From Waste to Resource: Compositional Analysis of Olive Cake’s Fatty Acids, Nutrients and Antinutrients
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples of Olive Cakes
2.2. Chemical Composition of Olive Cakes
2.3. Fatty Acid Profile
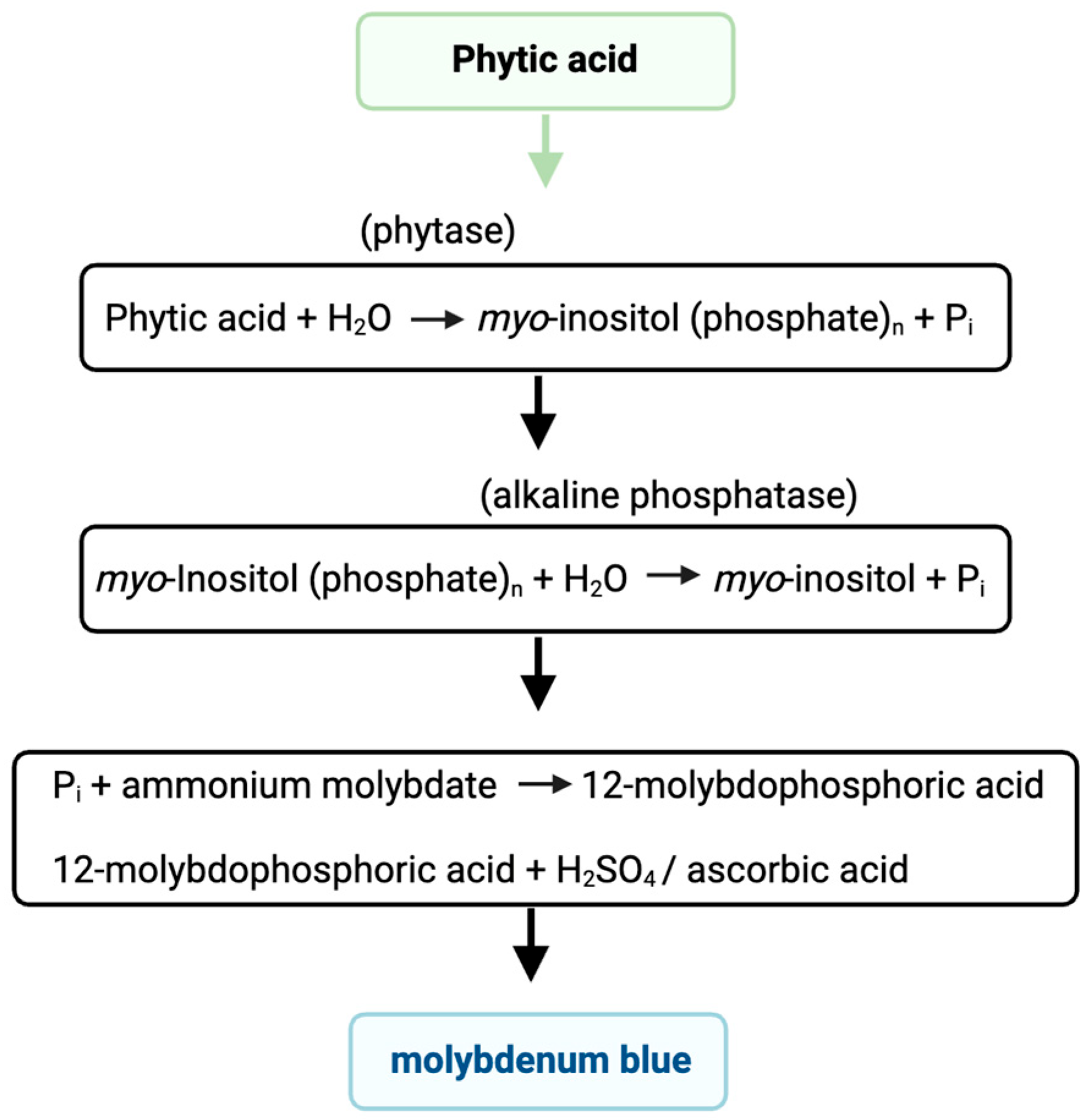
2.4. Phosphorus and Phytic Acid Content
2.5. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of Olive Cakes
3.2. Phosphorus and Phytic Acid Content
3.3. Fatty Acid Profile
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.H.; Saari, N. Valuable Nutrients and Functional Bioactives in Different Parts of Olive (Olea europaea L.)—A Review. Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef] [PubMed]
- Markhali, F.S.; Teixeira, J.A.; Rocha, C.M.R. Olive Tree Leaves—A Source of Valuable Active Compounds. Processes 2020, 8, 1177. [Google Scholar] [CrossRef]
- Ryan, D.; Robards, K. Critical Review. Phenolic Compounds in Olives. Analyst 1998, 123, 31R. [Google Scholar] [CrossRef]
- Otero, P.; Garcia-Oliveira, P.; Carpena, M.; Barral-Martinez, M.; Chamorro, F.; Echave, J.; Garcia-Perez, P.; Cao, H.; Xiao, J.; Simal-Gandara, J.; et al. Applications of By-Products from the Olive Oil Processing: Revalorization Strategies Based on Target Molecules and Green Extraction Technologies. Trends Food Sci. Technol. 2021, 116, 1084–1104. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, P.; Calvet, S.; Garciá-Rebollar, P.; De Blas, C.; Jiménez-Belenguer, A.I.; Hernández, P.; Piquer, O.; Cerisuelo, A. Partially Defatted Olive Cake in Finishing Pig Diets: Implications on Performance, Faecal Microbiota, Carcass Quality, Slurry Composition and Gas Emission. Animal 2020, 14, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Roig, A.; Cayuela, M.L.; Sánchez-Monedero, M.A. An Overview on Olive Mill Wastes and Their Valorisation Methods. Waste Manag. 2006, 26, 960–969. [Google Scholar] [CrossRef] [PubMed]
- De Blas, J.; Rodríguez, C.A.; Bacha, F.; Fernández, R.; Abad-Guamán, R. Nutritive Value of Co-Products Derived from Olivecake in Rabbit Feeding. World Rabbit Sci. 2015, 23, 255–262. [Google Scholar] [CrossRef]
- Restuccia, D.; Prencipe, S.A.; Ruggeri, M.; Spizzirri, U.G. Sustainability Assessment of Different Extra Virgin Olive Oil Extraction Methods through a Life Cycle Thinking Approach: Challenges and Opportunities in the Elaio-Technical Sector. Sustainability 2022, 14, 15674. [Google Scholar] [CrossRef]
- Otero, P.; Oliveira, F.M.; Lorini, A.; da Fonseca Antunes, B.; Oliveira, R.M.; Zambiazi, R.C. Oleuropein: Methods for extraction, purifying and applying. Rev. Ceres 2020, 67, 315–329. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Astray, G.; Carpena, M.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Valorization of By-Products from Olive Oil Industry and Added-Value Applications for Innovative Functional Foods. Food Res. Int. 2020, 137, 109683. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayiotou, M. Feeding Ensiled Crude Olive Cake to Lactating Chios Ewes, Damascus Goats and Friesian Cows. Livest. Prod. Sci. 1999, 59, 61–66. [Google Scholar] [CrossRef]
- Tufariello, M.; Durante, M.; Veneziani, G.; Taticchi, A.; Servili, M.; Bleve, G.; Mita, G. Patè Olive Cake: Possible Exploitation of a by-Product for Food Applications. Front. Nutr. 2019, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Souilem, S.; El-Abbassi, A.; Kiai, H.; Hafidi, A.; Sayadi, S.; Galanakis, C.M. Chapter 1—Olive oil production sector: Environmental effects and sustainability challenges. In Olive Mill Waste; Recent Advances for Sustainable Management; Galanakis, C.H., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Dahdouh, A.; Khay, I.; Bouizi, Y.; Kervern, G.; Pontvianne, S.; El Maakoul, A.; Bakhouya, M.; Le Brech, Y. Hydrothermal Carbonization of Two-Phase Olive Mill Waste (Alperujo): Effect of Aqueous Phase Recycling. Biomass Bioenergy 2024, 184, 107205. [Google Scholar] [CrossRef]
- Ferlisi, F.; Tang, J.; Cappelli, K.; Trabalza-Marinucci, M. Dietary Supplementation with Olive Oil Co-Products Rich in Polyphenols: A Novel Nutraceutical Approach in Monogastric Animal Nutrition. Front. Vet. Sci. 2023, 10, 1272274. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cruz, I.; Cara, C.; Romero, I.; Castro, E.; Gullón, B. Valorisation of Exhausted Olive Pomace by an Ecofriendly Solvent Extraction Process of Natural Antioxidants. Antioxidants 2020, 9, 1010. [Google Scholar] [CrossRef]
- Manzanares, P.; Ruiz, E.; Ballesteros, M.; Negro, M.J.; Gallego, F.J.; López-Linares, J.C.; Castro, E. Residual Biomass Potential in Olive Tree Cultivation and Olive Oil Industry in Spain: Valorization Proposal in a Biorefinery Context. Span. J. Agric. Res. 2017, 15, e0206. [Google Scholar] [CrossRef]
- Ollero, P.; Serrera, A.; Arjona, R.; Alcantarilla, S. The CO2 Gasiÿcation Kinetics of Olive Residue. Biomass Bioenergy 2003, 24, 151–161. [Google Scholar] [CrossRef]
- Medouni-Haroune, L.; Zaidi, F.; Medouni-Adrar, S.; Kecha, M. Olive Pomace: From An Olive Mill Waste To A Resource, An Overview of The New Treatments. J. Crit. Rev. 2018, 5, 1–6. [Google Scholar] [CrossRef]
- Xie, P.; Cecchi, L.; Bellumori, M.; Balli, D.; Giovannelli, L.; Huang, L.; Mulinacci, N. Phenolic Compounds and Triterpenes in Different Olive Tissues and Olive Oil By-Products, and Cytotoxicity on Human Colorectal Cancer Cells: The Case of Frantoio, Moraiolo and Leccino Cultivars (Olea europaea L.). Foods 2021, 10, 2823. [Google Scholar]
- Fainassi, F.; Taarji, N.; Benkhalti, F.; Hafidi, A.; Neves, M.A.; Isoda, H.; Nakajima, M. Emulsion Formation and Stabilizing Properties of Olive Oil Cake Crude Extracts. Processes 2021, 9, 633. [Google Scholar] [CrossRef]
- Lozano-Sánchez, J.; Castro-Puyana, M.; Mendiola, J.A.; Segura-Carretero, A.; Cifuentes, A.; Ibáñez, E. Recovering Bioactive Compounds from Olive Oil Filter Cake by Advanced Extraction Techniques. Int. J. Mol. Sci. 2014, 15, 16270–16283. [Google Scholar] [CrossRef] [PubMed]
- Suárez, M.; Romero, M.P.; Ramo, T.; Macià, A.; Motilva, M.J. Methods for Preparing Phenolic Extracts from Olive Cake for Potential Application as Food Antioxidants. J. Agric. Food Chem. 2009, 57, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Mojerlou, Z.; Elhamirad, A. Optimization of Ultrasound-Assisted Extraction (UAE) of Phenolic Compounds from Olive Cake. J. Food Sci. Technol. 2018, 55, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Amarni, F.; Kadi, H. Kinetics Study of Microwave-Assisted Solvent Extraction of Oil from Olive Cake Using Hexane. Comparison with the Conventional Extraction. Innov. Food Sci. Emerg. Technol. 2010, 11, 322–327. [Google Scholar] [CrossRef]
- Liu, J.J.; Gasmalla, M.A.A.; Li, P.; Yang, R. Enzyme-Assisted Extraction Processing from Oilseeds: Principle, Processing and Application. Innov. Food Sci. Emerg. Technol. 2016, 35, 184–193. [Google Scholar] [CrossRef]
- Herrero, M.; Temirzoda, T.N.; Segura-Carretero, A.; Quirantes, R.; Plaza, M.; Ibañez, E. New Possibilities for the Valorization of Olive Oil By-Products. J. Chromatogr. A 2011, 1218, 7511–7520. [Google Scholar] [CrossRef] [PubMed]
- Roselló-Soto, E.; Koubaa, M.; Moubarik, A.; Lopes, R.P.; Saraiva, J.A.; Boussetta, N.; Grimi, N.; Barba, F.J. Emerging Opportunities for the Effective Valorization of Wastes and By-Products Generated during Olive Oil Production Process: Non-Conventional Methods for the Recovery of High-Added Value Compounds. Trends Food Sci. Technol. 2015, 45, 296–310. [Google Scholar] [CrossRef]
- Donner, M.; Gohier, R.; de Vries, H. A New Circular Business Model Typology for Creating Value from Agro-Waste. Sci. Total Environ. 2020, 716, 137065. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants—Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef] [PubMed]
- Barbulova, A.; Colucci, G.; Apone, F. New Trends in Cosmetics: By-Products of Plant Origin and Their Potential Use as Cosmetic Active Ingredients. Cosmetics 2015, 2, 82–92. [Google Scholar] [CrossRef]
- Giromini, C.; Ottoboni, M.; Tretola, M.; Marchis, D.; Gottardo, D.; Caprarulo, V.; Baldi, A.; Pinotti, L. Nutritional Evaluation of Former Food Products (Ex-Food) Intended for Pig Nutrition. Food Addit. Contam. Part A 2017, 34, 1436–1445. [Google Scholar] [CrossRef] [PubMed]
- European Union. Commission Regulation (EU) Nº 68/2013 on the Catalogue of Feed Materials. Off. J. Eur. Union 2013, 3. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:029:0001:0064:EN:PDF (accessed on 20 June 2024).
- Donner, M.; Erraach, Y.; López-i-Gelats, F.; Manuel-i-Martin, J.; Yatribi, T.; Radić, I.; El Hadad-Gauthier, F. Circular Bioeconomy for Olive Oil Waste and By-Product Valorisation: Actors’ Strategies and Conditions in the Mediterranean Area. J. Environ. Manag. 2022, 321, 115836. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- ISO 12966-2:2017; Animal and Vegetable Fats and Oils-Gas Chromatography of Fatty Acid Methyl Esters-Part 2. International Standard. 2nd ed. CSA Group: Toronto, ON, Canada, 2017.
- Phytic Acid (Phytate)/Total Phosphorus Measured as Phosphorus Released by Phytase and Alkaline Phosphatase ASSAY PROCEDURE. Megazyme. 2019. Available online: https://d1kkimny8vk5e2.cloudfront.net/documents/Assay_Protocol/K-PHYT_DATA.pdf (accessed on 20 June 2024).
- Dorbane, Z.; Kadi, S.A.; Boudouma, D.; Gater-Belaid, N.; Bannelier, C.; Berchiche, M.; Gidenne, T. Nutritive Value of Two Types of Olive Cake (Olea europaea L.) for Growing Rabbit. World Rabbit Sci. 2019, 27, 69–75. [Google Scholar] [CrossRef]
- Ouaini, R.; Estephan, N.; Chebib, H. Chemical Composition of Olive Cakes Resulting from Various Mills in Lebanon. Agrochimica 2010, 54, 321–330. [Google Scholar]
- Molina Alcaide, E.; Nefzaou, A. Recycling of Olive Oil By-Products: Possibilities of Utilization in Animal Nutrition. Int. Bioterioration Biodegrad. 1996, 38, 227–235. [Google Scholar] [CrossRef]
- Araújo, M.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P.P. Phenolic Compounds from Olive Mill Wastes: Health Effects, Analytical Approach and Application as Food Antioxidants. Trends Food Sci. Technol. 2015, 45, 200–211. [Google Scholar] [CrossRef]
- Marđokić, A.; Maldonado, A.E.; Klosz, K.; Molnár, M.A.; Vatai, G.; Bánvölgyi, S. Optimization of Conditions for Microwave-Assisted Extraction of Polyphenols from Olive Pomace of Žutica Variety: Waste Valorization Approach. Antioxidants 2023, 12, 1175. [Google Scholar] [CrossRef] [PubMed]
- Dantas Palmeira, J.; Araújo, D.; Mota, C.C.; Alves, R.C.; Oliveira, M.B.P.P.; Ferreira, H.M.N. Fermentation as a Strategy to Valorize Olive Pomace, a By-Product of the Olive Oil Industry. Fermentation 2023, 9, 442. [Google Scholar] [CrossRef]
- Ying, D.Y.; Hlaing, M.M.; Lerisson, J.; Pitts, K.; Cheng, L.; Sanguansri, L.; Augustin, M.A. Physical Properties and FTIR Analysis of Rice-Oat Flour and Maize-Oat Flour Based Extruded Food Products Containing Olive Pomace. Food Res. Int. 2017, 100, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Cravotto, C.; Fabiano-Tixier, A.S.; Claux, O.; Rapinel, V.; Tomao, V.; Stathopoulos, P.; Skaltsounis, A.L.; Tabasso, S.; Jacques, L.; Chemat, F. Higher Yield and Polyphenol Content in Olive Pomace Extracts Using 2-Methyloxolane as Bio-Based Solvent. Foods 2022, 11, 1357. [Google Scholar] [CrossRef] [PubMed]
- Alburquerque, J.A.; Gonzálvez, J.; García, D.; Cegarra, J. Agrochemical Characterisation of “Alperujo”, a Solid by-Product of the Two-Phase Centrifugation Method for Olive Oil Extraction. Bioresour. Technol. 2004, 91, 195–200. [Google Scholar] [CrossRef] [PubMed]
- García-Rodríguez, J.; Mateos, I.; Saro, C.; González, J.S.; Carro, M.D.; Ranilla, M.J. Replacing Forage by Crude Olive Cake in a Dairy Sheep Diet: Effects on Ruminal Fermentation and Microbial Populations in Rusitec Fermenters. Animals 2020, 10, 2235. [Google Scholar] [CrossRef] [PubMed]
- Marcos, C.N.; de Evan, T.; García-Rebollar, P.; de Blas, C.; Carro, M.D. Influence of Storage Time and Processing on Chemical Composition and in Vitro Ruminal Fermentation of Olive Cake. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Ruiz, D.R.; Molina-Alcaide, E. A Comparative Study of the Effect of Two-Stage Olive Cake Added to Alfalfa on Digestion and Nitrogen Losses in Sheep and Goats. Animal 2007, 1, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Neifar, M.; Jaouani, A.; Ayari, A.; Abid, O.; Ben Salem, H.; Boudabous, A.; Najar, T.; Ghorbel, R.E. Improving the Nutritive Value of Olive Cake by Solid State Cultivation of the Medicinal Mushroom Fomes Fomentarius. Chemosphere 2013, 91, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Marcos, C.N.; García-Rebollar, P.; de Blas, C.; Carro, M.D. Variability in the Chemical Composition and in Vitro Ruminal Fermentation of Olive Cake By-Products. Animals 2019, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, P.; García-Rebollar, P.; Cerisuelo, A.; Ibáñez, M.A.; Rodríguez, C.A.; Calvet, S.; De Blas, C. Nutritional Value of Crude and Partially Defatted Olive Cake in Finishing Pigs and Effects on Nitrogen Balance and Gaseous Emissions. Anim. Feed Sci. Technol. 2018, 236, 131–140. [Google Scholar] [CrossRef]
- Molina-Alcaide, E.; Yáñez-Ruiz, D.R. Potential Use of Olive By-Products in Ruminant Feeding: A Review. Anim. Feed Sci. Technol. 2008, 147, 247–264. [Google Scholar] [CrossRef]
- Fathy, S.A.; Mahmoud, A.E.; Rashad, M.M.; Ezz, M.K.; Mohammed, A.T. Improving the Nutritive Value of Olive Pomace by Solid State Fermentation of Kluyveromyces Marxianus with Simultaneous Production of Gallic Acid. Int. J. Recycl. Org. Waste Agric. 2018, 7, 135–141. [Google Scholar] [CrossRef]
- Ahmed, R.I.; Shehata, S.; Bahgat, L.; Eid, E.; Al-Marakby, K. Effect of Some Chemical And Biological Treatments On The Chemical Composition, Cell Wall Constituents And In Situ Degradability of Olive Cake Nutrients. Zagazig J. Agric. Res. 2020, 47, 1201–1212. [Google Scholar] [CrossRef]
- Yansari, A.T.; Sadeghi, H.; Ansari-Pirsarai, Z.; Mohammad-Zadeh, H. Ruminal Dry Matter and Nutrient Degradability of Different Olive Cake By-Products after Incubation in the Rumen Using Nylon Bag Technique. Int. J. Agric. Biol. 2017, 9, 439–442. [Google Scholar]
- Mennane, Z.; Tada, S.; Aki, I.; Faid, M.; Hassani, S.; Salmaoui, S. Physicochemical and Microbiological Characterization of the Olive Residue of 26 Traditional Oil Mills in Beni Mellal (Morroco). Les Technol. De Lab. 2010, 5, 4–9. [Google Scholar]
- Hadi Alkarawi, H.; Zotz, G. Phytic Acid in Green Leaves. Plant Biol. 2014, 16, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Raboy, V. Approaches and Challenges to Engineering Seed Phytate and Total Phosphorus. Plant Sci. 2009, 177, 281–296. [Google Scholar] [CrossRef]
- Greenwood, J.S.; Gifford, D.J.; Bewley, J.D.; Greenwood, J.S. Accumulation of Phytic Acid in the Developing Endosperm and Embryo in Relation to the Deposition of Lipid, Protein, and Phosphorus. Can. J. Bot. 2011, 62, 255–261. [Google Scholar] [CrossRef]
- Alkarawi, H.H.; Zotz, G. Phytic Acid in Green Leaves of Herbaceous Plants-Temporal Variation in Situ and Response to Different Nitrogen/Phosphorus Fertilizing Regimes. AoB Plants 2014, 6, plu048. [Google Scholar] [CrossRef] [PubMed]
- Lott, J.N.A.; Ockenden, I.; Raboy, V.; Batten, G.D. Phytic Acid and Phosphorus in Crop Seeds and Fruits: A Global Estimate. Seed Sci. Res. 2000, 10, 11–33. [Google Scholar] [CrossRef]
- McKie, V.A.; McCleary, B.V. A Novel and Rapid Colorimetric Method for Measuring Total Phosphorus and Phytic Acid in Foods and Animal Feeds. J. AOAC Int. 2016, 99, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Kasim, A.B.; Edwards, H.M. The Analysis for Inositol Phosphate Forms in Feed Ingredients. J. Sci. Food Agric. 1998, 76, 1–9. [Google Scholar] [CrossRef]
- Lehrfeld, J.; Morris, E.R. Overestimation of Phytic Acid in Foods by the AOAC Anion-Exchange Method. J. Sci. Food Agric. 1992, 40, 2208–2210. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000; pp. 57–58. [Google Scholar]
- Špika, M.J.; Perica, S.; Žanetić, M.; Škevin, D. Virgin Olive Oil Phenols, Fatty Acid Composition and Sensory Profile: Can Cultivar Overpower Environmental and Ripening Effect? Antioxidants 2021, 10, 689. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.M.; Surh, J. Fatty Acid Composition as a Predictor for the Oxidation Stability of Korean Vegetable Oils with or without Induced Oxidative Stress. Prev. Nutr. Food Sci. 2012, 17, 158–165. [Google Scholar] [CrossRef]
- Li, H.; Fan, Y.W.; Li, J.; Tang, L.; Hu, J.N.; Deng, Z.Y. Evaluating and Predicting the Oxidative Stability of Vegetable Oils with Different Fatty Acid Compositions. J. Food Sci. 2013, 78, H633–H641. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.A.; Palmeira, J.D.; Melo, D.; Machado, S.; Lobo, J.C.; Costa, A.S.G.; Alves, R.C.; Ferreira, H.; Oliveira, M.B.P.P. Chemical Composition and Antimicrobial Activity of a New Olive Pomace Functional Ingredient. Pharmaceuticals 2021, 14, 913. [Google Scholar] [CrossRef] [PubMed]
- Antónia Nunes, M.; Costa, A.S.G.; Bessada, S.; Santos, J.; Puga, H.; Alves, R.C.; Freitas, V.; Oliveira, M.B.P.P. Olive Pomace as a Valuable Source of Bioactive Compounds: A Study Regarding Its Lipid- and Water-Soluble Components. Sci. Total Environ. 2018, 644, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Uribe, E.; Lemus-Mondaca, R.; Vega-Gálvez, A.; López, L.A.; Pereira, K.; López, J.; Ah-Hen, K.; Di Scala, K. Quality Characterization of Waste Olive Cake During Hot Air Drying: Nutritional Aspects and Antioxidant Activity. Food Bioprocess Technol. 2013, 6, 1207–1217. [Google Scholar] [CrossRef]
- Kotsampasi; Bampidis, V.A.; Tsiaousi, A.; Christodoulou, C.; Petrotos, K.; Amvrosiadis, I.; Fragioudakis, N.; Christodoulou, V. Effects of Dietary Partly Destoned Exhausted Olive Cake Supplementation on Performance, Carcass Characteristics and Meat Quality of Growing Lambs. Small Rumin. Res. 2017, 156, 33–41. [Google Scholar] [CrossRef]
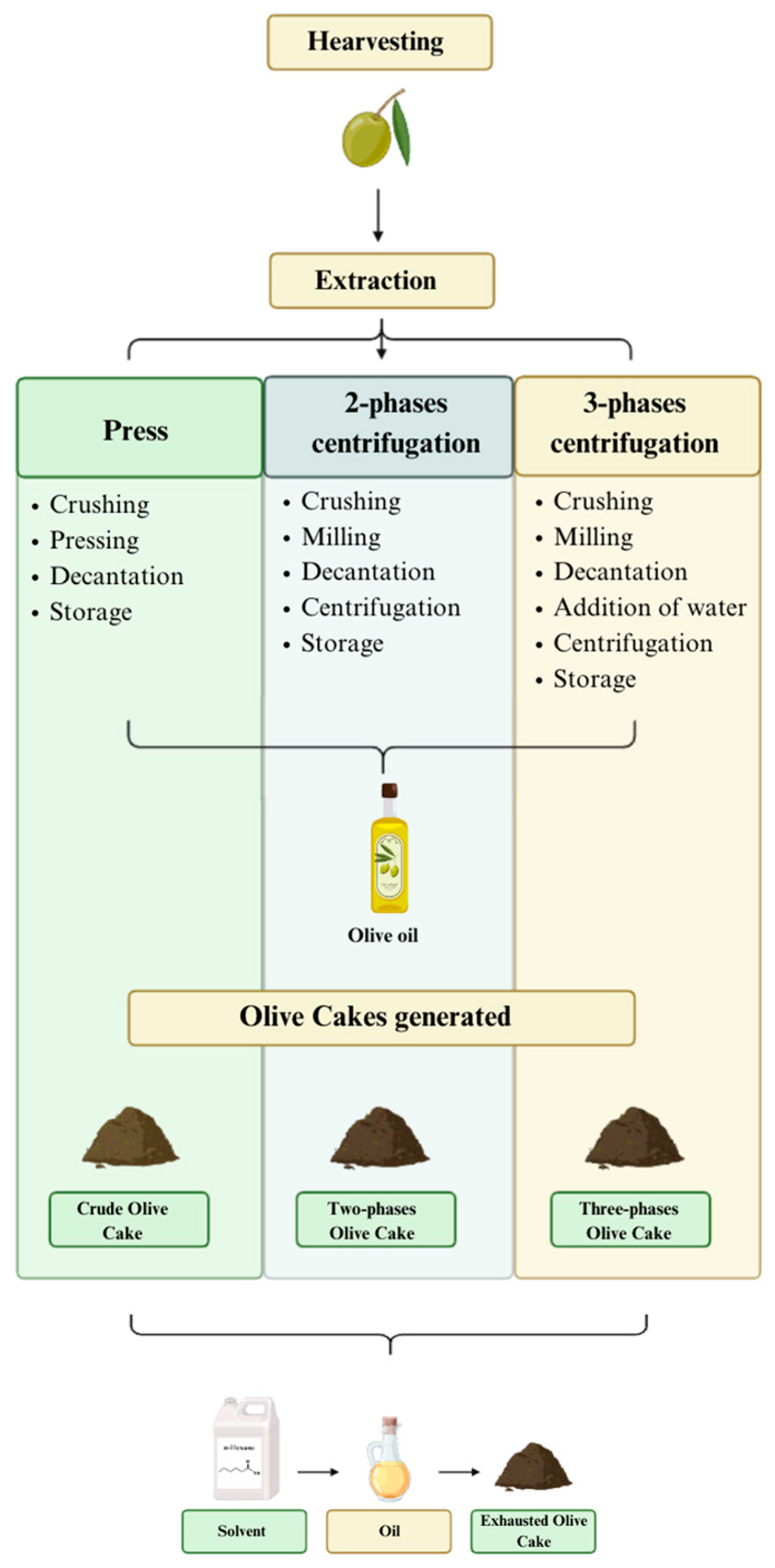
| Olive Cakes | Oil Mill | Campaigns |
|---|---|---|
| EOC 1 | Quinta das Covas | jan/21 |
| EOC 2 | Gimonde | mai/21 |
| TPOC (pitted) | Murça | mai/21 |
| TPOC (dehydrated) | Frechas | dez/21 |
| COC 1 | Baçal | mai/21 |
| COC 2 | Baçal | dez/21 |
| Chemical Composition (% DM) | Olive Cakes | |||||
|---|---|---|---|---|---|---|
| EOC 1 | EOC 2 | TPOC (Pitted) | TPOC (Dehydrated) | COC 1 | COC 2 | |
| DM | 90.16 ± 0.03 b | 89.79 ± 0.03 b | 35.41 ± 0.74 e | 96.76 ± 0.54 a | 69.62 ± 0.30 c | 65.53 ± 0.47 d |
| OM | 94.15 ± 0.01 d | 93.54 ± 0.01 e | 91.20 ± 0.00 f | 97.61 ± 0.00 b | 96.91 ± 0.10 c | 98.42 ± 0.02 a |
| NDF | 67.97 ± 0.16 b | 65.09 ± 0.92 c | 57.43 ± 0.02 d | 75.72 ± 0.98 a | 67.68 ± 0.27 b | 77.98 ± 0.25 a |
| ADF | 36.77 ± 1.26 c | 52.36 ± 0.51 b | 51.18 ± 0.3 b | 54.31 ± 1.57 ab | 52.27 ± 0.27 b | 57.68 ± 0.14 a |
| ADL | 24.19 ± 0.01 bc | 25.63 ± 0.23 ab | 23.32 ± 0.97 c | 25.87 ± 0.16 a | 26.16 ± 0.09 a | 26.53 ± 0.04 a |
| CEL | 25.67 ± 0.17 b | 26.74 ± 0.28 b | 27.87 ± 0.67 b | 28.45 ± 1.72 ab | 26.11 ± 0.18 b | 31.15 ± 0.17 a |
| CP | 6.84 ± 0.72 a | 7.26 ± 0.36 a | 6.75 ± 0.11 a | 6.33 ± 0.08 ab | 5.38 ± 0.02 b | 6.50 ± 0.13 ab |
| CF | 1.51 ± 0.05 d | 1.45 ± 0.04 d | 10.03 ± 0.06 b | 9.14 ± 0.08 bc | 14.5 ± 0.59 a | 8.43 ± 0.43 c |
| Olive Cakes | ||||||
|---|---|---|---|---|---|---|
| EOC 1 | EOC 2 | TPOC (Pitted) | TPOC (Dehydrated) | COC 1 | COC 2 | |
| Total Phosphorus | 0.07 ± 0.01 b | 0.14 ± 0.02 a | 0.13 ± 0.02 a | 0.11 ± 0.02 ab | 0.12 ± 0.01 a | 0.13 ± 0.01 a |
| Phytic Acid | 0.26 ± 0.04 b | 0.48 ± 0.06 a | 0.45 ± 0.08 a | 0.38 ± 0.07 ab | 0.43 ± 0.02 a | 0.46 ± 0.04 a |
| Fatty Acid Profile | Olive Cakes | |||||
|---|---|---|---|---|---|---|
| EOC 1 | EOC 2 | TPOC (Pitted) | TPOC (Dehydrated) | COC 1 | COC 2 | |
| C13:0 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| C14:0 | 0.05 ± 0.00 a | 0.05 ± 0.00 a | 0.02 ± 0.00 b | 0.03 ± 0.00 b | 0.02 ± 0.00 b | 0.03 ± 0.00 b |
| C16:0 | 10.63 ± 0.02 d | 11.42 ± 0.00 b | 11.28 ± 0.00 c | 12.50 ± 0.00 a | 10.68 ± 0.00 d | 11.47 ± 0.02 b |
| C16:1n-7 | 0,49 ± 0.01 c | 0.49 ± 0.00 c | 0.45 ± 0.00 d | 0.96 ± 0.01 a | 0.53 ± 0.00 b | 0.53 ± 0.01 b |
| C18:0 | 3.77 ± 0.03 a | 3.33 ± 0.00 c | 3.22 ± 0.00 | 2.44 ± 0.01 e | 3.69 ± 0.00 b | 3.25 ± 0.03 d |
| C18:1n-9 | 67.77 ± 0.02 e | 66.08 ± 0.00 f | 68.10 ± 0.00 d | 68.50 ± 0.08 c | 73.14 ± 0.00 a | 71.46 ± 0.02 b |
| C18:1n-7 | 1.72 ± 0.04 e | 1.95 ± 0.00 d | 1.90 ± 0.00 d | 3.06 ± 0.08 a | 2.18 ± 0.00 c | 2.42 ± 0.04 b |
| C18:2n-6 | 11.51 ± 0.00 c | 12.61 ± 0.00 a | 12.17 ± 0.00 b | 9.88 ± 0.01 d | 7.29 ± 0.00 f | 8.39 ± 0.00 e |
| C20:0 | 0.61 ± 0.00 a | 0.57 ± 0.00 b | 0.47 ± 0.00 c | 0.39 ± 0.00 e | 0.48 ± 0.00 c | 0.44 ± 0.00 d |
| C20:1n-9 | 0.39 ± 0.00 a | 0.40 ± 0.00 a | 0.34 ± 0.00 b | 0.31 ± 0.01 c | 0.28 ± 0.00 d | 0.29 ± 0.00 d |
| C22:0 | 0.63 ± 0.00 a | 0.43 ± 0.00 b | 0.23 ± 0.00 c | 0.00 ± 0.00 e | 0.20 ± 0.00 d | 0.00 ± 0.00 e |
| C22:1n-9 | 0.22 ± 0.00 a | 0.12 ± 0.00 c | 0.11 ± 0.00 d | 0.03 ± 0.00 e | 0.13 ± 0.00 b | 0.03 ± 0.00 e |
| C24:0 | 0.21 ± 0.01 b | 0.30 ± 0.00 a | 0.15 ± 0.00 c | 0.21 ± 0.01 b | 0.13 ± 0.00 c | 0.23 ± 0,01 b |
| ΣSFA | 16.32 ± 0.06 b | 16.56 ± 0.00 a | 15.66 ± 0.00 d | 15.83 ± 0.00 c | 15.50 ± 0.00 e | 15.70 ± 0.06 d |
| ΣMUFA | 70.98 ± 0.05 d | 69.30 ± 0.00 e | 71.00 ± 0.00 d | 72.90 ± 0.00 c | 76.36 ± 0.00 a | 74.72 ± 0.05 b |
| ΣPUFA | 12.70 ± 0.01 c | 14.14 ± 0.00 a | 13.34 ± 0.00 b | 11.28 ± 0.01 d | 8.13 ± 0.00 f | 9.58 ± 0.07 e |
| PUFA/SFA | 0.78 ± 0.00 d | 0.85 ± 0.00 c | 0.85 ± 0.00 c | 1.29 ± 0.00 a | 0.53 ± 0.00 e | 1.12 ± 0.00 b |
| n-6/n-3 | 10.57 ± 0.00 b | 9.29 ± 0.00 e | 11.30 ± 0.00 a | 9.99 ± 0.00 c | 9.73 ± 0.00 d | 8.46 ± 0.00 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paié-Ribeiro, J.; Baptista, F.; Teixeira, J.; Guedes, C.; Gomes, M.J.; Teixeira, A.; Barros, A.N.; Pinheiro, V.; Outor-Monteiro, D. From Waste to Resource: Compositional Analysis of Olive Cake’s Fatty Acids, Nutrients and Antinutrients. Appl. Sci. 2024, 14, 5586. https://doi.org/10.3390/app14135586
Paié-Ribeiro J, Baptista F, Teixeira J, Guedes C, Gomes MJ, Teixeira A, Barros AN, Pinheiro V, Outor-Monteiro D. From Waste to Resource: Compositional Analysis of Olive Cake’s Fatty Acids, Nutrients and Antinutrients. Applied Sciences. 2024; 14(13):5586. https://doi.org/10.3390/app14135586
Chicago/Turabian StylePaié-Ribeiro, Jessica, Filipa Baptista, José Teixeira, Cristina Guedes, Maria José Gomes, Alfredo Teixeira, Ana Novo Barros, Victor Pinheiro, and Divanildo Outor-Monteiro. 2024. "From Waste to Resource: Compositional Analysis of Olive Cake’s Fatty Acids, Nutrients and Antinutrients" Applied Sciences 14, no. 13: 5586. https://doi.org/10.3390/app14135586
APA StylePaié-Ribeiro, J., Baptista, F., Teixeira, J., Guedes, C., Gomes, M. J., Teixeira, A., Barros, A. N., Pinheiro, V., & Outor-Monteiro, D. (2024). From Waste to Resource: Compositional Analysis of Olive Cake’s Fatty Acids, Nutrients and Antinutrients. Applied Sciences, 14(13), 5586. https://doi.org/10.3390/app14135586










