Highly Efficient Blue Host Compound Based on an Anthracene Moiety for OLEDs
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Information and Device Fabrication
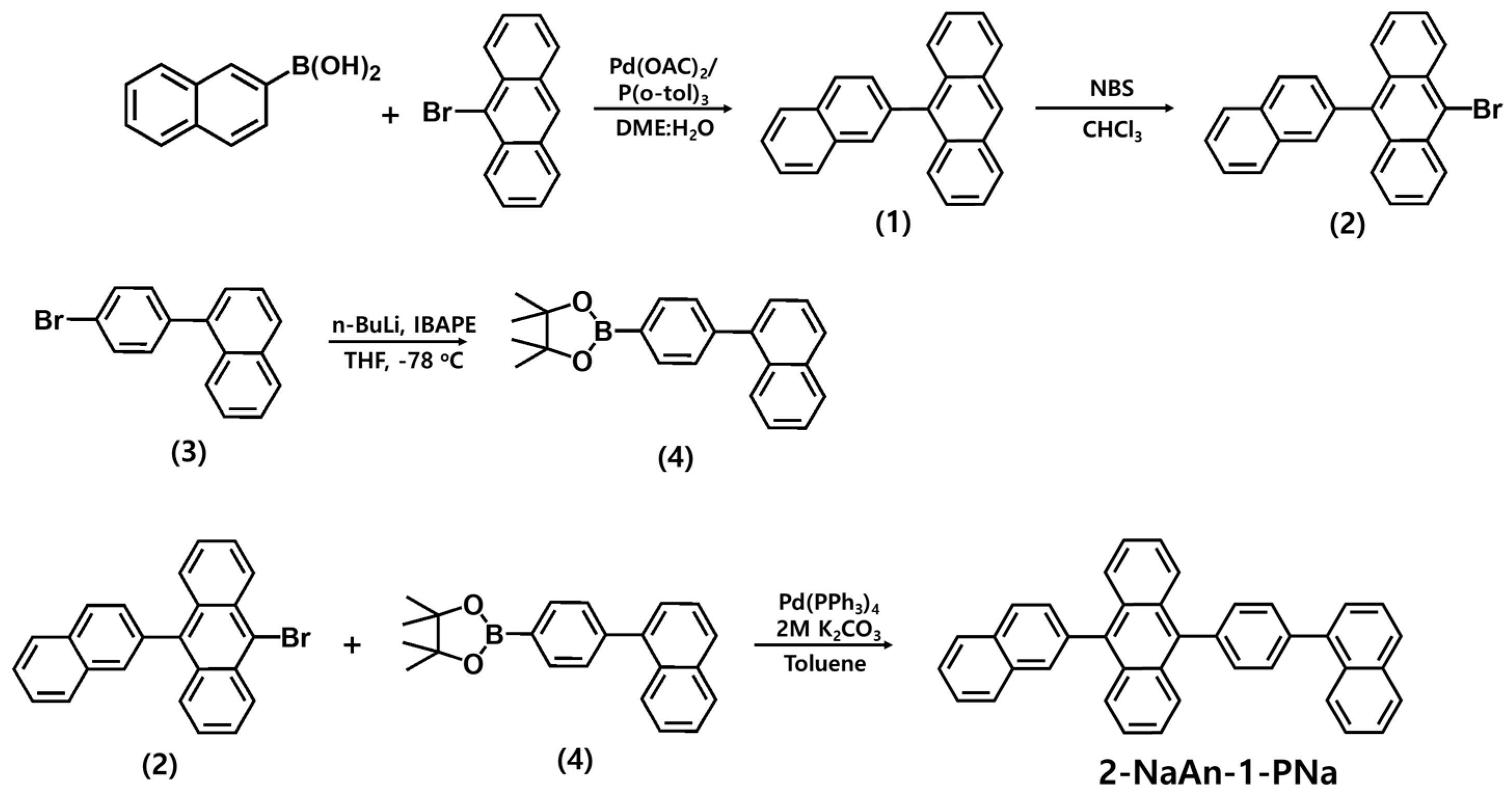
2.2. Synthesis of 10-(naphthalen-3-yl)anthracene (1)
2.3. Synthesis of 10-bromo-9-(naphthalen-3-yl)anthracene (2)
2.4. Synthesis of 1-(4-bromophenyl)naphthalene (3)
2.5. Synthesis of 4,4,5,5-tetramethyl-2-(4-(naphthalen-4-yl)phenyl)-1,3,2-dioxaborolane (4)
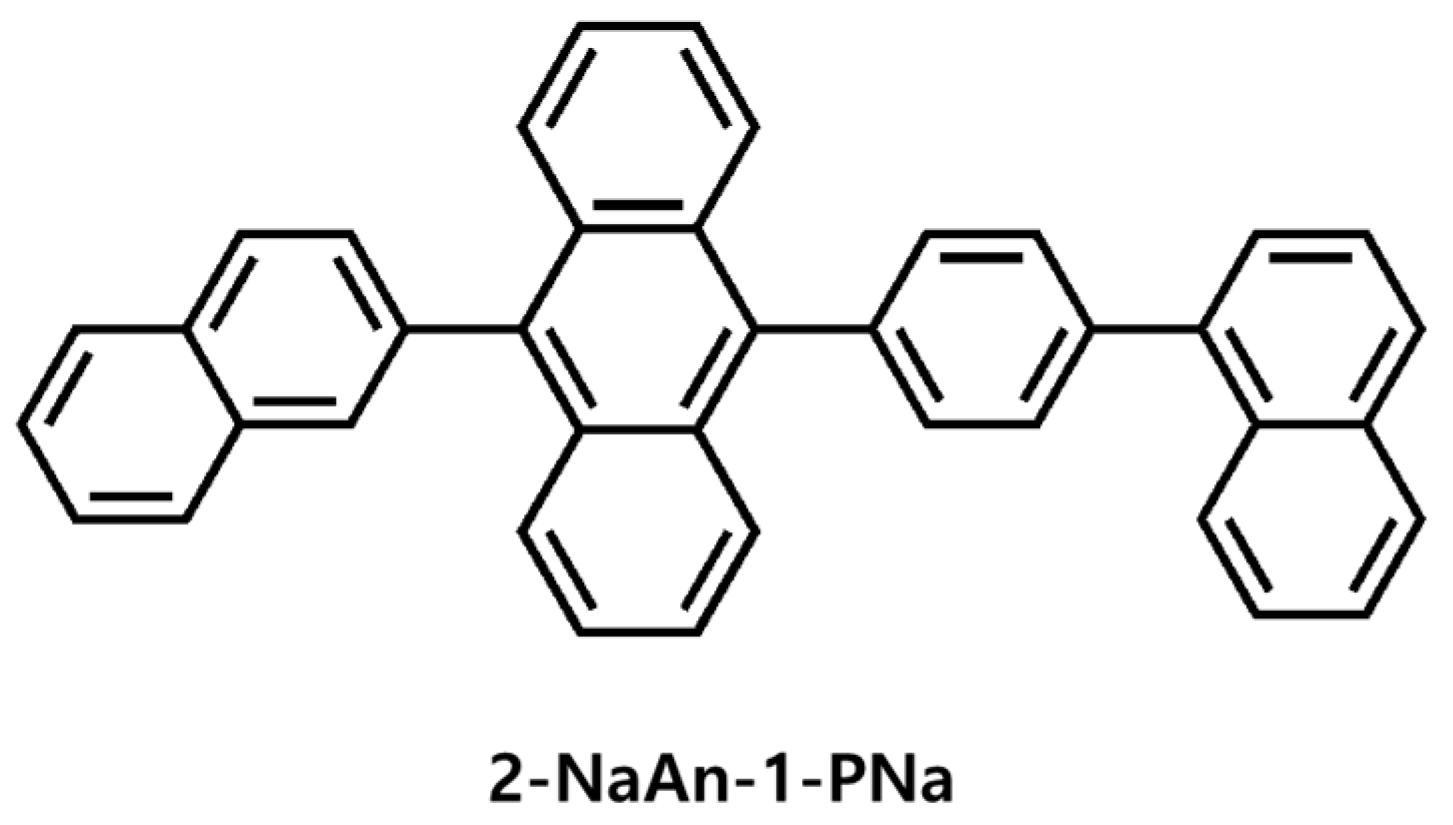
2.6. Synthesis of 10-(4-(naphthalen-1-yl)phenyl)-9-(naphthalen-3-yl)anthracene (2-NaAn-1-PNa)
3. Results and Discussion
3.1. Molecular Design, Synthesis, and Optical Properties
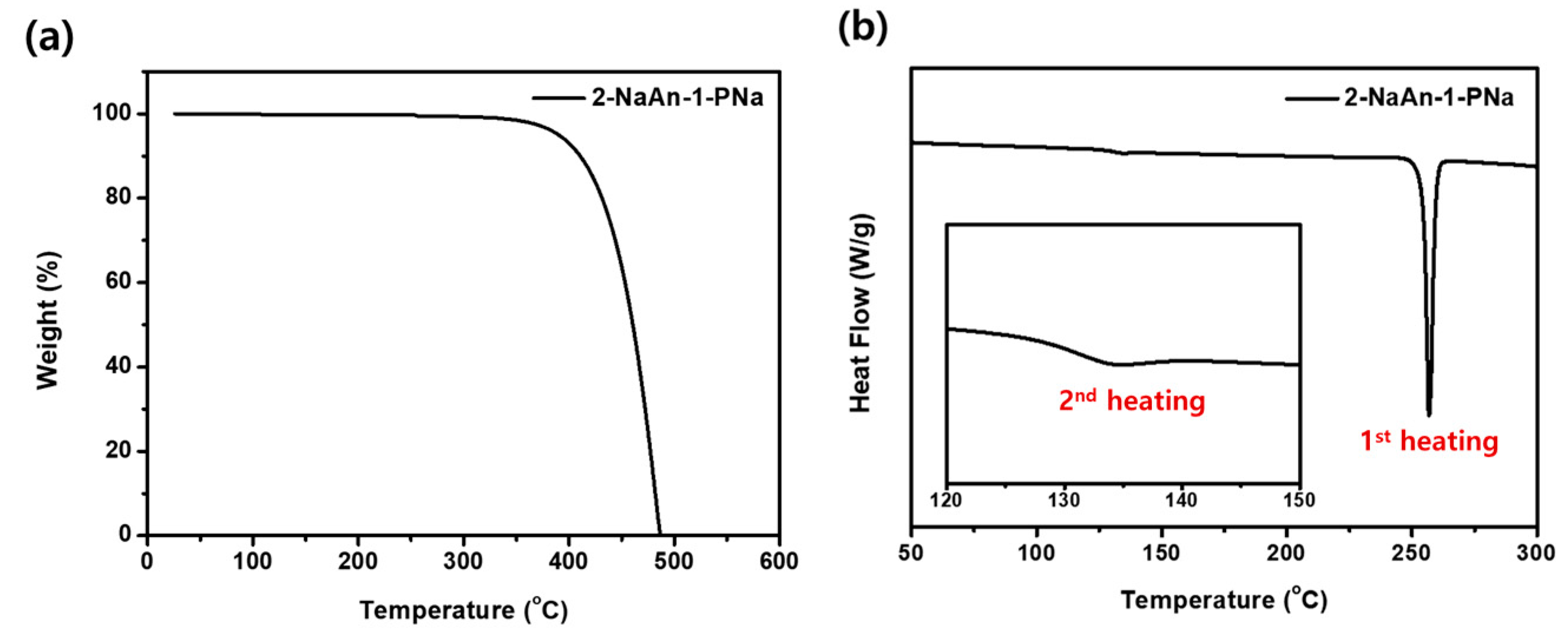
3.2. Thermal Properties
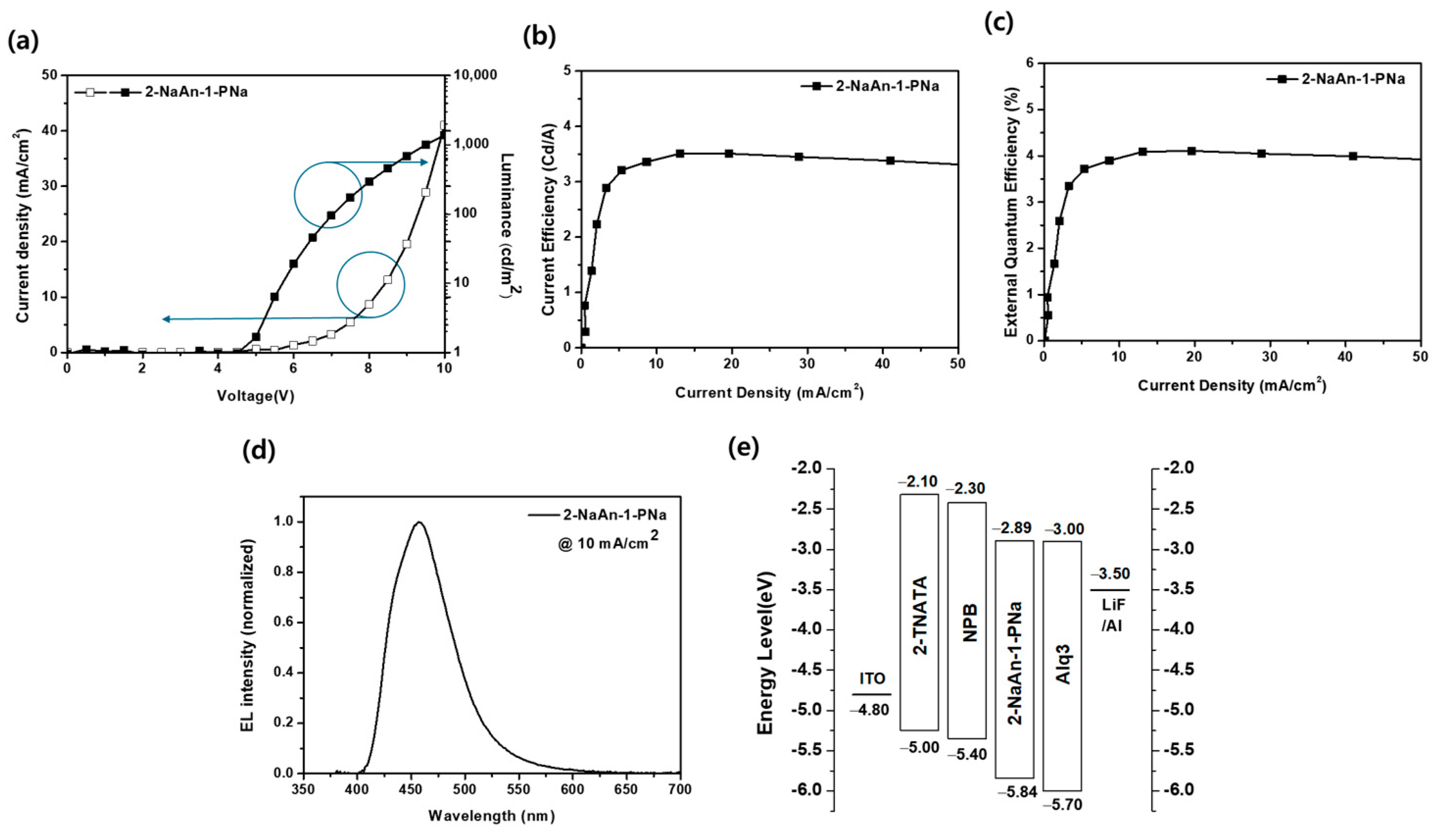
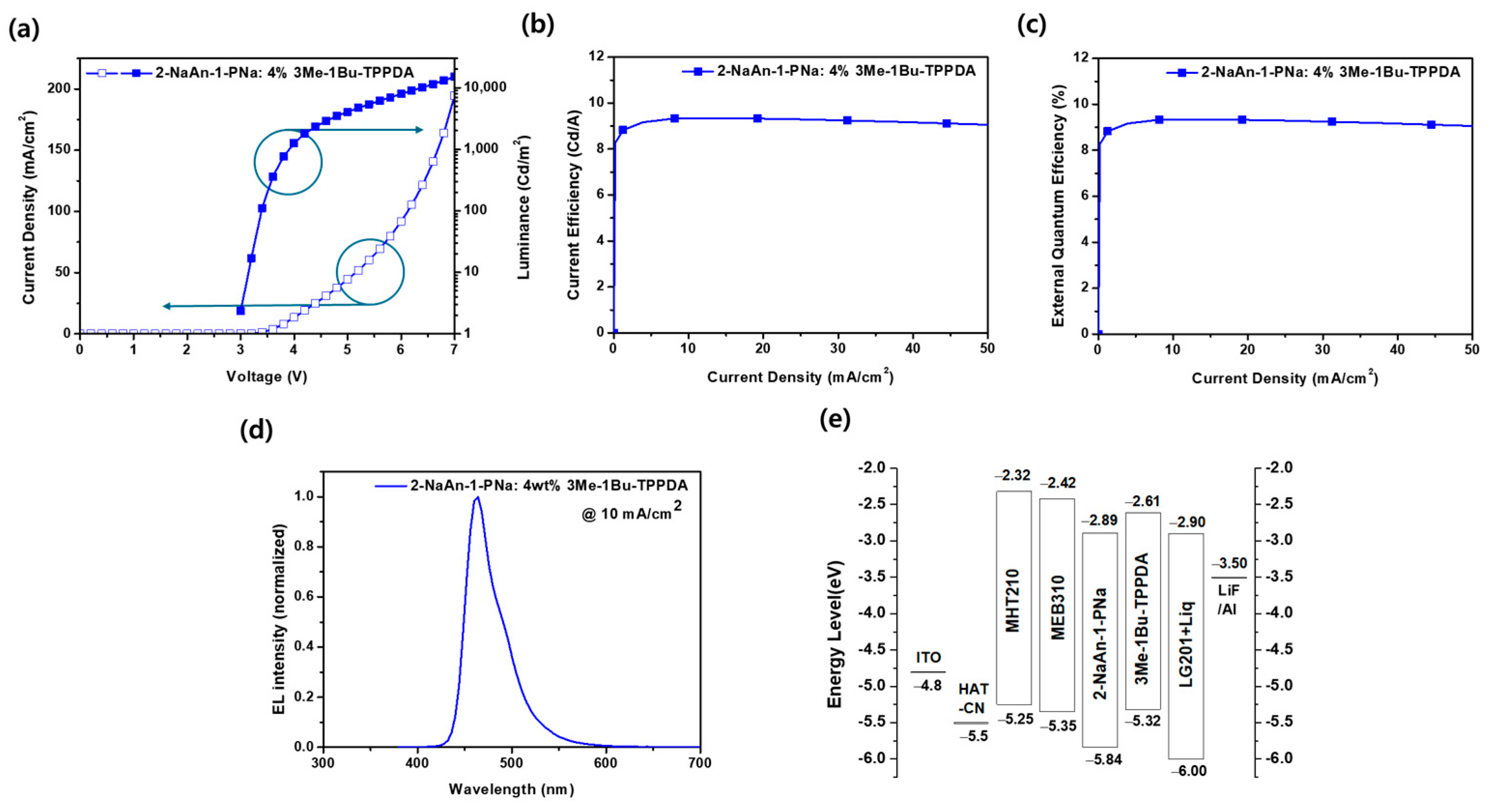
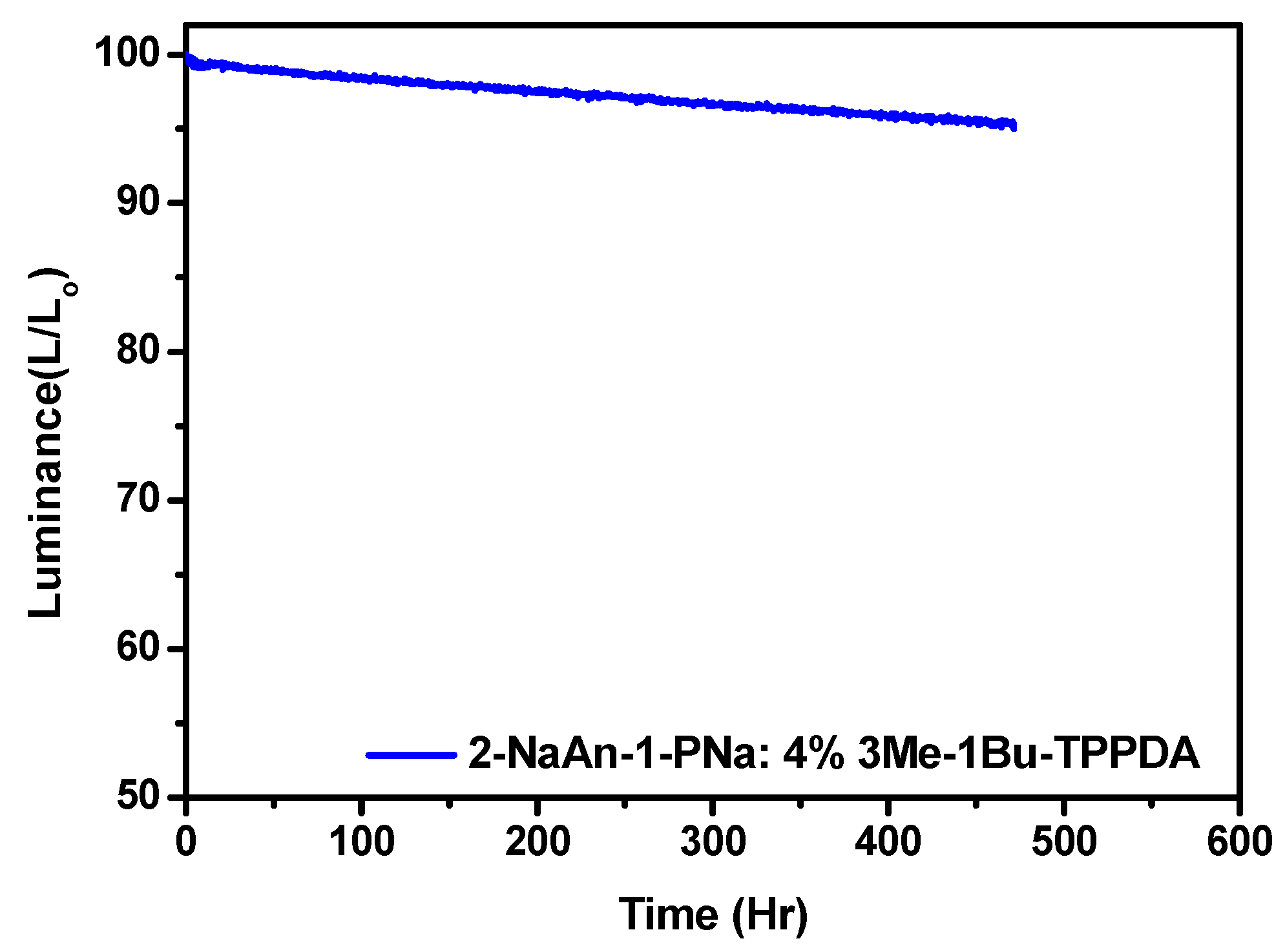
3.3. Electroluminescence Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pickup, D.F.; Yi, H.; Kun, H.; Iraqi, A.; Stevenson, M.; Lidzey, D.G. Alternating 2, 7-and 3, 6-linked carbazole copolymers as wide band gap energy transfer donors. Thin Solid Films 2009, 517, 2840–2844. [Google Scholar] [CrossRef]
- Liu, J.; Guo, X.; Bu, L.; Xie, Z.; Cheng, Y.; Geng, Y.; Wang, L.; Jing, X.; Wang, F. White Electroluminescence from a Single-Polymer System with Simultaneous Two-Color Emission: Polyfluorene Blue Host and Side-Chain-Located Orange Dopant. Adv. Funct. Mater. 2007, 17, 1917–1925. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kwon, Y.J.; Woo, J.W.; Moon, D.K. Synthesis and characterization of fluorine–thiophene-based π-conjugated polymers using coupling reaction. J. Ind. Eng. Chem. 2008, 14, 810–817. [Google Scholar] [CrossRef]
- Kang, I.; Hong, J.A.; Kim, R.; Jang, J.W.; Hwang, J.; Kim, J.H.; Kwon, S.K.; Kim, Y.H. Synthesis and characterization of a new π-conjugated polymer with cyanoacrylate side chain for organic thin film transistors. Macromol. Res. 2013, 21, 450–455. [Google Scholar] [CrossRef]
- Kang, S.W.; Kwon, H.M.; Pu, Y.-J.; Park, J. High-efficiency deep-blue emitter consisting of a chrysene core and optimized side groups. Mater. Today Energy 2021, 21, 100706. [Google Scholar]
- Yook, S.; Jeon, S.; Kim, O.; Lee, J. Lifetime study of red phosphorescent organic light-emitting diodes with a double doping structure. J. Ind. Eng. Chem. 2010, 16, 813–815. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, H.; Kang, S. Fabrication of highly efficient and stable doped red organic light-emitting device using 2-methyl-9,10-di(2-napthyl)anthracene and tris(8-hydroxyquinolinato)aluminum as cohost materials. Appl. Phys. Lett. 2006, 89, 183515. [Google Scholar] [CrossRef]
- Aydemir, M.; Haykır, C.; Battal, A.; Jankus, V.; Sugunan, S.K.; Dias, F.B.; Al-Attar, H.; Türksoy, F.; Tavaslı, M.; Monkman, A.P. High efficiency OLEDs based on anthracene derivatives: The impact of electron donating and withdrawing group on the performance of OLED. Org. Electron. 2016, 30, 49–157. [Google Scholar] [CrossRef]
- Hwang, H.; We, H.; Bian, G.; Song, L. An Anthracene-Based Bis-Stilbene Derivative as Luminescent Materials for Organic Light Emitting Diodes. Materials 2023, 16, 3685. [Google Scholar] [CrossRef]
- Zheng, C.-J.; Zhao, W.-M.; Wang, Z.-Q.; Huang, D.; Ye, J.; Qu, X.-M.; Zhang, X.-H.; Lee, C.-S.; Lee, S.-T. Highly efficient non-doped deep-blue organic light-emitting diodes based on anthracene derivatives. J. Mater. Chem. 2010, 20, 1560–1566. [Google Scholar] [CrossRef]
- Hu, J.-Y.; Pu, Y.-J.; Satoh, F.; Kawata, S.; Katagiri, H.; Sasabe, H.; Kido, J. Bisanthracene-Based Donor–Acceptor-type Light-Emitting Dopants: Highly Efficient Deep-Blue Emission in Organic Light-Emitting Devices. Adv. Funct. Mater. 2014, 24, 2064–2071. [Google Scholar] [CrossRef]
- Chiang, C.-J.; Kimyonok, A.; Etherington, M.K.; Griffiths, G.C.; Jankus, V.; Turksoy, F.; Monkman, A.P. Ultrahigh Efficiency Fluorescent Single and Bi-Layer Organic Light Emitting Diodes: The Key Role of Triplet Fusion. Adv. Funct. Mater. 2013, 23, 739–746. [Google Scholar] [CrossRef]
- Hayashi, H.; Kato, Y.; Matsumoto, A.; Shikita, S.; Aizawa, N.; Suzuki, M.; Aratani, N.; Yasuda, T.; Yamada, H. Synthesis of Anthracene Derivatives with Azaacene-Containing Iptycene Wings and the Utilization as a Dopant for Solution-Processed Organic Light-Emitting Diodes. Chem. Eur. J. 2019, 25, 15565–15571. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kang, S.; Park, S.; Kwon, H.; Lee, H.; Lee, K.; Park, J. Novel Fused Core Chromophore Incorporating Spirofluorene and Anthracene Groups for Sky-Blue Emission and Solution-Processed White Devices. Appl. Sci. 2023, 13, 1054. [Google Scholar] [CrossRef]
- Park, D.; Kang, S.; Ryoo, C.H.; Jhun, B.H.; Jung, S.; Le, T.N.; Suh, M.C.; Lee, J.; Jun, M.E.; Chu, C.; et al. High-performance blue OLED using multiresonance thermally activated delayed fluorescence host materials containing silicon atoms. Nat. Commun. 2023, 14, 5589. [Google Scholar] [CrossRef] [PubMed]
- Rothmann, M.M.; Hanader, S.; Como, E.D.; Lennartz, C.; Schildknecht, C.; Strohriegl, P. Donor-Substituted 1,3,5-Triazines as Host Materials for Blue Phosphorescent Organic Light-Emitting Diodes. Chem. Mater. 2010, 22, 2403. [Google Scholar] [CrossRef]
- Wee, J.-R.; Han, W.-S.; Kim, J.-E.; Kim, A.-L.; Kwon, S.; Kang, S.O. Asymmetric anthracene-based blue host materials: Synthesis and electroluminescence properties of 9-(2-naphthyl)-10-arylanthracenes. J. Mater. Chem. 2011, 21, 1115–1123. [Google Scholar] [CrossRef]
- Shirota, Y. Organic materials for electronic and optoelectronic devices. J. Mater. Chem. 2000, 10, 1–25. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, S.; You, J.; Zhang, Y.; Li, X. Solution-processed thermally stable amorphous films of small molecular hole injection/transport bi-functional materials and their application in high efficiency OLEDs. J. Mater. Chem. C 2015, 3, 11377–11384. [Google Scholar] [CrossRef]
- Lyu, Y.-Y.; Kwak, J.; Kwon, O.; Lee, S.-H.; Kim, D.; Lee, C.; Char, K. Silicon-Cored Anthracene Derivatives as Host Materials for Highly Efficient Blue Organic Light-Emitting Devices. Adv. Mater. 2008, 20, 2720–2729. [Google Scholar] [CrossRef]
- Sun, T.; Shui, X.; Chen, W.; Chen, Y.; Shi, W.; Huang, J.; Wei, B. Efficient and low roll-off deep-blue organic light-emitting diodes with anthracene-based compounds as hosts. New J. Chem. 2024, 48, 1867–1875. [Google Scholar] [CrossRef]
- Jung, H.; Kang, S.; Lee, H.; Yu, Y.-J.; Jeong, J.H.; Song, J.; Jeon, Y.; Park, J. High Efficiency and Long Lifetime of a Fluorescent Blue-Light Emitter Made of a Pyrene Core and Optimized Side Groups. Appl. Mater. Interfaces 2018, 10, 30022–30028. [Google Scholar] [CrossRef] [PubMed]
- Forrest, S.R.; Bradley, D.D.C.; Thompson, M.E. Measuring the Efficiency of Organic Light-Emitting Devices. Adv. Mater. 2003, 15, 1043–1048. [Google Scholar] [CrossRef]
- Shin, M.-G.; Kimg, S.O.; Park, H.T.; Park, S.J.; Yu, H.S.; Kim, Y.-H.; Kwon, S.-K. Synthesis and characterization of ortho-twisted asymmetric anthracene derivatives for blue organic light emitting diodes (OLEDs). Dyes. Pigments 2012, 92, 1075–1082. [Google Scholar] [CrossRef]
- Wang, L.; We, Z.-Y.; Wong, W.-Y.; Cheah, K.-W.; Huang, H.; Chen, C.H. New blue host materials based on anthracene-containing dibenzothiophene. Org. Electron. 2011, 12, 595–601. [Google Scholar] [CrossRef]
- Costa, J.C.S.; Taveira, J.S.; Lima, F.R.A.C.; Mendes, A.; Santos, M.N.B.F. Optical band gaps of organic semiconductor materials. Opt. Mater. 2016, 58, 51–60. [Google Scholar] [CrossRef]
- Onoda, M.; Tada, K. A consideration of thermochromic behavior in poly(p-phenylenevinylene) derivatives. Thin Solid Films 2003, 438–439, 187–194. [Google Scholar] [CrossRef]
- Park, Y.; Kim, B.; Lee, C.; Hyun, A.; Jang, S.; Lee, J.; Gal, Y.S.; Kim, T.; Kim, K.; Park, J. Highly efficient new hole injection materials for OLEDs based on dimeric phenothiazine and phenoxazine derivatives. J. Phys. Chem. C 2011, 115, 4843–4850. [Google Scholar] [CrossRef]
- Shin, J.; Lee, J. Hole injection control of electron blocking layer for broad recombination zone and low-efficiency roll-off in phosphorescent organic light emitting device. J. Inf. Disp. 2023, 1–10. [Google Scholar] [CrossRef]
- Fukagawa, H.; Shimizu, T.; Kamada, T.; Yui, S.; Hasegawa, M.; Morii, K.; Yamamoto, T. Highly efficient and stable organic light-emitting diodes with a greatly reduced amount of phosphorescent emitter. Sci. Rep. 2015, 5, 9855. [Google Scholar] [CrossRef]
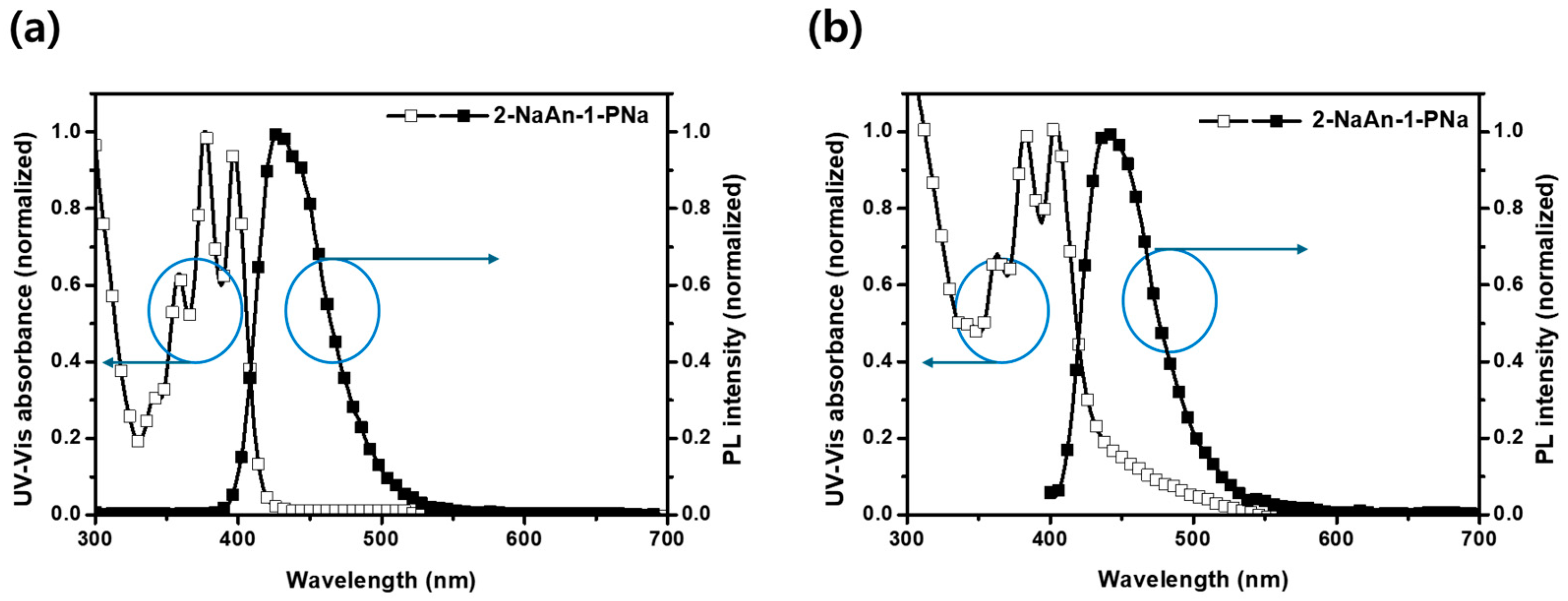
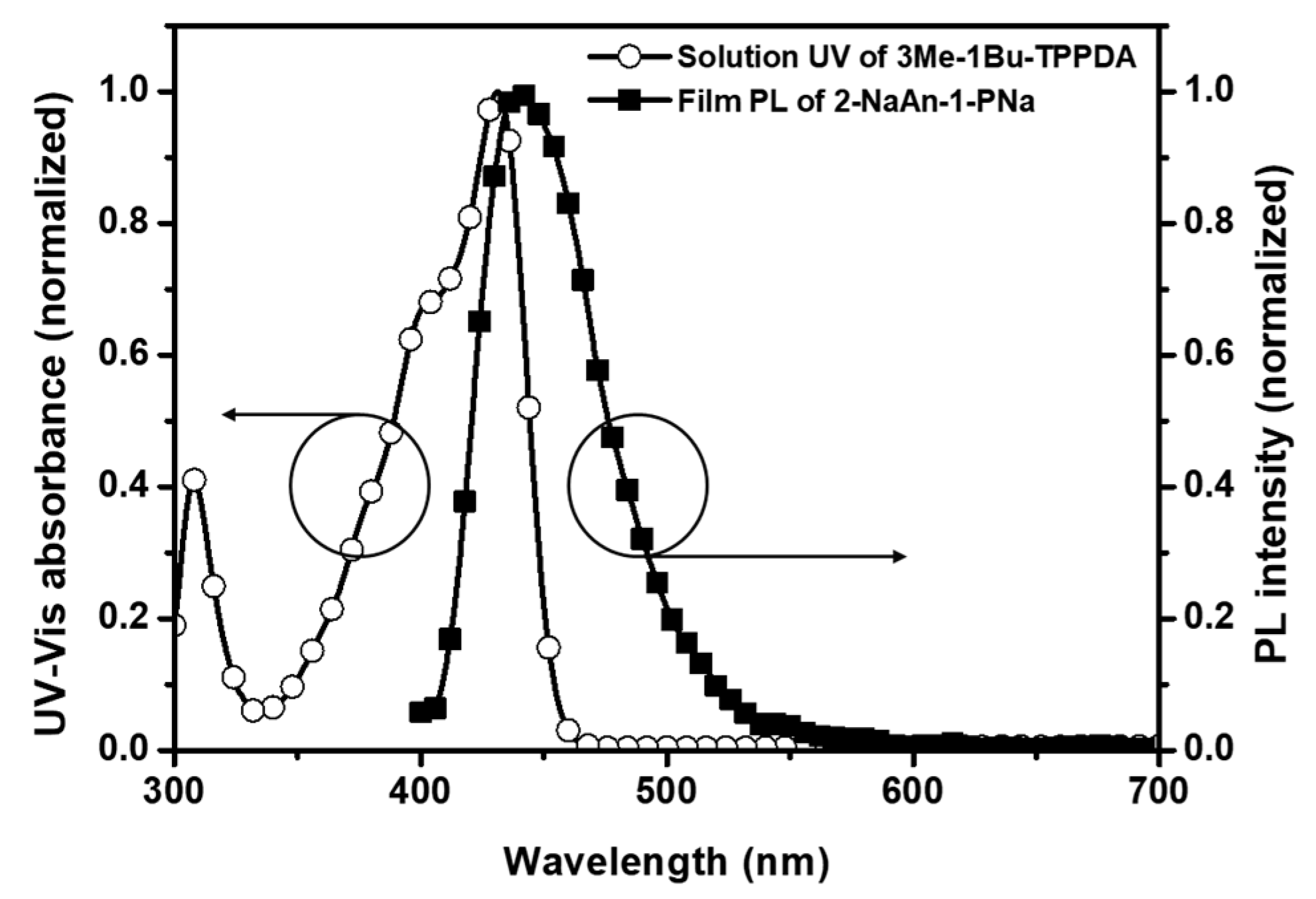
| Compound | Solution a | Film b | HO MO c (eV) | LU MO (eV) | Band Gap a/b (eV) | ||||
|---|---|---|---|---|---|---|---|---|---|
| λAbs (nm) | λPL (FWHM) (nm) | ΦF (%) | λAbs (nm) | λPL (FWHM) (nm) | ΦF (%) | ||||
| 2-NaAn-1-PNa | 340, 359, 376, 397 | 428 (53) | 73.1 | 363, 383, 403 | 440 (56) | 30.2 | −5.84 | −2.89 | 3.03/ 2.95 |
| Device | O.V. a (V) | Luminance (cd/m2) | CE (cd/A) | EQE (%) | ELmax (nm) | FWHM (nm) | CIE (x, y) |
|---|---|---|---|---|---|---|---|
| Non-doped | 8.1 | 335 | 3.4 | 3.9 | 457 | 65 | (0.150, 0.113) |
| Doped | 3.9 | 934 | 9.3 | 8.3 | 460 | 53 | (0.134, 0.139) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, H.; Oh, S.; Park, S.; Park, S.; Lee, H.; Park, J. Highly Efficient Blue Host Compound Based on an Anthracene Moiety for OLEDs. Appl. Sci. 2024, 14, 5716. https://doi.org/10.3390/app14135716
Kwon H, Oh S, Park S, Park S, Lee H, Park J. Highly Efficient Blue Host Compound Based on an Anthracene Moiety for OLEDs. Applied Sciences. 2024; 14(13):5716. https://doi.org/10.3390/app14135716
Chicago/Turabian StyleKwon, Hyukmin, Seyoung Oh, Sangwook Park, Sunwoo Park, Hayoon Lee, and Jongwook Park. 2024. "Highly Efficient Blue Host Compound Based on an Anthracene Moiety for OLEDs" Applied Sciences 14, no. 13: 5716. https://doi.org/10.3390/app14135716
APA StyleKwon, H., Oh, S., Park, S., Park, S., Lee, H., & Park, J. (2024). Highly Efficient Blue Host Compound Based on an Anthracene Moiety for OLEDs. Applied Sciences, 14(13), 5716. https://doi.org/10.3390/app14135716






