pH-Dependent Morphology of Copper (II) Oxide in Hydrothermal Process and Their Photoelectrochemical Application for Non-Enzymatic Glucose Biosensor
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Apparatus
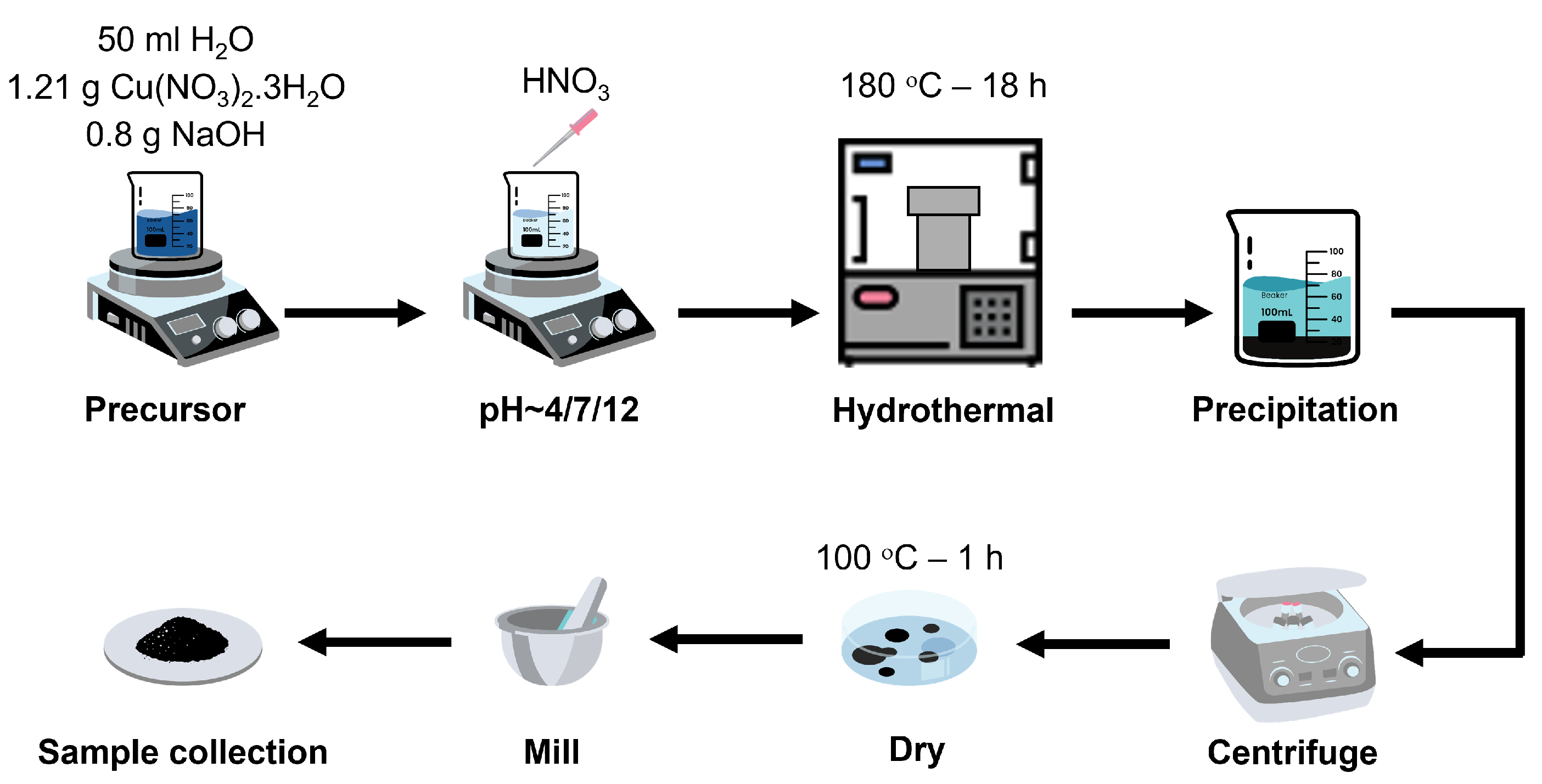
2.3. Synthesis of the Nanostructures and Working Electrode Preparation
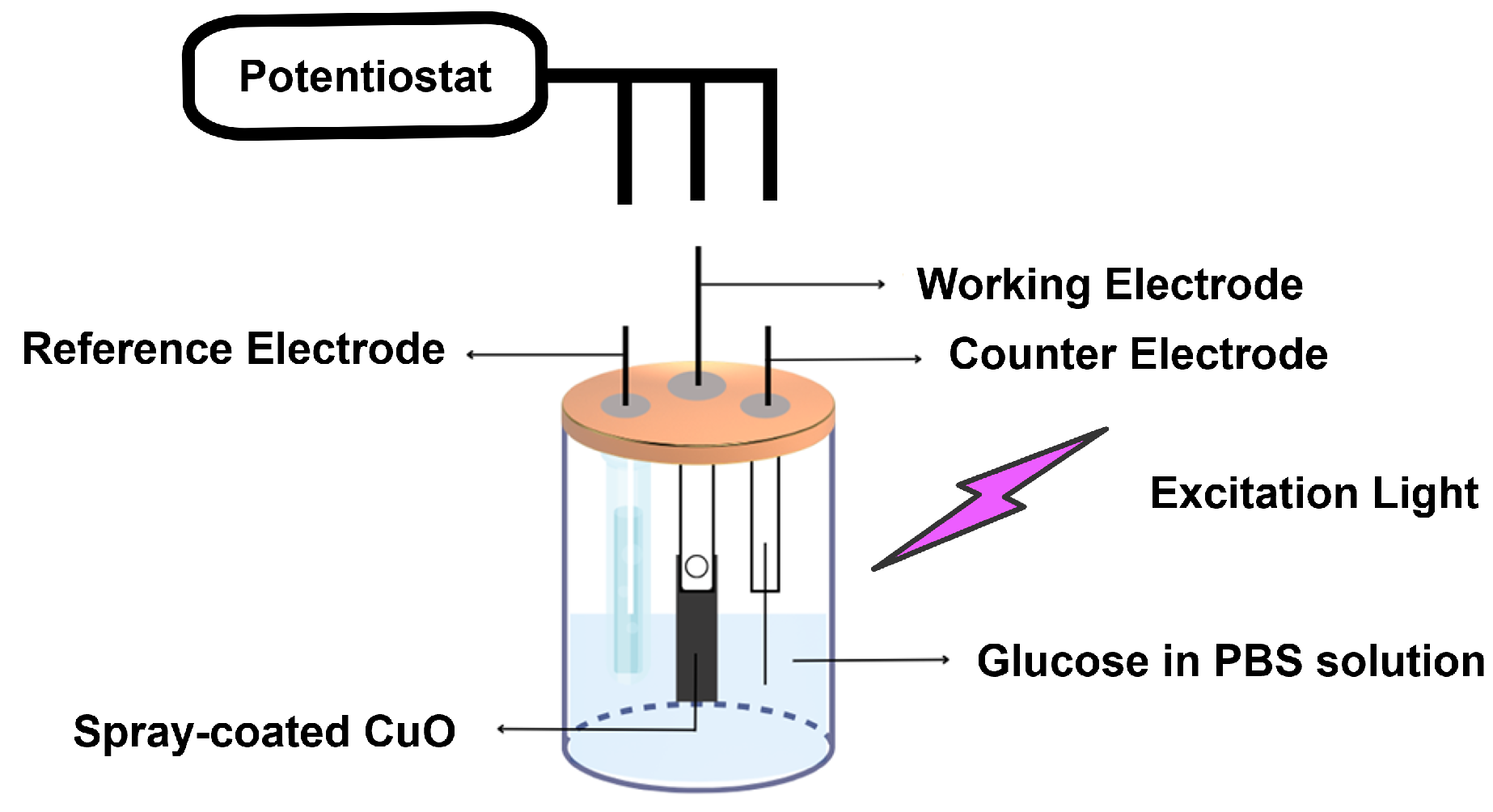
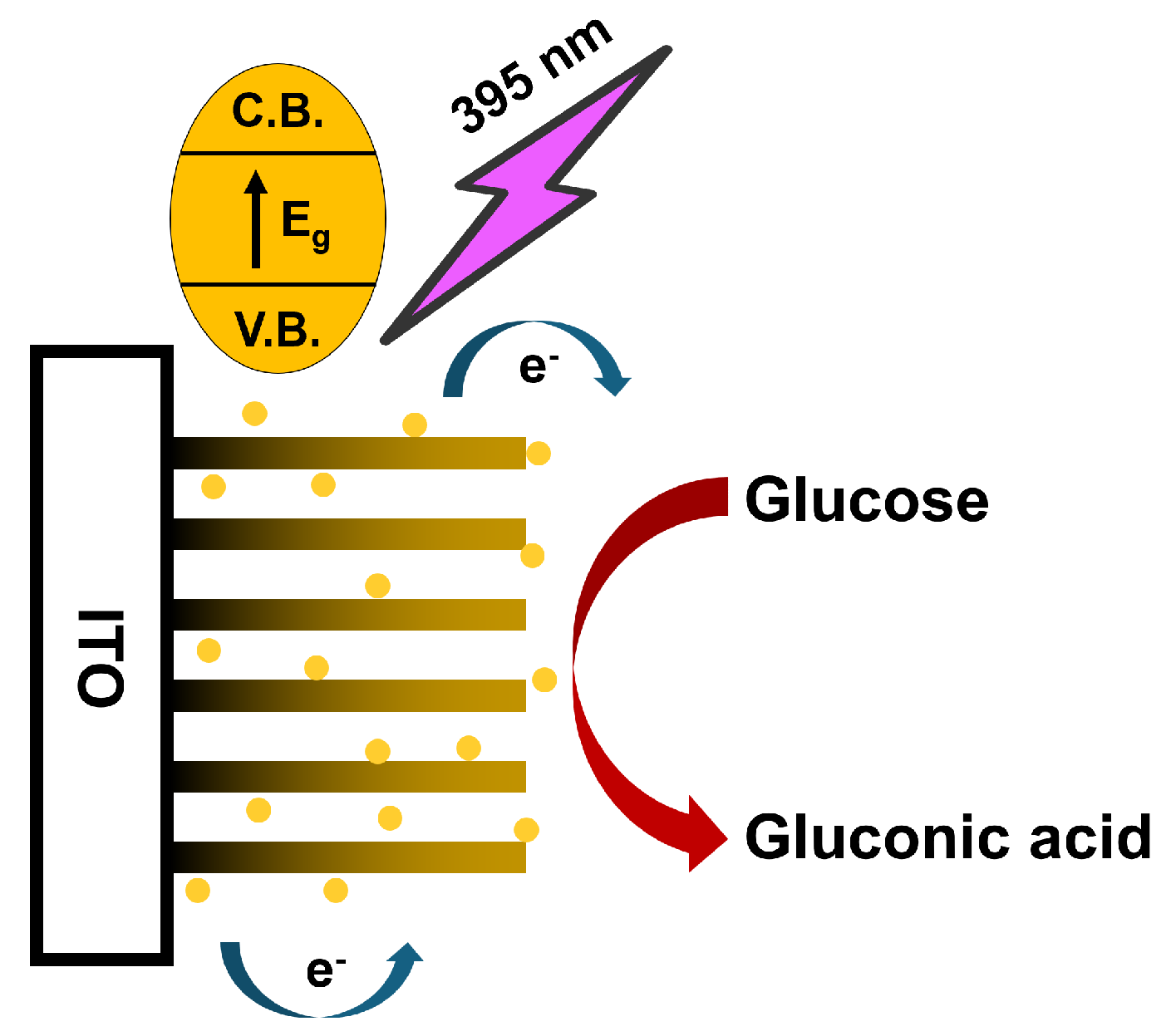
2.4. Photoelectrochemical Glucose Detection
3. Results and Discussions
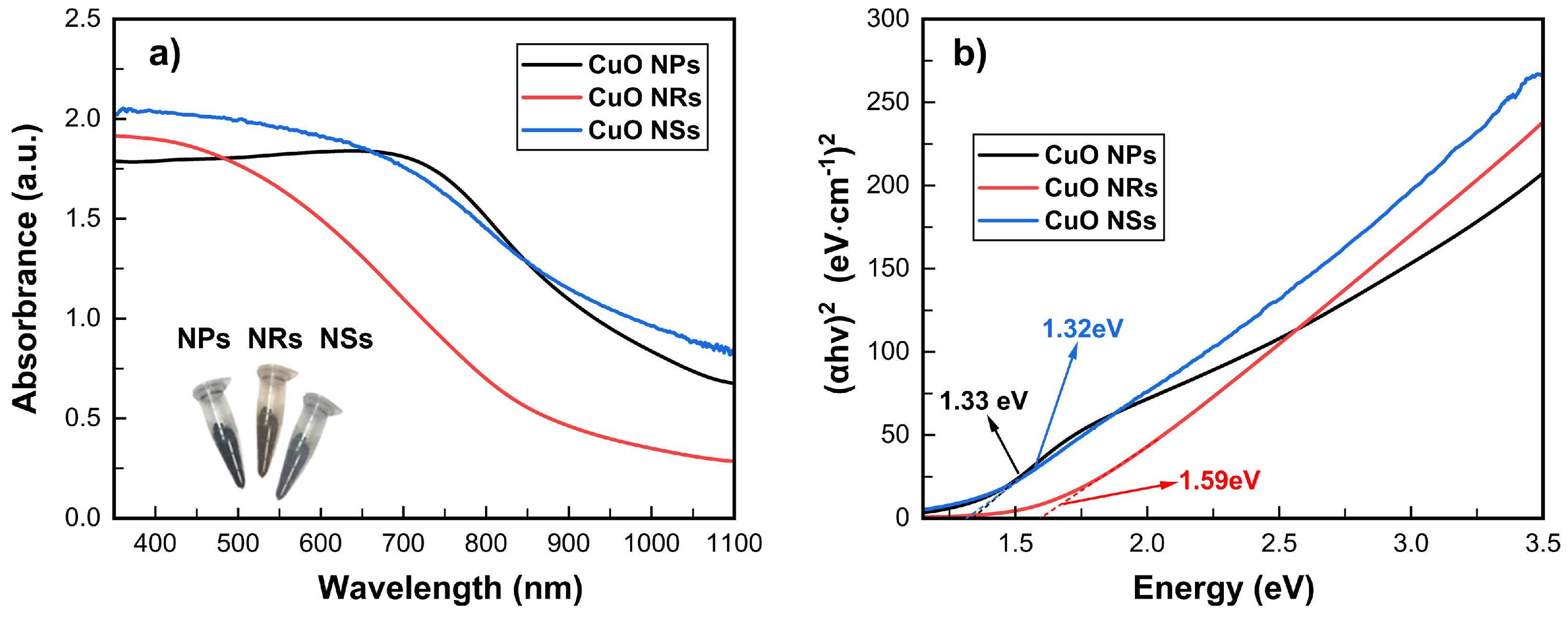
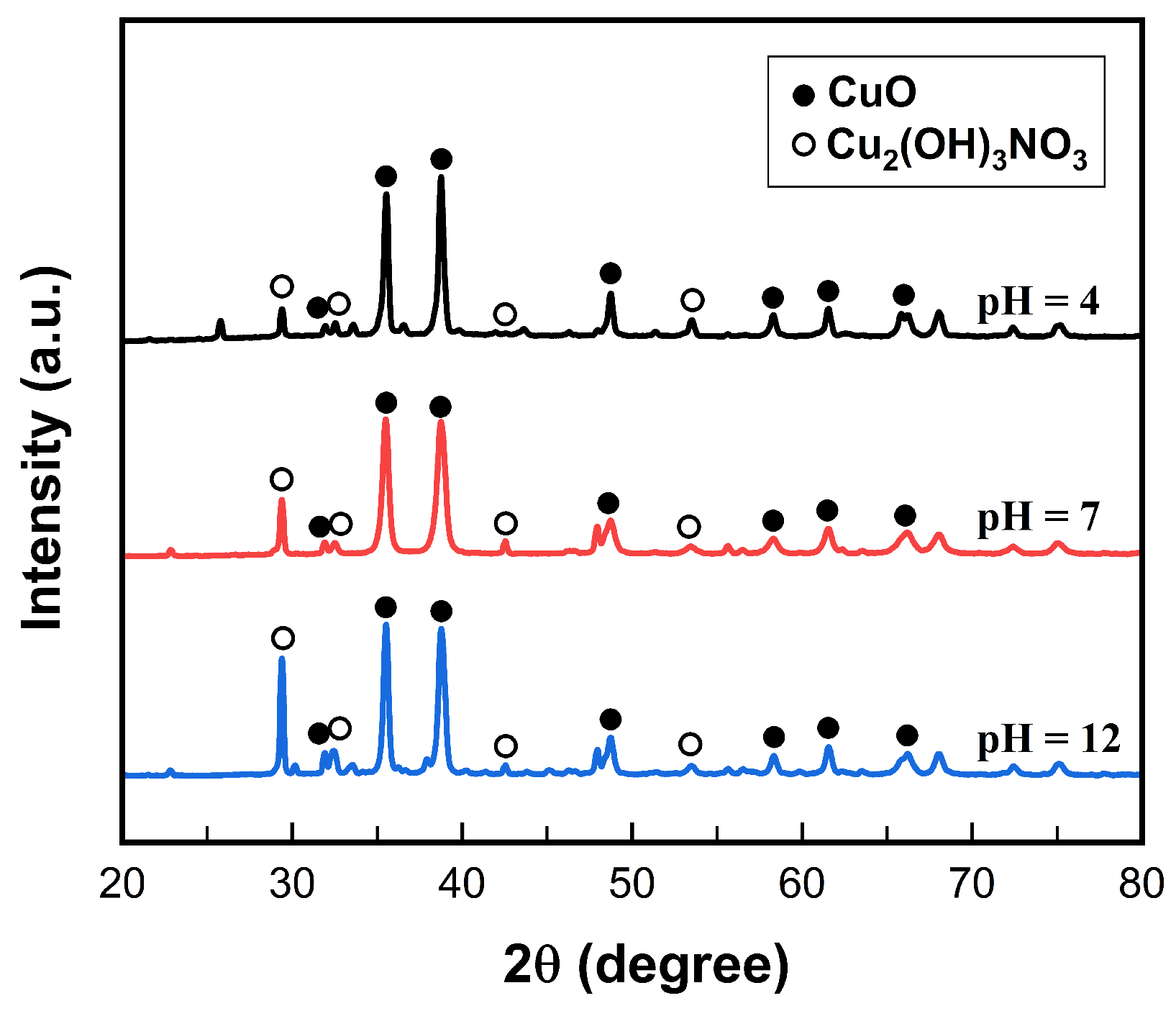
3.1. Characterization of Synthesized Nanostructures
3.2. Hypothesis on the Formation of in Different Forms
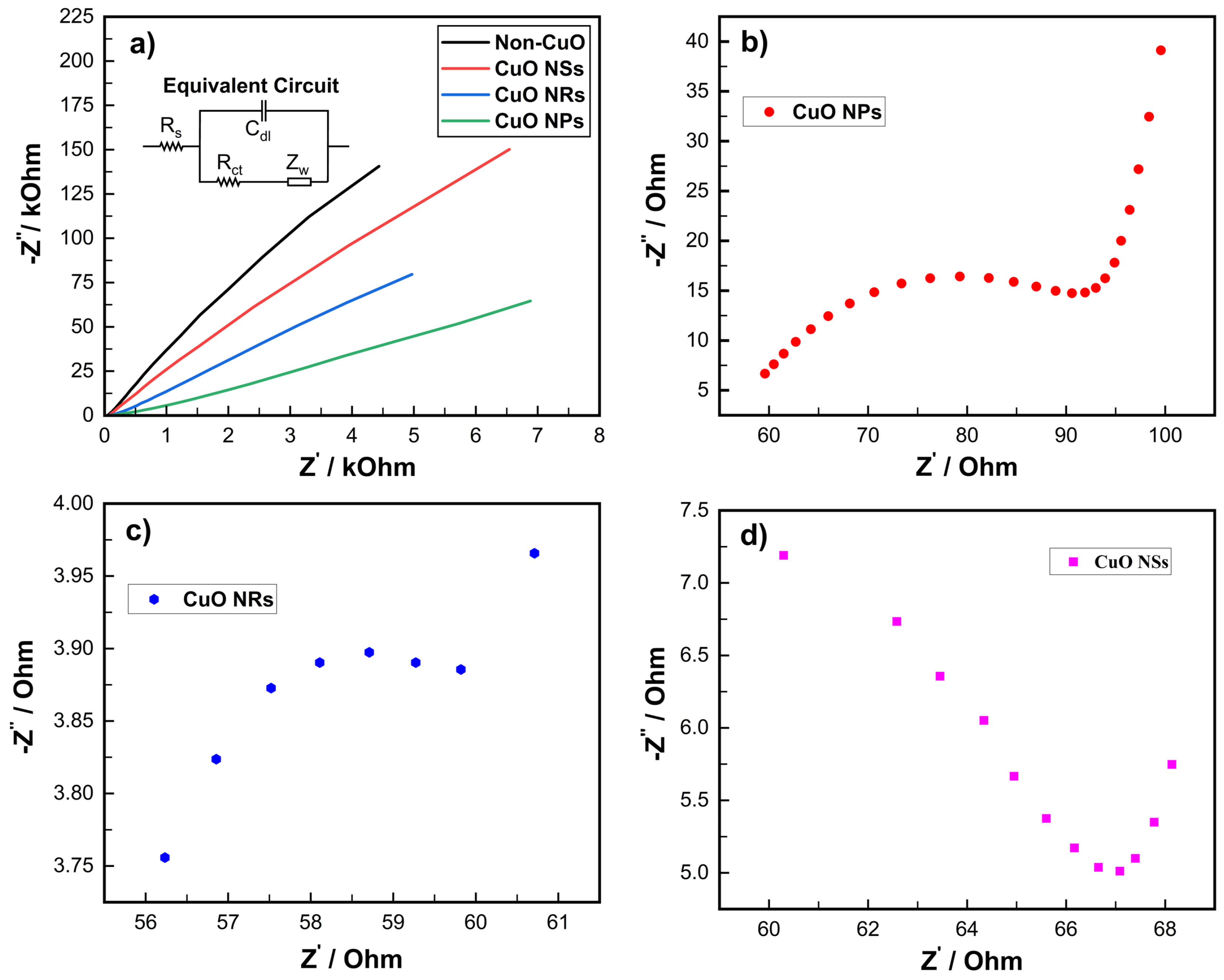
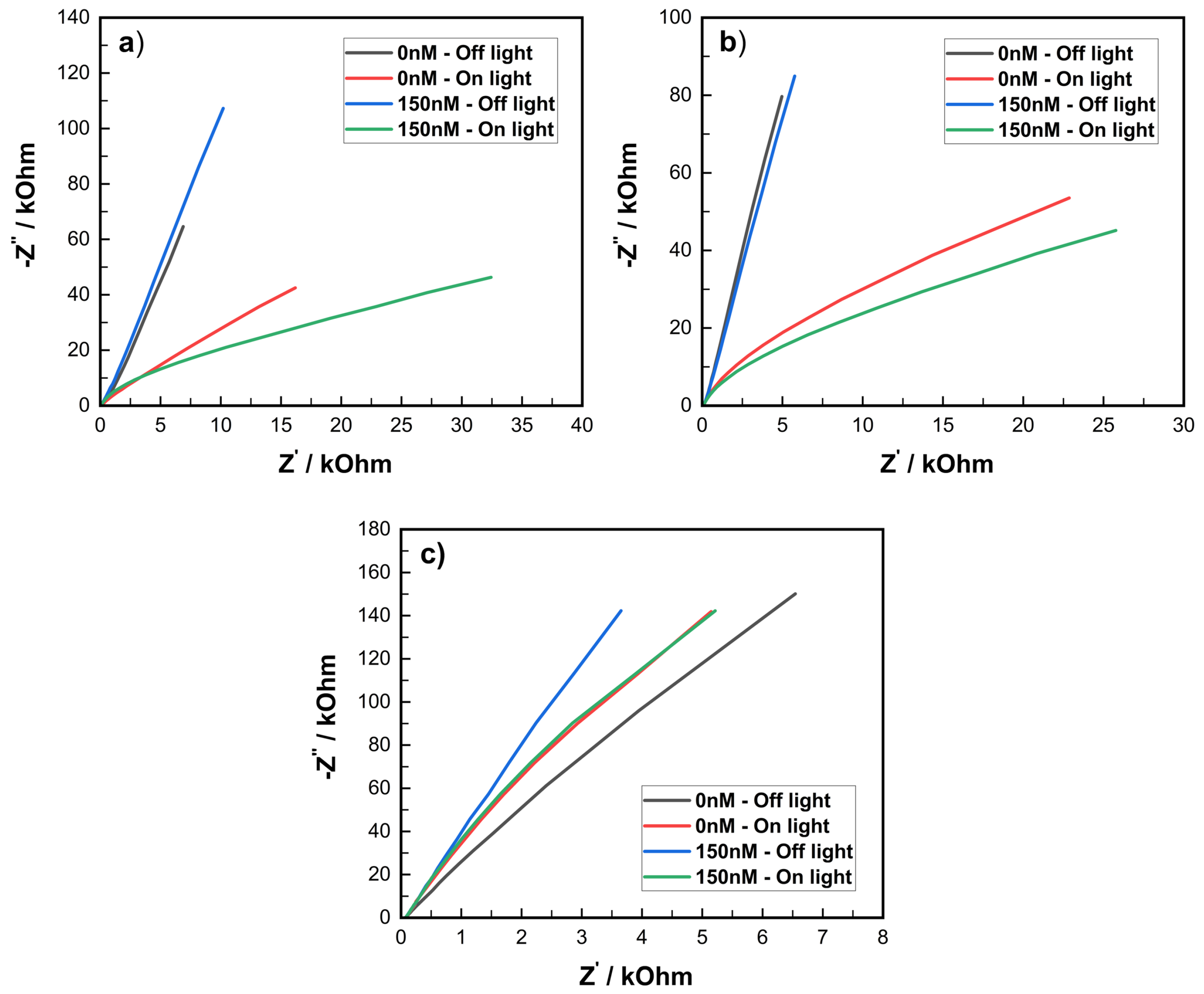
3.3. Electrochemical Impedance Spectroscopy of Electrodes
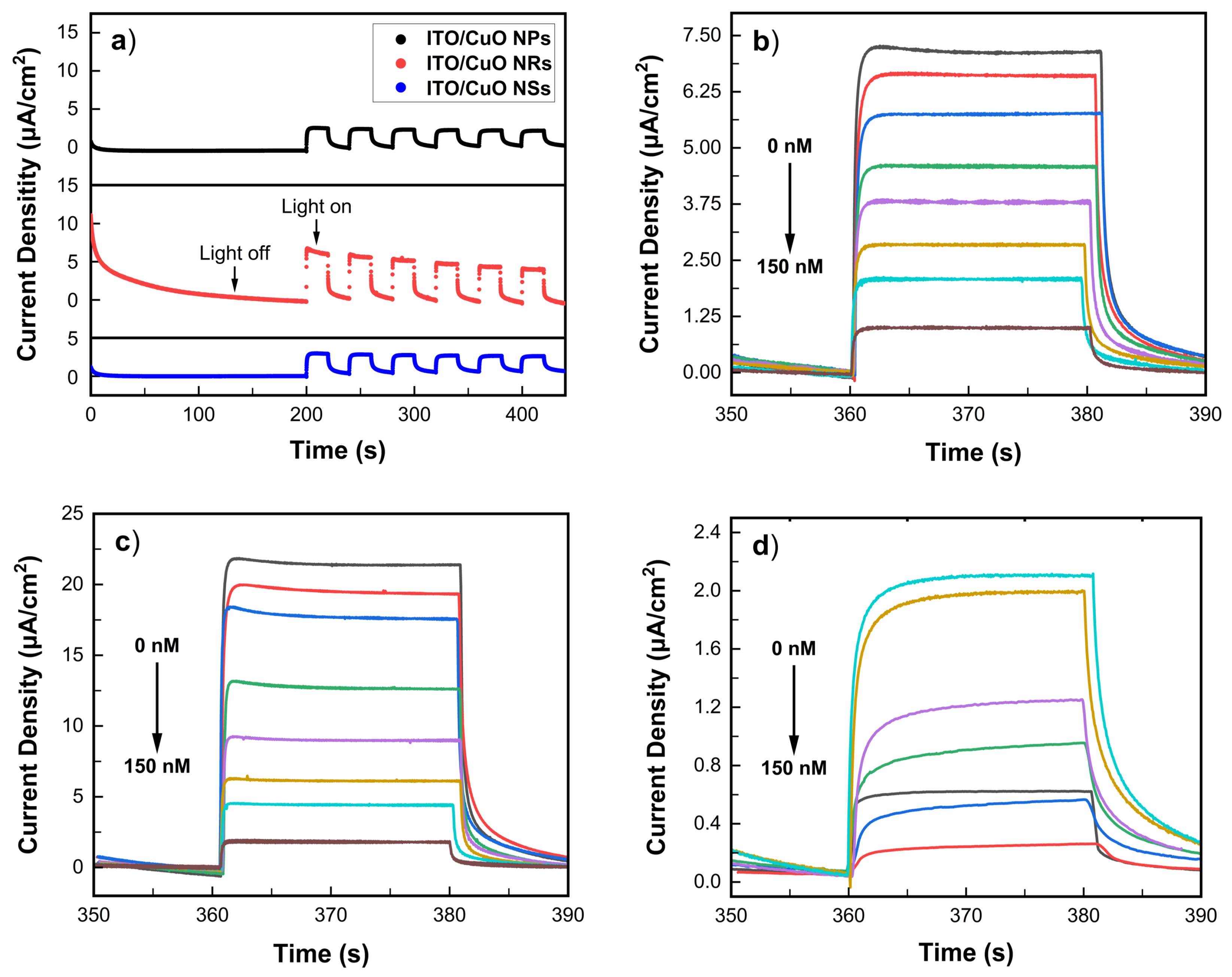
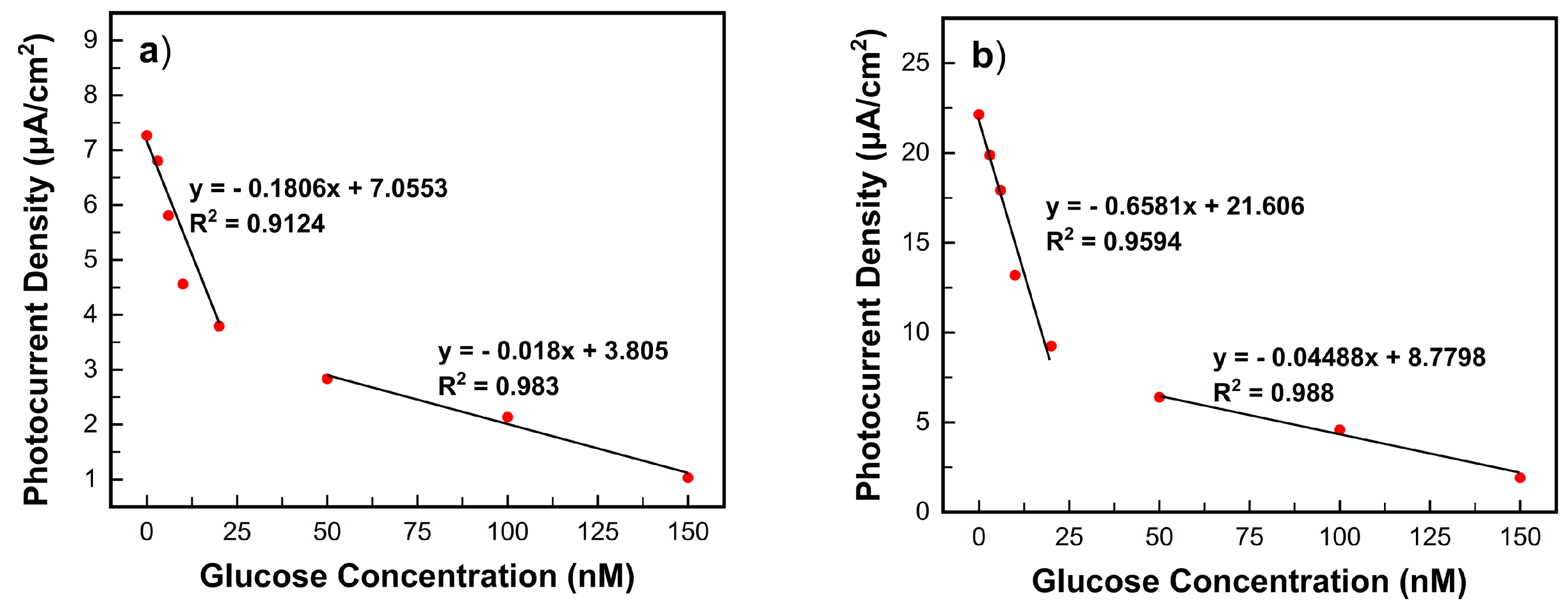
3.4. Photoamperometric Response with Glucose
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galant, A.L.; Kaufman, R.C.; Wilson, J.D. Glucose: Detection and analysis. Food Chem. 2015, 188, 149. [Google Scholar] [CrossRef]
- Steiner, M.-S.; Duerkop, A.; Wolfbeis, O.S. Optical methods for sensing glucose. Chem. Soc. Rev. 2011, 40, 4805. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour-Haratbar, A.; Mohammadpour-Haratbar, S.; Zare, Y.; Rhee, K.Y.; Park, S.-J. A Review on Non-Enzymatic Electrochemical Biosensors of Glucose Using Carbon Nanofiber Nanocomposites. Biosensors 2022, 12, 1004. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Glucose electrochemical biosensors: The past and current trends. Int. J. Electrochem. Sci. 2021, 16, 210719. [Google Scholar] [CrossRef]
- Juska, V.B.; Pemble, M.E. A Critical Review of Electrochemical Glucose Sensing: Evolution of Biosensor Platforms Based on Advanced Nanosystems. Sensors 2020, 20, 6013. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, H.A.; Mehaney, A. A new method for glucose detection using the one dimensional defective photonic crystals. Matter. Res. Express 2019, 6, 036201. [Google Scholar] [CrossRef]
- Sabu, C.; Henna, T.K.; Raphey, V.R.; Nivitha, K.P.; Pramod, K. Advanced biosensors for glucose and insulin. Biosens. Bioelectron. 2019, 141, 111201. [Google Scholar] [CrossRef]
- Wu, D.; Tang, D. Recent advances on portable photoelectrochemical biosensors for diagnostics. Electroanalysis 2023, 35, e202300265. [Google Scholar] [CrossRef]
- del Barrio, M.; Luna-López, G.; Pita, M. Enhancement of Biosensors by Implementing Photoelectrochemical Processes. Sensors 2020, 20, 3281. [Google Scholar] [CrossRef]
- Ge, L.; Liu, Q.; Hao, N.; Kun, W. Recent developments of photoelectrochemical biosensors for food analysis. J. Mater. Chem. B 2019, 7, 7283. [Google Scholar] [CrossRef]
- Devadoss, A.; Sudhagar, P.; Terashima, C.; Nakata, K.; Fujishima, A. Photoelectrochemical biosensors: New insights into promising photoelectrodes and signal amplification strategies. J. Photochem. Photobiol. C 2015, 24, 43. [Google Scholar] [CrossRef]
- Gopalan, A.I.; Muthuchamy, N.; Lee, K.P. A novel bismuth oxychloride-graphene hybrid nanosheets based non-enzymatic photoelectrochemical glucose sensing platform for high performances. Biosens. Bioelectron. 2017, 89, 352. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, H.; Chen, W.; Ma, B.; Ju, H. Device integration of electrochemical biosensors. Nat. Rev. Bioeng. 2023, 1, 346. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Xie, Z.; Xiao, G.; Sun, X.; Diao, H.; Zhou, X.; Yue, Z. Photoelectrochemical sensors based on heterogeneous nanostructures for in vitro diagnostics. Biosens. Bioelectron. X 2022, 11, 100200. [Google Scholar] [CrossRef]
- Li, Z.; Lu, J.; Wei, W.; Tao, M.; Wang, Z.; Dai, Z. Recent advances in electron manipulation of nanomaterials for photoelectrochemical biosensors. Chem. Commun. 2022, 58, 12418. [Google Scholar] [CrossRef]
- Devi, L.; Dalal, K.; Manchala, S.; Behera, K.; Sharma, K.N.; Das, A. 11—Nanostructured photoelectrochemical biosensors: Materials and applications. In Fundamentals of Sensor Technology; Barhoum, A., Altintas, Z., Eds.; Woodhead Publishing: Cambridge, UK, 2023; p. 265. [Google Scholar] [CrossRef]
- Cash, K.J.; Clark, H.A. Nanosensors and nanomaterials for monitoring glucose in diabetes. Trends Mol. Med. 2010, 16, 584. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Singh, J.; Goyat, R.; Saharan, Y.; Chaudhry, V.; Umar, A.; Ibrahim, A.A.; Akbar, S.; Ameen, S.; Baskoutas, S. Nanomaterials-based biosensor and their applications: A review. Heliyon 2023, 9, e19929. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Tai, N.-H. Carbon nanomaterials and their composites for electrochemical glucose biosensors: A review on fabrication and sensing properties. J. Taiwan Inst. Chem. E 2024, 154, 104957. [Google Scholar] [CrossRef]
- Ismail, W.; Ibrahim, G.; Atta, H.; Sun, B.; El-Shaer, A.; Abdelfatah, M. Improvement physical and photoelectrochemical properties of TiO2 nanorods toward biosensor and optoelectronic applications. Ceram. Int. 2024, 50, 17968. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Lakshmy, S.; Santhosh, S.; Kalarikkal, N.; Chakraborty, B.; Rout, C.S. Recent Developments and Future Perspective on Electrochemical Glucose Sensors Based on 2D Materials. Biosensors 2022, 12, 467. [Google Scholar] [CrossRef]
- Shengchen, K.; Ruoxi, Z.; Weijian, Z.; Wenxiang, L.; Lu, M.; Linling, Q.; Shaolong, W. Enzyme-free photoelectrochemical sensing of glucose based on the TiO2/CuO heterojunction. Proc. SPIE 2023, 12711, 127110R. [Google Scholar] [CrossRef]
- Chen, G.; Qin, Y.; Jiao, L.; Huang, J.; Wu, Y.; Hu, L.; Gu, W.; Xu, D.; Zhu, C. Nanozyme-Activated Synergistic Amplification for Ultrasensitive Photoelectrochemical Immunoassay. Anal. Chem. 2021, 93, 6881. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Du, Y.; Feng, J.; Xu, R.; Ren, X.; Fan, D.; Wei, Q.; Ju, H. A duple nanozyme stimulating tandem catalysis assisted multiple signal inhibition strategy for photoelectrochemical bioanalysis. Sens. Actuators B Chem. 2021, 334, 129608. [Google Scholar] [CrossRef]
- Cao, L.; Wang, P.; Chen, L.; Wu, Y.; Di, J. A photoelectrochemical glucose sensor based on gold nanoparticles as a mimic enzyme of glucose oxidase. RCS Adv. 2019, 9, 15307. [Google Scholar] [CrossRef] [PubMed]
- Devadoss, A.; Sudhagar, P.; Das, S.; Lee, S.Y.; Terashima, C.; Nakata, K.; Fujishima, A.; Choi, W.; Kang, Y.S.; Paik, U. Synergistic Metal–Metal Oxide Nanoparticles Supported Electrocatalytic Graphene for Improved Photoelectrochemical Glucose Oxidation. ACS Appl. Mater. Interfaces 2014, 6, 4864. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ke, S.; Lu, W.; Zhu, W.; Ma, L.; Qin, L.; Wu, S.; Li, X. Constructing a Si-CuO core-shell nanowire heterojunction photoanode for enzyme-free and highly-sensitive glucose sensing. App. Surf. Sci. 2023, 632, 157593. [Google Scholar] [CrossRef]
- Liu, F.; Wang, P.; Zhang, Q.; Wang, Z.; Liu, Y.; Zheng, Z.; Qin, X.; Zhang, X.; Dai, Y.; Huang, B. α-Fe2O3 Film with Highly Photoactivity for Non-enzymatic Photoelectrochemical Detection of Glucose. Electroanalysis 2019, 31, 1809. [Google Scholar] [CrossRef]
- Chen, L.; Miao, L.; Chen, Y.; Gao, Y.; Di, J. An enzyme-free photoelectrochemical glucose sensor based on coupling BiVO4 with gold nanoparticles. Mater. Sci. Semicond. Process. 2021, 125, 105632. [Google Scholar] [CrossRef]
- Ke, S.; Qin, L.; Zhang, R.; Zhu, W.; Lu, W.; Ma, L.; Wu, S.; Li, X. Towards self-driven and enzyme-free sweat glucose photoelectrochemical sensing via decorating CuO nanoparticles on TiO2 hierarchical nanotubes. Surf. Interfaces 2023, 40, 103102. [Google Scholar] [CrossRef]
- Sehit, E.; Altintas, Z. Significance of nanomaterials in electrochemical glucose sensors: An updated review (2016–2020). Biosens. Bioelectron. 2020, 159, 112165. [Google Scholar] [CrossRef]
- Syrek, K.; Skolarczyk, M.; Zych, M.; Sołtys-Mróz, M.; Sulka, G.D. A Photoelectrochemical Sensor Based on Anodic TiO2 for Glucose Determination. Sensors 2019, 19, 4981. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xu, W.; Yan, B.; Wu, B.; Ma, J.; Wang, X.; Qiao, B.; Tu, J.; Pei, H.; Chen, D.; et al. Gold and Platinum Nanoparticle-Functionalized TiO2 Nanotubes for Photoelectrochemical Glucose Sensing. ACS Omega 2022, 7, 2474. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, Y.; Li, J.; Da, P.; Geng, J.; Zheng, G. Sensitive enzymatic glucose detection by TiO2 nanowire photoelectrochemical biosensors. J. Mater. Chem. A 2014, 2, 6153. [Google Scholar] [CrossRef]
- Cory, N.J.; Visser, E.; Chamier, J.; Sackey, J.; Cummings, F.; Chowdhury, M. Electrodeposited CuO thin film for wide linear range photoelectrochemical glucose sensing. Appl. Surf. Sci. 2022, 576, 151822. [Google Scholar] [CrossRef]
- Yang, R.-F.; Zhang, S.-S.; Shi, D.-J.; Dong, J.-X.; Li, Y.-L.; Li, J.-X.; Guo, C.; Yue, Z.; Li, G.; Huang, W.-P.; et al. Glucose detection via photoelectrochemical sensitivity of 3D CuO-TiO2 heterojunction nanotubes/Ti combined with chemometric tools. Microchem. J. 2024, 199, 110017. [Google Scholar] [CrossRef]
- Lee, C.-T.; Chiu, Y.-S.; Ho, S.-C.; Lee, Y.-J. Investigation of a Photoelectrochemical Passivated ZnO-Based Glucose Biosensor. Sensors 2011, 11, 4648. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Han, N.; Hu, S.; Zhang, L.; Yi, S.; Zhang, Z.; Wang, Y.; Chen, D.; Gao, Y. Cu/ZnO Nano-Thorn with Modifiable Morphology for Photoelectrochemical Detection of Glucose. J. Electrochem. Soc. 2021, 168, 027516. [Google Scholar] [CrossRef]
- Liu, W.; Zhan, W.; Jia, X.; Liu, Q.; Chen, R.; Li, D.; Huang, Y.; Zhang, G.; Ni, H. Rapid synthesis of vertically-aligned zinc oxide nanorods on stainless steel for non-enzymatic glucose and H2O2 photoelectrochemical sensor. Appl. Surf. Sci. 2019, 480, 341. [Google Scholar] [CrossRef]
- Chen, D.; Zou, X.; Dong, F.; Zhen, C.; Xiao, D.; Wang, X.; Wu, Q.; Cao, Y.; Tu, J. Donor–Acceptor Compensated ZnO Semiconductor for Photoelectrochemical Biosensors. ACS Appl. Mater. Interfaces 2021, 13, 33006. [Google Scholar] [CrossRef]
- Han, Q.; Wang, H.; Wu, D.; Wei, Q. Preparation of PbSNPs/RGO/NiO nanosheet arrays heterostructure: Function-switchable self-powered photoelectrochemical biosensor for H2O2 and glucose monitoring. Biosens. Bioelectron. 2021, 173, 112803. [Google Scholar] [CrossRef]
- Jana, S.; Mondal, A.; Ghosh, A. Fabrication of stable NiO/Fe2O3 heterostructure: A versatile hybrid material for electrochemical sensing of glucose, methanol and enhanced photodecomposition and/photoreduction of water contaminants. Appl. Catal. B Environ. Energy 2018, 232, 26. [Google Scholar] [CrossRef]
- Khan, M.A.; Nayan, N.; Shadiullah; Ahmad, M.K.; Soon, C.F. Surface Study of CuO Nanopetals by Advanced Nanocharacterization Techniques with Enhanced Optical and Catalytic Properties. Nanomaterials 2020, 10, 1298. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.; Echabaane, M.; Omri, K.; El Mir, L.; Ben Chaabane, R. Development of an impedimetric non enzymatic sensor based on ZnO and Cu doped ZnO nanoparticles for the detection of glucose. J. Alloys Compd. 2019, 786, 960. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Chen, D.; Qiu, Y.; Wang, W. Dual-functional cubic cuprous oxide for non-enzymatic and oxygen-sensitive photoelectrochemical sensing of glucose. Sens. Actuators B Chem. 2015, 220, 441. [Google Scholar] [CrossRef]
- Singh, J.; Manna, A.K.; Soni, R.K. Sunlight driven photocatalysis and non-enzymatic glucose sensing performance of cubic structured CuO thin films. Appl. Surf. Sci. 2020, 530, 147258. [Google Scholar] [CrossRef]
- Santos, X.; Rodríguez, J.; Guillén, F.; Pozuelo, J.; Molina-Guijarro, J.M.; Videira-Quintela, D.; Martín, O. Capability of Copper Hydroxy Nitrate (Cu2(OH)3NO3) as an Additive to Develop Antibacterial Polymer Contact Surfaces: Potential for Food Packaging Applications. Polymers 2023, 15, 1661. [Google Scholar] [CrossRef] [PubMed]
- Morozov, I.V.; Znamenkov, K.O.; Korenev, Y.M.; Shlyakhtin, O.A. Thermal decomposition of Cu(NO3)2·3H2O at reduced pressures. Thermochim. Acta 2003, 403, 173. [Google Scholar] [CrossRef]
- Lazanas, A.C.; Prodromidis, M.I. Electrochemical Impedance Spectroscopy—A Tutorial. ACS Meas. Sci. 2023, 3, 162. [Google Scholar] [CrossRef] [PubMed]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef]
- Atchudan, R.; Muthuchamy, N.; Edison, T.N.J.I.; Perumal, S.; Vinodh, R.; Park, K.H.; Lee, Y.R. An ultrasensitive photoelectrochemical biosensor for glucose based on bio-derived nitrogen-doped carbon sheets wrapped titanium dioxide nanoparticles. Biosens. Bioelectron. 2019, 126, 160. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, L.; Zhang, S.; Wang, J.; Liu, Z. An ultrasensitive and wearable photoelectrochemical sensor for unbiased and accurate monitoring of sweat glucose. Sens. Actuators B Chem. 2022, 354, 131204. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, J.; Yin, Z.; Wang, X.; Li, Z.; Wang, X. Highly sensitive photoelectrochemical detection of glucose based on BiOBr/TiO2 nanotube array p-n heterojunction nanocomposites. Sens. Actuators B Chem. 2020, 312, 127978. [Google Scholar] [CrossRef]
- Mishra, A.K.; Mukherjee, B.; Kumar, A.; Jarwal, D.K.; Ratan, S.; Kumar, C.; Jit, S. Superficial fabrication of gold nanoparticles modified CuO nanowires electrode for non-enzymatic glucose detection. RSC Adv. 2019, 9, 1772. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guo, C.; Liu, C.; Ge, L.; Li, F. Laser-induced graphene hybrid photoelectrode for enhanced photoelectrochemical detection of glucose. Analyst 2020, 145, 4041. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, X.; Hao, W.; Wu, Q.; Peng, J.; Tu, J.; Cao, Y. 3D hollow-out TiO2 nanowire cluster/GOx as an ultrasensitive photoelectrochemical glucose biosensor. J. Mater. Chem. B 2020, 8, 2363. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Yan, B.; Lai, C.; Wang, X.; Cao, Y.; Tu, J.; Li, Y.; Wu, Q. Carbon Dot-Modified Branched TiO2 Photoelectrochemical Glucose Sensors with Visible Light Response. ACS Omega 2023, 8, 22099. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yin, L.; Wu, J.; Li, N.; He, N.; Zhao, H.; Wu, Q.; Li, X. Perovskite-SrTiO3/TiO2/PDA as photoelectrochemical glucose biosensor. Ceram. Int. 2021, 47, 29807. [Google Scholar] [CrossRef]
- Li, F.; Zhang, B.; Yan, B.; Chen, Q.; Wang, X.; Zhang, K.; Pei, H.; Wu, Q.; Chen, D.; Tu, J. Graphene-assisted titanium dioxide Z-mechanism photoelectrode as enzymatic glucose biosensor. AIP Adv. 2022, 12, 065208. [Google Scholar] [CrossRef]


| Materials | Limit of Detection | Linearity Range | Density of Photocurrent | References |
|---|---|---|---|---|
| NPs | 0.69 nM | 3–150 nM | 1–6 A · cm−2 | This work |
| NRs | 0.61 nM | 3–150 nM | 2–24 A · cm−2 | This work |
| NPs | 0.46 M | 1–1000 M | −1.4–−0.7 A · cm−2 | [25] |
| Cubic structured | 0.068 M | 0.001–1.8 mM | 0–2300 A · cm−2 | [46] |
| - NPs/ | 13 nM | 0.005–10 M | 0.5–3 A · cm−2 | [51] |
| -MWNT | 22.2 pM | 0.1 nM–1 mM | 1.3–3.5 A · cm−2 | [52] |
| nanotube arrays | 10 nM | 5 × 102–3 × 107 M | 270–1500 A · cm−2 | [53] |
| modified NWs | 0.5 M | 0.5 M–5.9 mM | 0–7 mA · cm−2 | [54] |
| 0.5 M | 1 M–1 mM | 20–90 A · cm−2 | [55] | |
| 3D hollow-out NWc/ | 8.7 M | 0–2 mM | 264.5–382.3 A · cm−2 | [56] |
| 43 M | 0–9 mM | 19.8–34.63 A · cm−2 | [57] | |
| 25.68 M | 0–32 mM | 601.9–773.74 A · cm−2 | [58] | |
| 0.034 M | 0–3 mM | 85.7–127.16 A · cm−2 | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, T.T.; Huynh Vo, A.H.; Nguyen, T.T.; Nguyen, A.D.; Huynh Tran, M.H.; Tran, V.C.; Tran, T.N. pH-Dependent Morphology of Copper (II) Oxide in Hydrothermal Process and Their Photoelectrochemical Application for Non-Enzymatic Glucose Biosensor. Appl. Sci. 2024, 14, 5688. https://doi.org/10.3390/app14135688
Tran TT, Huynh Vo AH, Nguyen TT, Nguyen AD, Huynh Tran MH, Tran VC, Tran TN. pH-Dependent Morphology of Copper (II) Oxide in Hydrothermal Process and Their Photoelectrochemical Application for Non-Enzymatic Glucose Biosensor. Applied Sciences. 2024; 14(13):5688. https://doi.org/10.3390/app14135688
Chicago/Turabian StyleTran, Trung Tin, Anh Hao Huynh Vo, Thien Trang Nguyen, Anh Duong Nguyen, My Hoa Huynh Tran, Viet Cuong Tran, and Trung Nghia Tran. 2024. "pH-Dependent Morphology of Copper (II) Oxide in Hydrothermal Process and Their Photoelectrochemical Application for Non-Enzymatic Glucose Biosensor" Applied Sciences 14, no. 13: 5688. https://doi.org/10.3390/app14135688
APA StyleTran, T. T., Huynh Vo, A. H., Nguyen, T. T., Nguyen, A. D., Huynh Tran, M. H., Tran, V. C., & Tran, T. N. (2024). pH-Dependent Morphology of Copper (II) Oxide in Hydrothermal Process and Their Photoelectrochemical Application for Non-Enzymatic Glucose Biosensor. Applied Sciences, 14(13), 5688. https://doi.org/10.3390/app14135688











