A Review of Impedance Spectroscopy Technique: Applications, Modelling, and Case Study of Relative Humidity Sensors Development
Abstract
1. Introduction
2. Impedance Spectroscopy
2.1. Scopes of Application
2.1.1. IS in Human Health Safety Due to Water Sources Contamination
2.1.2. IS Applied in Forest and Agriculture Management
2.1.3. IS in Materials Science and Applied Technologies
IS in Flexible Structures and Sensing Applications Assessment
IS Used on the Development/Optimization of Materials for Applications Related to Electrical Energy Production/Storage
IS Used on the Development/Optimization of Materials for Corrosion Processes Assessment
2.1.4. IS Summary
2.2. Basics of Impedance Spectroscopy and Modelling
2.3. Impedance Spectroscopy Modelling
2.4. Materials Modelling by IS
2.5. EleIS Modelling of Electrodeposited Ti-W Undoped and Vanadium Doped Humidity Sensor
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications, 3rd ed.; Barsoukov, E., Macdonald, J.R., Eds.; Wiley: Hoboken, NJ, USA, 2018; ISBN 9781119074083. [Google Scholar]
- El-Azazy, M. Electrochemical Impedance Spectroscopy (EIS) in Food, Water, and Drug Analyses: Recent Advances and Applications. In Electrochemical Impedance Spectroscopy; El-Azazy, M., Min, M., Annus, P., Eds.; IntechOpen: Rijeka, Croatia, 2020; ISBN 978-1-78985-216-5. [Google Scholar]
- Pejcic, B.; De Marco, R. Impedance Spectroscopy: Over 35 Years of Electrochemical Sensor Optimization. Electrochim. Acta 2006, 51, 6217–6229. [Google Scholar] [CrossRef]
- Warburg, E. Ueber Das Verhalten Sogenannter Unpolarisirbarer Elektroden Gegen Wechselstrom. Ann. Phys. 1899, 303, 493–499. [Google Scholar] [CrossRef]
- Bauerle, J.E. Study of Solid Electrolyte Polarization by a Complex Admittance Method. J. Phys. Chem. Solids 1969, 30, 2657–2670. [Google Scholar] [CrossRef]
- Macdonald, D.D. Reflections on the History of Electrochemical Impedance Spectroscopy. Electrochim. Acta 2006, 51, 1376–1388. [Google Scholar] [CrossRef]
- Pajkossy, T.; Jurczakowski, R. Electrochemical Impedance Spectroscopy in Interfacial Studies. Curr. Opin. Electrochem. 2017, 1, 53–58. [Google Scholar] [CrossRef]
- Lazanas, A.Ch.; Prodromidis, M.I. Electrochemical Impedance Spectroscopy—A Tutorial. ACS Meas. Sci. Au 2023, 3, 162–193. [Google Scholar] [CrossRef] [PubMed]
- Orazem, M.E.; Tribollet, B. A Tutorial on Electrochemical Impedance Spectroscopy. ChemTexts 2020, 6, 12. [Google Scholar] [CrossRef]
- Bandarenka, A.S. Exploring the Interfaces between Metal Electrodes and Aqueous Electrolytes with Electrochemical Impedance Spectroscopy. Analyst 2013, 138, 5540–5554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S.; Xu, K.; Jow, T.R. EIS Study on the Formation of Solid Electrolyte Interface in Li-Ion Battery. Electrochim. Acta 2006, 51, 1636–1640. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Gharbi, O.; Vivier, V.; Gao, M.; Orazem, M.E. Electrochemical Impedance Spectroscopy. Nat. Rev. Methods Primers 2021, 1, 41. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; White, H.S. Electrochemical Methods: Fundamentals and Applications, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; ISBN 978-1-119-33406-4. [Google Scholar]
- Shi, X. Experimental Studies and Modelling of Charge Transport and Defect States in Disordered Organic Semiconductors. Ph.D. Thesis, Imperial College London, London, UK, 2017. [Google Scholar]
- Popkirov, G.S.; Schindler, R.N. Optimization of the Perturbation Signal for Electrochemical Impedance Spectroscopy in the Time Domain. Rev. Sci. Instrum. 1993, 64, 3111–3115. [Google Scholar] [CrossRef]
- Parthasarathy, A.; Davé, B.; Srinivasan, S.; Appleby, A.J.; Martin, C.R. The Platinum Microelectrode/Nafion Interface: An Electrochemical Impedance Spectroscopic Analysis of Oxygen Reduction Kinetics and Nafion Characteristics. J. Electrochem. Soc. 1992, 139, 1634–1641. [Google Scholar] [CrossRef]
- Lindahl, P.A.; Cornachione, M.A.; Shaw, S.R. A Time-Domain Least Squares Approach to Electrochemical Impedance Spectroscopy. IEEE Trans. Instrum. Meas. 2012, 61, 3303–3311. [Google Scholar] [CrossRef]
- Al Nazer, R.; Cattin, V.; Granjon, P.; Montaru, M.; Ranieri, M.; Heiries, V. Classical EIS and Square Pattern Signals Comparison Based on a Well-Known Reference Impedance. In Proceedings of the 2013 World Electric Vehicle Symposium and Exhibition (EVS27), Barcelona, Spain, 17–20 November 2013; IEEE: Barcelona, Spain, 2013; pp. 1–7. [Google Scholar]
- Toll, J.S. Causality and the Dispersion Relation: Logical Foundations. Phys. Rev. 1956, 104, 1760–1770. [Google Scholar] [CrossRef]
- Kronig, R.D.L. On the Theory of Dispersion of X-rays. J. Opt. Soc. Am. 1926, 12, 547–557. [Google Scholar] [CrossRef]
- Kramers, M.H.A. La Diffusion de La Lumiere Par Les Atomes. Atti del Congresso Internazionale dei Fisici (Onoranze ad Alessandro Volta nel Primo Centenario Della Morte) Como-Pavia-Roma 1927, 2, 545–557. [Google Scholar]
- Agarwal, P.; Orazem, M.E.; Garcia-Rubio, L.H. Measurement Models for Electrochemical Impedance Spectroscopy: I. Demonstration of Applicability. J. Electrochem. Soc. 1992, 139, 1917–1927. [Google Scholar] [CrossRef]
- Boukamp, B.A. A Linear Kronig-Kramers Transform Test for Immittance Data Validation. J. Electrochem. Soc. 1995, 142, 1885–1894. [Google Scholar] [CrossRef]
- Randviir, E.P.; Banks, C.E. Electrochemical Impedance Spectroscopy: An Overview of Bioanalytical Applications. Anal. Methods 2013, 5, 1098–1115. [Google Scholar] [CrossRef]
- Rupp, G.M.; Opitz, A.K.; Nenning, A.; Limbeck, A.; Fleig, J. Real-Time Impedance Monitoring of Oxygen Reduction during Surface Modification of Thin Film Cathodes. Nat. Mater. 2017, 16, 640–645. [Google Scholar] [CrossRef]
- Song, D.; Wan, H.; Tu, X.; Li, W. A Better Understanding of Failure Process of Waterborne Coating/Metal Interface Evaluated by Electrochemical Impedance Spectroscopy. Prog. Org. Coat. 2020, 142, 105558. [Google Scholar] [CrossRef]
- Keddam, M.; Nóvoa, X.R.; Vivier, V. The Concept of Floating Electrode for Contact-Less Electrochemical Measurements: Application to Reinforcing Steel-Bar Corrosion in Concrete. Corros. Sci. 2009, 51, 1795–1801. [Google Scholar] [CrossRef]
- Alexander, C.L.; Orazem, M.E. Indirect Electrochemical Impedance Spectroscopy for Corrosion Detection in External Post-Tensioned Tendons: 1. Proof of Concept. Corros. Sci. 2020, 164, 108331. [Google Scholar] [CrossRef]
- Brett, C.M.A. Electrochemical Impedance Spectroscopy in the Characterisation and Application of Modified Electrodes for Electrochemical Sensors and Biosensors. Molecules 2022, 27, 1497. [Google Scholar] [CrossRef] [PubMed]
- Sankhala, D.; Muthukumar, S.; Prasad, S. A Four-Channel Electrical Impedance Spectroscopy Module for Cortisol Biosensing in Sweat-Based Wearable Applications. SLAS Technol. 2018, 23, 529–539. [Google Scholar] [CrossRef]
- Soares, C.; Tenreiro Machado, J.A.; Lopes, A.M.; Vieira, E.; Delerue-Matos, C. Electrochemical Impedance Spectroscopy Characterization of Beverages. Food Chem. 2020, 302, 125345. [Google Scholar] [CrossRef]
- Lopes, A.M.; Machado, J.A.T.; Ramalho, E.; Silva, V. Milk Characterization Using Electrical Impedance Spectroscopy and Fractional Models. Food Anal. Methods 2018, 11, 901–912. [Google Scholar] [CrossRef]
- Liu, X.; Duckworth, P.A.; Wong, D.K.Y. Square Wave Voltammetry versus Electrochemical Impedance Spectroscopy as a Rapid Detection Technique at Electrochemical Immunosensors. Biosens. Bioelectron. 2010, 25, 1467–1473. [Google Scholar] [CrossRef]
- Finšgar, M.; Xhanari, K.; Petovar, B. Copper-Film Electrodes for Pb(II) Trace Analysis and a Detailed Electrochemical Impedance Spectroscopy Study. Microchem. J. 2019, 147, 863–871. [Google Scholar] [CrossRef]
- Fan, L.; Zhao, G.; Shi, H.; Liu, M.; Li, Z. A Highly Selective Electrochemical Impedance Spectroscopy-Based Aptasensor for Sensitive Detection of Acetamiprid. Biosens. Bioelectron. 2013, 43, 12–18. [Google Scholar] [CrossRef]
- Yadav, A.K.; Jain, C.K.; Malik, D.S. Toxic Characterization of Textile Dyes and Effluents in Relation to Human Health Hazards. J. Sustain. Environ. Res. 2014, 3, 95–102. [Google Scholar]
- Islam, T.; Repon, M.R.; Islam, T.; Sarwar, Z.; Rahman, M.M. Impact of Textile Dyes on Health and Ecosystem: A Review of Structure, Causes, and Potential Solutions. Env. Sci. Pollut. Res. Int. 2023, 30, 9207–9242. [Google Scholar] [CrossRef] [PubMed]
- Tekaya, N.; Saiapina, O.; Ouada, H.B.; Lagarde, F.; Namour, P.; Ouada, H.B.; Jaffrezic-Renault, N. Bi-Enzymatic Conductometric Biosensor for Detection of Heavy Metal Ions and Pesticides in Water Samples Based on Enzymatic Inhibition in Arthrospira Platensis. J. Environ. Prot. 2014, 5, 441–453. [Google Scholar] [CrossRef]
- Avuthu, S.G.R.; Narakathu, B.B.; Eshkeiti, A.; Emamian, S.; Bazuin, B.J.; Joyce, M.; Atashbar, M.Z. Detection of Heavy Metals Using Fully Printed Three Electrode Electrochemical Sensor. In Proceedings of the IEEE SENSORS 2014 Proceedings, Valencia, Spain, 2–5 November 2014; IEEE: Valencia, Spain, 2014; Volume 2014, pp. 669–672. [Google Scholar]
- Liu, S.; Kang, M.; Yan, F.; Peng, D.; Yang, Y.; He, L.; Wang, M.; Fang, S.; Zhang, Z. Electrochemical DNA Biosensor Based on Microspheres of Cuprous Oxide and Nano-Chitosan for Hg(II) Detection. Electrochim. Acta 2015, 160, 64–73. [Google Scholar] [CrossRef]
- Castillo, G.; Lamberti, I.; Mosiello, L.; Hianik, T. Impedimetric DNA Aptasensor for Sensitive Detection of Ochratoxin A in Food. Electroanalysis 2012, 24, 512–520. [Google Scholar] [CrossRef]
- Castillo, G.; Spinella, K.; Poturnayová, A.; Šnejdárková, M.; Mosiello, L.; Hianik, T. Detection of Aflatoxin B1 by Aptamer-Based Biosensor Using PAMAM Dendrimers as Immobilization Platform. Food Control 2015, 52, 9–18. [Google Scholar] [CrossRef]
- Jiang, D.; Du, X.; Liu, Q.; Zhou, L.; Dai, L.; Qian, J.; Wang, K. Silver Nanoparticles Anchored on Nitrogen-Doped Graphene as a Novel Electrochemical Biosensing Platform with Enhanced Sensitivity for Aptamer-Based Pesticide Assay. Analyst 2015, 140, 6404–6411. [Google Scholar] [CrossRef] [PubMed]
- Labib, M.; Zamay, A.S.; Kolovskaya, O.S.; Reshetneva, I.T.; Zamay, G.S.; Kibbee, R.J.; Sattar, S.A.; Zamay, T.N.; Berezovski, M.V. Aptamer-Based Impedimetric Sensor for Bacterial Typing. Anal. Chem. 2012, 84, 8114–8117. [Google Scholar] [CrossRef]
- Ma, X.; Jiang, Y.; Jia, F.; Yu, Y.; Chen, J.; Wang, Z. An Aptamer-Based Electrochemical Biosensor for the Detection of Salmonella. J. Microbiol. Methods 2014, 98, 94–98. [Google Scholar] [CrossRef]
- Jahanbani, S.; Benvidi, A. Comparison of Two Fabricated Aptasensors Based on Modified Carbon Paste/Oleic Acid and Magnetic Bar Carbon Paste/Fe3O4@oleic Acid Nanoparticle Electrodes for Tetracycline Detection. Biosens. Bioelectron. 2016, 85, 553–562. [Google Scholar] [CrossRef]
- Wang, M.; Hu, B.; Yang, C.; Zhang, Z.; He, L.; Fang, S.; Qu, X.; Zhang, Q. Electrochemical Biosensing Based on Protein-Directed Carbon Nanospheres Embedded with SnO and TiO2 Nanocrystals for Sensitive Detection of Tobramycin. Biosens. Bioelectron. 2018, 99, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, G.C.; Eissa, S.; Ng, A.; Alhadrami, H.; Zourob, M.; Siaj, M. Aptamer-Based Label-Free Impedimetric Biosensor for Detection of Progesterone. Anal. Chem. 2015, 87, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-W.; Princy Merlin, J.; Chen, S.-M.; Anandaraj, S.; Elshikh, M.S.; Tseng, T.-W.; Wang, K.; Qi, D.; Jiang, J. Sonochemical Synthesis and Fabrication of Neodymium Sesquioxide Entrapped with Graphene Oxide Based Hierarchical Nanocomposite for Highly Sensitive Electrochemical Sensor of Anti-Cancer (Raloxifene) Drug. Ultrason. Sonochem 2020, 64, 104717. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Duan, F.; Zhang, S.; Tian, J.-Y.; Zhang, Z.; Wang, Z.-W.; Liu, C.-S.; Xu, W.-M.; Du, M. Iron Oxide@mesoporous Carbon Architectures Derived from an Fe(II)-Based Metal Organic Framework for Highly Sensitive Oxytetracycline Determination. J. Mater. Chem. A Mater. 2017, 5, 19378–19389. [Google Scholar] [CrossRef]
- Miele, G.; Bellucci, S.; Cataldo, A.; Di Tinno, A.; Ferrigno, L.; Maffucci, A.; Micheli, L.; Sibilia, S. Electrical Impedance Spectroscopy for Real-Time Monitoring of the Life Cycle of Graphene Nanoplatelets Filters for Some Organic Industrial Pollutants. IEEE Trans. Instrum. Meas. 2021, 70, 1–12. [Google Scholar] [CrossRef]
- Van Haeverbeke, M.; De Baets, B.; Stock, M. Plant Impedance Spectroscopy: A Review of Modeling Approaches and Applications. Front. Plant Sci. 2023, 14, 1187573. [Google Scholar] [CrossRef] [PubMed]
- Ozier-Lafontaine, H.; Bajazet, T. Analysis of Root Growth by Impedance Spectroscopy (EIS). Plant Soil. 2005, 277, 299–313. [Google Scholar] [CrossRef]
- Cao, Y.; Repo, T.; Silvennoinen, R.; Lehto, T.; Pelkonen, P. Analysis of the Willow Root System by Electrical Impedance Spectroscopy. J. Exp. Bot. 2011, 62, 351–358. [Google Scholar] [CrossRef]
- Repo, T.; Korhonen, A.; Lehto, T.; Silvennoinen, R. Assessment of Frost Damage in Mycorrhizal and Non-Mycorrhizal Roots of Scots Pine Seedlings Using Classification Analysis of Their Electrical Impedance Spectra. Trees 2016, 30, 483–495. [Google Scholar] [CrossRef]
- Vozáry, E.; Jócsák, I.; Droppa, M.; Bóka, K. Connection between Structural Changes and Electrical Parameters of Pea Root Tissue Under Anoxia. In Anoxia; Padilla, P., Ed.; InTech: Rijeka, Croatia, 2012; pp. 132–146. ISBN 978-953-307-664-5. [Google Scholar]
- Repo, T.; Korhonen, A.; Laukkanen, M.; Lehto, T.; Silvennoinen, R. Detecting Mycorrhizal Colonisation in Scots Pine Roots Using Electrical Impedance Spectra. Biosyst. Eng. 2014, 121, 139–149. [Google Scholar] [CrossRef]
- Bar-On, L.; Garlando, U.; Sophocleous, M.; Jog, A.; Motto Ros, P.; Sade, N.; Avni, A.; Shacham-Diamand, Y.; Demarchi, D. Electrical Modelling of In-Vivo Impedance Spectroscopy of Nicotiana Tabacum Plants. Front. Electron. 2021, 2, 1–12. [Google Scholar] [CrossRef]
- Tomkiewicz, D.; Piskier, T. A Plant Based Sensing Method for Nutrition Stress Monitoring. Precis. Agric. 2012, 13, 370–383. [Google Scholar] [CrossRef]
- Borges, E.; Matos, A.P.; Cardoso, J.M.; Correia, C.; Vasconcelos, T.; Gomes, N. Early Detection and Monitoring of Plant Diseases by Bioelectric Impedance Spectroscopy. In Proceedings of the 2012 IEEE 2nd Portuguese Meeting in Bioengineering (ENBENG), Coimbra, Portugal, 20–23 February 2012; IEEE: Coimbra, Portugal, 2012; pp. 1–4. [Google Scholar]
- Basak, R.; Wahid, K.A.; Dinh, A.; Soolanayakanahally, R.; Fotouhi, R.; Mehr, A.S. Rapid and Efficient Determination of Relative Water Contents of Crop Leaves Using Electrical Impedance Spectroscopy in Vegetative Growth Stage. Remote Sens. 2020, 12, 1753. [Google Scholar] [CrossRef]
- Basak, R.; Wahid, K.; Dinh, A. Determination of Leaf Nitrogen Concentrations Using Electrical Impedance Spectroscopy in Multiple Crops. Remote Sens. 2020, 12, 566. [Google Scholar] [CrossRef]
- Sugiyama, M.; Okajima, M. Application of Electrochemical Impedance Spectroscopy and Modeling of the Novel Equivalent Circuit for Monitoring Cellular Tissues. Theor. Exp. Plant Physiol. 2022, 34, 501–508. [Google Scholar] [CrossRef]
- Ivanovski, T.; Zhang, G.; Jemric, T.; Gulic, M.; Matetic, M. Fruit Firmness Prediction Using Multiple Linear Regression. In Proceedings of the 2020 43rd International Convention on Information, Communication and Electronic Technology (MIPRO), Opatija, Croatia, 28 September–2 October 2020; IEEE: Opatija, Croatia, 2020; pp. 1306–1311. [Google Scholar]
- Wu, L.; Ogawa, Y.; Tagawa, A. Electrical Impedance Spectroscopy Analysis of Eggplant Pulp and Effects of Drying and Freezing–Thawing Treatments on Its Impedance Characteristics. J. Food Eng. 2008, 87, 274–280. [Google Scholar] [CrossRef]
- Watanabe, T.; Nakamura, N.; Ando, Y.; Kaneta, T.; Kitazawa, H.; Shiina, T. Application and Simplification of Cell-Based Equivalent Circuit Model Analysis of Electrical Impedance for Assessment of Drop Shock Bruising in Japanese Pear Tissues. Food Bioproc. Technol. 2018, 11, 2125–2129. [Google Scholar] [CrossRef]
- Patil, A.C.; Fernández la Villa, A.; Mugilvannan, A.K.; Elejalde, U. Electrochemical Investigation of Edible Oils: Experimentation, Electrical Signatures, and a Supervised Learning–Case Study of Adulterated Peanut Oils. Food Chem. 2023, 402, 134143. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.K.; Sasmal, S. Nanoengineered Smart Cement Composite for Electrical Impedance-Based Monitoring of Corrosion Progression in Structures. Cem. Concr. Compos. 2022, 126, 104348. [Google Scholar] [CrossRef]
- Panda, M. Impedance Spectroscopy of Percolative Polymer Composites. In Percolation, Scaling, and Relaxation in Polymer Dielectrics; Springer Nature Switzerland AG: Cham, Switzerland, 2023; pp. 147–167. ISBN 978-3-031-27940-9. [Google Scholar]
- Chikara, K.S.; Bera, A.K.; Kumar, A.; Yusuf, S.M. Li-Ion Conduction Mechanism and Relaxation Dynamics in Sorosilicate Compound Li2Cu5(Si2O7)2. ACS Appl. Electron. Mater. 2023, 5, 5137–5150. [Google Scholar] [CrossRef]
- Pitruzzello, G.; Johnson, S.; Krauss, T.F. Exploring the Fundamental Limit of Antimicrobial Susceptibility by Near-Single-Cell Electrical Impedance Spectroscopy. Biosens. Bioelectron. 2023, 224, 115056. [Google Scholar] [CrossRef]
- Gul, M.M.; Ahmad, K.S.; Thomas, A.G.; Tawfeek, A.M. Advancing Photoelectrochemical Systems: Unleashing the Remarkable Performance of In:SnO2/Nd4In5S13 for Sustainable Energy Applications. ChemistrySelect 2024, 9, e202304530. [Google Scholar] [CrossRef]
- Mercante, L.A.; Andre, R.S.; Facure, M.H.M.; Correa, D.S.; Mattoso, L.H.C. Recent Progress in Conductive Electrospun Materials for Flexible Electronics: Energy, Sensing, and Electromagnetic Shielding Applications. Chem. Eng. J. 2023, 465, 142847. [Google Scholar] [CrossRef]
- Milkin, P.; Zhanbassynova, A.; Ionov, L. Superelastic, Soft, Stress-Healable, Recyclable Conductive Materials. Compos. Struct. 2024, 327, 117709. [Google Scholar] [CrossRef]
- Fernandes, I.J.; Aroche, A.F.; Schuck, A.; Lamberty, P.; Peter, C.R.; Hasenkamp, W.; Rocha, T.L.A.C. Silver Nanoparticle Conductive Inks: Synthesis, Characterization, and Fabrication of Inkjet-Printed Flexible Electrodes. Sci. Rep. 2020, 10, 8878. [Google Scholar] [CrossRef] [PubMed]
- Maged, A.; Madian, A.H.; Raafat, A.I.; Ali, A.E.-H. Cole-Cole Modelling of Reduced Graphene Oxide Decorated CuO Nano-Spheres Based Waterproof Conductive Ink for Flexible Electrodes. In Proceedings of the 2021 38th National Radio Science Conference (NRSC), Mansoura, Egypt, 27–29 July 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 207–214. [Google Scholar]
- Tai, Y.; Bera, T.K.; Lubineau, G.; Yang, Z. Combining the Converse Humidity/Resistance Response Behaviors of RGO Films for Flexible Logic Devices. J. Mater. Chem. C Mater. 2017, 5, 3848–3854. [Google Scholar] [CrossRef]
- Bhat, B.R.; Aravinda, L.S.; Bhat, K.U. Flexible Binder Free Functionalized Carbon Nanotube Electrodes for Ultracapacitor. In Proceedings of the SPIE 8987, Oxide-Based Materials and Devices V; Teherani, F.H., Look, D.C., Rogers, D.J., Eds.; SPIE: San Francisco, CA, USA, 2014; p. 89871K. [Google Scholar]
- Reshma, M.; Manu, R. Fabrication of Cu-WO3-Functionalised Multiwalled Carbon Nanotubes Composite Film Electrode by Direct Deposition for Sensing Application. Thin Solid Film. 2021, 737, 138940. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Han, H.S.; Shin, H.-Y.; Nguyen, C.T.; Phung, H.; Van Hoang, H.; Choi, H.R. Highly Sensitive Flexible Proximity Tactile Array Sensor by Using Carbon Micro Coils. Sens. Actuators A Phys. 2017, 266, 166–177. [Google Scholar] [CrossRef]
- Quan, J.; Shi, X.; Kim, K.N.; Oh, D.S.; Chua, B. Electrically Functional Water Sensing Duct Tape Suitable for Detection of Indoor Pinhole Leakages with High Spatial and Temporal Resolution. Sens. Actuators A Phys. 2023, 351, 114145. [Google Scholar] [CrossRef]
- Soares, A.C.; Soares, J.C.; dos Santos, D.M.; Migliorini, F.L.; Popolin-Neto, M.; dos Santos Cinelli Pinto, D.; Carvalho, W.A.; Brandão, H.M.; Paulovich, F.V.; Correa, D.S.; et al. Nanoarchitectonic E-Tongue of Electrospun Zein/Curcumin Carbon Dots for Detecting Staphylococcus Aureus in Milk. ACS Omega 2023, 8, 13721–13732. [Google Scholar] [CrossRef]
- Yu, J.M.; Jang, J.-W. Organic Semiconductor-Based Photoelectrochemical Cells for Efficient Solar-to-Chemical Conversion. Catalysts 2023, 13, 814. [Google Scholar] [CrossRef]
- Lo Vecchio, C.; Giacoppo, G.; Barbera, O.; Carbone, A.; Baglio, V.; Aricò, A.S.; Monforte, G.; Trocino, S. Scalability and Investigation of the Geometrical Features and Shapes of a Tandem Photo-Electrolysis Cell Based on Non-Critical Raw Materials. Catalysts 2024, 14, 98. [Google Scholar] [CrossRef]
- Gul, M.M.; Ahmad, K.S.; Thomas, A.G.; Ali, D. In:SnO2/Yb2S3:Cu2S:ZnS: A Rare Earth Metal Sulfide-Conjugated Transition Metal Sulfide Photoactive Electrode. Energy Technol. 2023, 11, 2300062. [Google Scholar] [CrossRef]
- Gul, M.M.; Ahmad, K.S.; Thomas, A.G.; Almanqur, L.; Alderhami, S.A.; Alharbi, Y.T. A Clear Path to Enhanced Performance: In:SnO2/(Er2S3–ZrS3–NiS2)GO as an Effective Transparent Electrode in Photoelectrochemical Cells. Mater. Chem. Phys. 2023, 309, 128445. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, B.; Shi, T.; Tang, Z.; Liao, G. Enhanced Photovoltaic Performance and Stability of Carbon Counter Electrode Based Perovskite Solar Cells Encapsulated by PDMS. J. Mater. Chem. A Mater. 2016, 4, 10700–10709. [Google Scholar] [CrossRef]
- Gul, M.M.; Ahmad, K.S.; Thomas, A.G.; Almanqur, L.; Alderhami, S.A. Rare In:SnO2/NdYbS3 Metal Sulphide: A Novel Photoactive Electrode for Sustainable Energy Applications. Ceram. Int. 2023, 49, 32874–32884. [Google Scholar] [CrossRef]
- Manohar, A.; Vijayakanth, V.; Prabhakar Vattikuti, S.V.; Kim, K.H. Electrochemical Investigation on Nickel-Doped Spinel Magnesium Ferrite Nanoparticles for Supercapacitor Applications. Mater. Chem. Phys. 2023, 301, 127601. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, X.; Zhou, Y.; Zhuo, C.; Huang, J.; Li, S. Facile Solvothermal Synthesis of Porous ZnFe2O4 Microspheres for Capacitive Pseudocapacitors. RSC Adv. 2015, 5, 39270–39277. [Google Scholar] [CrossRef]
- Das, S.; Manuraj, M.; Rakhi, R.B.; Ajayaghosh, A. High-Frequency Electrochemical Double Layer Capacitor Based on Carbon Nanotubes Ink Coated Eggshell Membrane Electrodes. J. Energy Storage 2022, 45, 103799. [Google Scholar] [CrossRef]
- Figueroa-González, E.; Oliva, A.I.; Rodriguez-Gonzalez, V.; Gomez-Solis, C.; Garcia, C.R.; Oliva, J. Using Recycled Tetrapak and Ag/BaMoO4 Nanoparticles to Make Efficient and Flexible Solid State Supercapacitors. J. Energy Storage 2022, 47, 103544. [Google Scholar] [CrossRef]
- Rammelt, U.; Reinhard, G. Application of Electrochemical Impedance Spectroscopy (EIS) for Characterizing the Corrosion-Protective Performance of Organic Coatings on Metals. Prog. Org. Coat. 1992, 21, 205–226. [Google Scholar] [CrossRef]
- Roggero, A.; Caussé, N.; Dantras, E.; Villareal, L.; Santos, A.; Pébère, N. Thermal Activation of Impedance Measurements on an Epoxy Coating for the Corrosion Protection: 2. Electrochemical Impedance Spectroscopy Study. Electrochim. Acta 2019, 305, 116–124. [Google Scholar] [CrossRef]
- Narozny, M.; Zakowski, K.; Darowicki, K. Application of Electrochemical Impedance Spectroscopy to Evaluate Cathodically Protected Coated Steel in Seawater. Constr. Build. Mater. 2018, 181, 721–726. [Google Scholar] [CrossRef]
- Šoić, I.; Šoljić, I.; Eškinja, M.; Mujezinović, A.; Martinez, S. The Novel Paste Electrolyte Measuring Cell for EIS Testing of the Commonly Used Surface Protection on Bronze. Prog. Org. Coat. 2023, 177, 107442. [Google Scholar] [CrossRef]
- Almufarij, R.S. Facile Formulation of New Innovative Eco-Friendly Hybrid Protective Coating for Mild Steel in Acidic Media. Sustainability 2023, 15, 2779. [Google Scholar] [CrossRef]
- Bifano, L.; Fischerauer, A.; Liedtke, A.; Fischerauer, G. Characterization of Sand and Sand–Binder Systems from the Foundry Industry with Electrical Impedance Spectroscopy. J. Sens. Sens. Syst. 2021, 10, 43–51. [Google Scholar] [CrossRef]
- Mishra, S.K.; Kumar, D.; Biradar, A.M.; Rajesh. Electrochemical Impedance Spectroscopy Characterization of Mercaptopropionic Acid Capped ZnS Nanocrystal Based Bioelectrode for the Detection of the Cardiac Biomarker—Myoglobin. Bioelectrochemistry 2012, 88, 118–126. [Google Scholar] [CrossRef]
- Bonavita, A.; Caddemi, A.; Donato, N.; Accordino, P.; Galvagno, S.; Neri, G. Electrical Characterization and Modeling of Thin-Film Humidity Sensors. In Proceedings of the ICECS 2001, 8th IEEE International Conference on Electronics, Circuits and Systems (Cat. No.01EX483), Malta, 2–5 September 2001; IEEE: Malta, 2001; Volume 2, pp. 673–676. [Google Scholar]
- Faia, P.M.; Furtado, C.S.; Ferreira, A.J. AC Impedance Spectroscopy: A New Equivalent Circuit for Titania Thick Film Humidity Sensors. Sens. Actuators B Chem. 2005, 107, 353–359. [Google Scholar] [CrossRef]
- Maritinsen, O.G.; Grimnes, S.; Schwan, H.P. Interface Phenomena and Dielectric Properties of Biological Tissue. In Encyclopedia of Surface and Colloid Science; Hubbard, A.T., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 2002; ISBN 9780824707965. [Google Scholar]
- Kerner, Z.; Pajkossy, T. On the Origin of Capacitance Dispersion of Rough Electrodes. Electrochim. Acta 2000, 46, 207–211. [Google Scholar] [CrossRef]
- Huang, V.M.-W.; Vivier, V.; Orazem, M.E.; Pébère, N.; Tribollet, B. The Apparent Constant-Phase-Element Behavior of an Ideally Polarized Blocking Electrode: A Global and Local Impedance Analysis. J. Electrochem. Soc. 2007, 154, C81–C88. [Google Scholar] [CrossRef]
- Orazem, M.E.; Frateur, I.; Tribollet, B.; Vivier, V.; Marcelin, S.; Pébère, N.; Bunge, A.L.; White, E.A.; Riemer, D.P.; Musiani, M. Dielectric Properties of Materials Showing Constant-Phase-Element (CPE) Impedance Response. J. Electrochem. Soc. 2013, 160, C215–C225. [Google Scholar] [CrossRef]
- Drüschler, M.; Huber, B.; Passerini, S.; Roling, B. Hysteresis Effects in the Potential-Dependent Double Layer Capacitance of Room Temperature Ionic Liquids at a Polycrystalline Platinum Interface. J. Phys. Chem. C 2010, 114, 3614–3617. [Google Scholar] [CrossRef]
- Lensch, H.; Bastuck, M.; Baur, T.; Schütze, A.; Sauerwald, T. Impedance Model for a High-Temperature Ceramic Humidity Sensor. J. Sens. Sens. Syst. 2019, 8, 161–169. [Google Scholar] [CrossRef]
- Rammelt, U.; Reinhard, G. On the Applicability of a Constant Phase Element (CPE) to the Estimation of Roughness of Solid Metal Electrodes. Electrochim. Acta 1990, 35, 1045–1049. [Google Scholar] [CrossRef]
- Zelinka, S.L.; Rammer, D.R.; Stone, D.S. Impedance Spectroscopy and Circuit Modeling of Southern Pine above 20% Moisture Content. Holzforschung 2008, 62, 737–744. [Google Scholar] [CrossRef]
- Park, Y.-J.; Lee, S.; Kim, B.; Kim, J.-H.; So, J.-H.; Koo, H.-J. Impedance Study on Humidity Dependent Conductivity of Polymer Composites with Conductive Nanofillers. Compos. B Eng. 2020, 202, 108412. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Breitkopf, C. Determination of Diffusion Coefficients Using Impedance Spectroscopy Data. J. Electrochem. Soc. 2018, 165, E826–E831. [Google Scholar] [CrossRef]
- Xie, D.-M.; Wang, T.-Y.; Xie, J.-P.; Cao, F.-Y.; Wang, Y.-G. Comparative Study of Two Equivalent Circuit Models for Electrochemical Impedance Spectroscopy Analysis of Epoxy Zinc-Rich Coatings. Corros. Eng. Sci. Technol. Int. J. Corros. Process. Corros. Control. 2023, 58, 734–746. [Google Scholar] [CrossRef]
- Kemp, N.T. A Tutorial on Electrochemical Impedance Spectroscopy and Nanogap Electrodes for Biosensing Applications. IEEE Sens. J. 2021, 21, 22232–22245. [Google Scholar] [CrossRef]
- Nam, I.; Kim, G.-P.; Park, S.; Han, J.W.; Yi, J. All-Solid-State, Origami-Type Foldable Supercapacitor Chips with Integrated Series Circuit Analogues. Energy Env. Sci. 2014, 7, 1095–1102. [Google Scholar] [CrossRef]
- Kampouris, D.K.; Ji, X.; Randviir, E.P.; Banks, C.E. A New Approach for the Improved Interpretation of Capacitance Measurements for Materials Utilised in Energy Storage. RSC Adv. 2015, 5, 12782–12791. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Qian, J.; Di, B.; Zhang, G.; Ren, Z. Electrical Impedance Spectroscopy (EIS) in Plant Roots Research: A Review. Plant Methods 2021, 17, 118. [Google Scholar] [CrossRef] [PubMed]
- Schipani, F.; Miller, D.R.; Ponce, M.A.; Aldao, C.M.; Akbar, S.A.; Morris, P.A. Electrical Characterization of Semiconductor Oxide-Based Gas Sensors Using Impedance Spectroscopy: A Review. Rev. Adv. Sci. Eng. 2016, 5, 86–105. [Google Scholar] [CrossRef]
- Elseady, A.A.E.; Lee, I.; Zhuge, Y.; Ma, X.; Chow, C.W.K.; Gorjian, N. Piezoresistivity and AC Impedance Spectroscopy of Cement-Based Sensors: Basic Concepts, Interpretation, and Perspective. Materials 2023, 16, 768. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liang, F.; Lu, W.; Zhao, Y.; Fan, G.; Wang, X. Improved Dielectric Properties of Ag@TiO2/PVDF Nanocomposites Induced by Interfacial Polarization and Modifiers with Different Carbon Chain Lengths. Appl. Phys. Lett. 2018, 112, 162902. [Google Scholar] [CrossRef]
- Abouelamaiem, D.I.; He, G.; Neville, T.P.; Patel, D.; Ji, S.; Wang, R.; Parkin, I.P.; Jorge, A.B.; Titirici, M.-M.; Shearing, P.R.; et al. Correlating Electrochemical Impedance with Hierarchical Structure for Porous Carbon-Based Supercapacitors Using a Truncated Transmission Line Model. Electrochim. Acta 2018, 284, 597–608. [Google Scholar] [CrossRef]
- Abouelamaiem, D.I.; He, G.; Parkin, I.; Neville, T.P.; Jorge, A.B.; Ji, S.; Wang, R.; Titirici, M.-M.; Shearing, P.R.; Brett, D.J.L. Synergistic Relationship between the Three-Dimensional Nanostructure and Electrochemical Performance in Biocarbon Supercapacitor Electrode Materials. Sustain. Energy Fuels 2018, 2, 772–785. [Google Scholar] [CrossRef]
- Mikolajek, M.; Reinheimer, T.; Bohn, N.; Kohler, C.; Hoffmann, M.J.; Binder, J.R. Fabrication and Characterization of Fully Inkjet Printed Capacitors Based on Ceramic/Polymer Composite Dielectrics on Flexible Substrates. Sci. Rep. 2019, 9, 13324. [Google Scholar] [CrossRef]
- Abdelrahman, A.A.; Bendary, S.H.; Mahmoud, S.A. Synthesis and Electrochemical Properties of Ni-Al LDH@RGO Hierarchical Nanocomposite as a Potential Counter Electrode in Dye Sensitized Solar Cells. Diam. Relat. Mater. 2023, 134, 109738. [Google Scholar] [CrossRef]
- Kononov, N.; Yagudaeva, E.; Voronov, V.; Dorofeev, S.; Malakhova, Y.; Cerven, V.; Zubov, V. Phase Transition in a Tetraaniline/Nanosilicon Composite Film Detected by Impedance Spectroscopy. J. Phys. Chem. C 2023, 127, 17063–17077. [Google Scholar] [CrossRef]
- Kowsari, E. High-Performance Supercapacitors Based on Ionic Liquids and a Graphene Nanostructure. In Ionic Liquids—Current State of the Art; Handy, S., Ed.; InTech: Rijeka, Croatia, 2015. [Google Scholar]
- Hammons, J.A.; Ustarroz, J.; Muselle, T.; Torriero, A.A.J.; Terryn, H.; Suthar, K.; Ilavsky, J. Supported Silver Nanoparticle and Near-Interface Solution Dynamics in a Deep Eutectic Solvent. J. Phys. Chem. C 2016, 120, 1534–1545. [Google Scholar] [CrossRef]
- Fletcher, S.; Black, V.J.; Kirkpatrick, I. A Universal Equivalent Circuit for Carbon-Based Supercapacitors. J. Solid State Electrochem. 2014, 18, 1377–1387. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Sun, X.; An, Y.; Song, S.; Li, C.; Wang, K.; Su, F.; Chen, C.-M.; Liu, F.; et al. Electrochemical Impedance Spectroscopy Study of Lithium-Ion Capacitors: Modeling and Capacity Fading Mechanism. J. Power Sources 2021, 488, 229454. [Google Scholar] [CrossRef]
- Niklasson, G.A.; Qiu, Z.; Bayrak Pehlivan, I.; Edvinsson, T. Impedance Spectroscopy of Water Splitting Reactions on Nanostructured Metal-Based Catalysts. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Functional Materials and Nanotechnologies (FM&NT 2018), Riga, Latvia, 2–5 October 2018; Volume 503, p. 012005. [Google Scholar]
- Tolouei, N.E.; Mazrouei, R.; Shavezipur, M. Three-Dimensional Impedance-Based Sensors for Detection of Chemicals in Aqueous Solutions. Int. J. Anal. Bioanal. Methods 2020, 2, 35840. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Y.; Lassiter, K.; Li, Y.; Hargis, B.; Tung, S.; Berghman, L.; Bottje, W. Interdigitated Array Microelectrode Based Impedance Immunosensor for Detection of Avian Influenza Virus H5N1. Talanta 2009, 79, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Faia, P.M.; Libardi, J.; Barbosa, I.; Araújo, E.S.; de Oliveira, H.P. Preparation, Characterization, and Evaluation of Humidity-Dependent Electrical Properties of Undoped and Niobium Oxide-Doped TiO2:WO3 Mixed Powders. Adv. Mater. Sci. Eng. 2017, 2017, 2808262. [Google Scholar] [CrossRef]
- Araújo, E.S.; Libardi, J.; Faia, P.M.; de Oliveira, H.P. Characterization and Electrical Response to Humidity of Sintered Polymeric Electrospun Fibers of Vanadium Oxide-TiO2/WO3. J. Electron. Mater. 2018, 47, 2710–2717. [Google Scholar] [CrossRef]

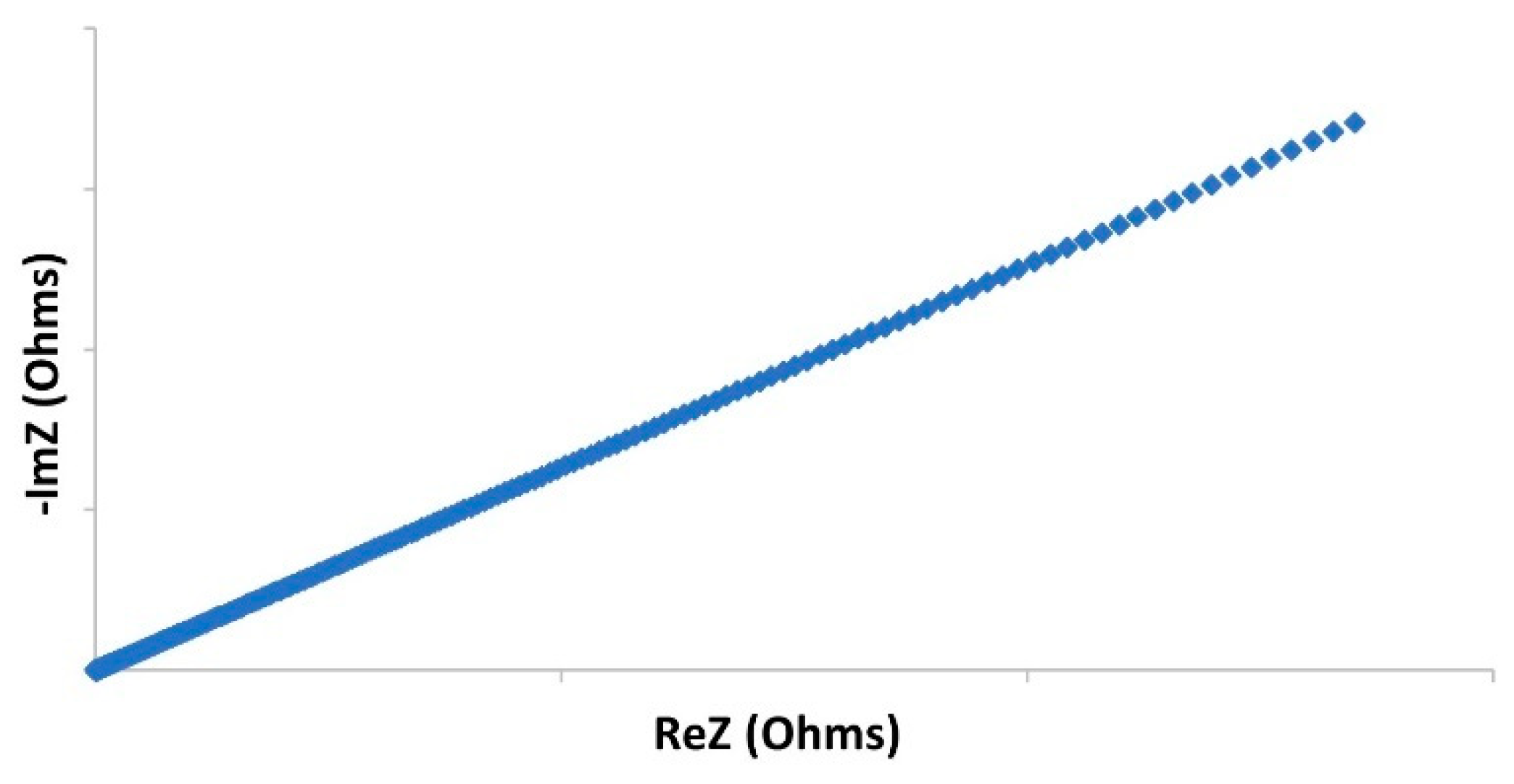
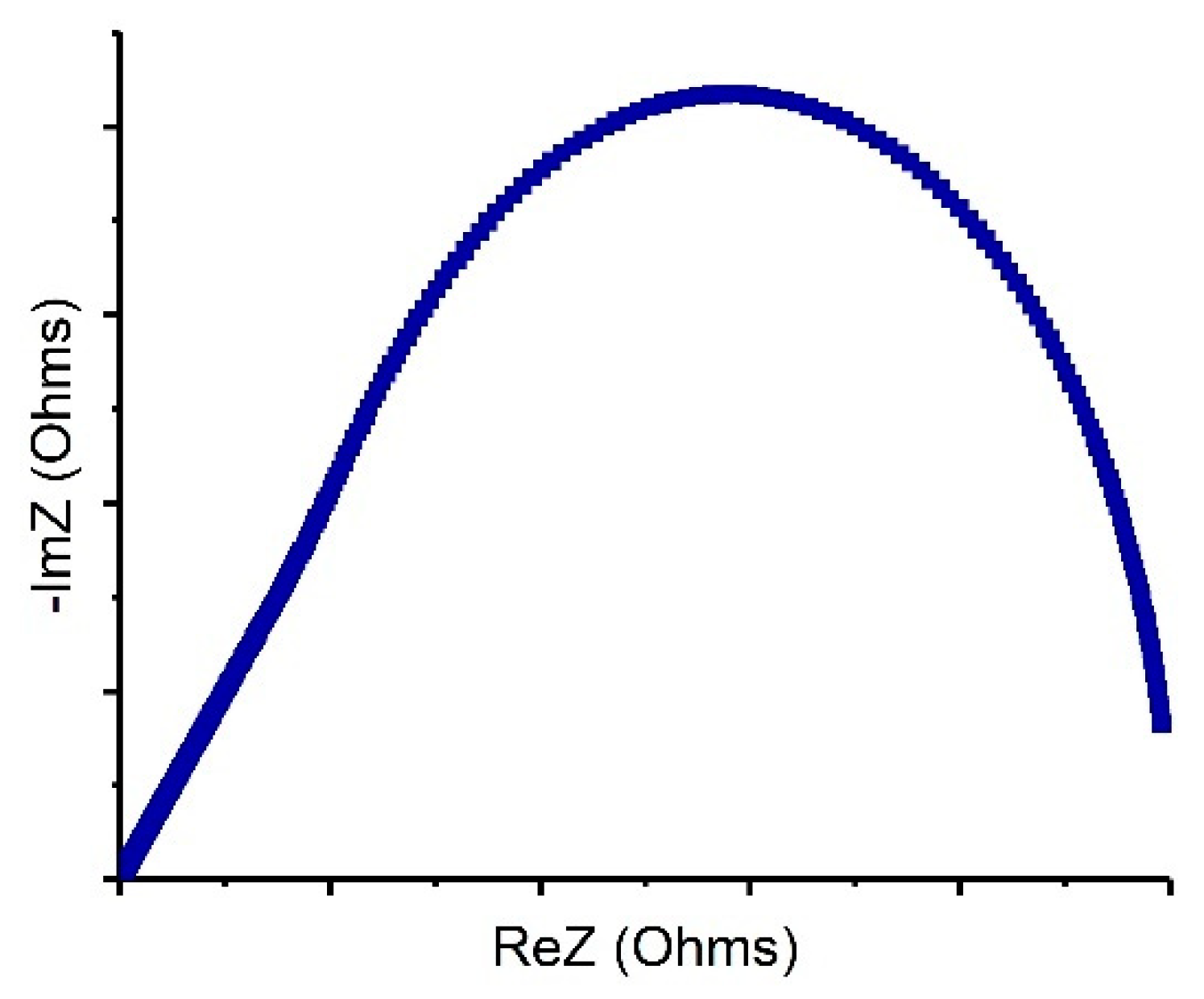
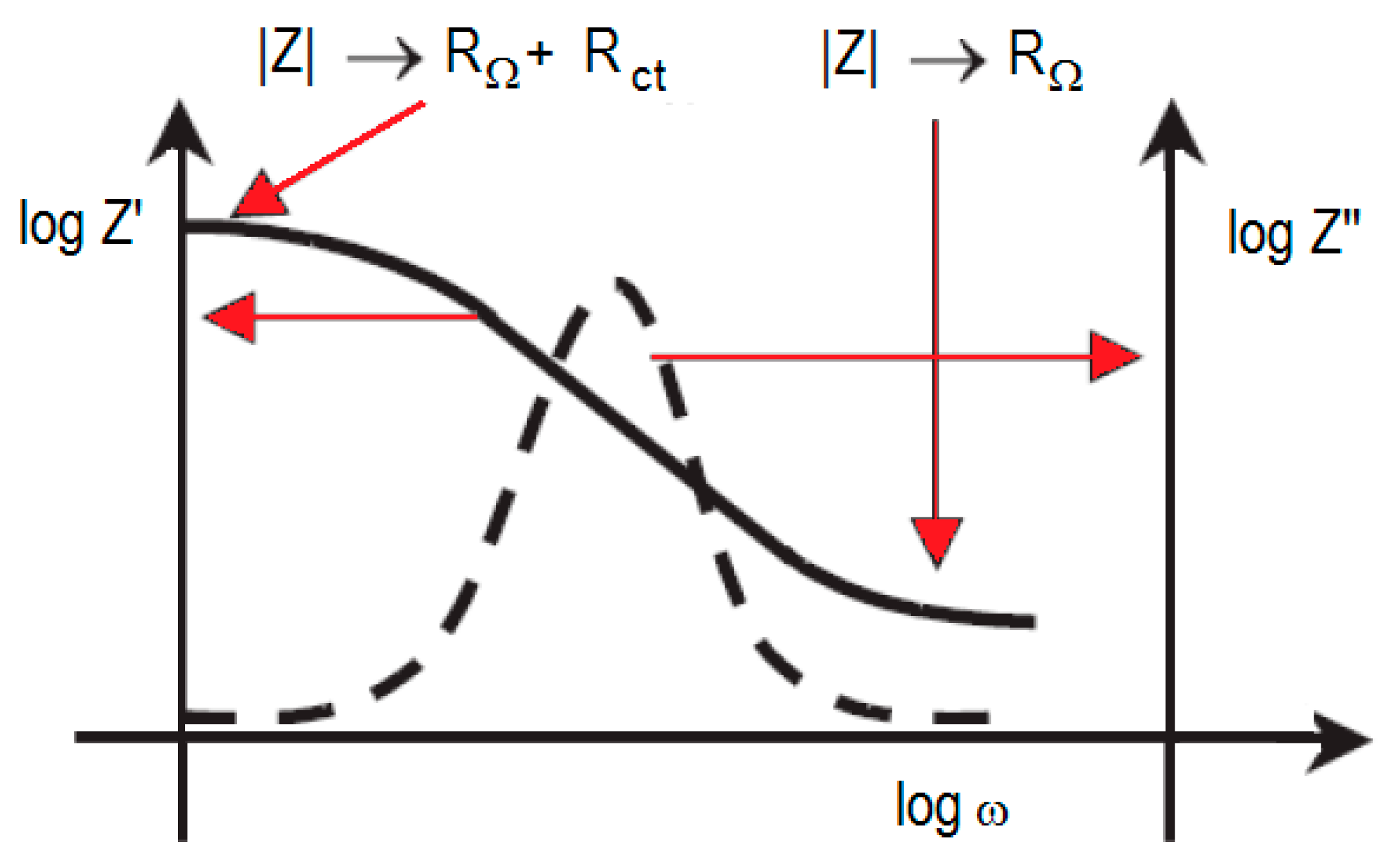
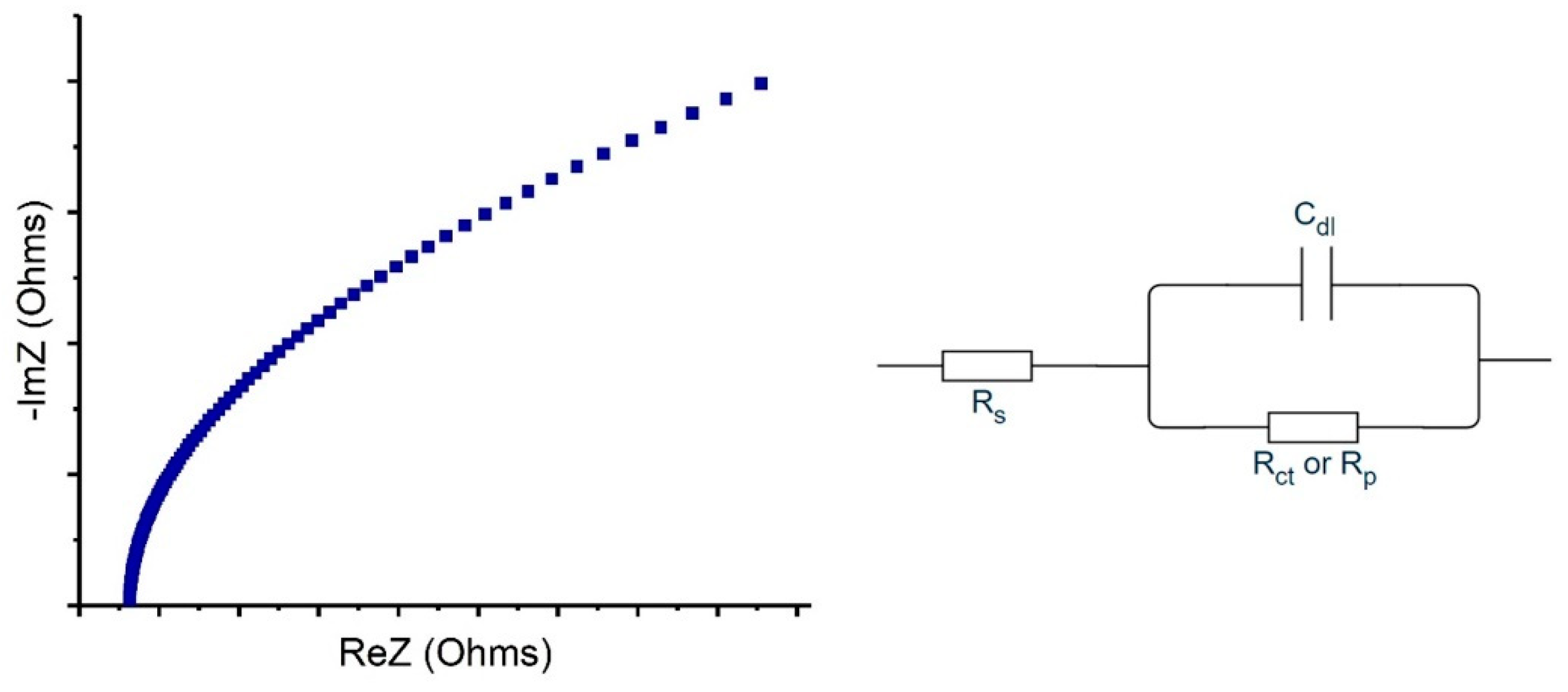

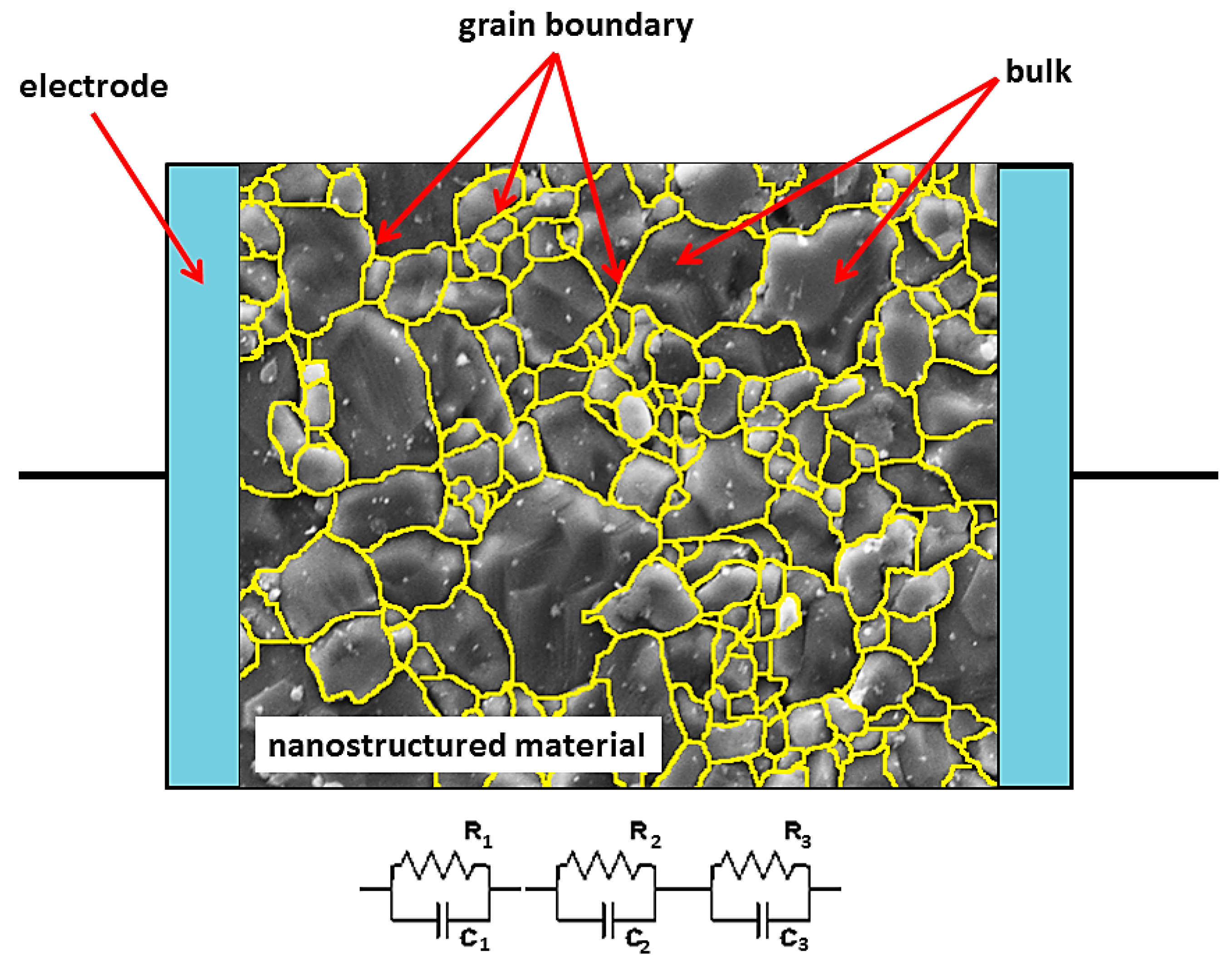
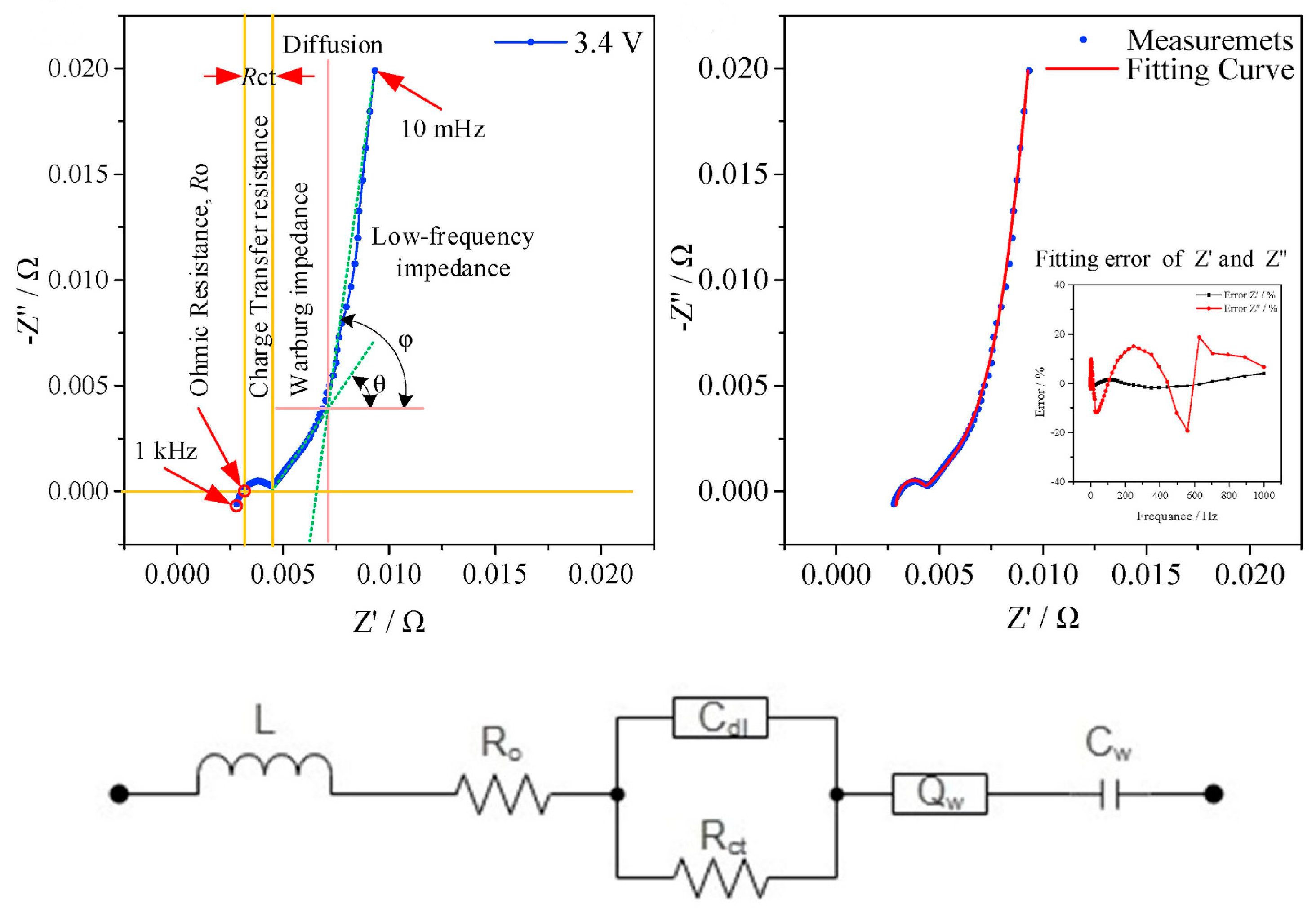
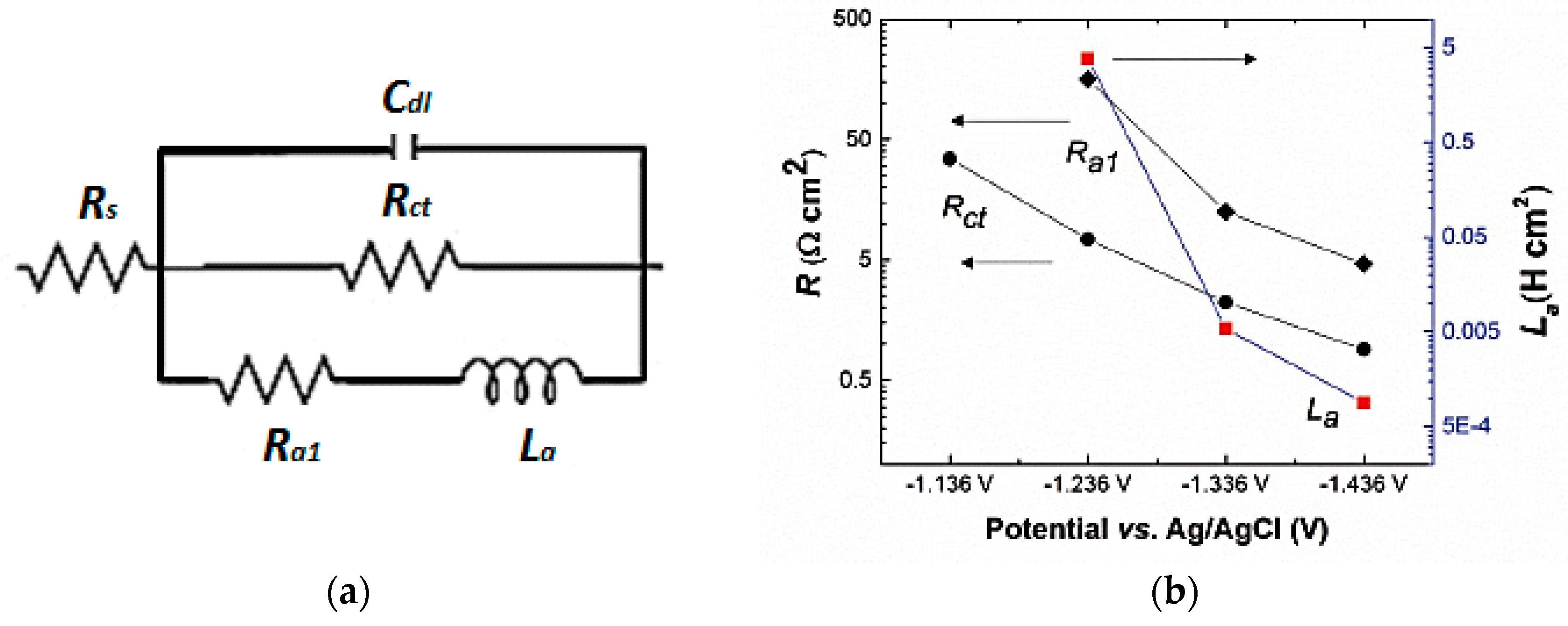


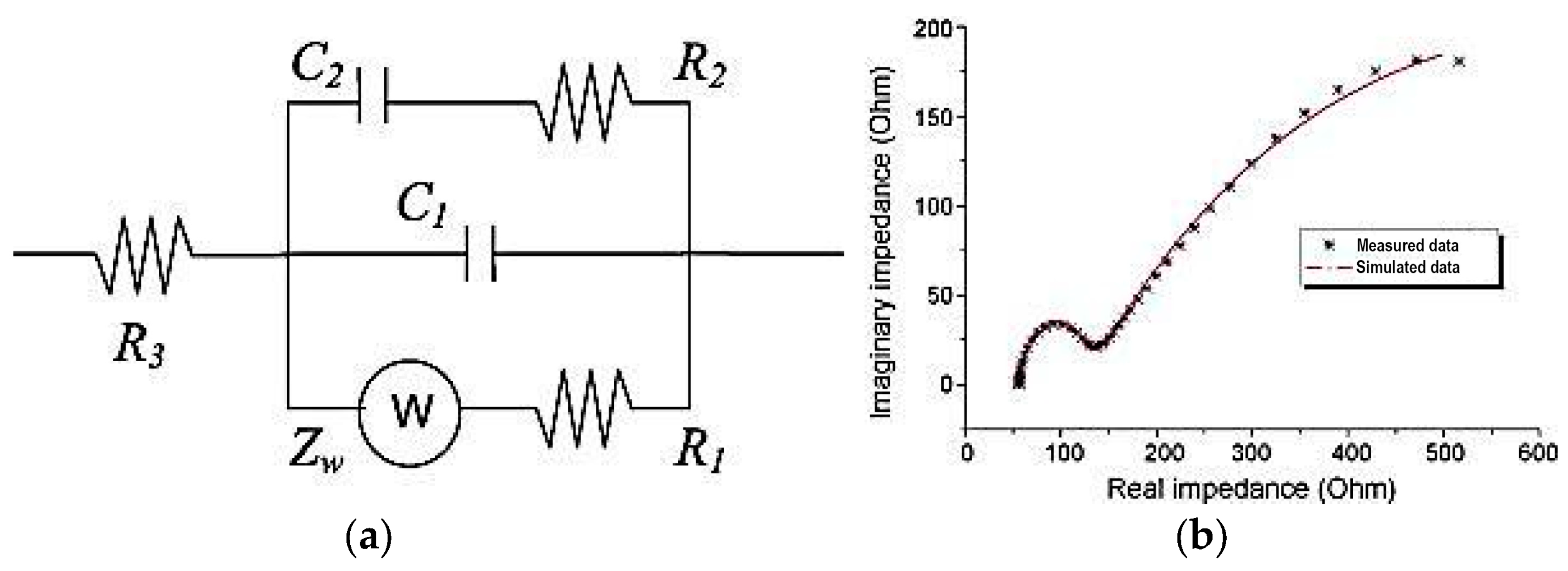
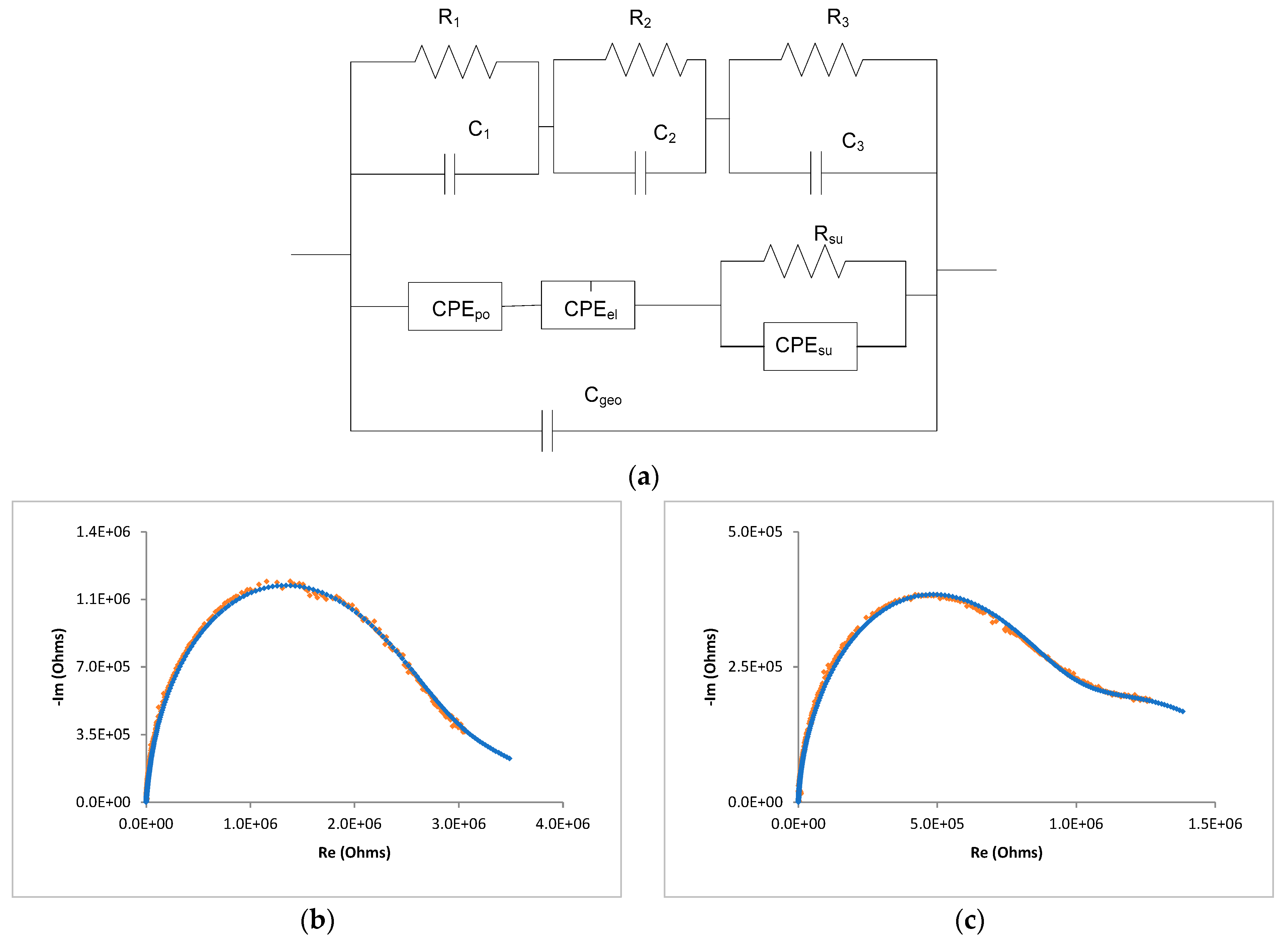
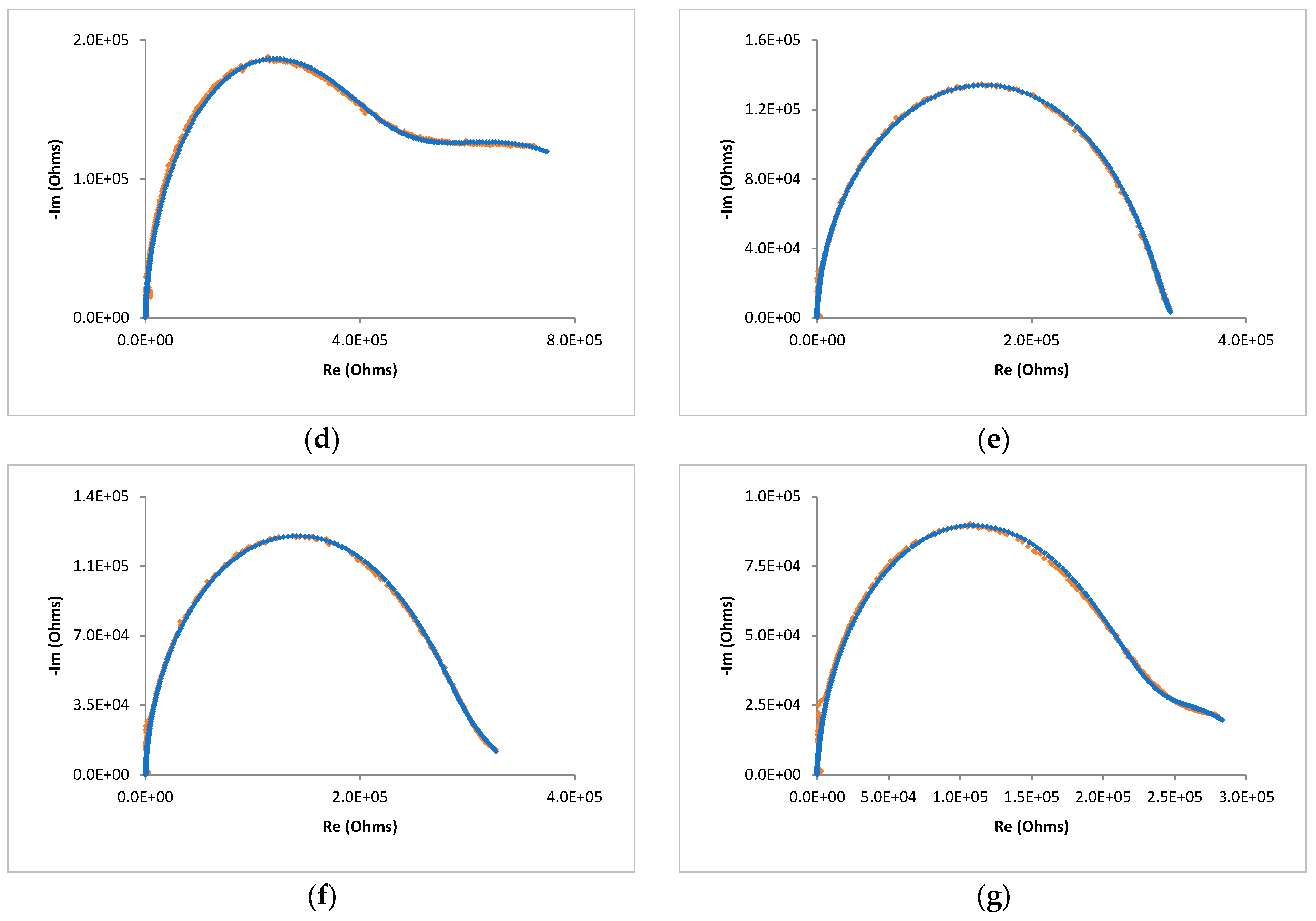


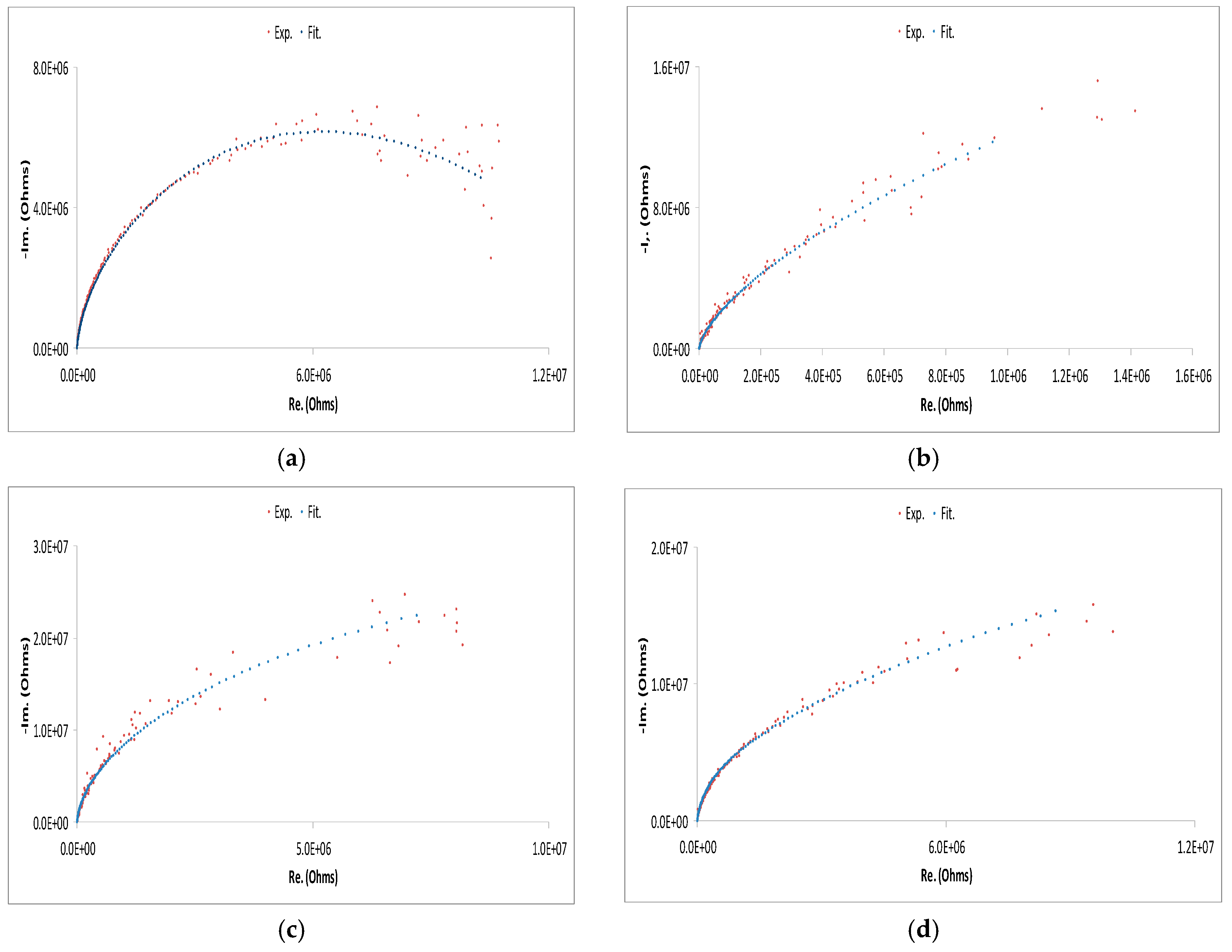
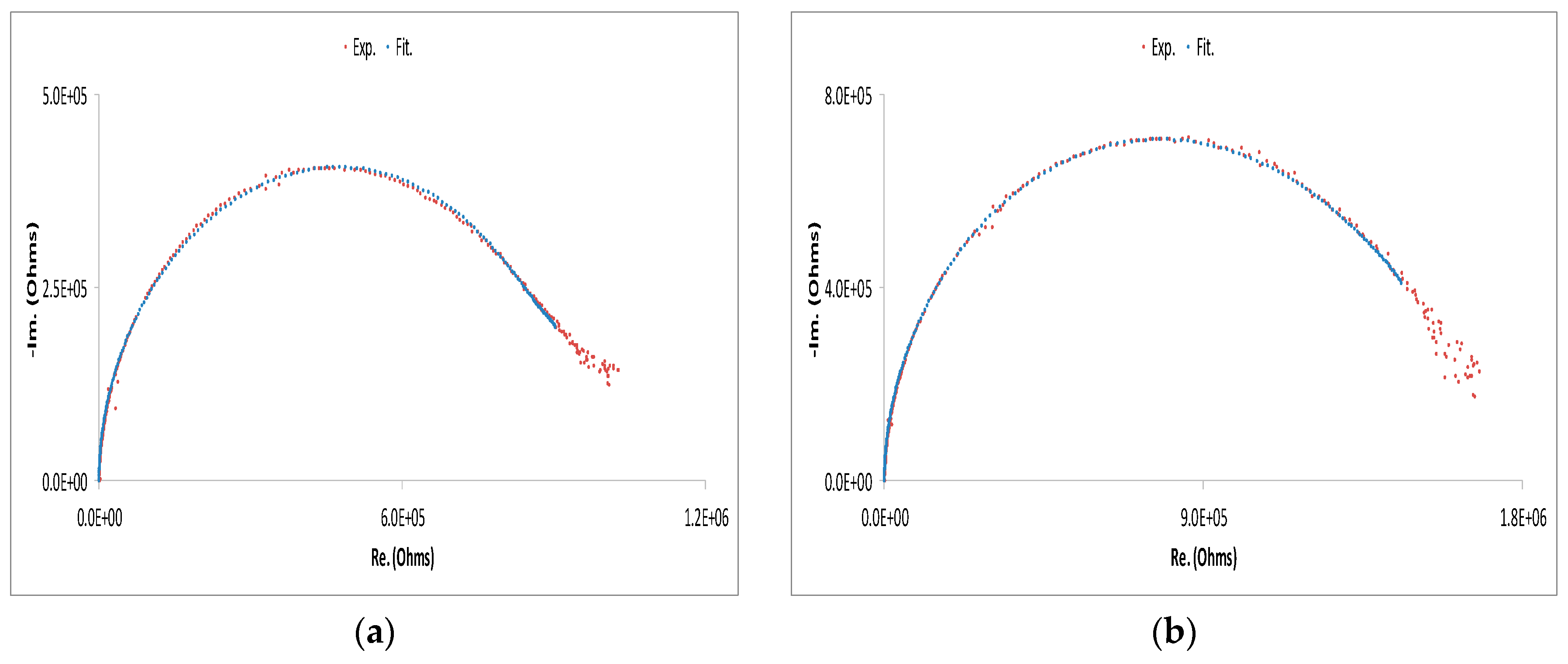

| Cell Configuration Approach (n° of Points) | Frequency Range (Hz) | Use of Bias Signal | Reported in |
|---|---|---|---|
| EleIS | Starting from 0.01 Hz up to 10 MHz | No | [38,51,53,54,55,56,57,58,61,62,63,65,66,75,76,77,78,79,82,85,87,88,91,92,93,96,98] |
| EIS | Starting from 0.01 Hz up to 100 kHz | Yes | [41,42,43,44,46,47,48,50,89,94,95] |
| RH (%) | R1 (Ω) | C1 (F) | R2 (Ω) | C2 (F) | Ypo (Ω) | npo (a.u.) | Yel (Ω) | nel (a.u.) | Cgeo (F) | R3 (Ω) | C3 (F) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 1.2 × 107 | 8.0 × 10−12 | 2.1 × 106 | 6.0 × 10−12 | 1.2 × 10−9 | 5.1 × 10−1 | 4.0 × 10−9 | 6.1 × 10−1 | 3.2 × 10−11 | 2.3 × 107 | 6.0 × 10−12 |
| 20 | 3.4 × 107 | 2.0 × 10−11 | 2.2 × 107 | 8.1 × 10−11 | 1.2 × 10−9 | 4.9 × 10−1 | 6.0 × 10−9 | 6.1 × 10−1 | 2.3 × 10−11 | 3.5 × 107 | 6.0 × 10−12 |
| 30 | 3.6 × 107 | 2.5 × 10−11 | 9.2 × 106 | 1.1 × 10−10 | 1.1 × 10−9 | 4.9 × 10−1 | 6.0 × 10−9 | 5.8 × 10−1 | 2.3 × 10−11 | 5.0 × 107 | 7.0 × 10−12 |
| 40 | 3.6 × 107 | 2.5 × 10−11 | 3.2 × 106 | 1.1 × 10−10 | 1.1 × 10−9 | 4.9 × 10−1 | 6.0 × 10−9 | 5.8 × 10−1 | 2.3 × 10−11 | 5.0 × 107 | 7.0 × 10−12 |
| 50 | 1.6 × 107 | 2.5 × 10−11 | 3.2 × 106 | 1.1 × 10−10 | 1.2 × 10−9 | 4.9 × 10−1 | 6.0 × 10−9 | 5.8 × 10−1 | 2.3 × 10−11 | 3.6 × 107 | 1.3 × 10−11 |
| 60 | 1.6 × 107 | 2.5 × 10−11 | 3.2 × 106 | 1.1 × 10−10 | 1.2 × 10−9 | 4.9 × 10−1 | 6.0 × 10−9 | 5.8 × 10−1 | 2.3 × 10−11 | 3.6 × 107 | 1.3 × 10−11 |
| 70 | 1.4 × 107 | 2.2 × 10−11 | 1.7 × 106 | 2.3 × 10−10 | 1.6 × 10−9 | 4.9 × 10−1 | 7.0 × 10−9 | 5.9 × 10−1 | 2.3 × 10−11 | 2.1 × 107 | 1.4 × 10−11 |
| 80 | 1.1 × 107 | 2.2 × 10−11 | 1.1 × 106 | 2.3 × 10−10 | 1.6 × 10−9 | 5.0 × 10−1 | 1.0 × 10−8 | 6.2 × 10−1 | 2.3 × 10−11 | 1.1 × 107 | 1.8 × 10−11 |
| 90 | 1.0 × 107 | 2.2 × 10−11 | 9.2 × 105 | 2.3 × 10−10 | 1.6 × 10−9 | 5.0 × 10−1 | 1.0 × 10−8 | 6.2 × 10−1 | 2.3 × 10−11 | 9.3 × 106 | 1.9 × 10−11 |
| 100 | 6.5 × 106 | 2.2 × 10−11 | 8.4 × 105 | 2.3 × 10−10 | 1.6 × 10−9 | 5.0 × 10−1 | 1.0 × 10−8 | 6.2 × 10−1 | 2.3 × 10−11 | 6.3 × 106 | 2.1 × 10−11 |
| RH (%) | R1 (Ω) | C1 (F) | R2 (Ω) | C2 (F) | Ypo (Ω) | npo (a.u.) | Yel (Ω) | nel (a.u.) | Cgeo (F) | R3 (Ω) | C3 (F) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 1.6 × 107 | 1.4 × 10−11 | 1.7 × 106 | 1.7 × 10−10 | 7.2 × 10−10 | 5.2 × 10−1 | 1.9 × 10−9 | 6.5 × 10−1 | 1.2 × 10−11 | 2.1 × 107 | 9.1 × 10−12 |
| 20 | 7.6 × 107 | 2.4 × 10−11 | 1.7 × 107 | 1.7 × 10−9 | 4.1 × 10−10 | 5.2 × 10−1 | 9.9 × 10−9 | 6.2 × 10−1 | 5.2 × 10−11 | 3.7 × 108 | 7.2 × 10−12 |
| 30 | 7.6 × 107 | 2.1 × 10−11 | 1.7 × 107 | 1.7 × 10−9 | 1.6 × 10−10 | 5.2 × 10−1 | 9.9 × 10−9 | 7.2 × 10−1 | 3.2 × 10−11 | 3.7 × 108 | 6.0 × 10−12 |
| 40 | 7.6 × 107 | 2.1 × 10−11 | 1.7 × 107 | 1.7 × 10−9 | 1.6 × 10−10 | 5.2 × 10−1 | 9.9 × 10−9 | 7.2 × 10−1 | 3.2 × 10−11 | 3.7 × 108 | 4.0 × 10−12 |
| 50 | 7.6 × 107 | 2.1 × 10−11 | 1.7 × 107 | 1.7 × 10−9 | 1.6 × 10−10 | 5.0 × 10−1 | 9.9 × 10−9 | 7.2 × 10−1 | 3.2 × 10−11 | 1.8 × 108 | 6.0 × 10−12 |
| 60 | 7.6 × 107 | 6.1 × 10−11 | 3.7 × 107 | 3.7 × 10−11 | 1.6 × 10−10 | 4.9 × 10−1 | 9.9 × 10–10 | 6.0 × 10−1 | 0.7 × 10−11 | 1.8 × 108 | 3.0 × 10−11 |
| 70 | 3.1 × 107 | 6.1 × 10−11 | 5.7 × 107 | 2.7 × 10−11 | 1.3 × 10−10 | 4.9 × 10−1 | 2.0 × 10−8 | 3.0 × 10−1 | 0.1 × 10−11 | 2.0 × 107 | 3.1 × 10−11 |
| 80 | 3.1 × 107 | 5.1 × 10−11 | 6.7 × 107 | 1.6 × 10−11 | 2.3 × 10−10 | 4.9 × 10−1 | 2.0 × 10−8 | 3.0 × 10−1 | 1.0 × 10−11 | 2.7 × 107 | 3.6 × 10−11 |
| 90 | 4.1 × 107 | 5.1 × 10−11 | 6.7 × 107 | 1.6 × 10−11 | 3.2 × 10−10 | 4.9 × 10−1 | 2.0 × 10−8 | 3.0 × 10−1 | 0.8 × 10−11 | 3.5 × 107 | 2.5 × 10−11 |
| 100 | 5.1 × 106 | 5.1 × 10−11 | 6.7 × 107 | 1.6 × 10−11 | 3.2 × 10−10 | 4.9 × 10−1 | 10.0 × 10−8 | 3.0 × 10−1 | 1.0 × 10−11 | 3.8 × 106 | 2.2 × 10−11 |
| RH (%) | R1 (Ω) | C1 (F) | R2 (Ω) | C2 (F) | Ypo (Ω) | npo (a.u.) | Yel (Ω) | nel (a.u.) | Cgeo (F) | R3 (Ω) | C3 (F) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 2.3 × 105 | 1.1 × 10−10 | 7.2 × 105 | 4.5 × 10−12 | 9.0 × 10−12 | 8.8 × 10−1 | 2.9 × 10−7 | 2.1 × 10−2 | 4.1 × 10−11 | 2.7 × 105 | 1.8 × 10−9 |
| 20 | 2.3 × 105 | 1.1 × 10−10 | 1.2 × 106 | 4.5 × 10−12 | 9.0 × 10−12 | 8.8 × 10−1 | 2.9 × 10−7 | 2.1 × 10−2 | 4.1 × 10−11 | 3.9 × 105 | 1.2 × 10−9 |
| 30 | 2.3 × 105 | 1.1 × 10−10 | 1.4 × 106 | 4.5 × 10−12 | 9.0 × 10−12 | 8.8 × 10−1 | 2.9 × 10−7 | 2.1 × 10−2 | 4.1 × 10−11 | 4.3 × 105 | 1.1 × 10−9 |
| 40 | 2.3 × 105 | 1.1 × 10−10 | 1.4 × 106 | 4.5 × 10−12 | 9.0 × 10−12 | 8.8 × 10−1 | 2.9 × 10−7 | 2.1 × 10−2 | 4.1 × 10−11 | 4.5 × 105 | 9.2 × 10−10 |
| 50 | 2.3 × 105 | 1.1 × 10−10 | 1.2 × 106 | 4.5 × 10−12 | 9.0 × 10−12 | 8.8 × 10−1 | 2.9 × 10−7 | 2.1 × 10−2 | 4.1 × 10−11 | 4.6 × 105 | 9.5 × 10−10 |
| 60 | 2.3 × 105 | 1.1 × 10−10 | 1.2 × 106 | 4.5 × 10−12 | 9.0 × 10−12 | 8.8 × 10−1 | 2.9 × 10−7 | 2.1 × 10−2 | 4.1 × 10−11 | 4.6 × 105 | 9.5 × 10−10 |
| 70 | 2.0 × 105 | 1.1 × 10−10 | 8.4 × 105 | 4.5 × 10−12 | 9.0 × 10−12 | 8.8 × 10−1 | 2.9 × 10−7 | 2.1 × 10−2 | 4.1 × 10−11 | 3.6 × 105 | 1.1 × 10−9 |
| 80 | 1.9 × 105 | 1.1 × 10−10 | 6.4 × 105 | 4.5 × 10−12 | 9.0 × 10−12 | 8.8 × 10−1 | 2.9 × 10−7 | 2.1 × 10−2 | 4.1 × 10−11 | 2.5 × 105 | 1.6 × 10−9 |
| 90 | 1.6 × 105 | 1.2 × 10−10 | 5.2 × 105 | 4.5 × 10−12 | 9.0 × 10−12 | 9.0 × 10−1 | 2.9 × 10−7 | 2.1 × 10−2 | 4.1 × 10−11 | 2.1 × 105 | 1.6 × 10−9 |
| 100 | 1.4 × 105 | 1.5 × 10−10 | 4.6 × 105 | 4.7 × 10−12 | 4.0 × 10−11 | 9.0 × 10−1 | 5.0 × 10−7 | 1.9 × 10−2 | 4.1 × 10−11 | 1.6 × 105 | 2.0 × 10−9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, G.M.G.; Faia, P.M.; Mendes, S.R.; Araújo, E.S. A Review of Impedance Spectroscopy Technique: Applications, Modelling, and Case Study of Relative Humidity Sensors Development. Appl. Sci. 2024, 14, 5754. https://doi.org/10.3390/app14135754
da Silva GMG, Faia PM, Mendes SR, Araújo ES. A Review of Impedance Spectroscopy Technique: Applications, Modelling, and Case Study of Relative Humidity Sensors Development. Applied Sciences. 2024; 14(13):5754. https://doi.org/10.3390/app14135754
Chicago/Turabian Styleda Silva, Georgenes M. G., Pedro M. Faia, Sofia R. Mendes, and Evando S. Araújo. 2024. "A Review of Impedance Spectroscopy Technique: Applications, Modelling, and Case Study of Relative Humidity Sensors Development" Applied Sciences 14, no. 13: 5754. https://doi.org/10.3390/app14135754
APA Styleda Silva, G. M. G., Faia, P. M., Mendes, S. R., & Araújo, E. S. (2024). A Review of Impedance Spectroscopy Technique: Applications, Modelling, and Case Study of Relative Humidity Sensors Development. Applied Sciences, 14(13), 5754. https://doi.org/10.3390/app14135754







