Sequential Extraction of Incineration Bottom Ash: Conclusions Regarding Ecotoxicity
Abstract
:1. Introduction
- (1)
- Σ c(H400);
- (2)
- 100 × Σ c(H410) + 10 × Σ c(H411) + Σ c(H412);
- (3)
- Σ c(H410) + Σ c(H411) + Σ c(H412).
2. Materials and Methods
3. Results
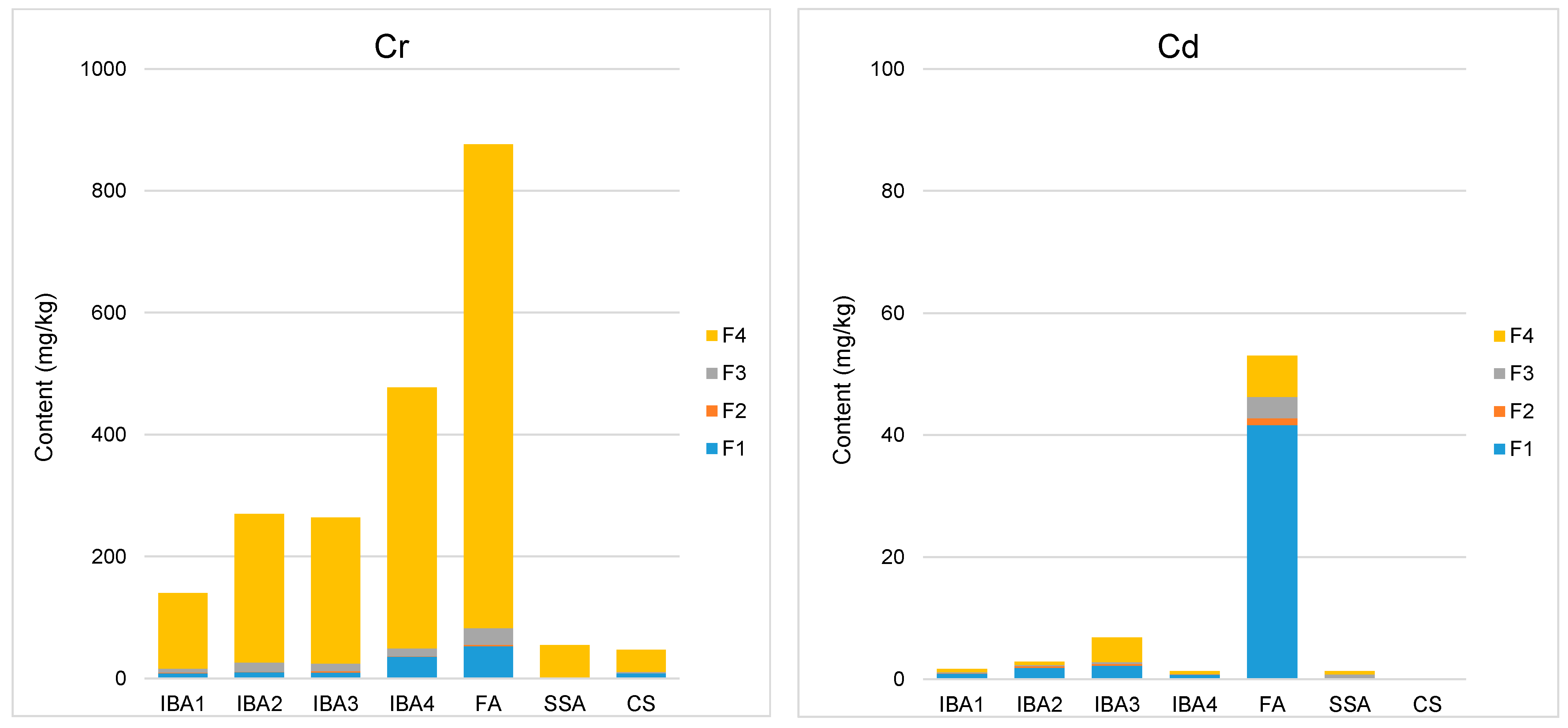
3.1. Sequential Extraction
3.2. Calcualtion of Synthesis Toxicity Index (STI)
3.3. Selective Extraction with Maleic Acid
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Suppliers of Waste-to-Energy Technology (ESWET). Municipal Waste Treatment in the EU, Fact Sheet. 2021. Available online: https://eswet.eu/wp-content/uploads/2021/07/05_ESWET-Fact-Sheet_Municipal-Waste-Treatment-in-the-EU_Update-2021.pdf (accessed on 25 June 2024).
- Blasenbauer, D.; Huber, F.; Lederer, J.; Quina, M.J.; Blanc-Biscarat, D.; Bogush, A.; Bontempi, E.; Blondeau, J.; Chimenos, J.M.; Dahlbo, H.; et al. Legal situation and current practice of waste incineration bottom ash utilisation in Europe. Waste Manag. 2020, 102, 868–883. [Google Scholar] [CrossRef]
- Di Gianfilippo, M.; Hyks, J.; Verginelli, I.; Costa, G.; Hjelmar, O.; Lombardi, F. Leaching behaviour of incineration bottom ash in a reuse scenario: 12years-field data vs. lab test results. Waste Manag. 2018, 73, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Hyks, J.; Syc, M. Utilisation of Incineration Bottom Ash in Road Construction. In Waste Mangement; Waste-to-Energy; Thiel, S., Thomé-Kozmiensky, E., Winter, F., Juchelkova, D., Eds.; TK-Verlag: Nietwerder, Germany, 2019; Volume 9, pp. 731–741. [Google Scholar]
- Pecqueur, G.; Crignon, C.; Quénée, B. Behaviour of cement-treated MSWI bottom ash. Waste Manag. 2001, 21, 229–233. [Google Scholar] [CrossRef]
- Dou, X.; Ren, F.; Nguyen, M.Q.; Ahamed, A.; Yin, K.; Chan, W.P.; Chang, V.W.-C. Review of MSWI bottom ash utilization from perspectives of collective characterization, treatment and existing application. Renew. Sustain. Energy Rev. 2017, 79, 24–38. [Google Scholar] [CrossRef]
- Clavier, K.A.; Paris, J.M.; Ferraro, C.C.; Townsend, T.G. Opportunities and challenges associated with using municipal waste incineration ash as a raw ingredient in cement production—A review. Resour. Conserv. Recycl. 2020, 160, 104888. [Google Scholar] [CrossRef]
- European Parliament and Council of the EU. Directive of 19 November 2008 on Waste and repealing certain Directives. Off. J. Eur. Union 2008, L312, 3–30. [Google Scholar]
- European Commission, Commission Regulation (EU). No 1357/2014 of 18 December 2014 replacing Annex III to Directive 2008/98/EC of the European Parliament and of the Council on waste and repealing certain Directives. Off. J. Eur. Union 2014, L365, 89–96. [Google Scholar]
- European Parliament and Council of the EU. Regulation (EC) No 1272/2008 of the European parliament and of the council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. Off. J. Eur. Union 2008, L353, 1–1355. [Google Scholar]
- Stark, C.; Koch-Jugl, J. Leitfaden zur Anwendung der CLP-Verordnung, das neue Einstufungs- und Kennzeichnungssystem für Chemikalien nach GHS; Umweltbundesamt: Dessau-Roßlau, Germany, 2013. [Google Scholar]
- Nordsieck, H.; Wambach, K.; Thiel, N.; Warnecke, R.; Rommel, W. Gefährliche Eigenschaft HP14 von Rostaschen. In Mineralische Nebenprodukte und Abfälle, Aschen, Schlacken Stäube und Baurestmassen; Thiel, S., Thomé-Kozmiensky, E., Pretz, T., Senk, D.G., Wotruba, H., Eds.; TK-Verlag: Neuruppin, Germany, 2019; Volume 6, pp. 98–112. [Google Scholar]
- Nordsieck, H.; Wambach, K.; Rommel, W. Hazardous Property HP14 of MSWI Bottom Ash. In Waste Management; Thiel, S., Thomé-Kozmiensky, E., Winter, F., Juchelková, D., Eds.; TK-Verlag: Nietwerder, Germany, 2018; Volume 8, pp. 287–300. [Google Scholar]
- Hennebert, P. Hazard classification of waste: Review of available practical methods and tools. Detritus 2019, 7, 13–28. [Google Scholar] [CrossRef]
- Council of the European Union. Council regulation (EU) 2017/997 of 8 June 2017 amending Annex III to Directive 2008/98/EC of the European Parliament and of the Council as regards the hazardous property HP 14 ‘Ecotoxic’. Off. J. Eur. Union 2017, L150, 1–4. [Google Scholar]
- Hjelmar, O.; van der Sloot, H.A.; van Zomeren, A. Hazard property classification of high temperature waste materials. In Proceedings of the Fourteenth International Waste Management and Landfill Symposium, Sardinia_2013; CISA Publisher: S. Margerita di Pula, Italy, 2013. [Google Scholar]
- Vateva, I.; Laner, D. Grain-Size Specific Characterisation and Resource Potentials of Municipal Solid Waste Incineration (MSWI) Bottom Ash: A German Case Study. Resources 2020, 9, 66. [Google Scholar] [CrossRef]
- Huber, F.; Blasenbauer, D.; Aschenbrenner, P.; Fellner, J. Chemical composition and leachability of differently sized material fractions of municipal solid waste incineration bottom ash. Waste Manag. 2019, 95, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, L.; Tribaudino, M.; Matteis, C.D.; Funari, V. Particle Size and Potential Toxic Element Speciation in Municipal Solid Waste Incineration (MSWI) Bottom Ash. Sustainability 2021, 13, 1911. [Google Scholar] [CrossRef]
- Holm, O.; Simon, F.G. Innovative treatment trains of bottom ash (BA) from municipal solid waste incineration (MSWI) in Germany. Waste Manag. 2017; 59, 229–236. [Google Scholar] [CrossRef]
- Simon, F.-G.; Scholz, P. Assessment of the Long-Term Leaching Behavior of Incineration Bottom Ash: A Study of Two Waste Incinerators in Germany. Appl. Sci. 2023, 13, 3228. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Quevauviller, P.; Rauret, G.; López-Sánchez, J.F.; Rubio, R.; Ure, A.; Muntau, H. Certification of trace metal extractable contents in a sediment reference material (CRM 601) following a three-step sequential extraction procedure. Sci. Total Env. Environ. 1997, 205, 223–234. [Google Scholar] [CrossRef]
- Haberl, J.; Schuster, M. Solubility of elements in waste incineration fly ash and bottom ash under various leaching conditions studied by a sequential extraction procedure. Waste Manag. 2019, 87, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Filgueiras, A.V.; Lavilla, I.; Bendicho, C. Chemical sequential extraction for metal partitioning in environmental solid samples. J. Environ. Monit. 2002, 4, 823–857. [Google Scholar] [CrossRef]
- De Matteis, C.; Mantovani, L.; Tribaudino, M.; Bernasconi, A.; Destefanis, E.; Caviglia, C.; Toller, S.; Dinelli, E.; Funari, V. Sequential extraction procedure of municipal solid waste incineration (MSWI) bottom ash targeting grain size and the amorphous fraction. Front. Environ. Sci. 2023, 11, 1254205. [Google Scholar] [CrossRef]
- He, Y.; Kasina, M. The Sequential Extraction of Municipal Solid Waste Incineration Bottom Ash: Heavy Metals Mobility and Sustainable Application of Ashes. Sustainability 2023, 15, 4638. [Google Scholar] [CrossRef]
- Ma, W.; Shi, W.; Shi, Y.; Chen, D.; Liu, B.; Chu, C.; Li, D.; Li, Y.; Chen, G. Plasma vitrification and heavy metals solidification of MSW and sewage sludge incineration fly ash. J. Hazard. Mater. 2021, 408, 124809. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Chai, M.; Li, R. Heavy Metal Migration and Potential Environmental Risk Assessment During the Washing Process of MSW Incineration Fly Ash and Molten Slag. Procedia Environ. Sci. 2016, 31, 351–360. [Google Scholar] [CrossRef]
- Luan, J.; Chai, M.; Liu, Y.; Ke, X. Heavy-metal speciation redistribution in solid phase and potential environmental risk assessment during the conversion of MSW incineration fly ash into molten slag. Environ. Sci. Pollut. Res. 2018, 25, 3793–3801. [Google Scholar] [CrossRef] [PubMed]
- IGAM; ITAD. Einstufung von Hausmüllverbrennungsschlacken in das Abfallverzeichnis anhand der Gefahrenrelevanten Eigenschaften HP1–HP15, Praxisleitfaden der Verbände IGAM und ITAD e.V., Version 2.1 vom 24.04.2020. Available online: https://www.itad.de/wissen/praxisleitfaden-zur-einstufung-von-hmv-schlacken (accessed on 23 June 2024).
- Brunner, P.H.; Mönch, H. The Flux of Metals Through Municipal Solid Waste Incinerators. Waste Manag. Res. 1986, 4, 105–119. [Google Scholar] [CrossRef]
- Abanades, S.; Flamant, G.; Gagnepain, B.; Gauthier, D. Fate of heavy metals during municipal solid waste incineration. Waste Manag. Res. 2002, 20, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Pienkoß, F.; Kalbe, U.; Simon, F.G. Nassmechanische Aufbereitung von Bauschutt-Brechsand mit der Setztechnik—Ein Schritt auf dem Weg zum Ersatzbaustoff. Chem. Ing. Tech. 2023, 95, 1916–1924. [Google Scholar] [CrossRef]
- Krüger, O.; Grabner, A.; Adam, C. Complete Survey of German Sewage Sludge Ash. Env. Environ. Sci. Technol. 2014, 48, 11811–11818. [Google Scholar] [CrossRef] [PubMed]
- Bandarra, B.S.; Mesquita, C.; Passos, H.; Martins, R.C.; Coelho, P.A.L.F.; Pereira, J.L.; Quina, M.J. An integrated characterisation of incineration bottom ashes towards sustainable application: Physicochemical, ecotoxicological, and mechanical properties. J. Hazard. Mater. 2023, 455, 131649. [Google Scholar] [CrossRef]
- Kraus, J. Ökotoxizität mineralischer Abfälle—Erfahrung in Österreich mit der Konkretisierung des HP14 Kriteriums. In Mineralische Nebenprodukte und Abfälle, Aschen, Schlacken Stäube und Baurestmassen; Thiel, S., Thomé-Kozmiensky, E., Pretz, T., Senk, D.G., Wotruba, H., Eds.; TK-Verlag: Neuruppin, Germany, 2019; Volume 6, pp. 88–97. [Google Scholar]
- Rissler, J.; Klementiev, K.; Dahl, J.; Steenari, B.-M.; Edo, M. Identification and Quantification of Chemical Forms of Cu and Zn in MSWI Ashes Using XANES. Energy Fuels 2020, 34, 14505–14514. [Google Scholar] [CrossRef]
- Tiberg, C.; Sjöstedt, C.; Karlfeldt Fedje, K. Speciation of Cu and Zn in bottom ash from solid waste incineration studied by XAS, XRD, and geochemical modelling. Waste Manag. 2021, 119, 389–398. [Google Scholar] [CrossRef]
- De Matteis, C.; Pollastri, S.; Mantovani, L.; Tribaudino, M. Potentially toxic elements speciation in bottom ashes from a municipal solid waste incinerator: A combined SEM-EDS, µ-XRF and µ-XANES study. Environ. Adv. 2024, 15, 100453. [Google Scholar] [CrossRef]
- Bundesregierung. Verordnung über Deponien und Langzeitlager (Deponieverordnung—DepV). Bundesgesetzblatt 2009, I22, 900–950. [Google Scholar]
- Bundesregierung. Verordnung zur Einführung einer Ersatzbaustoffverordnung, zur Neufassung der Bundes-Bodenschutz- und Altlastenverordnung und zur Änderung der Deponieverordnung und der Gewerbeabfallverordnung. Bundesgesetzblatt 2021, I43, 2598–2752. [Google Scholar]
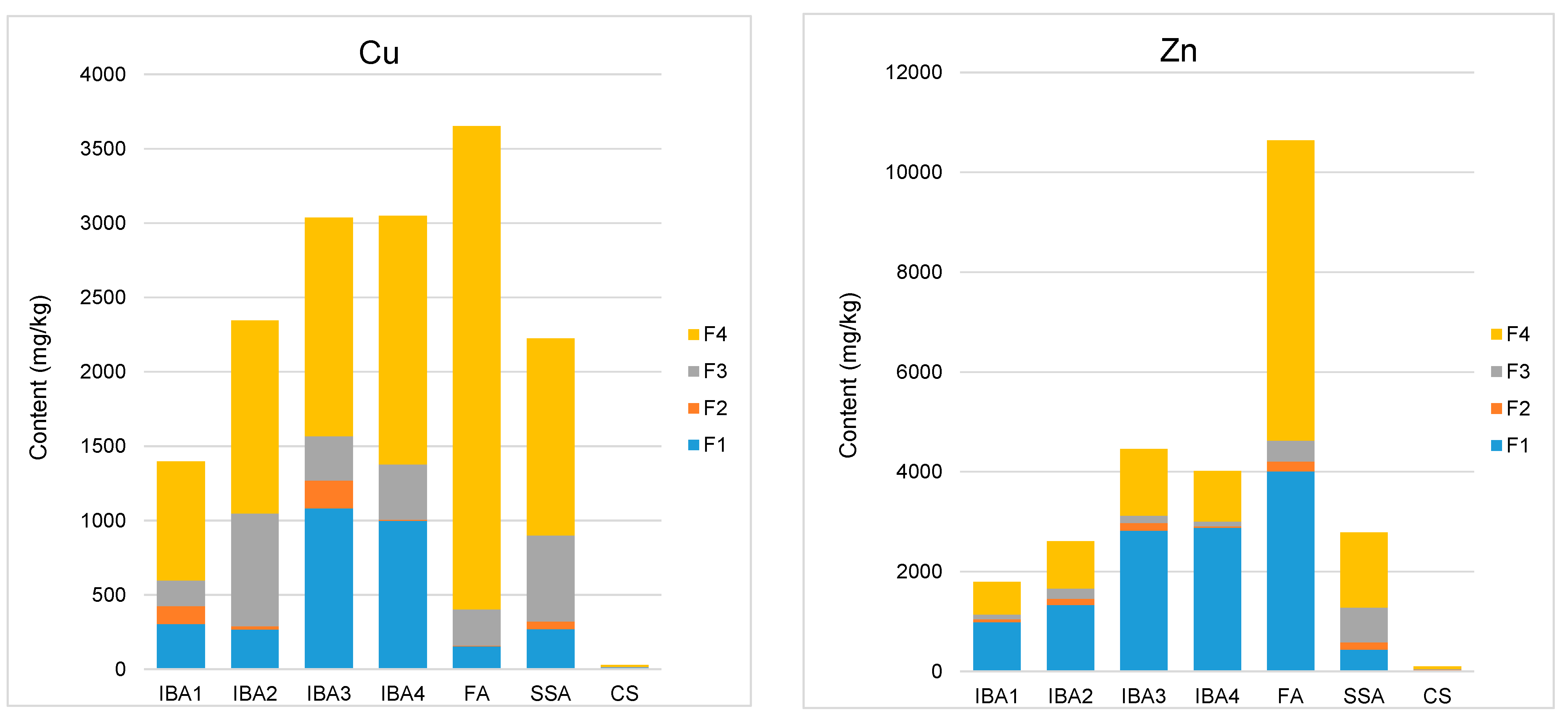

| Heavy Metals | Cu | Zn | Pb | Ni | Cr | Cd |
|---|---|---|---|---|---|---|
| Ti | 5 | 1 | 5 | 5 | 2 | 40 |
| CN (mg/kg) | 200 | 300 | 240 | 190 | 350 | 0.8 |
| Bioavalaibility coefficient | F1 | F2 | F3 | F4 | ||
| Ej | 7 | 5 | 2 | 0 | ||
| Sample | STI |
|---|---|
| IBA1 | 494 |
| IBA2 | 882 |
| IBA3 | 1218 |
| IBA4 | 576 |
| FA | 15,366 |
| SSA | 219 |
| CS | 38 |
| Sample | Cu (mg/kg) | Zn (mg/kg) | Cu2CO3(OH)2 (mg/kg) | ZnO (mg/kg) | HP14 Sum (%) |
|---|---|---|---|---|---|
| IBA1 | 430.82 | 1113.02 | 749.62 | 1380.14 | 13.8 |
| IBA2 | 597.34 | 1132.59 | 1039.37 | 1404.41 | 24.4 |
| IBA3 | 559.60 | 1231.01 | 973.71 | 1526.45 | 15.3 |
| IBA4 | 746.52 | 886.56 | 1298.95 | 1099.33 | 24.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simon, F.-G.; Scholz, P. Sequential Extraction of Incineration Bottom Ash: Conclusions Regarding Ecotoxicity. Appl. Sci. 2024, 14, 5541. https://doi.org/10.3390/app14135541
Simon F-G, Scholz P. Sequential Extraction of Incineration Bottom Ash: Conclusions Regarding Ecotoxicity. Applied Sciences. 2024; 14(13):5541. https://doi.org/10.3390/app14135541
Chicago/Turabian StyleSimon, Franz-Georg, and Philipp Scholz. 2024. "Sequential Extraction of Incineration Bottom Ash: Conclusions Regarding Ecotoxicity" Applied Sciences 14, no. 13: 5541. https://doi.org/10.3390/app14135541








