chemical analysis was described in a previous study by Przemieniecki et al. [30]. Prior to analysis, the soil samples underwent sieving through a 2 mm mesh and were subsequently ground using a mortar. Soil pH was assessed using 1 mol/dm KCl solution (at a soil-to-solution ratio of 1:2.5 w/v) employing a potentiometer. Magnesium extraction was carried out using a 0.0125 M CaCl2 solution (with a soil-to-solvent ratio of 1:10) following the procedure [31]. Phosphorus and potassium levels were determined utilizing the Egner–Riehm method [32] with buffered calcium lactate and lactic acid (pH 3.5). Total nitrogen content was analyzed using the Kjeldahl method [33].
Nitrate nitrogen and ammonium nitrogen were analyzed on an SKW 500 station (Palintest, Gateshead, United Kingdom). Ready reagent stocks and kits were provided by the manufacturer.
2.4. Soil DNA Isolation and Nanopore Sequencing
The microbiome structure was assessed using the MinION nanopore sequencer, developed by Oxford Nanopore, employing a commercial kit with the “16S Barcoding Kit” methodology, available at https://community.nanoporetech.com (accessed on 5 April 2023).
The protocol for nanopore sequencing entails several main steps. Following DNA isolation, 10 µL of the isolated DNA (containing 10 ng of genomic DNA) was used for library preparation. The key steps of library preparation included PCR amplification with specific cycling conditions, purification of amplicons using magnetic beads and 70% ethanol, fluorometric and spectrophotometric measurement for assessing DNA quality and quantity, and pooling of barcoded libraries in desired ratios to a total of 50–100 fmoles in 10 μL of 10 mM Tris-HCl pH 8.0 with 50 mM NaCl, followed by the addition of RAP solution. The Flongle Flow Cell was utilized for sequencing. Sequencing and basecalling were conducted using MinKnow software. Data filtration, post-processing, and the generation of the BIOM format table were performed on the BugSeq platform (https://bugseq.com/, accessed on 10 June 2023).
2.5. Statistical Analysis
The taxonomic diversities of the analyzed OTUs were determined with the use of Chao 1, Simpson dominance (λ), Shannon diversity (H′), and Pielou’s evenness index (J′). Diversity indices and rarefaction curves were obtained by the PAST 4.13 program [34]. Domination classes were determined according to previous work [35].
The MACADAM database [36] was used for determining the predicted function of the microbial community (MetaCyc subdatabase for Plant Growth Promoting (PGP) properties and FAPROTAX for metabolic function).
The obtained raw data of chemical and biometric measurements were analyzed for normality of distribution using the Shapiro–Wilk test and homogeneity of variances using the Levene test. Then, one-way ANOVA for parametric tests, or the Kruskal–Wallis test, was performed. Post hoc tests were performed using Tukey’s test or Dunn’s test with Bonferroni correction.
In order to determine the differences between the abundance of OTUs relative to the control, the Fisher’s exact test was used, while the t test was used for diversity indices. The Bray–Curtis dissimilarity test was used to test differences between variables, and hierarchical dendrograms were prepared to visualize the obtained results. Among the multivariate tests, principal component analysis (PCA) based on the Pearson correlation matrix and nonmetric multidimensional scaling (nMDS) were used. A heatmap was created on the basis of n-standardized data without reduction. Network analysis was performed using the Fruchterman–Reingold algorithm and the correlation index for combining points for R > 0.8. In this study, XLSTAT [37] and PAST 4.13 [34] were used.
3. Results
3.1. Structure of the Rhizosphere Bacteriobiome
The examination of microbiota diversity conducted at the phylum level revealed that Proteobacteria constituted the most abundant phylum of microorganisms (averaging 43.0%), with the highest prevalence observed in the ZM-w variant (68.6%) and the lowest frequency following the control (31.9%). The second most prevalent phylum was Firmicutes, making up about 19.1% on average. They were most abundant in the TM treatment (27.0%) and least abundant in the ZM-w one (13.5%). Planctomycetota, the third most dominant phylum, with an average of 9.1%, showed the highest frequency in the TM variant (12.7%) and the lowest in the ZM-d variant (4.8%). The frequency of subsequent phyla, i.e., Bacteroidetes and Acidobacteria, fluctuated over 7.0%.
Detailed analysis (Table 1) revealed a large taxonomic rearrangement compared with the control. On average, the most abundant OTU was the genus Bacillus, which was eudominant in each variant. Another OTU, the order Rhizobiales, was eudominant only in the case of the ZM-w variant, and dominated the microbiome, with more than half of the percentage share. In the remaining variants, this OTU was almost absent. In the case of the order Burkholderiales, a significant increase in the share of this OTU was observed from the occasional class in the control to the eudominant class in the VC and ZM-w variants. OTUs Gemmataceae and Xanthobacteraceae decreased their shares under the influences of the ZM-d and ZM-w treatments. The Comamonadaceae, Rhizobiaceae, and Flavitalea OTUs reached the eudominant classes, and significant increases in these OTUs, compared with the control, were observed in the ZM-d treatment. The genus Paenibacillus was an occasional OTU under almost every variant, but after the application of TM, it reached the eudominant class. Eudominant taxa in the control, such as Nitrosomonadaceae, Geoalkalibacter, and Terrimonas, significantly reduced their shares, or at least their dominance classes, after the application of any of the tested fertilizers. Other important OTUs, such as Acicmicrobiia, occurred only in the VC variant, Noviherbaspirillum in TM, Gemmatimonadaceae in the ZM-d treatment, and Pirellulaceae in the ZM-w treatment.

Table 1.
The structure and diversity of bacteriobiomes depending on the fertilizer used *.
Based on the diversity results, it was observed that, in terms of Simpson’s dominance, the TM variant, in which dominance decreased, and the ZM-w variant, which achieved an almost-twice higher dominance index, differed significantly from the control. The Shannon diversity and Pielou evenness indices were significantly higher in the TM and ZM-d variants, and lower in the ZM-w variants (Table 1).
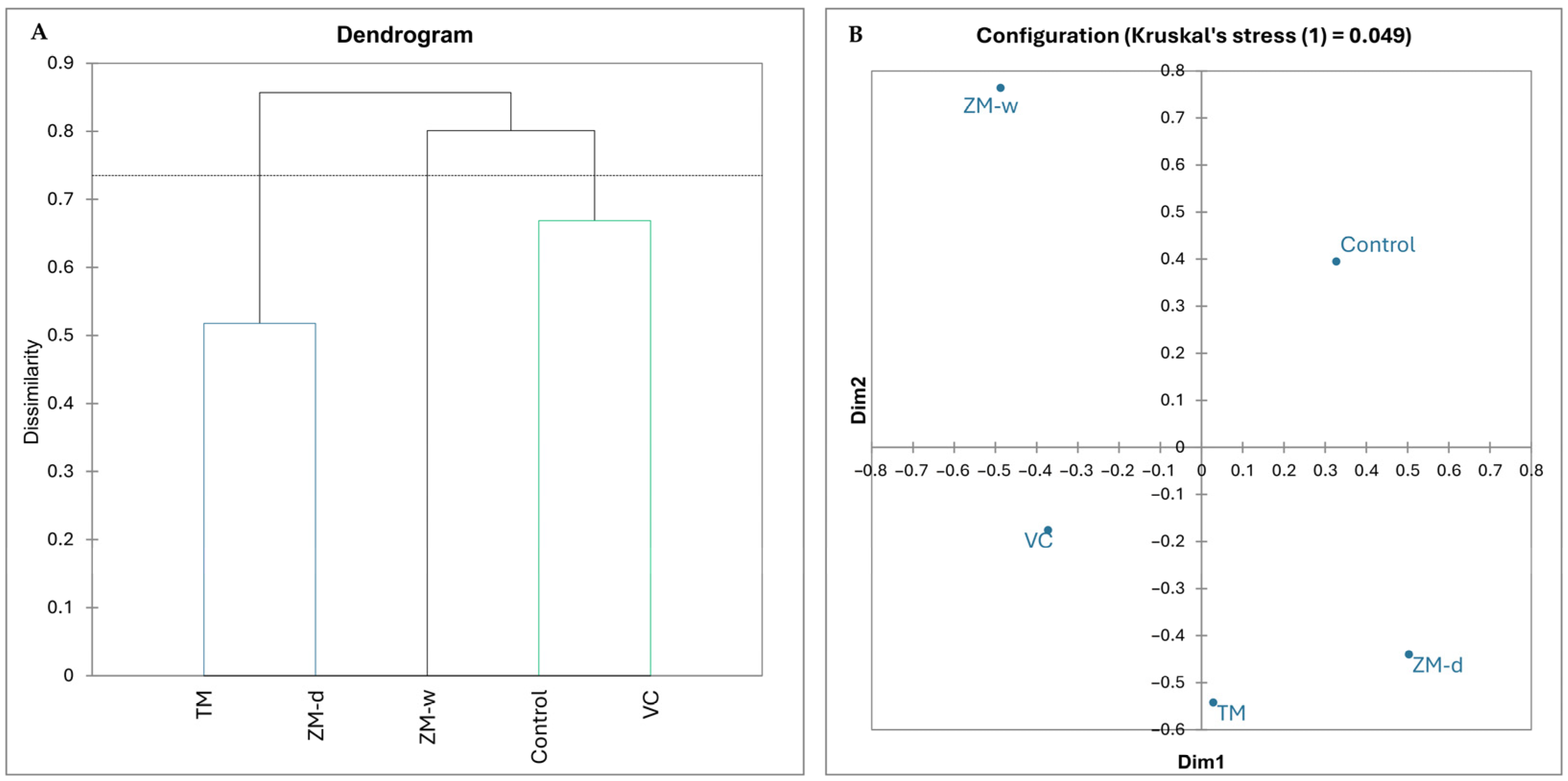
Based on the results of the hierarchical analysis and nMDS (Figure 1), it was observed that only the VC treatment had a largely similar bacteriobiome structure compared to the control. In contrast, the bacteriobiome structures of the other treatments were much more different from the bacteriobiome in the control. The TM and ZM-d variants were approximately half similar to each other (0.01), whereas the formation of a unique rhizobium, different from those in the other treatments, was observed in the ZM-w treatment.
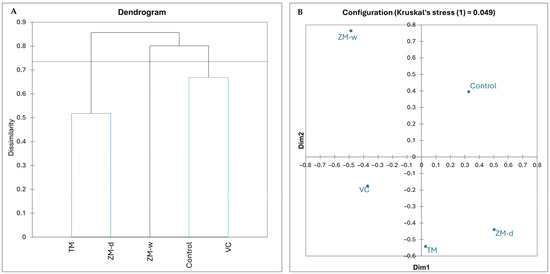
Figure 1.
Hierarchical dendrogram presenting differences between microbiomes; variants whose branches are located below the threshold line and are marked with the same color do not show significant differences (A); non-metric multidimensional scaling (B).
3.2. Chemical Properties of Soils
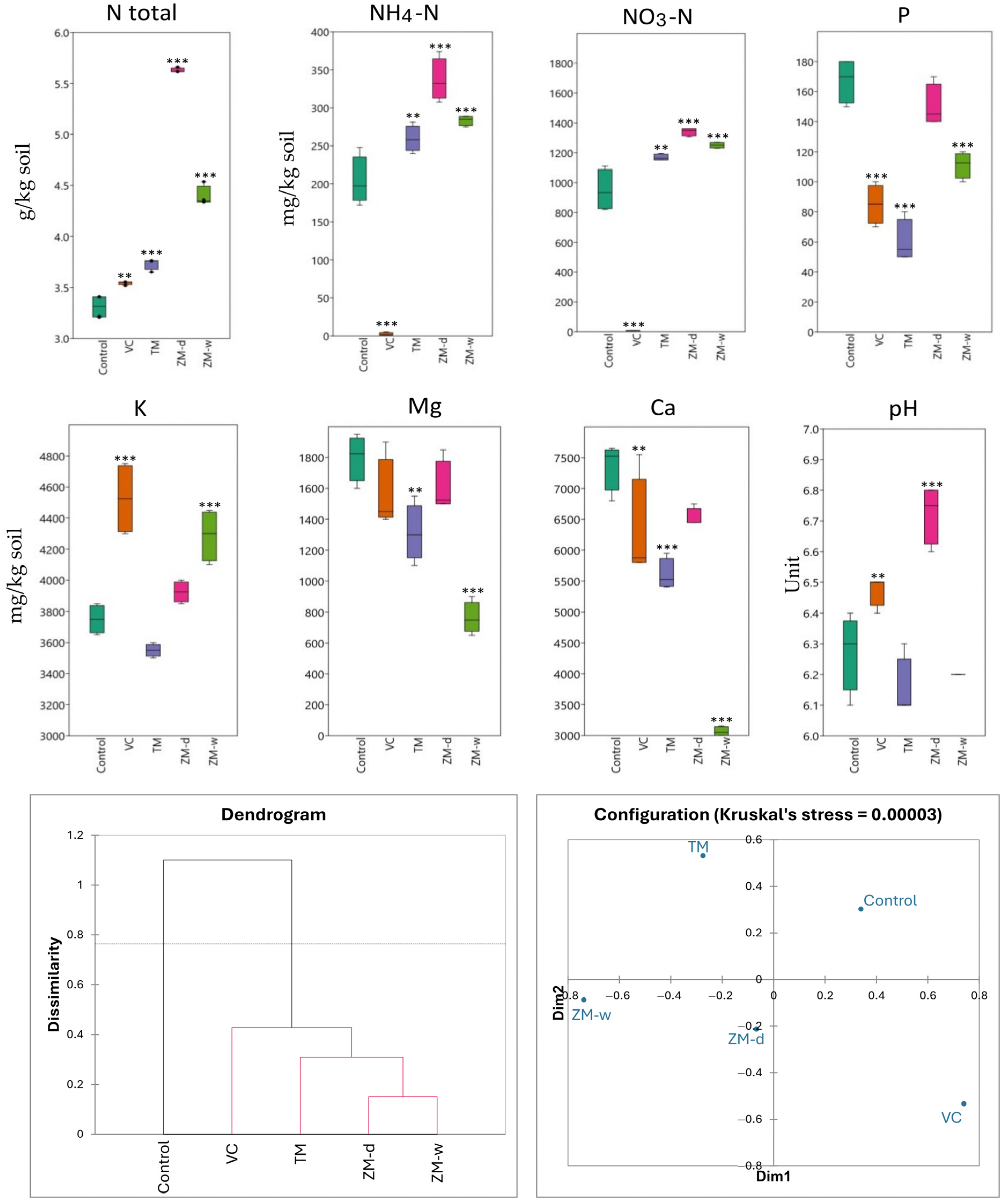
As part of the analysis of the impact of various fertilization methods on soil chemical parameters, a detailed examination of soil chemical components was performed after 60 days of beet cultivation. The results show significant differences in the concentrations of key soil components (Table 2, Figure 2).

Table 2.
Analysis of the impacts of different fertilization treatments on soil properties and the NDVI index of plants.
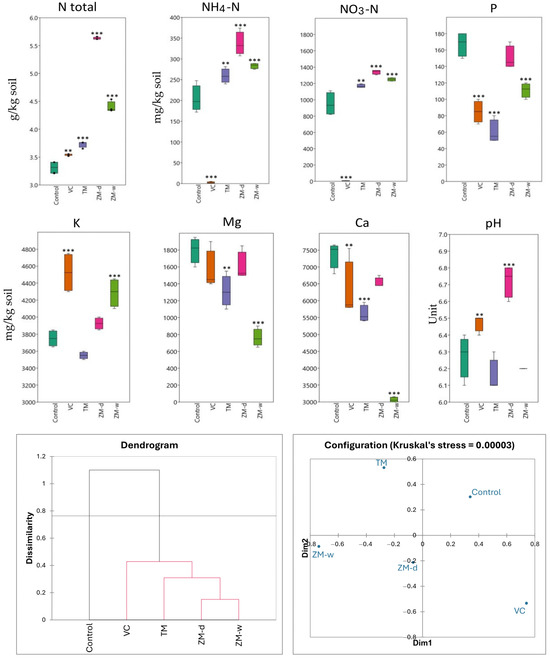
Figure 2.
The chemical parameters (macro- and microelements) of the rhizosphere after 60 days of beet cultivation, and Bray–Curtis hierarchical clustering and nMDS showing dissimilarity between treatments. Significance levels are denoted by asterisk as follows: ** p < 0.01, *** p < 0.001.
Soils fertilized with vermicompost (VC) and dry superworm frass (ZM-d) had the highest concentrations of most of the tested components. In turn, the lowest values of most chemical parameters were recorded in the control soils, except for the P content, which was the lowest in the soil with the addition of mealworm frass, TM. The total nitrogen content (Ntotal) indicates higher concentrations for fertilization with dry superworm frass (ZM-d). In the case of ammonium nitrogen (NH4-N) and nitrate nitrogen (NO3-N), the highest values were also observed for fertilization with ZM-d, at 336.38 and 1341.80 mg/kg, respectively, significantly higher compared with the control, where the concentration of these ingredients were 203.58 and 949.01 mg/kg. Similarly, the highest concentrations of potassium (K) and calcium (Ca) were also observed in VC-fertilized soils, at 4525 mg/kg and 6275 mg/kg, respectively. Magnesium (Mg) shows less variation between the fertilization methods used, but the ranges on the graph show some variability. The soil fertilized with ZM-w had the lowest Mg content among the tested soils, and the soil without fertilization had the highest. In this study, soil pH changed depending on the fertilizer used. The lowest pH value, of approximately 6.15, was recorded for TM fertilization, whereas the highest pH, of 6.73, was observed in the soil with the addition of ZM-d. The use of vermicompost (VC) also contributed to the increase in pH, reaching a value of 6.48. Analyzing the P content, control soil samples had the highest levels of phosphorus, whereas the soil fertilized with mealworm frass (TM) showed the lowest concentration of this component (Table 2, Figure 2).
The statistical analysis ANOVA (analysis of variance) showed that, for all nutrients tested, there were significant differences between the groups (p < 0.05). For total nitrogen (Ntotal), the F value was 674.84, indicating a very high statistical significance of differences between the means. Similarly high F values for ammonium nitrogen (NH4-N) and nitrate nitrogen (NO3-N) confirm the significant impacts of fertilization on these parameters (Table 2). The statistical significance of differences, marked with asterisk symbols on the charts, highlights which differences in the concentrations of chemical components are significant, enabling a more precise assessment of the effectiveness of the fertilization methods used, in the context of their impact on soil chemistry (Figure 2).
Analyzing the dendrogram showing the differences in the impacts of different fertilization methods on the chemical properties of the soil (Figure 2), the formation of two main groups was observed. The first group includes only the control (no fertilization), and the second group consists of all types of fertilization used, i.e., VC, TM, ZM-d, and ZM-w. The analysis shows that the control soil and the soil supplemented with vermicompost (VC) are the most chemically distinct. The closest in terms of chemical similarity are the dry (ZM-d) and wet (ZM-w) soils with frass. The soil with the addition of mealworm (TM) is positioned between the superworm frass and VC groups.
Based on the results of hierarchical grouping, the treatments were divided into two groups of differences. The first group included only the control, while the remaining variants formed a common second group. The Bray–Curtis dissimilarity between the groups was 1.1, while the intra-group dissimilarity of the second group ranged from approximately 0.41 to 0.17.
The nMDS results indicated much greater dissimilarity between the fertilized variants and less dissimilarity between the controls than indicated by the hierarchical clustering dendrogram. Kruskal’s stress value was <0.0001, indicating high reliability of the obtained results. Despite differences in the degree of dissimilarity, both methods indicated that the ZM-w and ZM-d variants are most similar to each other, while the TM, VC, and control variants are clearly different from each other.
3.3. Biometrical Parameters of Plants
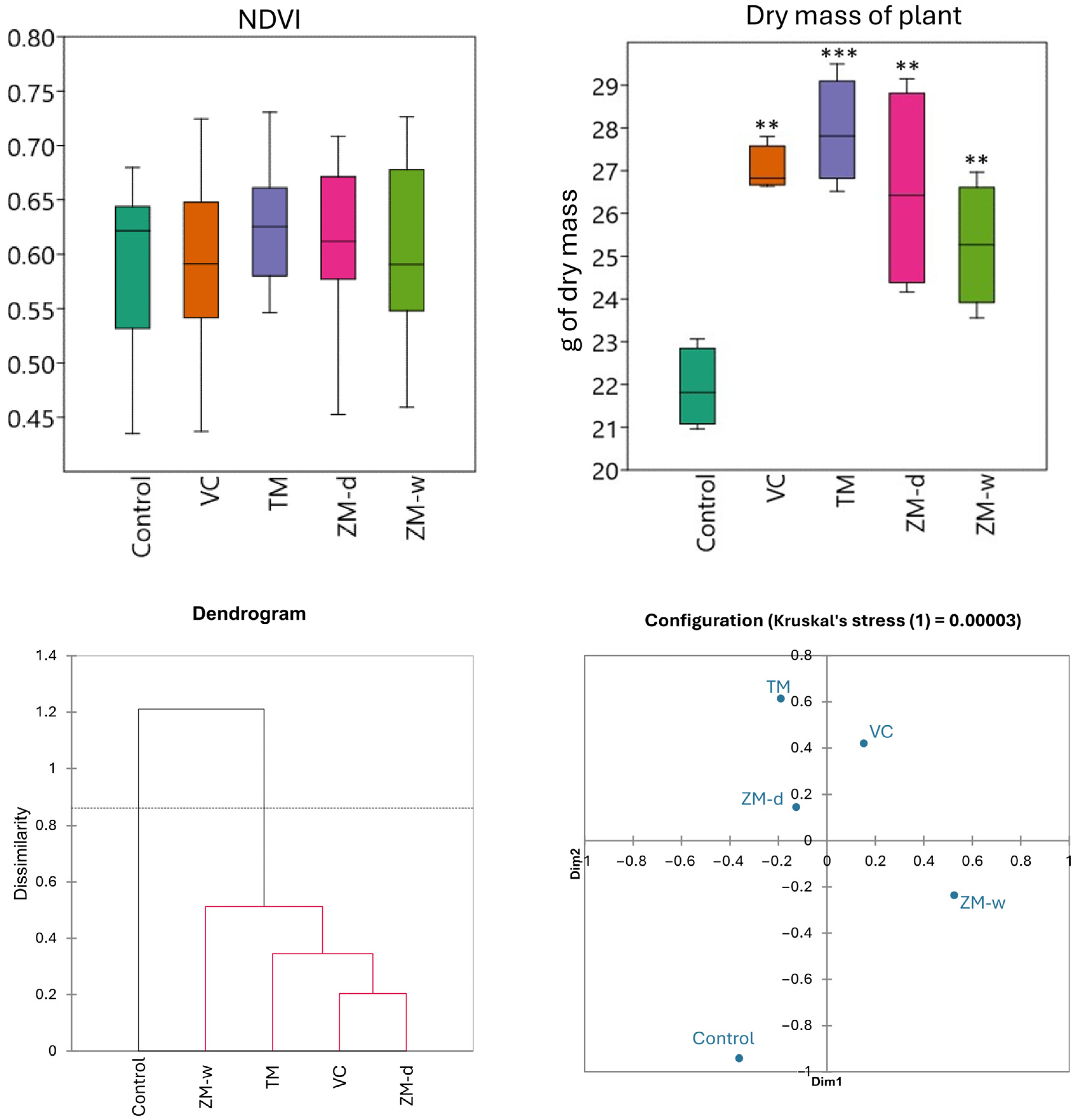
Analyzing the vegetation index of plants and beet yield after 60 days of beet vegetation (Figure 3), it was observed that the NDVI values for all groups were similar, with slight differences between individual fertilizers. They also did not show statistically significant differences. This index ranged from 0.59 to 0.62, depending on the type of fertilizer used. The control reached a value of about 0.60, while soils fertilized with TM and ZM-d showed a slight increase in NDVI. Fertilization with ZM-w achieved the same result as the control, while fertilization with vermicompost (VC) reached the lowest score, at 0.59. However, statistically significant differences were observed in the case of beet yield, measured as plant biomass. The control group showed the lowest biomass values, 21.91 units, while the application of vermicompost (VC) and mealworm frass (TM) resulted in significantly higher values, reaching 27.02 and 27.91 units, respectively. Wet (ZM-w) and dry (ZM-d) frass from superworms also contributed to biomass growth, with a value of about 26 units (Table 2, Figure 3). Based on the differences visualized on the nMDS plot (Figure 3), a significant overlap with the data obtained on the hierarchical dendrogram was observed. In the case of nMDS, treatments TM, VC, and ZM-d showed slight differences, while the ZM-w treatment and the control were different from both the aforementioned group of treatments and from each other.
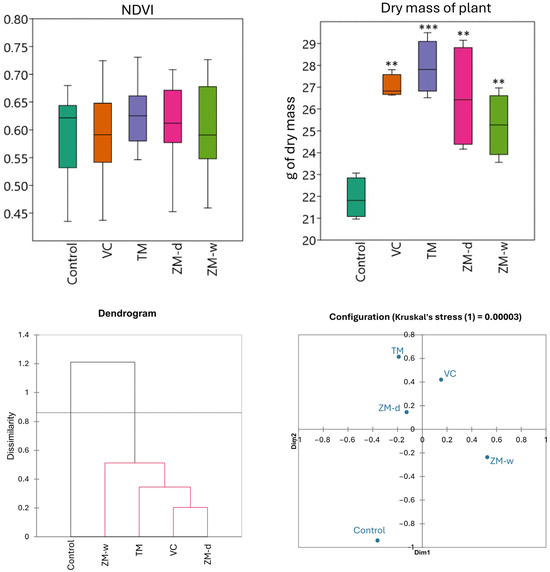
Figure 3.
The vegetation index of plants and beet yield after 60 days of beet vegetation and Bray–Curtis hierarchical clustering and nMDS, showing dissimilarity between treatments. Significance levels are denoted by asterisk as follows: ** p < 0.01, *** p < 0.001.
3.4. Overall Relationships
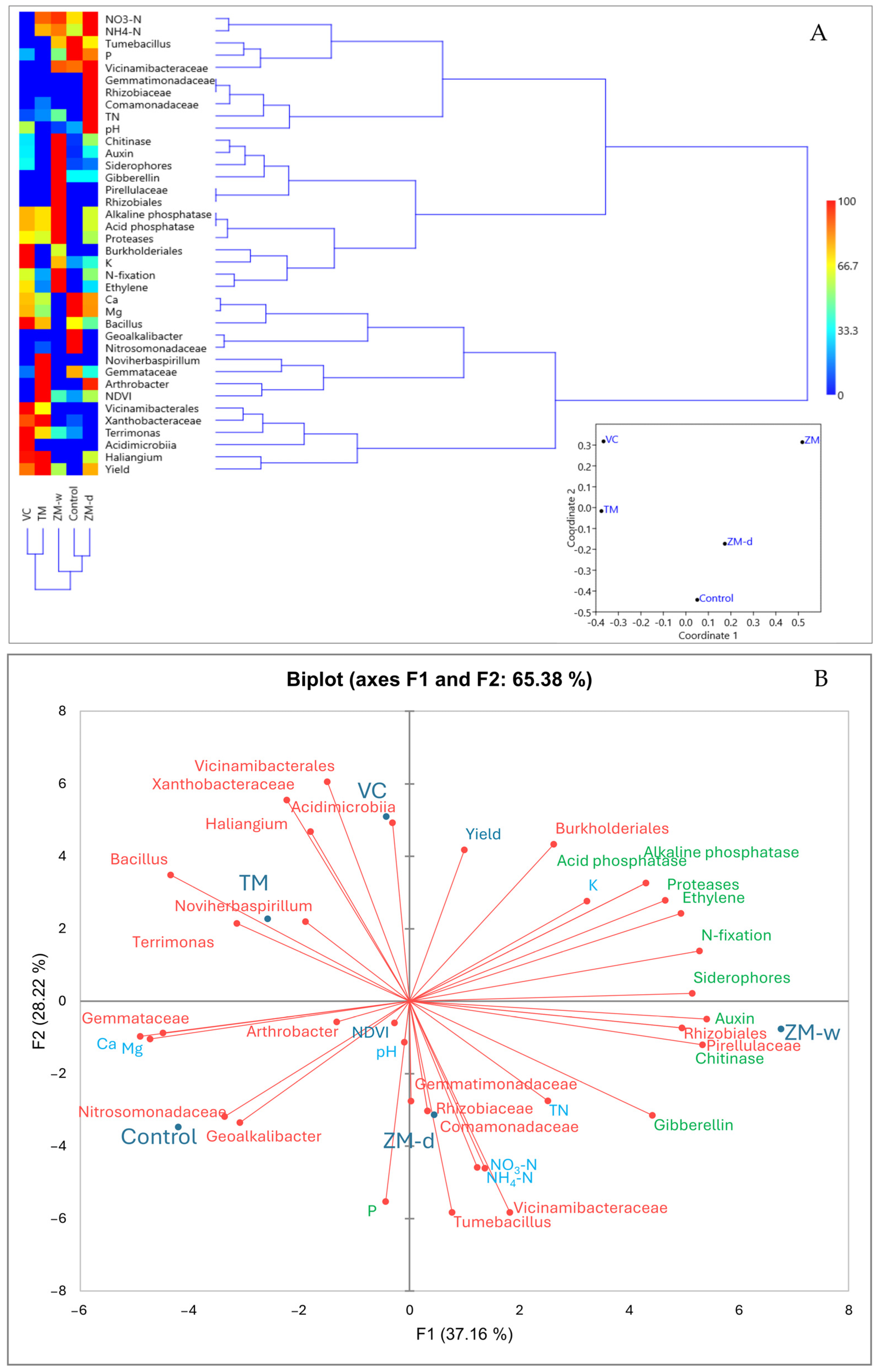
Analyzing the similarity of treatments based on the analyzed main characteristics, such as the chemical composition of the rhizosphere, biometric parameters, bacterial microbiome structure, and PGP feature load, three groups were observed on the dendrogram. The first group includes VC and TM, the second includes only ZM-w, and the third includes control and ZM-d. These relationships align with the nMDS scatterplot, confirming the validity of the obtained results.
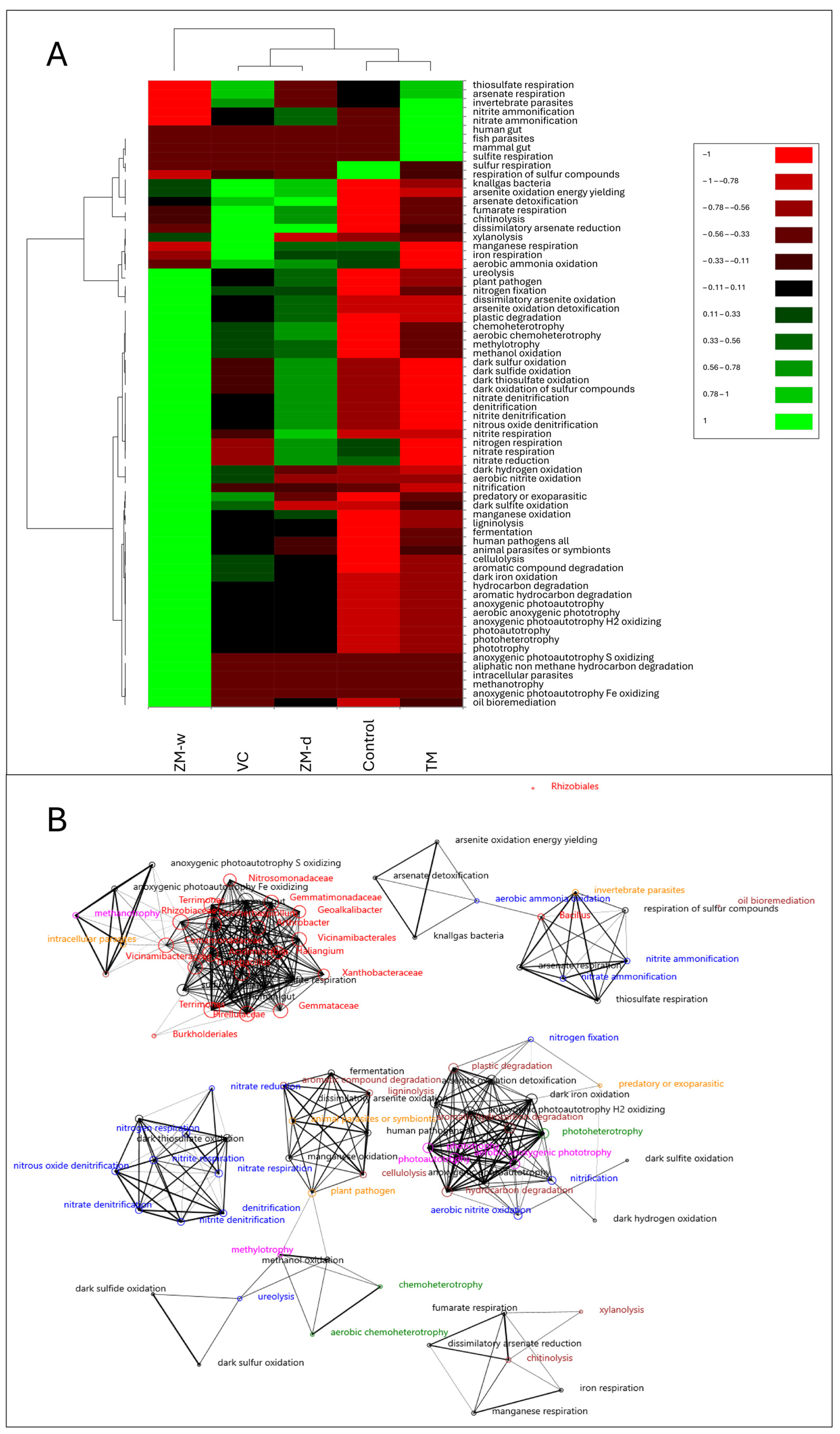
Examining the similarity of traits (Figure 4A and Figure S1), it was observed that the analyzed traits were separated into seven clusters on the dendrogram. The first cluster comprised traits such as Ca, Mg, Bacillus, Geoalkalibacter, and Nitrosomondaceae. According to the heatmap, high values of these variables were characteristic of the control and moderately present in the other variants, except ZM-w. The second cluster included Tumebacillus, P, Vicimibacteraceae, NO3-N, and NH4-N, which were characteristic of the control and ZM-d. The third cluster consisted of Gemmatimonadaceae, Rhizobiaceae, Comamonadaceae, TN, and pH, characteristic of ZM-d. The fourth cluster comprised Haliangium, yield, Vicinamibacterales, Xanthobacteraceae, and Acidimicrobia, mostly characteristic of VC and TM. The fifth cluster included NDVI, Noviherbaspirillum, Gemmataceae, and Arthrobacter, characteristic of TM. The sixth cluster comprised chitinase, auxin, siderophores, Gibberellin, Pirellulaceae, and Rhizobiales, most characteristic of ZM-w. The last cluster included alkaline and acid phosphatases, proteases, Butkholderiales, K, N-fixation, and ethylene, which were most characteristic of ZM-w and, to a lesser extent, present in other treatments except the control.
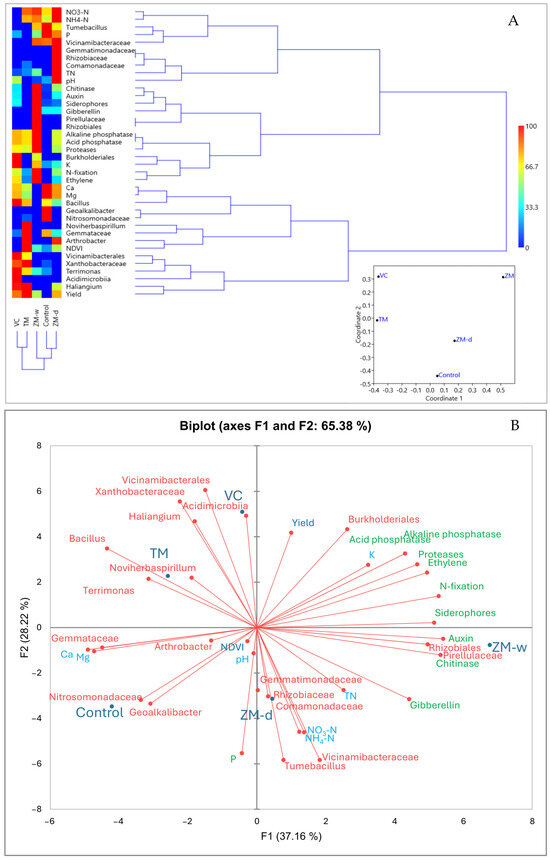
Figure 4.
A dendrogram showing the similarities between all analyzed features in the experiment, created on the basis of the Manhattan coefficient, and an nMDS plot created on the basis of the Bray–Curtis dissimilarity matrix (A). Principal component analysis, created on the basis of the Pearson correlation matrix, showing the relationships between the observed traits and the rhizobiome (B).
This study also analyzed the influences of different types of organic fertilization on soil microbiological composition and their potential impacts on plant productivity using principal component analysis (PCA). Relationships between applied fertilizers, specific microbial groups, and biochemical processes in the soil were identified (Figure 4B). Two main components, explaining 65.38% of the variability, were identified, indicating an explanation of the high level of variance. The PCA biplot clearly shows the distribution of microbial groups and chemical parameters, depending on the type of fertilization applied. TM, VC, ZM-d, and ZM-w fertilization are distributed in different parts of the biplot, suggesting unique impacts for each type of fertilization on microbiota composition and soil chemistry. ZM-d fertilization, characterized by the increased availability of nitrate and ammonium nitrogen, was correlated with Comamonadaceae, Gemmatimonadaceae, Tumebacillus, Rhizobiaceae, and Vicinamibacteraceae. ZM-w treatment correlated with Rhizobiales, Pirellulaceae, and chitinase enzyme. Additionally, hormonal compounds, such as gibberellins and auxins, essential for plant growth and development, were observed. TM fertilization correlated with Bacillus, Noviherbaspirillum, and Terrimonas bacteria. VC fertilization was closely associated with Acidimicrobiia and Vicinamibacterales activity. The control group was mainly correlated with Nitrosomonadaceae and Geoalkalibacter, which were either not observed or significantly reduced in other variants. The control showed the lowest correlation with these beneficial properties.
Based on the results presented on the heatmap (Figure 5A), it was observed that the control and TM were similar in terms of characteristics and contained the lowest overall physiological feature loads. The next group consisted of VC and ZM-d, which were characterized by the higher activities of their traits. The highest potential activity of physiological features was demonstrated by the rhizobiome treated with ZM-w, which was most distinct from the other variants. The control showed activities of sulphur respiration and moderate activity of magnesium, iron, nitrogen, nitrate, aerobic ammonia oxidation, and nitrate reduction. The TM variant showed activities of thiosulphate, sulphite, and arsenate respiration, as well as activity typical of gut microbiota and mammalian and invertebrate pathogens. In the ZM-d variant, activities were observed in traits such as gas-producing bacteria, arsenic detoxification, fumarate respiration, chitinolysis, xylanolysis, aerobic ammonia oxidation, and nitrate reduction. Additionally, ureolytic activity, moderate nitrogen binding, phytopathogenicity, a set of organic compound degradation traits, a set of traits associated with sulfur metabolism in the dark, denitrification capability, and a set of traits associated with respiration and nitrogen compound reduction and chemoheterotrophy were observed. The VC variant was partly similar to ZM-d, but did not show such clear metabolic activity in the dark, instead being characterized by thiosulphate and arsenate respiration activity, as well as invertebrate pathogenicity. In contrast to ZM-d, it showed higher activity of parasitic bacteria, dark sulphite and iron oxidation, and high cellulolytic and aromatic compound degradation activities. The ZM-w variant showed high activities in 42 out of 60 analyzed physiological traits. Traits with low potential load included thiosulphate, arsenate, manganese, iron, and sulphur-containing compound respiration, as well as invertible parasites, nitrate, and nitrite ammonification.
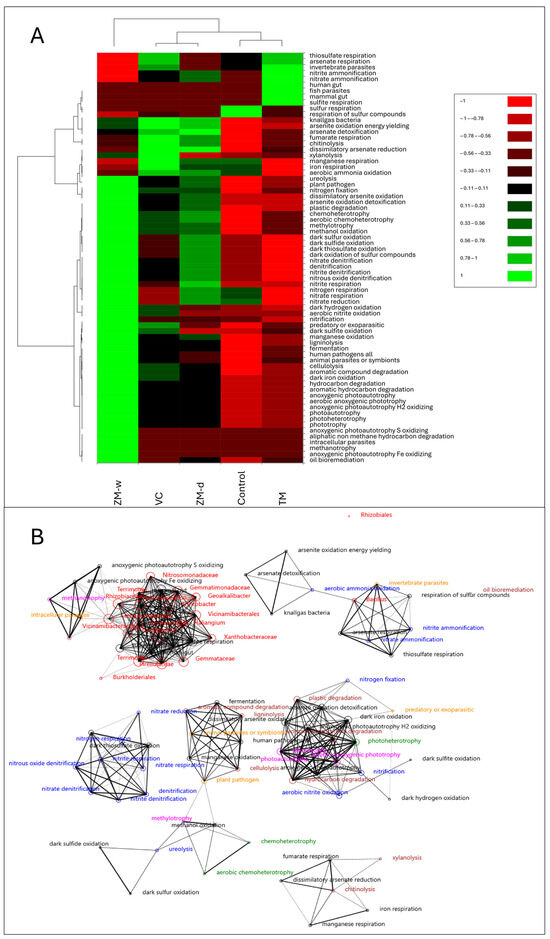
Figure 5.
Heatmap (A) and network analysis (B) (co-occurrence analysis), representing relationships between bacterial microbiomes and their metabolic features (r > 0.8).
Based on the results of rigorous network analysis (Figure 5B), it was observed that individual OTUs of bacteria were directly correlated with a small number of metabolic features. Among the few traits correlated with a specific OTU were anoxygenic photoautotrophy S and Fe oxidation, methanotrophy, and intercellular parasites correlated with Rhizobiaceae and Vicinamibacteraceae. Most OTUs were directly associated with typical gut bacteria and correlated with sulphate and sulphite respiration. Two exceptions to the relationship observed in other rhizobiome OTUs were Rhizobiales and Bacillus, whose changes in abundance were not related to rhizobiome abundance. Representatives of the Rhizobiales order were a completely different variable, unrelated to any observed variable. The Bacillus was correlated with the detoxification, oxidation, and respiration of arsenic, the respiration of thiosulphate and sulphur compounds, aerobic ammonification, as well as nitrite and nitrate ammonification.
4. Discussion
The composition of bacterial communities mainly consisted of Proteobacteria and Firmicutes across all treatments used (ranging from 56.30% in VC to 82.10% in ZM-w). Proteobacteria are primarily responsible for nitrogen fixation in soil [38], whereas Firmicutes have been identified as disease-suppressing microbes [39]. The presence of these two dominant bacterial phyla constitutes a significant part of the bacterial community in vermicompost [40], as was also recorded in the bacterial profile of the TM variant [41].
Integrated and biological methods are increasingly used in agriculture, replacing chemical-based solutions and sometimes proving more effective. Using natural sources from earthworms and their products through vermicomposting can offer natural alternatives to synthetic chemicals, like fertilizers and pesticides. These alternatives not only benefit plant cultivation, but also help combat various soil pathogenic fungi, such as Rhizoctonia solani, Alternaria solani, Aspergillus niger, A. flavus, Fusarium oxysporum, and F. graminearum [42]. Moreover, increasing the share of vermicompost has been shown to enhance the survival of bacteria used as biofertilizers, such as Azotobacter chroococcum, Bacillus megaterium, and Rhizobium leguminosarum [43]. The application of vermicompost biofortified with selected biological control agents (BCAs), i.e., Trichoderma harzianum, Pseudomonas fluorescens, and Bacillus subtilis, resulted in a lower incidence of disease symptoms, in addition to improving plant growth and yield [44]. Our results demonstrated a high abundance of genus Bacillus (18.2% in VC and 16.4% in TM) (Table 1). It is worth bearing in mind that fungi of the genera Trichoderma and Pseudomonas have positive effects on the increasingly important threat in rapeseed cultivation, which is the fungus of the genus Verticillium [45]. Interestingly, in the ZM-w variant, there was a large number of bacteria from the order Rhizobiales (52.2%), which shows a strong dependence on chitinases (Figure 4B), known for their ability to degrade fungal cell walls [46]. This might have ecological significance in its interactions with pathogenic fungi as biological control agents. Moreover, organic compounds resulting from the use of vermicompost can stimulate microbial activities and increase mineralizing and nitrifying enzymes [47], thus promoting nutrient turnover [48]. We previously showed that increased nitrogen levels encouraged the proliferation of aerobic spore-forming bacteria, which generate antibiotics useful as biocontrol agents. Moderate fertilizer levels led to the accumulation of nitrogen in bacterial biomass [49]. Additionally, the activities of symbiotic microbes, alongside gut enzymes like cellulase, protease, chitinase, and acid phosphatase, play crucial roles in converting ingested soil and organic matter into a valuable product rich in essential nutrients and active components of microbial biomass [50]. We also showed that higher frass rates increased the abundance of microorganisms supporting organic matter mineralization [27]. Symbiotic microbes living in the soil play key roles in the life, growth, and health of most plants. Some host plants can attract beneficial microorganisms through their roots, which helps them fight pathogens [51].
The use of vermicompost (VC) and mealworm frass (TM) resulted in the significantly highest values of red beet biomass in this research (Table 2, Figure 3). Both vermicompost and frass from mealworm feeding have properties that accelerate the decomposition of organic matter and increase the content of available nutrients for plants (N, P, and K). In addition to nitrogen and potassium, phosphorus is one of the key elements for the proper development of plants, especially at their initial stage. Unfortunately, this ingredient is often found in the soil in a form that is inaccessible to plants. This is due to its poor solubility in soil solution, low concentration, and low mobility. Using vermicompost or frass can increase the amount of phosphorus available to plants, potentially resulting in better yields and a healthier field. This is an important aspect of sustainable agriculture, allowing for a reduction in the use of mineral fertilizers [52]. To date, limited research has been conducted on the utilization of mealworm frass in plant applications, but some evidence suggests that it promotes plant growth and induces tolerance to abiotic stress [41,53]. This is important because the agricultural sector is highly sensitive to weather changes. Stress caused by rainfall patterns, drought, or frost affects plants and determines the final yield. In previous studies, we also confirmed that higher frass rates increased the abundance of plant-available nutrients (N, P, Mg, K, and Na) in peat [27,49]. Vermicompost (VC) enhances the presence of stable humic and fulvic acids in the compost, which are beneficial for plant growth and soil health [54,55]. Humic and fulvic acids, as the basic building blocks of humus in the soil, have structure-forming properties. They positively influence water–air relations in the soil solution, increasing its water retention capacity [56]
Vermicompost significantly affects plant growth by increasing the seed germination rate, seedling index, shoot biomass, root biomass, and total biomass [57]. We also recorded higher abundances of orders Rhizobiales (ZM-w) and Burkholderiales (VC and ZM-w), which have well-documented advantageous relationships with plants, showing a positive association between their presence and plant biomass growth [58,59]. Interestingly, the case of the ZM-w variant showed the greatest potential activity of physiological features, and it was the most distinct from the other variants (Figure 4A). In ZM-w, the Rhizobiales order was eudominant, comprising over half of the microbiome (52.2%). Additionally, hormonal compounds, including gibberellins and auxins, were clearly connected in ZM-w (Figure 4B). Several studies have suggested that certain types of Rhizobium function as biocontrol agents by generating siderophores [60]. Our results support this, showing strong relation between Rhizobiales and siderophores in PCA (Figure 4B). All these relationships may contribute to better the growth and development of plants using the ZM-w variant. This can help regulate plant metabolism, stimulate root development, improve soil structure, and increase nutrient uptake by plants. Increasing the presence of Proteobacteria, a phylum often associated with enhancing plant growth, reinforces findings regarding the beneficial effects of applied treatments on promoting plant growth and suppressing plant diseases.
Insect frass and vermicompost both hold potential as fertilizers and phytofortifiers, offering the capacity to enhance plant tolerance to abiotic stresses and bolster resistance against biotic stresses. The locations of TM, VC, ZM-d, and ZM-w in different parts of the biplot, suggesting the unique impact of each type of fertilization on microbiota composition and soil chemistry (Figure 4B). Additionally, recent research has shown that mealworms and superworms can use biodegradable materials (such as polyethylene and polystyrene) as carbon sources in food and degrade them without toxic effects. This can result in a sustainable, waste-free bioremediation cycle [61,62], and the gut microbiota can also degrade polymer waste [63]. Advantages of using insect frass and vermicompost include improving the soil structure, positively impacting plant health, increasing plant resistance to pathogens, and being safe for the environment as an alternative to chemical solutions. These benefits are attributed to its direct nutrient provision to the soil and plants, along with the presence of valuable biomolecules and beneficial microorganisms, such as plant growth promoters.
The comparison of the network analysis results with other results allowed only a partial correlation between the variables and their connections with the treatments used (Figure 5B). The lack of association between Rhizobiales and other studied OTUs, and the physiological features of the bacteriobiome, indicate its unique properties, and completely different relationships with treatments than the other OTUs. The completely unique metabolism of this order of bacteria is due to its tendency toward symbiosis with plants. The failure to obtain associations was mainly due to the lack of information about metabolic pathways in the database used. Bacillus spp. were also correlated with a unique set of characteristics, indicating the different impacts of the fertilizers used. In the case of Bacillus, its higher abundance is characteristic of almost every variant with the highest potential activity for TM. However, the load of Bacillus and ammonification was the lowest for ZM-w. In this variant, the ammonification and nitrogen acquisition processes, the metabolism of sulfur and arsenic, and the detoxification process were the lowest, but as heatmap analysis showed, this variant tends toward nitrification. Going further, with respect to the remaining data, it can be stated that the connections with heterotrophy and degradation of organic substances, as well as other features, e.g., nitrification, are not associated with any OTU, which indicates that, during rearrangement under the influence of fertilization in the rhizosphere bacteriobiome, the OTUs are replaced with others with similar properties. Moreover, the obtained results showed that ZM-w, VC, and ZM-d all showed high chemoheterotrophic potential, which indicates an r-selection strategy and high adaptive flexibility of the bacterial rhizobium. As a result, both life in the root zone and the microbiome itself may be better adapted to sudden, unfavorable environmental changes, or attack by plant pathogens [29]. Previously, we demonstrated that fertilizing wheat with TM has a positive effect on nitrogen transformations in the wheat rhizosphere, but it does not completely equate to the efficacy of mineral fertilizer [27]. In this study, TM also stimulated nitrogen availability. However, the wet fertilizer from ZM frass possesses multi-level positive properties for both soil and plants.
5. Conclusions
Recently, there has been increasing interest in using insect frass and vermicompost as agricultural resources. Soils fertilized with vermicompost and dry superworm frass had the highest concentrations of most of the tested chemical components. Dry superworm frass fertilization was characterized by increased availability levels of total nitrogen, NH4-N, and NO3-N. In this research, utilizing vermicompost and mealworm frass led to significantly higher red beet biomass values. However, the presence of important hormonal substances in plant development (auxins, gibberellins) was demonstrated using wet superworm frass. In this variant, the greatest improvement in the microbial activity of the rhizosphere and the highest nitrogen fixation potential were also observed. The presented data suggest that the effects of different types of treatments on the rhizosphere microbiome may result from a complex interplay of the rhizosphere, direct colonization of taxa from treatments, changes induced by these microorganisms in the entire rhizosphere community, and indirect changes in the rhizosphere community through secondary metabolites or nutrients. These results can lead to more sustainable and eco-friendly practices in agriculture and could have significant positive implications for both environmental health and crop productivity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app14135539/s1, Figure S1: The variability of plant growth-promoting characteristics in relation to treatments. Table S1. Chemical properties of vermicompost and insect frass.
Author Contributions
Conceptualization, S.W.P.; methodology, S.W.P. and O.K.; software, S.W.P.; validation, M.D., B.P. and K.W.; formal analysis, S.W.P., M.D., O.K., B.P., K.W., M.B.-S. and A.K.; investigation, S.W.P., M.D., O.K., B.P., K.W. and M.B.-S.; data curation, S.W.P., O.K., B.P. and K.W.; writing—original draft preparation, S.W.P., M.D., O.K., B.P. and K.W.; writing—review and editing, S.W.P., M.D., O.K., B.P., K.W., M.B.-S. and A.K.; visualization, S.W.P. and O.K.; supervision, S.W.P.; project administration, S.W.P.; funding acquisition, S.W.P. and A.K. All authors have read and agreed to the published version of the manuscript.
Funding
The results presented in this paper were obtained as part of a comprehensive study financed by the University of Warmia and Mazury in Olsztyn, Faculty of Agriculture and Forestry nos. 30.610.010-110 and 30.610.011-110.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available on request.
Acknowledgments
We would like to thank Magdalena Głąb and Jędrzej Mastalerz for technical support during experimentation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gableta, M.; Dziuba, S. Organic Food Production from the Standpoint of Employee Potential. Mark. Manag. 2018, 51, 63–72. [Google Scholar] [CrossRef]
- Góralczyk, K. Is Organic Food Really the Best? Stud. Ecol. Et Bioethicae 2018, 16, 51–56. [Google Scholar] [CrossRef]
- Fehse, K.; Simmank, F.; Gutyrchik, E.; Sztrókay-Gaul, A. Organic or Popular Brands—Food Perception Engages Distinct Functional Pathways. An FMRI Study. Cogent. Psychol. 2017, 4, 1284392. [Google Scholar] [CrossRef]
- Żelezik, M. Why Ecological Agriculture? Świętokrzyskie Yearbook. B-Nat. Sci. 2009, 30, 155–166. [Google Scholar]
- Golik, D.; Żmija, D. Organic Agriculture and Prospects of Its Development in Poland in the Light of EU Experience. Sci. J. Krakow Univ. Econ. 2017, 1, 117–129. [Google Scholar] [CrossRef][Green Version]
- Zuba, M. Chances and Barriers in the Integration of the Chain of the Organic Food in Poland. WSEI Sci. J. Econ. Ser. 2011, 3, 261–288. [Google Scholar]
- Kazimierczak, R.; Salach, K.; Rembiałkowska, E. Distribution Channels of Organic Agricultural Products in Poland—An Example of the Producers from the Masovian Voivodeship. J. Res. Appl. Agric. Eng. 2014, 59, 103–107. [Google Scholar]
- Pawlewicz, A.; Gotkiewicz, W. Channels of distribution of raw materials from organic farms in the voivodeship of Warmia and Mazury. Logistyka 2012, 4, 1168–1174. [Google Scholar]
- Skłodowski, P.; Bielska, A. Properties and Fertility of Polish Soils—The Basis for Shaping Agri-Environmental Relations. Water-Environment-Rural 2009, 9, 203–214. [Google Scholar]
- Harinarayanan, U.N.D.; Lakshmanan, P. Organic Vegetable Cultivation. In Vegetable Crops; Yildirim, E., Ekinci, M., Eds.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Akan, S.; Tuna Gunes, N.; Erkan, M. Red Beetroot: Health Benefits, Production Techniques, and Quality Maintaining for Food Industry. J. Food Process. Preserv. 2021, 45, e15781. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, Y.; Hu, Z.; Wu, S.; Jin, C. Beetroot as a Functional Food with Huge Health Benefits: Antioxidant, Antitumor, Physical Function, and Chronic Metabolomics Activity. Food Sci. Nutr. 2021, 9, 6406–6420. [Google Scholar] [CrossRef] [PubMed]
- Hlisnikovský, L.; Menšík, L.; Křížová, K.; Kunzová, E. The Effect of Farmyard Manure and Mineral Fertilizers on Sugar Beet Beetroot and Top Yield and Soil Chemical Parameters. Agronomy 2021, 11, 133. [Google Scholar] [CrossRef]
- Alves, A.U.; Prado, R.d.M.; Gondim, A.R.d.O.; Fonseca, I.M.; Cecílio Filho, A.B. Desenvolvimento e Estado Nutricional Da Beterraba Em Função Da Omisão de Nutrientes. Hortic. Bras. 2008, 26, 292–295. [Google Scholar] [CrossRef]
- Martin, H.L. Management of Soilborne Diseases of Beetroot in Australia: A Review. Aust. J. Exp. Agric. 2003, 43, 1281–1292. [Google Scholar] [CrossRef]
- Cervenski, J.; Gvozdanović-Varga, J.; Vasić, M.; Stojanović, A.; Medić-Pap, S.; Danojević, D.; Savić, A. Home Gardens and Backyards-Suitable Area for Vegetable Production. Acta Hortic. 2016, 1142, 179–186. [Google Scholar] [CrossRef]
- Kosewska, A.; Nijak, K.; Nietupski, M.; Kędzior, R.; Ludwiczak, E. Effect of Plant Protection on Assemblages of Ground Beetles (Coleoptera, Carabidae) in Sugar Beet Crops in Four-Year Rotation. Acta Zool. Acad. Sci. Hung. 2020, 66, 49–68. [Google Scholar] [CrossRef]
- Menino, R.; Murta, D. The Insects as a Workforce for Organic Fertilizers Production–Insect Frass. New Gener. Org. Fertil. 2022, 1–20. [Google Scholar] [CrossRef]
- Newton, G.L.; Booram, C.V.; Barker, R.W.; Hale, O.M. Dried Hermetia Illucens Larvae Meal as a Supplement for Swine. J. Anim. Sci. 1977, 44, 395–400. [Google Scholar] [CrossRef]
- Medvedev, A.Y.; Volgina, N.V.; Smetankina, V.G.; Matkovskaya, A.A.; Zelenkov, A.P.; Zelenkova, G.A. Biological Features of Tenebrio molitor, Zophobas morio and Hermetia illucens Larvae as the Source of Feed Protein for Animals. Vet. Pathol. 2023, 22, 19–25. [Google Scholar] [CrossRef]
- Van Huis, A. Potential of Insects as Food and Feed in Assuring Food Security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-Art on Use of Insects as Animal Feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Houben, D.; Daoulas, G.; Faucon, M.P.; Dulaurent, A.M. Potential Use of Mealworm Frass as a Fertilizer: Impact on Crop Growth and Soil Properties. Sci. Rep. 2020, 10, 4659. [Google Scholar] [CrossRef] [PubMed]
- Menino, R.; Felizes, F.; Castelo-Branco, M.A.; Fareleira, P.; Moreira, O.; Nunes, R.; Murta, D. Agricultural Value of Black Soldier Fly Larvae Frass as Organic Fertilizer on Ryegrass. Heliyon 2021, 7, e05855. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, E.; de Vries, W. Potential Benefits of Using Hermetia illucens Frass as a Soil Amendment on Food Production and for Environmental Impact Reduction. Curr. Opin. Green Sustain. Chem. 2020, 25, 100335. [Google Scholar] [CrossRef]
- Antoniadis, V.; Molla, A.; Grammenou, A.; Apostolidis, V.; Athanassiou, C.G.; Rumbos, C.I.; Levizou, E. Insect Frass as a Novel Organic Soil Fertilizer for the Cultivation of Spinach (Spinacia oleracea): Effects on Soil Properties, Plant Physiological Parameters, and Nutrient Status. J. Soil Sci. Plant Nutr. 2023, 23, 5935–5944. [Google Scholar] [CrossRef]
- Nogalska, A.; Przemieniecki, S.W.; Krzebietke, S.J.; Załuski, D.; Kosewska, A.; Skwierawska, M.; Sienkiewicz, S. The Effect of Mealworm Frass on the Chemical and Microbiological Properties of Horticultural Peat in an Incubation Experiment. Int. J. Environ. Res. Public Health 2023, 20, 21. [Google Scholar] [CrossRef] [PubMed]
- Przemieniecki, S.W.; Ruraż, K.; Kosewska, O.; Oćwieja, M.; Gorczyca, A. The Impact of Various Forms of Silver Nanoparticles on the Rhizosphere of Wheat (Triticum aestivum L.)–Shifts in Microbiome Structure and Predicted Microbial Metabolic Functions. Sci. Total Environ. 2024, 914, 169824. [Google Scholar] [CrossRef] [PubMed]
- Ling, N.; Wang, T.; Kuzyakov, Y. Rhizosphere Bacteriome Structure and Functions. Nat. Commun. 2022, 13, 836. [Google Scholar] [CrossRef]
- Przemieniecki, S.W.; Kosewska, A.; Purwin, C.; Zapałowska, A.; Mastalerz, J.; Kotlarz, K.; Kolaczek, K. Biometric, Chemical, and Microbiological Evaluation of Common Wheat (Triticum aestivum L.) Seedlings Fertilized with Mealworm (Tenebrio molitor L.) Larvae Meal. Appl. Soil Ecol. 2021, 167, 104037. [Google Scholar] [CrossRef]
- Schachtschabel, P. The magnesium of the soil available to plants and its purpose. J. Plant Nutr. Fert. Soil Sci. 1954, 67, 9–23. [Google Scholar] [CrossRef]
- Egńer, H.; Riehm, H.; Domingo, W.R. Studies on chemical soil analysis as a basis for assessing the nutrient status of the soil. II. Chemical extraction methods for determining phosphorus and potassium. K. Lantbrukshogskolans Ann. 1960, 26, 199–215. [Google Scholar]
- Kjeldahl, J. New Method for the Determination of Nitrogen in Organic Substances. Z. Anal. Chem. 1883, 22, 366–383. [Google Scholar] [CrossRef]
- Hammer, D.A.T.; Ryan, P.D.; Hammer, Ø.; Harper, D.A.T. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontologia. Electronica. 2001, 4, 1–9. [Google Scholar]
- Przemieniecki, S.W.; Damszel, M.; Ciesielski, S.; Kubiak, K.; Mastalerz, J.; Sierota, Z.; Gorczyca, A. Bacterial Microbiome in Armillaria Ostoyae Rhizomorphs Inhabiting the Root Zone during Progressively Dying Scots Pine. Appl. Soil Ecol. 2021, 164, 103929. [Google Scholar] [CrossRef]
- Le Boulch, M.; Déhais, P.; Combes, S.; Pascal, G. The MACADAM database: A MetAboliC pAthways DAtabase for Microbial taxonomic groups for mining potential metabolic capacities of archaeal and bacterial taxonomic groups. Database. 2019, 2019, baz049. [Google Scholar] [CrossRef] [PubMed]
- Lumivero XLSTAT Basic Solutions. Available online: https://www.xlstat.com/en/solutions/basic (accessed on 20 June 2023).
- Delgado-Baquerizo, M.; Oliverio, A.M.; Brewer, T.E.; Benavent-González, A.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K.; Fierer, N. A Global Atlas of the Dominant Bacteria Found in Soil. Science 2018, 359, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Kong, H.G.; Song, G.C.; Ryu, C.M. Disruption of Firmicutes and Actinobacteria Abundance in Tomato Rhizosphere Causes the Incidence of Bacterial Wilt Disease. ISME J. 2021, 15, 330–347. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, D.P.; Tiwari, R.; Kumar, K.; Singh, R.V.; Singh, S.; Prasanna, R.; Saxena, A.K.; Nain, L. Taxonomic and Functional Annotation of Gut Bacterial Communities of Eisenia Foetida and Perionyx Excavatus. Microbiol. Res. 2015, 175, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.; Jiménez-Gómez, A.; Saati-Santamaría, Z.; Usategui-Martín, R.; Rivas, R.; García-Fraile, P. Mealworm Frass as a Potential Biofertilizer and Abiotic Stress Tolerance-Inductor in Plants. Appl. Soil Ecol. 2019, 142, 110–122. [Google Scholar] [CrossRef]
- Gudeta, K.; Bhagat, A.; Julka, J.M.; Sinha, R.; Verma, R.; Kumar, A.; Kumari, S.; Ameen, F.; Bhat, S.A.; Amarowicz, R.; et al. Vermicompost and Its Derivatives against Phytopathogenic Fungi in the Soil: A Review. Horticulturae 2022, 8, 311. [Google Scholar] [CrossRef]
- Raja Sekar, K.; Karmegam, N. Earthworm Casts as an Alternate Carrier Material for Biofertilizers: Assessment of Endurance and Viability of Azotobacter chroococcum, Bacillus megaterium and Rhizobium leguminosarum. Sci. Hortic. 2010, 124, 286–289. [Google Scholar] [CrossRef]
- Basco, M.J.; Bisen, K.; Keswani, C.; Singh, H.B. Biological Management of Fusarium Wilt of Tomato Using Biofortified Vermicompost. Mycosphere 2017, 8, 467–483. [Google Scholar] [CrossRef]
- Zusková, E.; Konradyová, V.; Ryšánek, P.; Kazda, J. Bioproducts and Their Potential in Protection of Brassica napus L. against Verticillium Longisporum. Plant Soil Environ. 2024, 70, 188–194. [Google Scholar] [CrossRef]
- Sridevi, M.; Mallaiah, K.V. Factors Effecting Chitinase Activity of Rhizobium Sp. from Sesbania Sesban. Biologia 2008, 63, 307–312. [Google Scholar] [CrossRef]
- Adnan, M.; Joshi, N. The Uniqueness of Microbial Diversity from the Gut of Earthworm and Its Importance. J. Microbiol. Biotechnol. Res. 2013, 3, 111–115. [Google Scholar]
- Zhang, W.; Wang, C.; Liu, M.; Yu, Y. Integrated Reclamation of Saline Soil Nitrogen Transformation in the Hyphosphere by Earthworms and Arbuscular Mycorrhizal Fungus. Appl. Soil Ecol. 2019, 135, 137–146. [Google Scholar] [CrossRef]
- Nogalska, A.; Przemieniecki, S.W.; Krzebietke, S.J.; Kosewska, A.; Załuski, D.; Kozera, W.J.; Żarczyński, P.J. Farmed Insect Frass as a Future Organic Fertilizer. Appl. Sci. 2024, 14, 2380. [Google Scholar] [CrossRef]
- Zhang, B.-G.; Li, G.-T.; Shen, T.-S.; Wang, J.-K.; Sun, Z. Changes in Microbial Biomass C, N, and P and Enzyme Activities in Soil Incubated with the Earthworms Metaphire guillelmi or Eisenia fetida. Soil Biol. Biochem. 2000, 32, 2055–2062. [Google Scholar] [CrossRef]
- Cao, Y.H.; Zhao, X.W.; Nie, G.; Wang, Z.Y.; Song, X.; Zhang, M.X.; Hu, J.P.; Zhao, Q.; Jiang, Y.; Zhang, J.L. The Salt-Tolerance of Perennial Ryegrass Is Linked with Root Exudate Profiles and Microflora Recruitment. Sci. Total Environ. 2024, 916, 170205. [Google Scholar] [CrossRef]
- Młodzińska-Michta, E. Regulation of Growth and Development of the Roots by Selected Environmental and Endogenous Factors. Kosmos 2019, 67, 801–811. [Google Scholar] [CrossRef]
- Blakstad, J.I.; Strimbeck, R.; Poveda, J.; Bones, A.M.; Kissen, R. Frass from Yellow Mealworm (Tenebrio molitor) as Plant Fertilizer and Defense Priming Agent. Biocatal. Agric. Biotechnol. 2023, 53, 102862. [Google Scholar] [CrossRef]
- Atiyeh, R.M.; Lee, S.; Edwards, C.A.; Arancon, N.Q.; Metzger, J.D. The Influence of Humic Acids Derived from Earthworm-Processed Organic Wastes on Plant Growth. Bioresour. Technol. 2002, 84, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Ievinsh, G.; Vikmane, M.; Iirse, A.; Karlsons, A. Effect of Vermicompost Extract and Vermicompostderived Humic Acids on Seed Germination and Seedling Growth of Hemp. Proc. Latv. Acad. Sci. Sect. B Nat. Exact Appl. Sci. 2017, 71, 286–292. [Google Scholar] [CrossRef]
- Ukalska-Jaruga, A.; Debaene, G.; Smreczak, B. Particle and structure characterization of fulvic acids from agricultural soils. J. Soils Sediments 2018, 18, 2833–2843. [Google Scholar] [CrossRef]
- Blouin, M.; Barrere, J.; Meyer, N.; Lartigue, S.; Barot, S.; Mathieu, J. Vermicompost Significantly Affects Plant Growth. A Meta-Analysis. Agron. Sustain. Dev. 2019, 39, 34. [Google Scholar] [CrossRef]
- Rostami, M.; Karegar, A.; Taghavi, S.M.; Ghasemi-Fasaei, R.; Ghorbani, A. Effective Combination of Arugula Vermicompost, Chitin and Inhibitory Bacteria for Suppression of the Root-Knot Nematode Meloidogyne javanica and Explanation of Their Beneficial Properties Based on Microbial Analysis. PLoS ONE 2023, 18, e0289935. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Von-Wobeser, E.; Rocha-Estrada, J.; Shapiro, L.R.; De La Torre, M. Enrichment of Verrucomicrobia, Actinobacteria and Burkholderiales Drives Selection of Bacterial Community from Soil by Maize Roots in a Traditional Milpa Agroecosystem. PLoS ONE 2018, 13, e0208852. [Google Scholar] [CrossRef]
- Datta, B.; Chakrabartty, P.K. Siderophore Biosynthesis Genes of Rhizobium sp. Isolated from Cicer Arietinum L. 3 Biotech. 2014, 4, 391–401. [Google Scholar] [CrossRef]
- Przemieniecki, S.W.; Oćwieja, M.; Ciesielski, S.; Halecki, W.; Matras, E.; Gorczyca, A. Chemical Structure of Stabilizing Layers of Negatively Charged Silver Nanoparticles as an Effector of Shifts in Soil Bacterial Microbiome under Short-Term Exposure. Int. J. Environ. Res. Public Health 2022, 19, 14438. [Google Scholar] [CrossRef]
- Jung, H.; Shin, G.; Park, S.B.; Jegal, J.; Park, S.A.; Park, J.; Oh, D.X.; Kim, H.J. Circular Waste Management: Superworms as a Sustainable Solution for Biodegradable Plastic Degradation and Resource Recovery. Waste Manag. 2023, 171, 568–579. [Google Scholar] [CrossRef]
- Przemieniecki, S.W.; Kosewska, A.; Ciesielski, S.; Kosewska, O. Changes in the Gut Microbiome and Enzymatic Profile of Tenebrio Molitor Larvae Biodegrading Cellulose, Polyethylene and Polystyrene Waste. Environ. Pollut. 2020, 256, 113265. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).