An Evaluation of the Fire Safety of Waste Paper-Based Internal Finishing Materials Combined with Expandable Graphite According to Changes in Magnesium Hydroxide Content
Abstract
:1. Introduction
2. Description of Specimens and Test
2.1. Description of Process to Make Specimens
- Expandable graphite has been utilized as a flame retardant based on its physical mechanism in various architectural materials research studies [15,16,17,18]. Expandable graphite expands into a carbon layer upon heating, thus protecting the bottom of the specimen and simultaneously inhibiting the external spread of fire [18,19,20]. An 80-mesh expandable graphite was chosen in this study due to its significant reduction in total heat release compared to expandable graphite of other particle sizes [21];
- Magnesium hydroxide has been utilized as a flame retardant with a chemical mechanism in various studies [22,23,24,25,26]. Magnesium hydroxide was added to enhance the fire performance through an endothermic reaction during the heat reaction. The endothermic reaction of magnesium hydroxide is expressed in Equation (1) [27,28,29].
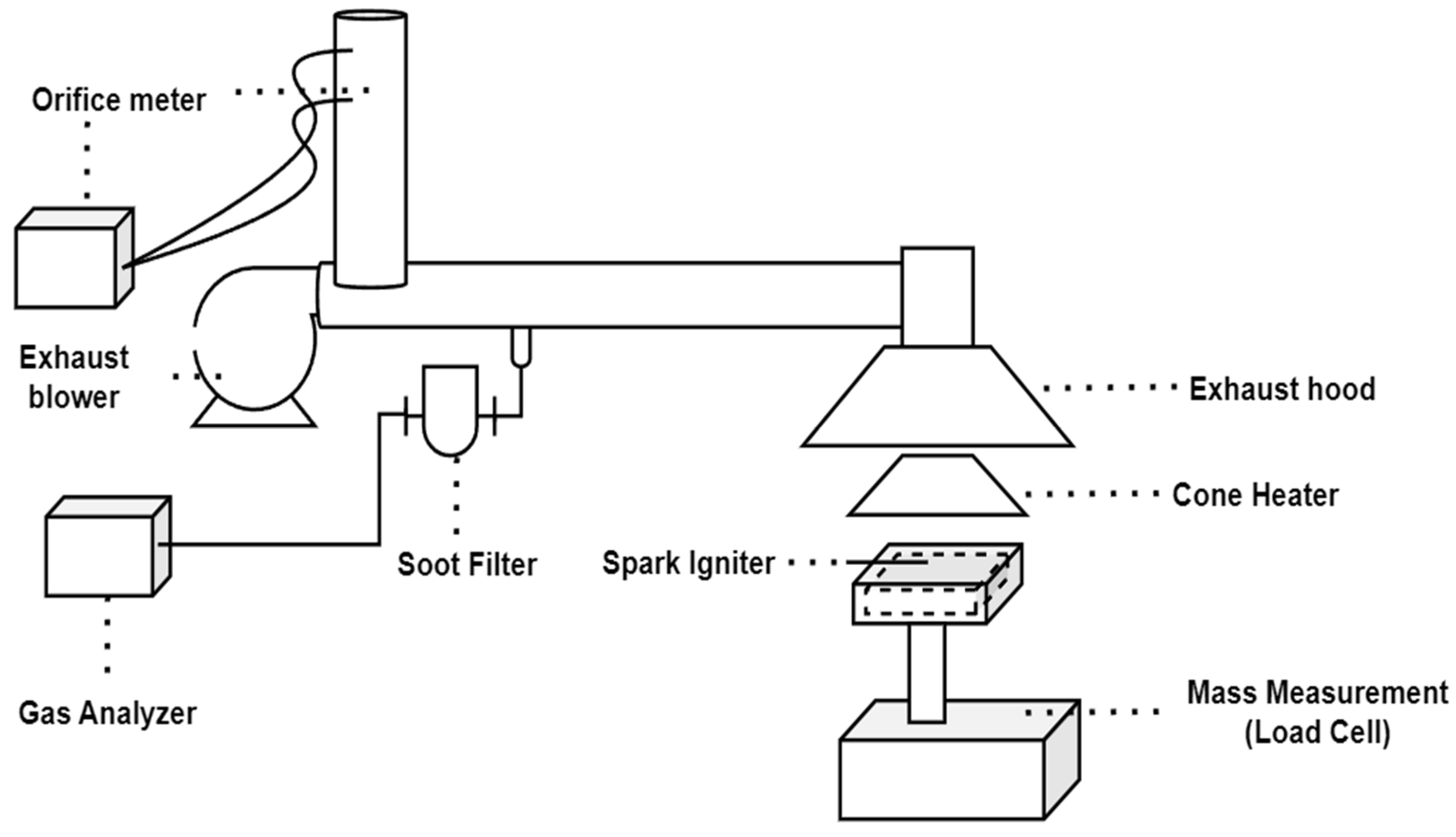
2.2. Description of Heat Release Rate Test
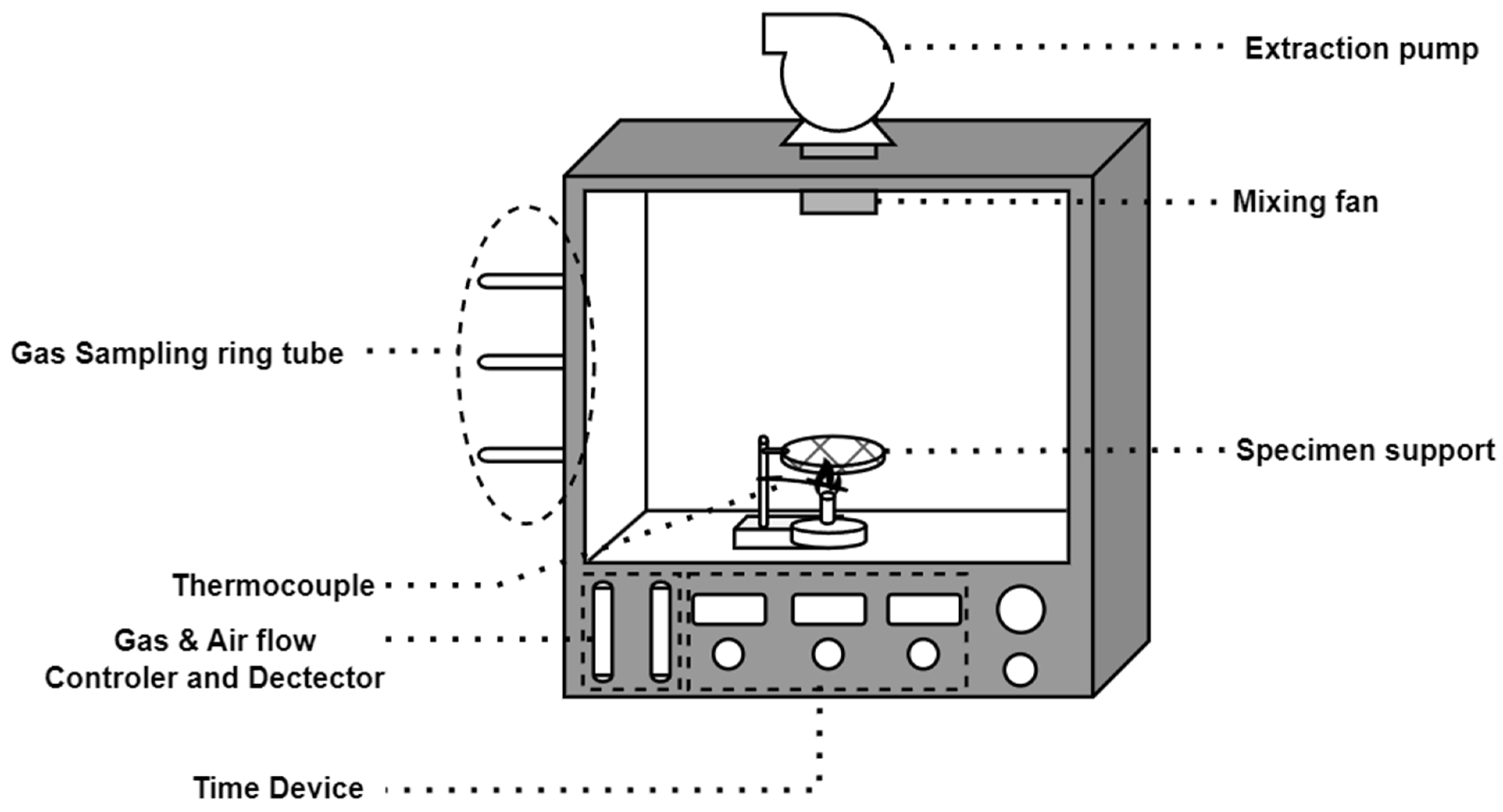
2.3. Description of NES 713 Test
3. Test Results
3.1. Result of ISO 5660-1
3.2. Result of NES 713
4. Discussion
4.1. ISO 5660-1 Test
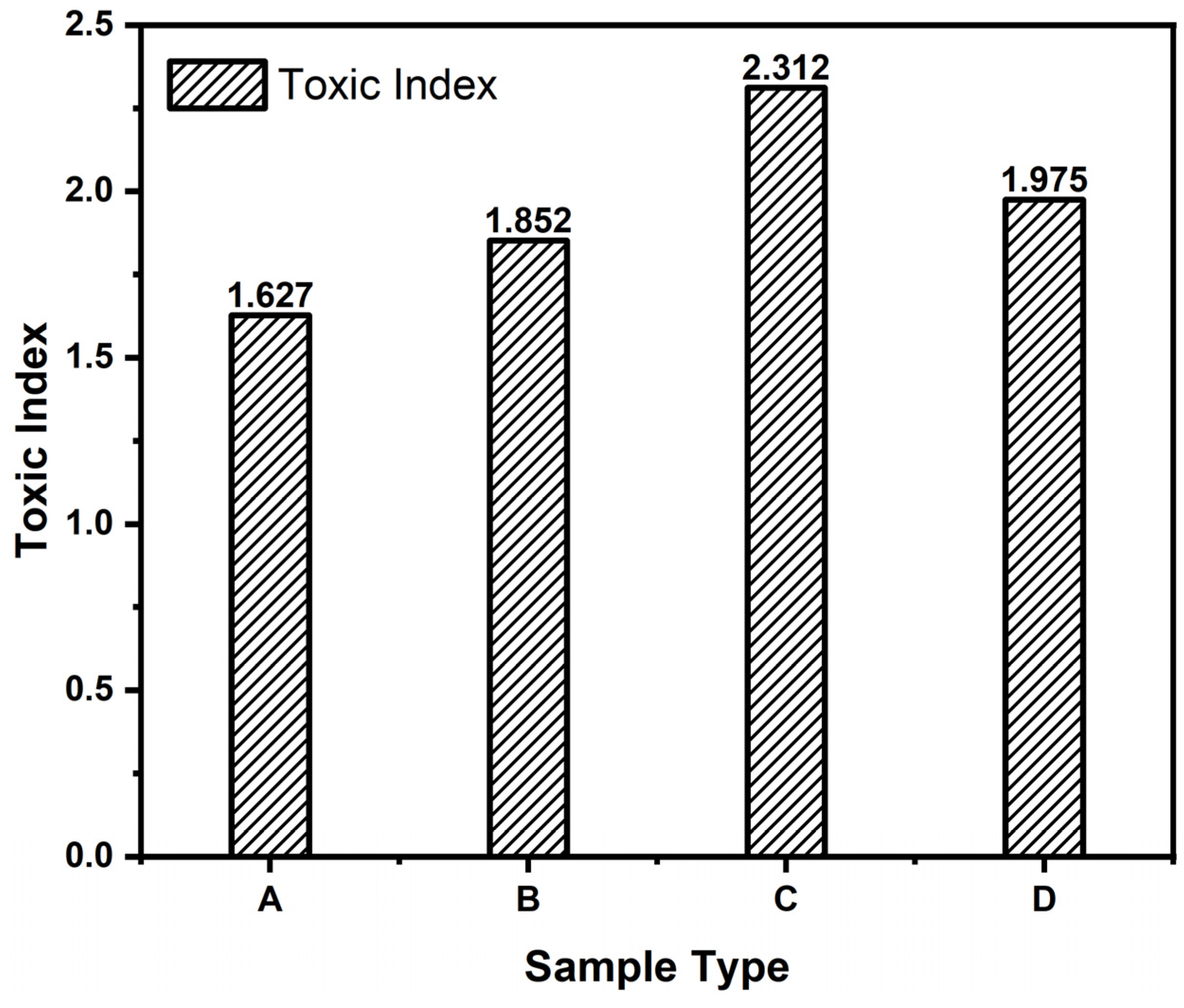
4.2. Toxic Index
5. Conclusions
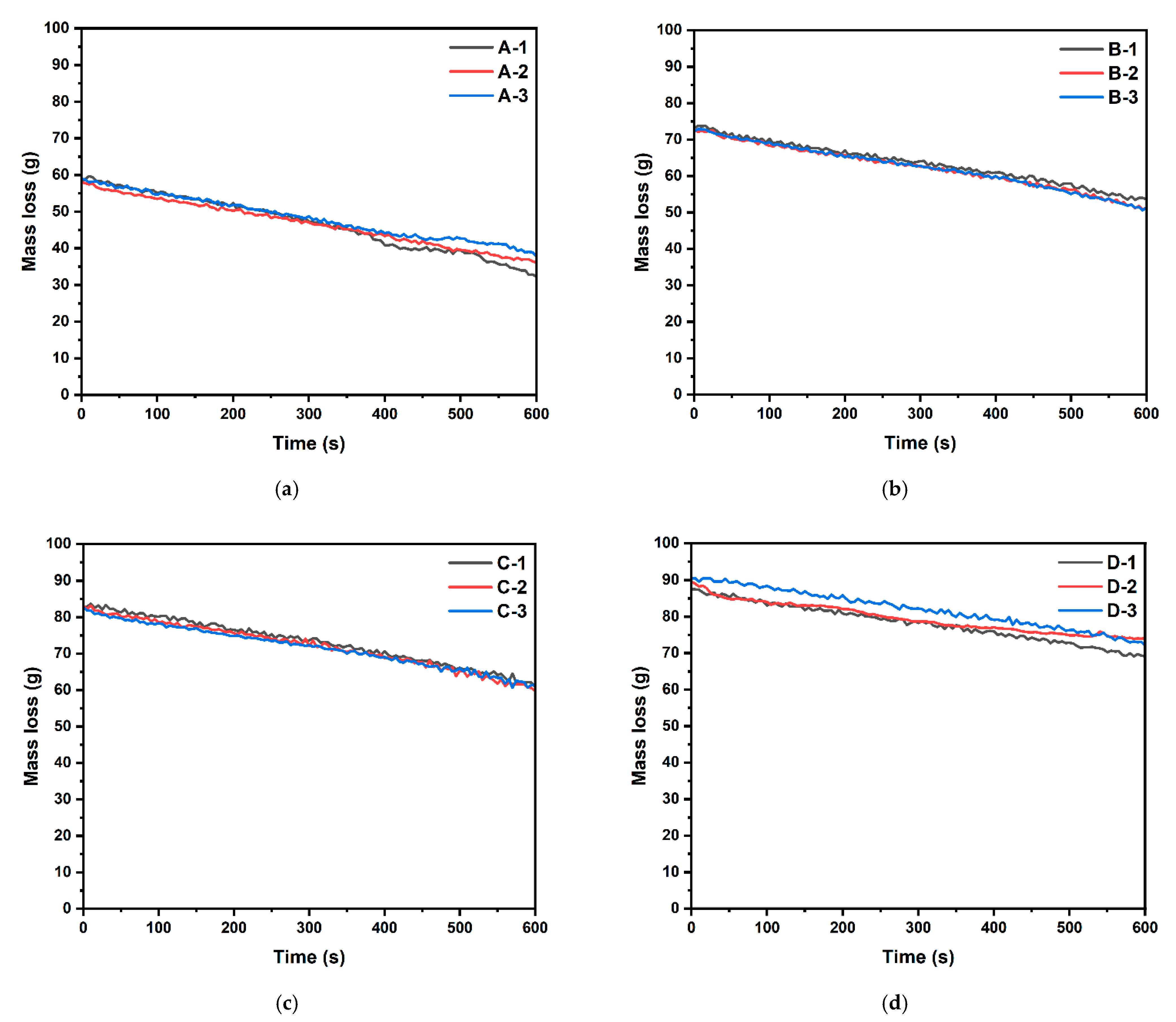
- The cellulose building finishing material manufactured using a small press exhibited uniform total heat release, consistent CO and CO2 generation, and uniform mass loss;
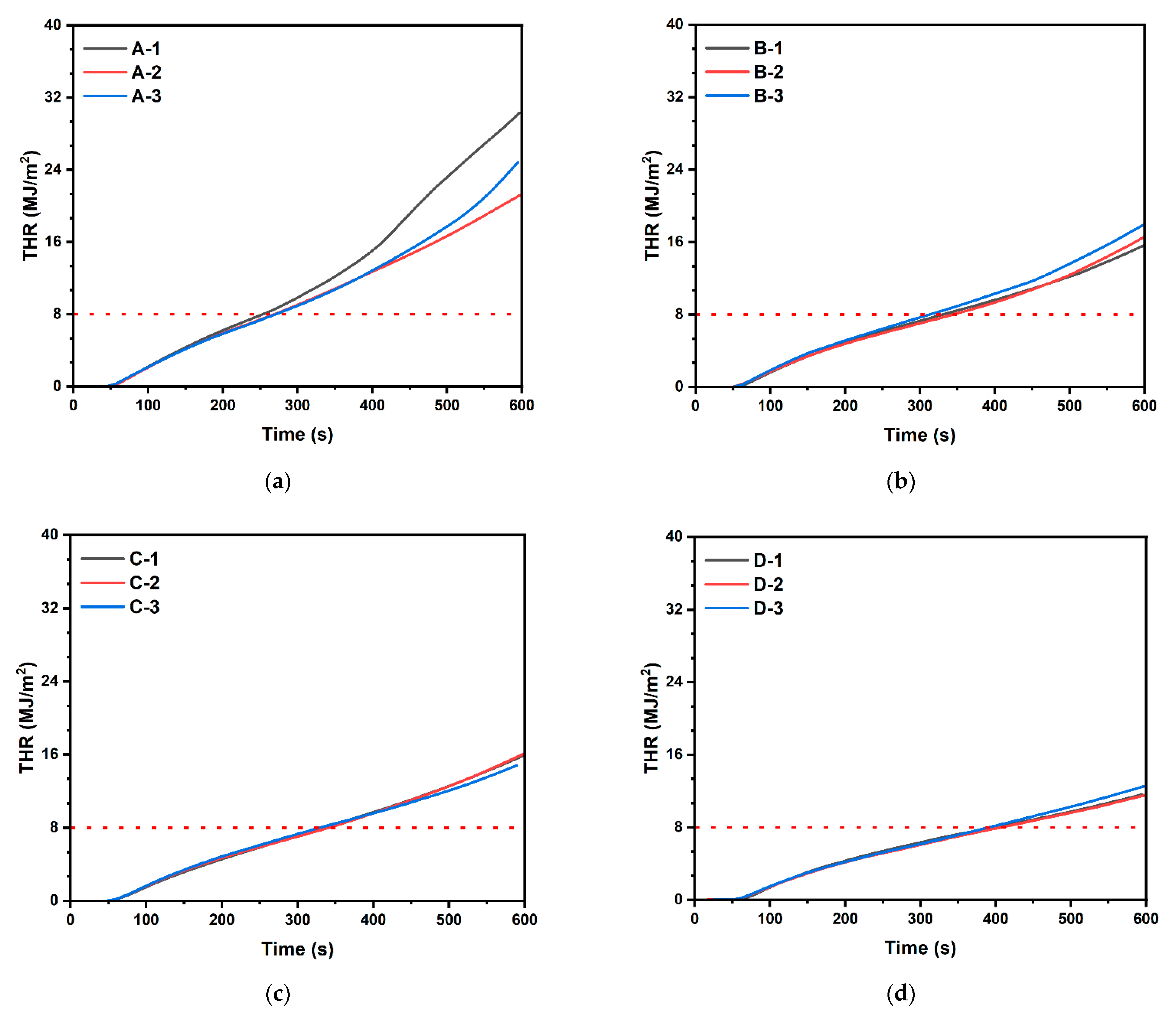
- ISO 5660-1 tests conducted on specimens produced by varying magnesium hydroxide additions revealed that as the magnesium hydroxide content increased, the total heat release rate of the cellulose building finishing material decreased;
- Based on the results of ISO 5660-1 tests, it was confirmed that cellulose building finishing material, composed of 100 g of cellulose and 20 g of expanded graphite along with the addition of at least 10 g of magnesium hydroxide, did not exceed a THR of 8 MJ/m2 at 300 s. This affirms its potential utility as a fire-resistant building finishing material;
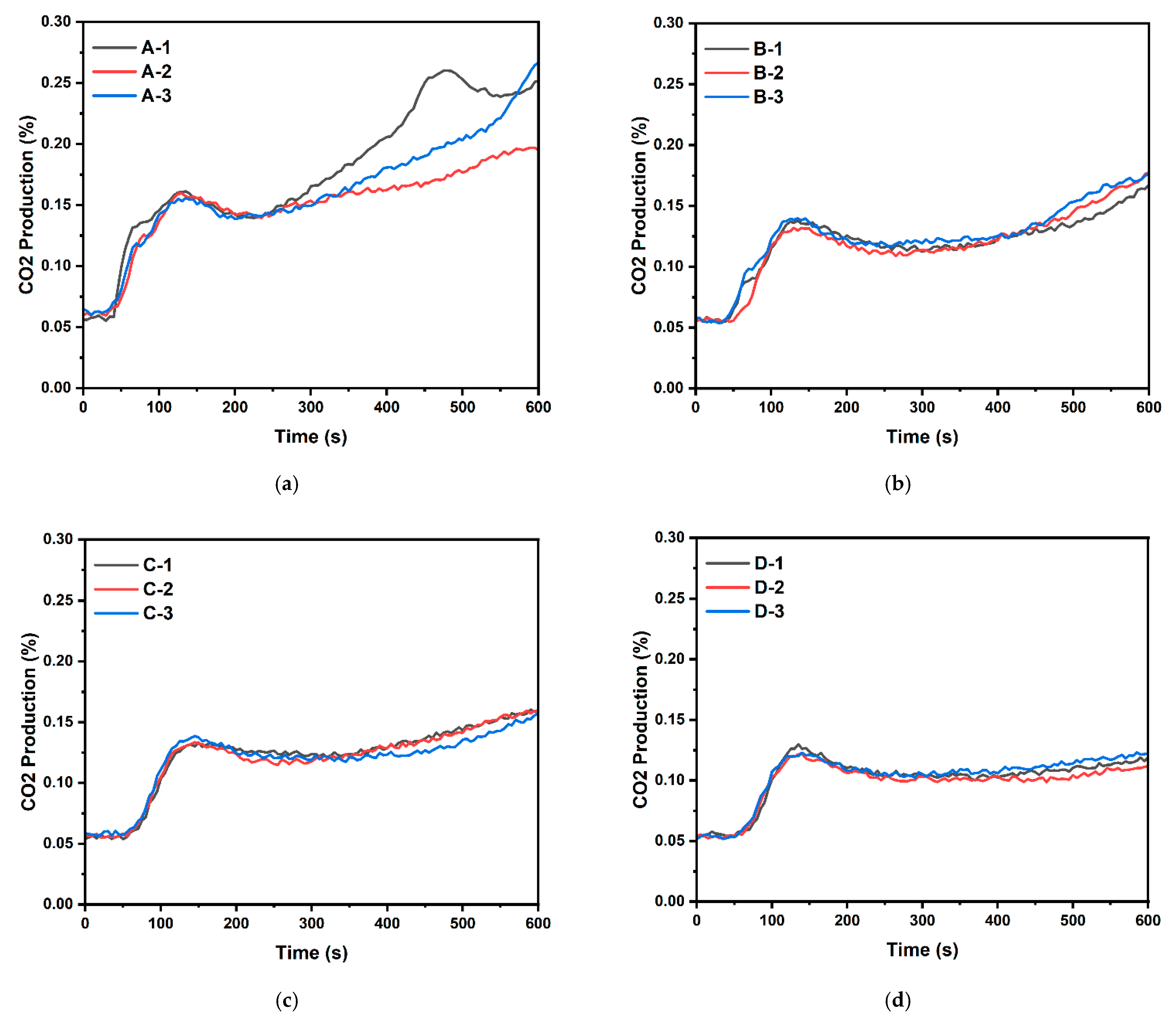
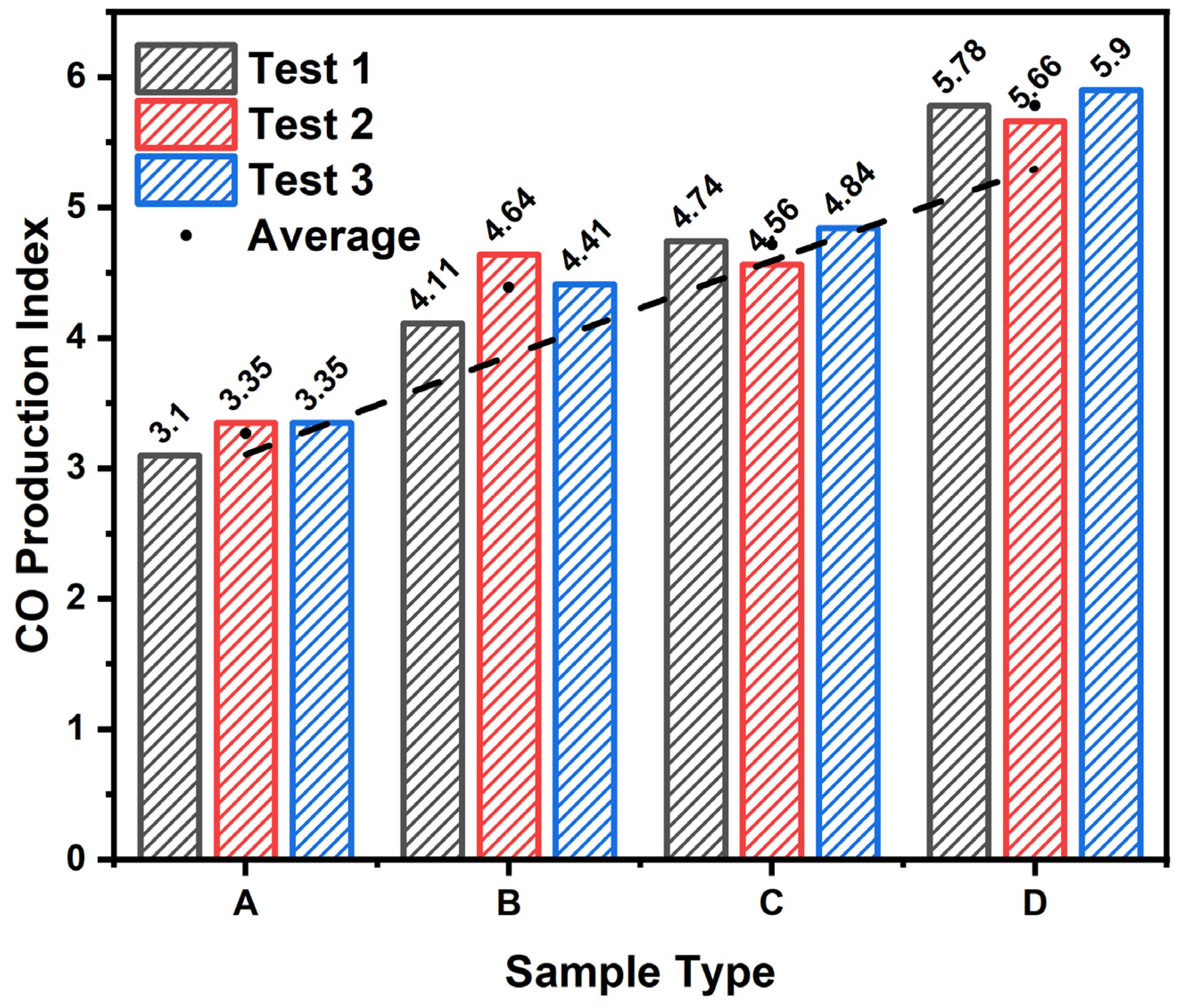
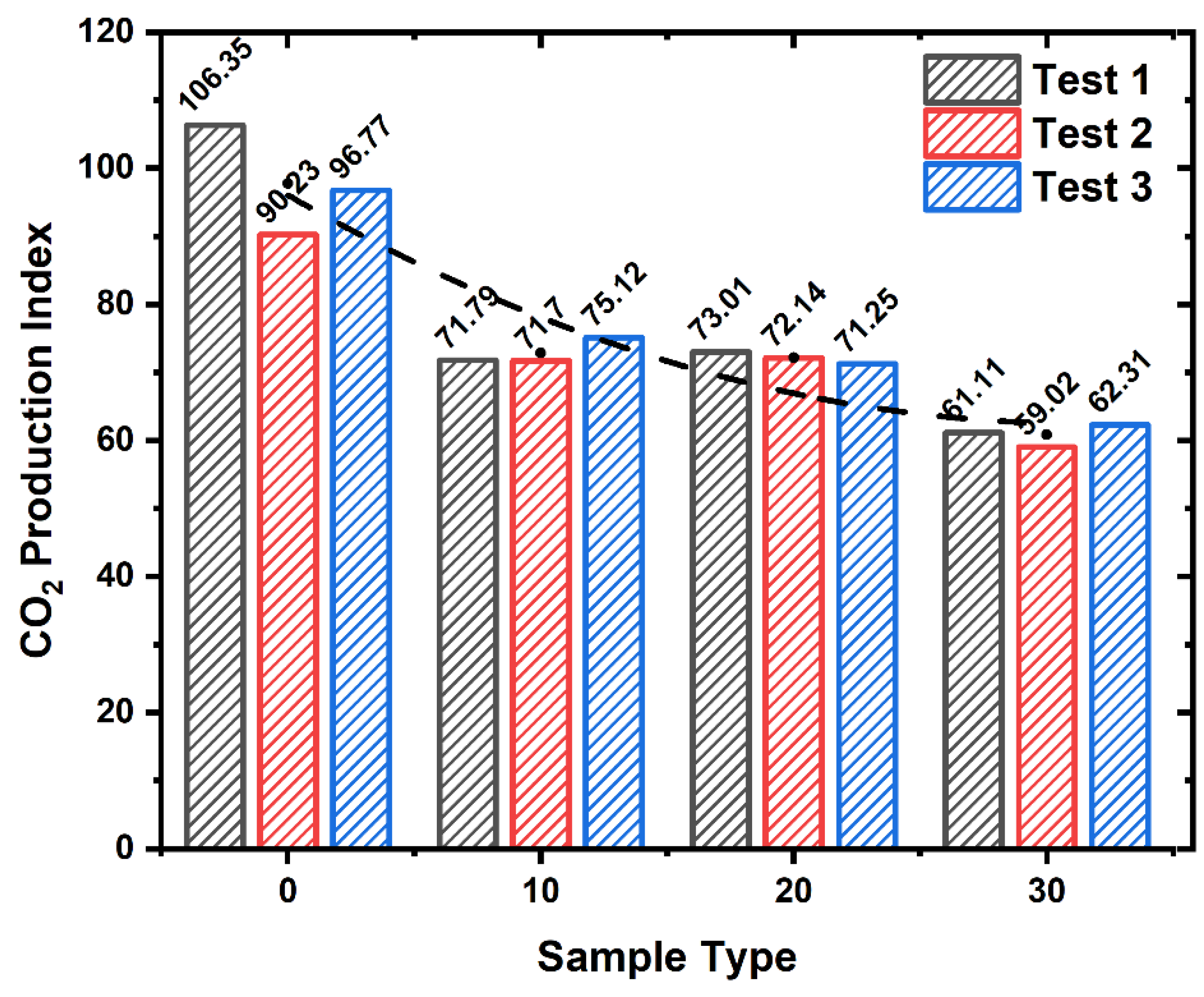
- The investigation of CO and CO2 emissions with varying magnesium hydroxide additions through both ISO 5660-1 tests and NES 713 toxicity tests revealed that, as the magnesium hydroxide content increased in cellulose building finishing material, there was an increase in CO emissions and a decrease in CO2 emissions during fire conditions;
- The architectural finishing material produced was confirmed not to emit harmful levels of CO to humans by comparing the CO emissions generated over 10 min in the ISO 5660-1 experiment and over 2 min in the NES 713 experiment, with the levels specified in the NES standard for 30 min of exposure;
- Through the analysis of Toxic Index values from the NES 713 toxicity test, it was observed that the Toxic Index was high when the magnesium hydroxide addition was 20 g. The Toxic Index decreased above that due to a reduction in CO2 generation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, T.B.Y.; Yuen, A.C.Y.; Yeoh, G.H.; Yang, W.; Chan, Q.N. Fire Risk Assessment of Combustible Exterior Cladding Using a Collective Numerical Database. Fire 2019, 2, 11. [Google Scholar] [CrossRef]
- Ha, J.; Kim, H.J.; Kim, J.H.; Park, D. Experience of Disaster Response Team in Jecheon Sports Center Fire. J. Korean Soc. Integr. Med. 2022, 10, 39–48. [Google Scholar]
- Hassan, M.K.; Hossain, M.D.; Gilvonio, M.; Rahnamayiezekavat, P.; Douglas, G.; Pathirana, S.; Saha, S. Numerical Investigations on the Influencing Factors of Rapid Fire Spread of Flammable Cladding in a High-Rise Building. Fire 2022, 5, 149. [Google Scholar] [CrossRef]
- Hossain, M.D.; Hassan, M.K.; Yuen, A.C.Y.; He, Y.; Saha, S.; Hittini, W. Flame behaviour, fire hazard and fire testing approach for lightweight composite claddings—A review. J. Struct. Fire Eng. 2021, 12, 257–292. [Google Scholar] [CrossRef]
- Hossain, M.D.; Saha, S.; Hassan, M.K.; Yuen, A.C.Y.; Wang, C.; Hittini, W.; George, L.; Wuhrer, R. Testing of aluminium composite panels in a cone calorimeter: A new specimen preparation method. Polym. Test. 2022, 106, 107454. [Google Scholar] [CrossRef]
- NFPA 255; Standard Method of Test of Surface Burning Characteristics of Building Materials. NFPA (National Fire Protection Association): Quincy, MA, USA, 2006.
- Nussbaumer, M.; Van Opdenbosch, D.; Engelhardt, M.; Briesen, H.; Benz, J.P.; Karl, T. Material characterization of pressed and unpressed wood–mycelium composites derived from two Trametes species. Environ. Technol. Innov. 2023, 30, 103063. [Google Scholar] [CrossRef]
- DIN4102-1: Fire Test to Building Material–Classification Building Material Fire Test Center_FireTC.net. Available online: http://www.firetc.net/firetesting/show.php?itemid=663 (accessed on 2 November 2021).
- Kwark, J.H.; Choi, J.M.; Ku, J.H. A Study on Evaluation Methods for the Fire-retardant Performance of Hanok Components. Fire Sci. Eng. 2011, 25, 1–7. [Google Scholar]
- Ministry of Land, Infrastructure and Transport. Code for the Flame—Retardant Performance of Building Finishing Materials and the Fire Preventing Structure. 2022, No. 2022-1106. Available online: https://www.law.go.kr/admRulLsInfoP.do?chrClsCd=&admRulSeq=2100000195901 (accessed on 29 March 2021).
- Hwang, J.; Park, D.; Kim, S.; Rie, D. A Study on the Flame-Retardant Performance of Recycled Paper Building Materials Manufactured by 3D Printer. Sustainability 2022, 14, 4798. [Google Scholar] [CrossRef]
- Ahn, C.; Park, D.; Hwang, J.; Rie, D. A Study on the Reliability of Mass, Density, and Fire Performance of Recycled Wastepaper Building Finishing Material Made with Large Wet Cellulose 3D Printers. Sustainability 2022, 14, 13090. [Google Scholar] [CrossRef]
- Kim, Y.; Park, D.; Kim, S.; Rie, D. A Study on the Quantitative Fire Performance Evaluation Method of Building Finishing Materials with a Focus on Medical Facilities. Sustainability 2023, 15, 9373. [Google Scholar] [CrossRef]
- Kim, Y.; Park, D.; Rie, D. Evaluation of the Flame-Retardant Performance and Fire Risk of Cellulose Building Finishing Material Due to the Particle Size of Expandable Graphite. Sustainability 2023, 15, 5426. [Google Scholar] [CrossRef]
- Mazela, B.; Batista, A.; Grześkowiak, W. Expandable graphite as a fire retardant for cellulosic materials—A review. Forests 2020, 11, 755. [Google Scholar] [CrossRef]
- Wi, S.; Kim, Y.U.; Choi, J.Y.; Shin, B.; Kim, S. Active protection against fire: Enhancing the flame retardancy of sandwich panels using an expandable graphite layer formation. Int. J. Therm. Sci. 2024, 195, 108658. [Google Scholar] [CrossRef]
- Kabir, I.I.; Carlos Baena, J.; Wang, W.; Wang, C.; Oliver, S.; Nazir, M.T.; Khalid, A.; Fu, Y.; Yuen, A.C.Y.; Yeoh, G.H. Optimisation of Additives to Maximise Performance of Expandable Graphite-Based Intumescent-Flame-Retardant Polyurethane Composites. Molecules 2023, 28, 5100. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Sun, Y.P.; Sheng, J.; Geng, T.; Turng, L.S.; Guo, Y.G.; Liu, X.H.; Liu, C.T. Effects of expandable graphite on the flame-retardant and mechanical performances of rigid polyurethane foams. J. Phys. Condens. Matter 2021, 34, 084002. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Chai, W.; Liu, Y.; Su, X.; Liao, C.; Gao, M.; Zheng, Z. Facile fabrication of starch-based, synergistic intumescent and halogen-free flame-retardant strategy with expandable graphite in enhancing the fire safety of polypropylene. Ind. Crops Prod. 2022, 184, 115002. [Google Scholar] [CrossRef]
- Chun, K.O.; Rie, D.H. A study for the fire retardant-characteristics of expandable graphite composite materials. J. Korean Soc. Saf. 2017, 32, 28–33. [Google Scholar] [CrossRef]
- Chan, Y.Y.; Korwitz, A.; Pospiech, D.; Schartel, B. Flame Retardant Combinations with Expandable Graphite/Phosphorus/CuO/Castor Oil in Flexible Polyurethane Foams. ACS Appl. Polym. Mater. 2023, 5, 1891–1901. [Google Scholar] [CrossRef]
- Tang, H.; Chen, K.; Li, X.; Ao, M.; Guo, X.; Xue, D. Environment-friendly, flame retardant thermoplastic elastomer–magnesium hydroxide composites. Funct. Mater. Lett. 2017, 10, 1750042. [Google Scholar] [CrossRef]
- Pan, F.; Jia, H.; Huang, Y.; Chen, Z.; Liang, S.; Jiang, P. Analyzing Temperature Distribution Patterns on the Facing and Backside Surface: Investigating Combustion Performance of Flame-Retardant Particle Boards Using Aluminum Hypophosphite, Intumescent, and Magnesium Hydroxide Flame Retardants. Polymers 2023, 15, 4479. [Google Scholar] [CrossRef]
- Piperopoulos, E.; Scionti, G.; Atria, M.; Calabrese, L.; Proverbio, E. Flame-retardant performance evaluation of functional coatings filled with Mg(OH)2 and Al(OH)3. Polymers 2022, 14, 372. [Google Scholar] [CrossRef]
- Zaghloul, M.M.Y.; Zaghloul, M.M.Y. Influence of flame retardant magnesium hydroxide on the mechanical properties of high density polyethylene composites. J. Reinf. Plast. Compos. 2017, 36, 1802–1816. [Google Scholar] [CrossRef]
- Kang, M.; Wang, G.; Liu, W.; Yu, D.; Li, G.; Song, Z.; Liu, X.; Wang, H. Fabrication of highly flame-retardant paper by in situ loading of magnesium hydroxide/basic magnesium chloride onto cellulose fibers. Cellulose 2023, 30, 7295–7312. [Google Scholar] [CrossRef]
- Chung, Y.J.; Lim, H.M.; Jin, E.; Oh, J. Combustion-retardation properties of low density polyethylene and ethylene vinyl acetate mixtures with magnesium hydroxide. Appl. Chem. Eng. 2011, 22, 439–443. [Google Scholar]
- Meucci, M.; Haveriku, S.; Badalassi, M.; Cardelli, C.; Ruggeri, G.; Pucci, A. Effect of Polyolefin Elastomers’ Characteristics and Natural Magnesium Hydroxide Content on the Properties of Halogen-Free Flame-Retardant Polyolefin Composites. Micro 2022, 2, 164–182. [Google Scholar] [CrossRef]
- Jiao, L.L.; Zhao, P.C.; Liu, Z.Q.; Wu, Q.S.; Yan, D.Q.; Li, Y.L.; Chen, Y.N.; Li, J.S. Preparation of magnesium hydroxide flame retardant from hydromagnesite and enhance the flame-retardant performance of EVA. Polymers 2022, 14, 1567. [Google Scholar] [CrossRef] [PubMed]
- British Standards Institution Reaction-to-Fire Tests. Heat Release, Smoke Production and Mass Loss Rate. Part 1, Heat Release Rate (Cone Calorimeter Method) and Smoke Production Rate (Dynamic Measurement). 2015. Available online: https://www.iso.org/standard/57957.html (accessed on 20 May 2024).
- Zhang, W.; Chen, X.; Chen, Q.; Ding, C.; Liu, J.; Chen, M.; Wang, J. Combustion calorimetry of carbonate electrolytes used in lithium-ion batteries. J. Fire Sci. 2015, 33, 22–36. [Google Scholar] [CrossRef]
- Nazare, S.; Pitts, W.M.; Shields, J.; Knowlton, E.; De Leon, B.; Zammarano, M.; Davis, R. Assessing fire-blocking effectiveness of barrier fabrics in the cone calorimeter. J. Fire Sci. 2019, 37, 340–376. [Google Scholar] [CrossRef]
- Babrauskas, V. The Early History of the Cone Calorimeter. Fire Sci. Technol. 2022, 41, 21–31. [Google Scholar] [CrossRef]
- NES 713; Determination of the Toxicity Index of the Products of Combustion from Small Specimens of Materials. Ministry of Defense Naval Engineering Standards (NES): Washington, DC, USA, 1990.
- Naval Engineering Standards NES 713 Determination of the Toxicity Index of the Products of Combustion from Small Specimens of Materials; UK Ministry of Defence: London, UK, 2013.
- Seo, H.J.; Kim, N.K.; Lee, M.C.; Lee, S.K.; Moon, Y.S. Investigation into the toxicity of combustion products for CR/EPR cables based on aging period. J. Mech. Sci. Technol. 2020, 34, 1785–1794. [Google Scholar] [CrossRef]
- Hyun Jeong, S.; Dong Won, S. Hazard assessment of combustion gases from interior materials. Fire Sci. Eng. 2015, 29, 49–56. [Google Scholar]
- Kim, M.H.; Lee, S.H.; Jeong, S.Y.; Lee, S.K.; Lee, J.E.; Kwark, J.H.; Lee, M.C. Investigation of combustion, smoke, and toxicity characteristics of flame-retardant and fiber-optic cables used in nuclear power plants. J. Mech. Sci. Technol. 2023, 37, 987–999. [Google Scholar] [CrossRef]
- Seo, H.J.; Chung, Y.H.; Song, T.J. An Experimental Study for Deriving Fire Risk Evaluation Factors for Cables in Utility Tunnels. Fire 2023, 6, 342. [Google Scholar] [CrossRef]







| Specimens Name | Basic Material | Flame Retardant | After Drying Mass (g) | |
|---|---|---|---|---|
| Waste Paper | Expandable Graphite (EG) | Magnesium Hydroxide (Mg(OH)2) | ||
| A | 100 | 30 | 0 | 56 |
| 55.9 | ||||
| 56.6 | ||||
| B | 100 | 10 | 69 | |
| 69.8 | ||||
| 69.4 | ||||
| C | 100 | 20 | 79.7 | |
| 79.3 | ||||
| 79.5 | ||||
| D | 100 | 30 | 85.1 | |
| 86.2 | ||||
| 87.5 | ||||
| Case | Details |
|---|---|
| Heat flux of cone heater | 50 kW |
| Specimen distance of cone heater | 22.5 mm |
| C-factor | 0.0411 |
| Duct flow rate | 24 ± 0.1 L/s |
| Specimens size | 100 mm × 100 mm × 20 mm |
| Number of tests per specimen type | 3 |
| Test time | 600 s |
| Standard | Class | Evaluation Criteria |
|---|---|---|
| ISO 5660-1 (Cone Calorimeter Method) | Semi- non-combustible Material |
|
| ISO 5660-1 (Cone Calorimeter Method) | Fire Retardant Material |
|
| Specimens | |
| Mass | 1 ± 0.05 g |
| Sample type | A, B, C, D |
| Number per sample type | 3 |
| Mechanical | |
| Chamber volume | 800 × 800 × 1000 (W × D × H) mm 0.7 m3 |
| Exhaust | 50 L/s |
| Calibration Test | |
| Calibration gas | CO, CO2, NOx |
| Calibration test time | 2 min |
| Test | |
| Flame temperature | 1150 ± 50 °C |
| Flame height | 100 ± 10 mm |
| Test time | 2 min |
| Stirring time | 30 s |
| Ventilation time | at least 5 min |
| Toxic Gas | Cf (ppm) |
|---|---|
| Carbon Dioxide (CO2) | 100,000 |
| Carbon Monoxide (CO) | 4000 |
| Hydrogen Sulfide (H2S) | 750 |
| Ammonia (NH3) | 750 |
| Formaldehyde (HCHO) | 500 |
| Hydrogen Chloride (HCl) | 500 |
| Sulfur Dioxide (SO2) | 400 |
| Acrylonitrile (CH2CHCN) | 400 |
| Nitrogen Oxides (NOx) | 250 |
| Phenol (C6H5OH) | 250 |
| Hydrogen Cyanide (HCN) | 150 |
| Hydrogen Bromide (HBr) | 150 |
| Hydrogen Fluoride (HF) | 100 |
| Sample Type | No. | Ignition Time | Peak Heat Release Rate (kW/m2) | THR (MJ/m2) | Toxic Gas Generated | Mass Loss (g) | ||
|---|---|---|---|---|---|---|---|---|
| at 300 s | at 600 s | CO (%) | CO2 (%) | |||||
| A | 1 | 12 s | 87.259 | 9.854 | 30.31 | 0.0069 | 0.204 | 25.76 |
| 2 | 16 s | 48.999 | 9.05 | 21.217 | 0.0065 | 0.137 | 21.93 | |
| 3 | 11 s | 90.972 | 8.853 | 24.842 | 0.0070 | 0.202 | 21.15 | |
| B | 1 | 21 s | 41.262 | 7.293 | 15.678 | 0.0076 | 0.111 | 19.52 |
| 2 | 14 s | 47.873 | 7.052 | 16.581 | 0.0084 | 0.122 | 21.54 | |
| 3 | 14 s | 48.554 | 7.691 | 17.973 | 0.0086 | 0.119 | 20.94 | |
| C | 1 | 16 s | 38.64 | 7.123 | 15.958 | 0.0097 | 0.106 | 21.81 |
| 2 | 16 s | 39.713 | 7.028 | 16.138 | 0.0093 | 0.103 | 23.35 | |
| 3 | 11 s | 39.245 | 7.307 | 15.918 | 0.0096 | 0.098 | 21.22 | |
| D | 1 | 17 s | 38.535 | 6.34 | 12.183 | 0.0114 | 0.077 | 21.04 |
| 2 | 16 s | 33.795 | 6.078 | 11.575 | 0.0112 | 0.068 | 18.48 | |
| 3 | 15 s | 34.495 | 6.171 | 12.594 | 0.0113 | 0.071 | 15.59 | |
| Sample Type | Toxic Gas | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO2 | CO | H2S | NH3 | HCHO | HCl | SO2 | CH2CHCN | NOx | C6H5OH | HCN | HBr | HF | |
| A-1 | 3300 | 80 | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | 0 | 0.2 | 0 | 0 |
| A-2 | 3700 | 60 | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | 0 | 0.2 | 0 | 0 |
| A-3 | 3500 | 70 | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | 0 | 0.2 | 0 | 0 |
| B-1 | 2950 | 110 | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | 0 | 0.2 | 0 | 0 |
| B-2 | 3150 | 120 | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | 0 | 0.2 | 0 | 0 |
| B-3 | 2900 | 130 | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | 0 | 0.2 | 0 | 0 |
| C-1 | 2350 | 220 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.5 | 0 | 0 |
| C-2 | 2500 | 185 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.5 | 0 | 0 |
| C-3 | 2650 | 180 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.5 | 0 | 0 |
| D-1 | 800 | 240 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.5 | 0 | 0 |
| D-2 | 1300 | 190 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.5 | 0 | 0 |
| D-3 | 900 | 200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.5 | 0 | 0 |
| Sample Type | Test No. | Initial Mass (g) | After Test Mass (g) | Mass Loss (%) | Standard Deviation |
|---|---|---|---|---|---|
| A | 1 | 56 | 30.24 | 46 | 3.71 |
| 2 | 55.9 | 33.97 | 39.23 | ||
| 3 | 56.6 | 35.45 | 37.37 | ||
| B | 1 | 69 | 49.48 | 28.29 | 1.09 |
| 2 | 69.8 | 48.26 | 30.86 | ||
| 3 | 69.4 | 48.46 | 30.17 | ||
| C | 1 | 79.7 | 57.89 | 27.37 | 1.17 |
| 2 | 79.3 | 55.95 | 29.45 | ||
| 3 | 79.5 | 58.28 | 26.69 | ||
| D | 1 | 85.1 | 64.06 | 24.72 | 2.82 |
| 2 | 86.2 | 67.72 | 21.44 | ||
| 3 | 87.5 | 71.91 | 17.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, D.; Kim, Y.; Rie, D. An Evaluation of the Fire Safety of Waste Paper-Based Internal Finishing Materials Combined with Expandable Graphite According to Changes in Magnesium Hydroxide Content. Appl. Sci. 2024, 14, 5758. https://doi.org/10.3390/app14135758
Park D, Kim Y, Rie D. An Evaluation of the Fire Safety of Waste Paper-Based Internal Finishing Materials Combined with Expandable Graphite According to Changes in Magnesium Hydroxide Content. Applied Sciences. 2024; 14(13):5758. https://doi.org/10.3390/app14135758
Chicago/Turabian StylePark, Dongin, Yongjoo Kim, and Dongho Rie. 2024. "An Evaluation of the Fire Safety of Waste Paper-Based Internal Finishing Materials Combined with Expandable Graphite According to Changes in Magnesium Hydroxide Content" Applied Sciences 14, no. 13: 5758. https://doi.org/10.3390/app14135758
APA StylePark, D., Kim, Y., & Rie, D. (2024). An Evaluation of the Fire Safety of Waste Paper-Based Internal Finishing Materials Combined with Expandable Graphite According to Changes in Magnesium Hydroxide Content. Applied Sciences, 14(13), 5758. https://doi.org/10.3390/app14135758







