Antibiotic Resistance in the Farming Environment
Abstract
1. Introduction
2. Antibiotics in Farming Practices
3. Farmed Animals as Reservoirs of Antibiotic Resistance
4. Husbandry Wastes as a Reservoir of Antibiotic Resistance and Its Carrier in the Environment
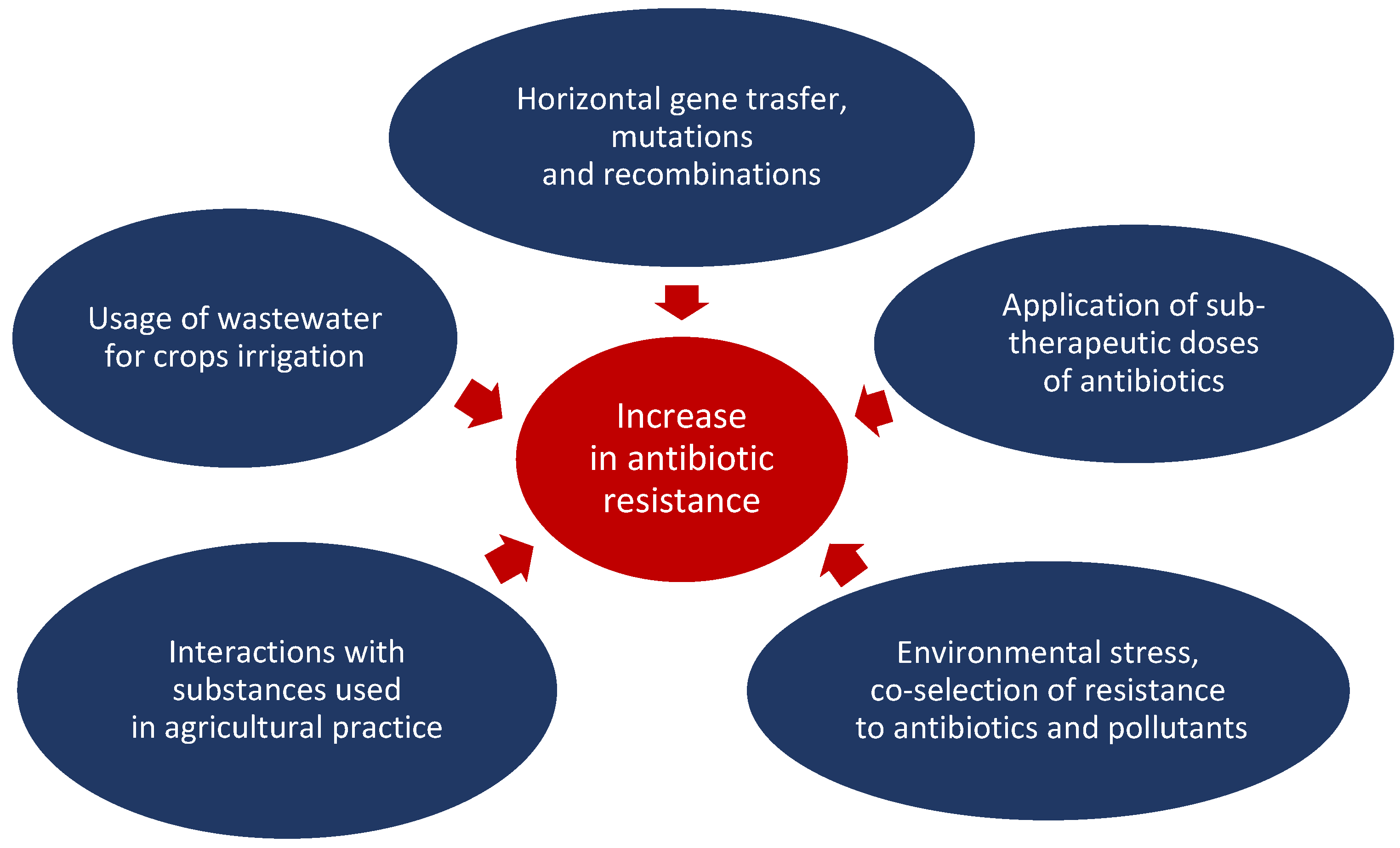
5. Determinants of Antibiotic Resistance in Farming
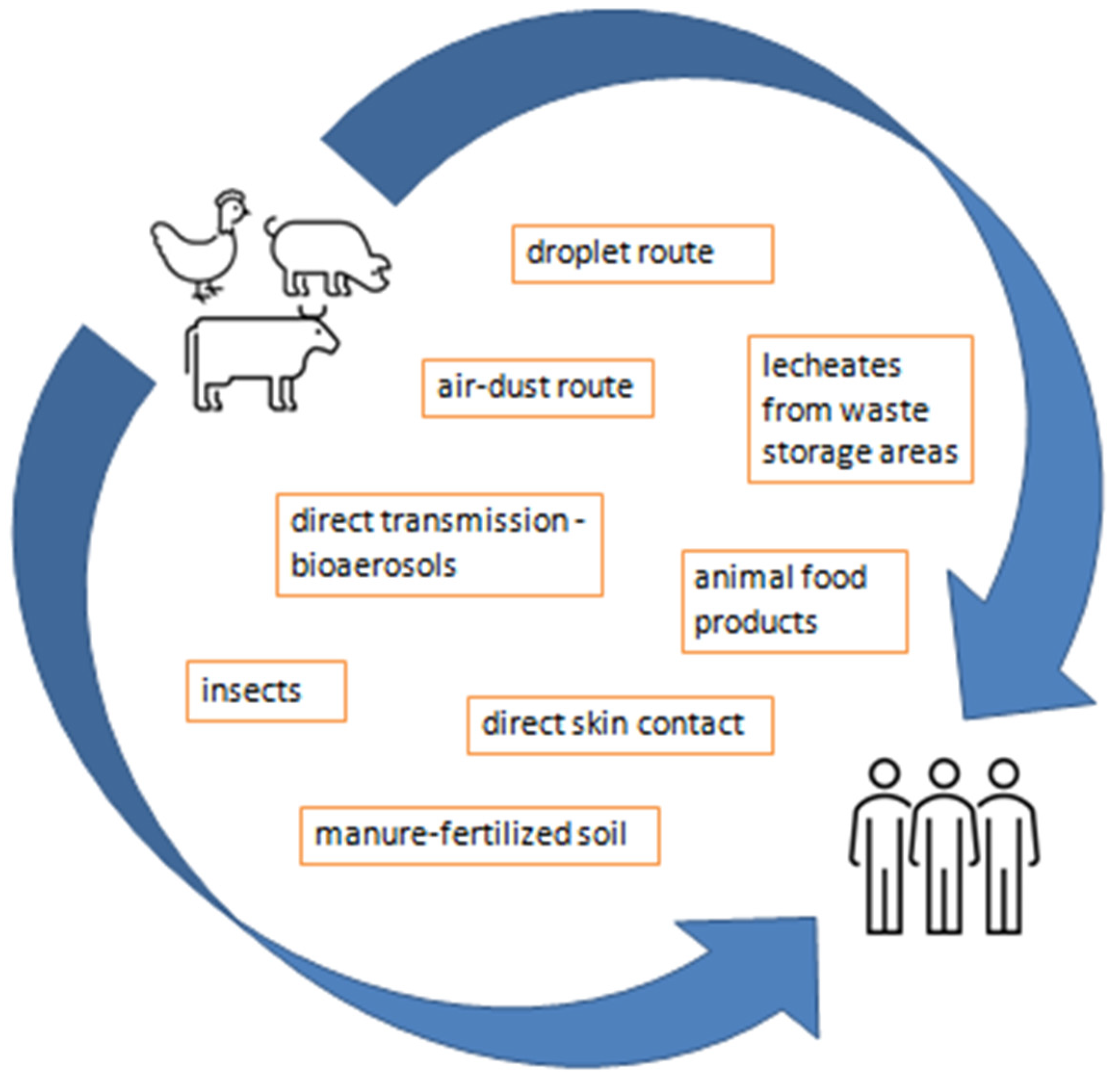
6. Environmental Emissions and Human Health Risks
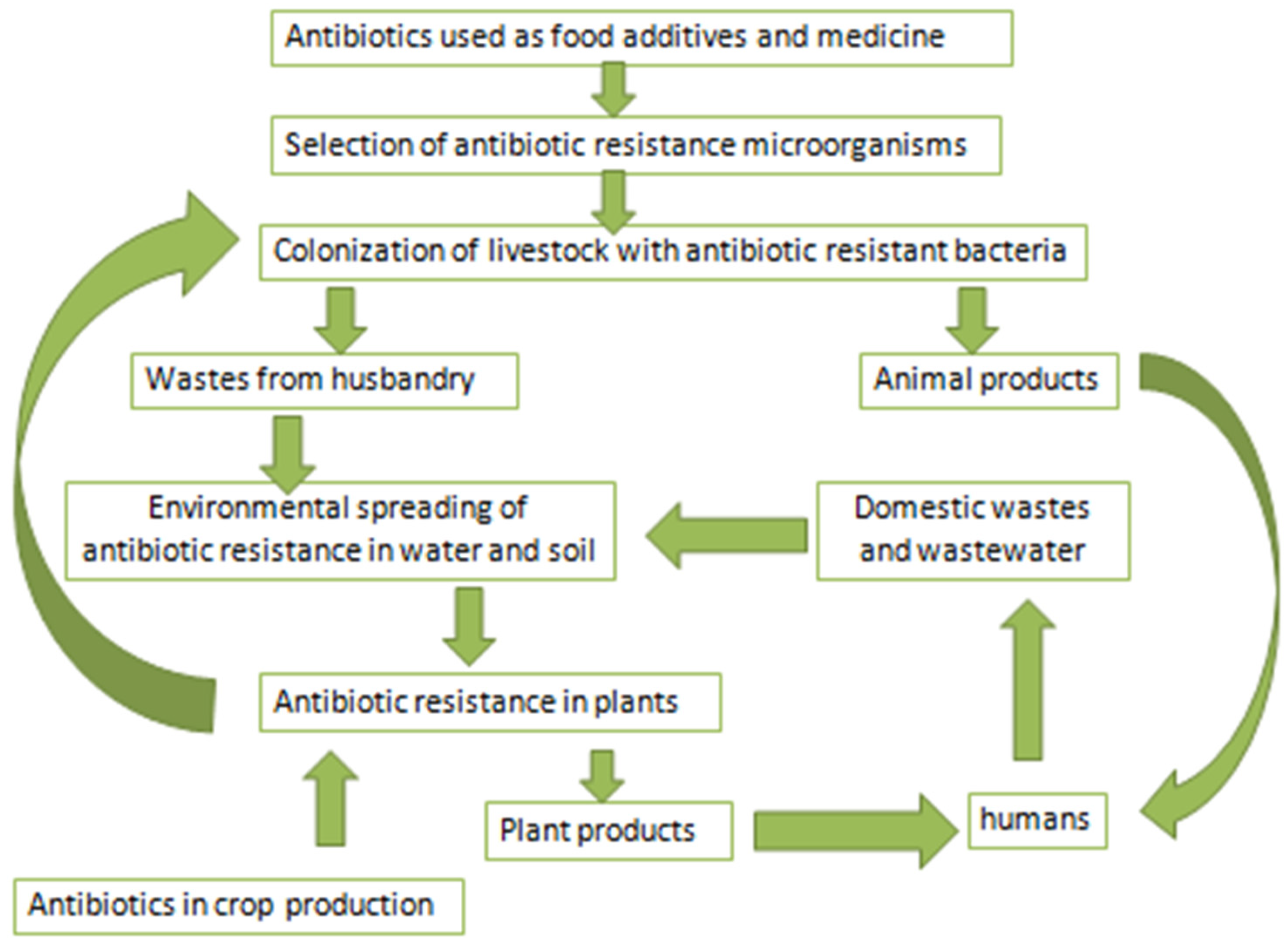
7. Antibiotic Resistance in Animal and Plant Products
8. Summary and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Serwecińska, L. Antimicrobials and antibiotic-resistant bacteria: A risk to the environment and to public health. Water 2020, 12, 3313. [Google Scholar] [CrossRef]
- Stanton, I.C.; Bethel, A.; Leonard, A.F.; Gaze, W.H.; Garside, R. Existing evidence on antibiotic resistance exposure and transmission to humans from the environment: A systematic map. Environ. Evid. 2022, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Landers, T.F.; Cohen, B.; Wittum, T.E.; Larson, E.L. A review of antibiotic use in food animals: Perspective, policy, and potential. Public Health Rep. 2012, 127, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Iwu, C.D.; Korsten, L.; Okoh, A.I. The incidence of antibiotic resistance within and beyond the agricultural ecosystem: A concern for public health. Microbiologyopen 2020, 9, e1035. [Google Scholar] [CrossRef] [PubMed]
- Haenni, M.; Dagot, C.; Chesneau, O.; Bibbal, D.; Labanowski, J.; Vialette, M.; Bouchard, D.; Martin -Laurent, F.; Calsat, L.; Nazaret, S.; et al. Environmental contamination in a high-income country (France) by antibiotics, antibiotic-resistant bacteria, and antibiotic resistance genes: Status and possible causes. Environ. Int. 2022, 159, 107047. [Google Scholar] [CrossRef] [PubMed]
- Waglechner, N.; Wright, G.D. Antibiotic resistance: It’s bad, but why isn’t it worse? BMC Biol. 2017, 15, 84. [Google Scholar] [CrossRef] [PubMed]
- Tiedje, J.M.; Wang, F.; Manaia, C.M.; Virta, M.; Sheng, H.; Liping, M.; Zhang, T.; Edward, T. Antibiotic resistance genes in the human-impacted environment: A one health perspective. Pedosphere 2019, 29, 273–282. [Google Scholar] [CrossRef]
- Wang, F.; Xu, M.; Stedtfeld, R.D.; Sheng, H.J.; Fan, J.; Liu, M.; Chai, B.; de Carvalho, T.S.; Li, H.; Li, Z.; et al. Long-term effect of different fertilization and cropping systems on the soil antibiotic resistome. Environ. Sci. Technol. 2018, 52, 13037–13046. [Google Scholar] [CrossRef] [PubMed]
- Walsh, F. Investigating antibiotic resistance in non-clinical environments. Front. Microbiol. 2013, 4, 39553. [Google Scholar] [CrossRef]
- Busch, G.; Kassas, B.; Palma, M.A.; Risius, A. Perceptions of antibiotic use in livestock farming in Germany, Italy and the United States. Livest. Sci. 2020, 241, 104251. [Google Scholar] [CrossRef]
- Helliwell, R.; Morris, C.; Raman, S. Antibiotic stewardship and its implications for agricultural animal-human relationships: Insights from an intensive dairy farm in England. J. Rural Stud. 2020, 78, 447–456. [Google Scholar] [CrossRef]
- Regan, Á.; Sweeney, S.; McKernan, C.; Benson, T.; Dean, M. Consumer perception and understanding of the risks of antibiotic use and antimicrobial resistance in farming. Agric. Hum. Values 2022, 40, 989–1001. [Google Scholar] [CrossRef]
- Bombaywala, S.; Mandpe, A.; Paliya, S.; Kumar, S. Antibiotic resistance in the environment: A critical insight on its occurrence, fate, and eco-toxicity. Environ. Sci. Pollut. Res. 2021, 28, 24889–24916. [Google Scholar] [CrossRef] [PubMed]
- Eltayb, A.; Barakat, S.; Marrone, G.; Shaddad, S.; Stålsby Lundborg, C. Antibiotic use and resistance in animal farming: A quantitative and qualitative study on knowledge and practices among farmers in Khartoum, Sudan. Zoonoses Public Health 2012, 59, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.Y.; Yannarell, A.C.; Dai, Q.; Ekizoglu, M.; Mackie, R.I. Monitoring the perturbation of soil and groundwater microbial communities due to pig production activities. Appl. Environ. Microbiol. 2013, 79, 2620–2629. [Google Scholar] [CrossRef] [PubMed]
- Skandalis, N.; Maeusli, M.; Papafotis, D.; Miller, S.; Lee, B.; Theologidis, I.; Luna, B. Environmental spread of antibiotic resistance. Antibiotics 2021, 10, 640. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Antimicrobials in Agriculture and the Environment: Reducing Unnecessary Use and Waste: The Review on Antimicrobial Resistance; Wellcometrust: London, UK, 2015. [Google Scholar]
- Ma, Z.; Lee, S.; Jeong, K.C. Mitigating antibiotic resistance at the livestock-environment interface: A review. J. Microbiol. Biotechnol. 2019, 29, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ahn, J. Emergence and spread of antibiotic-resistant foodborne pathogens from farm to table. Food Sci. Biotechnol. 2022, 31, 1481–1499. [Google Scholar] [CrossRef]
- Zalewska, M.; Blazejewska, A.; Czapko, A.; Popowska, M. Antibiotics and antibiotic resistance genes in animal manure—Consequences of its application in agriculture. Front. Microbiol. 2021, 12, 610656. [Google Scholar] [CrossRef]
- Williams-Nguyen, J.; Sallach, J.B.; Bartelt-Hunt, S.; Boxall, A.B.; Durso, L.M.; McLain, J.E.; Singer, R.S.; Snow, D.D.; Zilles, J.L. Antibiotics and antibiotic resistance in agroecosystems: State of the science. J. Environ. Qual. 2016, 45, 394–406. [Google Scholar] [CrossRef]
- Ebmeyer, S.; Kristiansson, E.; Larsson, D.J. A framework for identifying the recent origins of mobile antibiotic resistance genes. Commun. Biol. 2021, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Chekabab, S.M.; Lawrence, J.R.; Alvarado, A.; Predicala, B.; Korber, D.R. A health metadata-based management approach for comparative analysis of high-throughput genetic sequences for quantifying antimicrobial resistance reduction in Canadian hog barns. Comput. Struct. Biotechnol. J. 2020, 18, 2629–2638. [Google Scholar] [CrossRef] [PubMed]
- Chekabab, S.M.; Lawrence, J.R.; Alvarado, A.C.; Predicala, B.Z.; Korber, D.R. Piglet gut and in-barn manure from farms on a raised without antibiotics program display reduced antimicrobial resistance but an increased prevalence of pathogens. Antibiotics 2021, 10, 1152. [Google Scholar] [CrossRef] [PubMed]
- Berkner, S.; Konradi, S.; Schönfeld, J. Antibiotic resistance and the environment—There and back again. Science & Society series on Science and Drugs. EMBO Rep. 2014, 15, 740–744. [Google Scholar] [PubMed]
- Monger, X.C.; Gilbert, A.A.; Saucier, L.; Vincent, A.T. Antibiotic resistance: From pig to meat. Antibiotics 2021, 10, 1209. [Google Scholar] [CrossRef]
- Lekagul, A.; Tangcharoensathien, V.; Yeung, S. Patterns of antibiotic use in global pig production: A systematic review. Vet. Anim. Sci. 2019, 7, 100058. [Google Scholar] [CrossRef] [PubMed]
- Callens, B.; Persoons, D.; Maes, D.; Laanen, M.; Postma, M.; Boyen, F.; Haesebrouck, F.; Butaye, P.; Catry, B.; Dewulf, J. Prophylactic and metaphylactic antimicrobial use in Belgian fattening pig herds. Prev. Vet. Med. 2012, 106, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, W.; Korzeniewska, E.; Harnisz, M.; Drzymała, J.; Felis, E.; Bajkacz, S. Wastewater treatment plants as a reservoir of integrase and antibiotic resistance genes—An epidemiological threat to workers and environment. Environ. Int. 2021, 156, 106641. [Google Scholar] [CrossRef]
- Hong, P.Y.; Al-Jassim, N.; Ansari, M.I.; Mackie, R.I. Environmental and public health implications of water reuse: Antibiotics, antibiotic resistant bacteria, and antibiotic resistance genes. Antibiotics 2013, 2, 367–399. [Google Scholar] [CrossRef]
- Karkman, A.; Pärnänen, K.; Larsson, D.J. Fecal pollution can explain antibiotic resistance gene abundances in anthropogenically impacted environments. Nat. Commun. 2019, 10, 80. [Google Scholar] [CrossRef]
- Paltansing, S.; Vlot, J.A.; Kraakman, M.E.; Mesman, R.; Bruijning, M.L.; Bernards, A.T.; Visser, L.G.; Veldkamp, K.E. Extended-spectrum βlactamase–producing Enterobacteriaceae among travelers from the Netherlands. Emerg Infect Dis. 2013, 19, 1206. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.G.; Johnson, T.A.; Su, J.Q.; Qiao, M.; Guo, G.X.; Stedtfeld, R.D.; Hashsham, S.A.; Tiedje, J.M. Diverse and abundant antibiotic resistance genes in Chinese swine farms. Proc. Natl. Acad. Sci. USA 2013, 110, 3435–3440. [Google Scholar] [CrossRef] [PubMed]
- Berendonk, T.U.; Manaia, C.M.; Merlin, C.; Fatta-Kassinos, D.; Cytryn, E.; Walsh, F.; Bürgmann, H.; Sørum, H.; Norström, M.; Pons, M.-N.; et al. Tackling antibiotic resistance: The environmental framework. Nat. Rev. Microbiol. 2015, 13, 310–317. [Google Scholar] [CrossRef]
- Rousham, E.K.; Unicomb, L.; Islam, M.A. Human, animal and environmental contributors to antibiotic resistance in low-resource settings: Integrating behavioural, epidemiological and One Health approaches. Proc. R. Soc. B 2018, 285, 20180332. [Google Scholar] [CrossRef] [PubMed]
- Zibo, L.; Yuan, T.; Zhou, L.; Sen, C.; Qu, X.; Lu, P.; Qiyan, F. Impact factors of the accumulation, migration and spread of antibiotic resistance in the environment. Environ. Geochem. Health 2021, 43, 1741–1758. [Google Scholar]
- Bai, H.; He, L.Y.; Wu, D.L.; Gao, F.Z.; Zhang, M.; Zou, H.Y.; Yao, M.S.; Ying, G.G. Spread of airborne antibiotic resistance from animal farms to the environment: Dispersal pattern and exposure risk. Environ. Int. 2022, 158, 106927. [Google Scholar] [CrossRef] [PubMed]
- Salerno, B.; Furlan, M.; Sabatino, R.; Di Cesare, A.; Leati, M.; Volanti, M.; Barco, L.; Orsini, M.; Losasso, C.; Cibin, V. Antibiotic resistance genes load in an antibiotic free organic broiler farm. Poult. Sci. 2022, 101, 101675. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.Z.; He, L.Y.; Bai, H.; He, L.X.; Zhang, M.; Chen, Z.Y.; Liu, Y.S.; Ying, G.G. Airborne bacterial community and antibiotic resistome in the swine farming environment: Metagenomic insights into livestock relevance, pathogen hosts and public risks. Environ. Int. 2023, 172, 107751. [Google Scholar] [CrossRef] [PubMed]
- Pormohammad, A.; Nasiri, M.J.; Azimi, T. Prevalence of antibiotic resistance in Escherichia coli strains simultaneously isolated from humans, animals, food, and the environment: A systematic review and meta-analysis. Infect. Drug Resist. 2019, 12, 1181–1197. [Google Scholar] [CrossRef]
- Caruso, G. Antibiotic resistance in Escherichia coli from farm livestock and related analytical methods: A review. J. AOAC Int. 2018, 101, 916–922. [Google Scholar] [CrossRef]
- Amador, P.; Fernandes, R.; Prudencio, C.; Duarte, I. Prevalence of antibiotic resistance genes in multidrug-resistant Enterobacteriaceae on portuguese livestock manure. Antibiotics 2019, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Akond, M.A.; Alam, S.; Hassan, S.M.R.; Shirin, M. Antibiotic resistance of Escherichia coli isolated from poultry and poultry environment of Bangladesh. Internet J. Food Saf. 2009, 11, 19–23. [Google Scholar]
- Mohammad, A.A. Colonization and antibiotic susceptibility pattern of methicillin resistance Staphylococcus aureus (MRSA) among farm animals in Saudi Arabia. J. Bacteriol. Res. 2011, 3, 63–68. [Google Scholar]
- Sobur, M.A.; Sabuj, A.A.M.; Sarker, R.; Rahman, A.T.; Kabir, S.L.; Rahman, M.T. Antibiotic-resistant Escherichia coli and Salmonella spp. associated with dairy cattle and farm environment having public health significance. Vet. World 2019, 12, 984. [Google Scholar] [CrossRef]
- Liu, J.; Taft, D.H.; Maldonado-Gomez, M.X.; Johnson, D.; Treiber, M.L.; Lemay, D.G.; De Peters, E.J.; Mills, D.A. The fecal resistome of dairy cattle is associated with diet during nursing. Nat. Commun. 2019, 10, 4406. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, Z.; Avillan, J.J.; Call, D.R.; Davis, M.; Sischo, W.M.; Zhang, A. Dairy farm soil presents distinct microbiota and varied prevalence of antibiotic resistance across housing areas. Environ. Pollut. 2019, 254, 113058. [Google Scholar] [CrossRef]
- Tang, M.; Zhou, Q.; Zhang, X.; Zhou, S.; Zhang, J.; Tang, X.; Lu, J.; Gao, Y. Antibiotic resistance profiles and molecular mechanisms of Campylobacter from chicken and pig in China. Front. Microbiol. 2020, 11, 592496. [Google Scholar] [CrossRef] [PubMed]
- Holmer, I.; Salomonsen, C.M.; Jorsal, S.E.; Astrup, L.B.; Jensen, V.F.; Hog, B.B.; Pedersen, K. Antibiotic resistance in porcine pathogenic bacteria and relation to antibiotic usage. BMC Vet. Res. 2019, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Butaye, P.; Devriese, L.A.; Haesebrouck, F. Differences in antibiotic resistance patterns of Enterococcus faecalis and Enterococcus faecium strains isolated from farm and pet animals. Antimicrob. Agents Chemother. 2001, 45, 1374–1378. [Google Scholar] [CrossRef]
- Dohmen, W.; Schmitt, H.; Bonten, M.; Heederik, D. Air exposure as a possible route for ESBL in pig farmers. Environ. Res. 2017, 155, 359–364. [Google Scholar] [CrossRef]
- Lau, C.H.F.; van Engelen, K.; Gordon, S.; Renaud, J.; Topp, E. Novel antibiotic resistance determinants from agricultural soil exposed to antibiotics widely used in human medicine and animal farming. Appl. Environ. Microbiol. 2017, 83, e00989-17. [Google Scholar] [CrossRef]
- Farooq, M.; Smoglica, C.; Ruffini, F.; Soldati, L.; Marsilio, F.; Di Francesco, C.E. Antibiotic Resistance Genes Occurrence in Conventional and Antibiotic-Free Poultry Farming, Italy. Animals 2022, 12, 2310. [Google Scholar] [CrossRef] [PubMed]
- Udikovic-Kolic, N.; Wichmann, F.; Broderick, N.A.; Handelsman, J. Bloom of resident antibiotic-resistant bacteria in soil following manure fertilization. Proc. Natl. Acad. Sci. USA 2014, 111, 15202–15207. [Google Scholar] [CrossRef] [PubMed]
- Di Labio, E.; Regula, G.; Steiner, A.; Miserez, R.; Thomann, A.; Ledergerber, U. Antimicrobial resistance in bacteria from Swiss veal calves at slaughter. Zoonoses Public Health 2007, 54, 344–352. [Google Scholar] [CrossRef]
- Braykov, N.P.; Eisenberg, J.N.S.; Grossman, M.; Zhang, L.; Vasco, K.; Cevallos, W.; Muñoz, D.; Acevedo, A.; Moser, K.A.; Marrs, C.F.; et al. Antibiotic resistance in animal and environmental samples associated with small-scale poultry farming in northwestern Ecuador. mSphere 2016, 10, e00021-15. [Google Scholar] [CrossRef] [PubMed]
- Österberg, J.; Wingstrand, A.; Nygaard Jensen, A.; Kerouanton, A.; Cibin, V.; Barco, L.; Denis, M.; Aabo, S.; Bengtsson, B. Antibiotic resistance in Escherichia coli from pigs in organic and conventional farming in four European countries. PLoS ONE 2016, 11, e0157049. [Google Scholar] [CrossRef]
- Bengtsson-Palme, J.; Larsson, D.G.J. Concentrations of antibiotics predicted to select for resistant bacteria: Proposed limits for environmental regulation. Environ. Int. 2016, 86, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Smalla, K.; Cook, K.; Djordjevic, S.P.; Klümper, U.; Gillings, M. Environmental dimensions of antibiotic resistance: Assessment of basic science gaps. FEMS Microbiol. Ecol. 2018, 94, fiy195. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Chen, T.; Cao, Z.; Zhong, S.; Wen, X.; Mi, J.; Ma, B.; Zou, Y.; Zhang, N.; Liao, X.; et al. Antibiotic resistance genes in layer farms and their correlation with environmental samples. Poult. Sci. 2021, 100, 101485. [Google Scholar] [CrossRef]
- Lima, T.; Domingues, S.; Da Silva, G.J. Manure as a potential hotspot for antibiotic resistance dissemination by horizontal gene transfer events. Vet. Sci. 2020, 7, 110. [Google Scholar] [CrossRef]
- Xie, W.Y.; Shen, Q.; Zhao, F.J. Antibiotics and antibiotic resistance from animal manures to soil: A review. Eur. J. Soil Sci. 2018, 69, 181–195. [Google Scholar] [CrossRef]
- Bhushan, C.; Khurana, A.; Sinha, R.; Nagaraju, M. Antibiotic Resistance in Poultry Environment: Spread of Resistance from Poultry Farm to Agricultural Field; Centre for Science and Environment: New Delhi, India, 2017; pp. 1–36. [Google Scholar]
- Argudin, M.; Deplano, A.; Meghraoui, A.; Dodemont, M.; Heinrichs, A.; Denis, O.; Nonhoff, C.; Roisin, S. Bacteria from animals as a pool of antimicrobial resistance genes. Antibiotics 2017, 6, 12. [Google Scholar] [CrossRef]
- Tien, Y.C.; Li, B.; Zhang, T.; Scott, A.; Murray, R.; Sabourin, L.; Marti, R.; Topp, E. Impact of dairy manure pre-application treatment on manure composition, soil dynamics of antibiotic resistance genes, and abundance of antibiotic-resistance genes on vegetables at harvest. Sci. Total Environ. 2017, 581, 32–39. [Google Scholar] [CrossRef]
- Chen, C.; Pankow, C.A.; Oh, M.; Heath, L.S.; Zhang, L.; Du, P.; Xia, K.; Pruden, A. Effect of antibiotic use and composting on antibiotic resistance gene abundance and resistome risks of soils receiving manure-derived amendments. Environ. Int. 2019, 128, 233–243. [Google Scholar] [CrossRef]
- Wang, F.; Fu, Y.H.; Sheng, H.J.; Topp, E.; Jiang, X.; Zhu, Y.G.; Tiedje, J.M. Antibiotic resistance in the soil ecosystem: A One Health perspective. Curr. Opin. Environ. Sci. Health 2021, 20, 100230. [Google Scholar] [CrossRef]
- Flores-Vargas, G.; Bergsveinson, J.; Lawrence, J.R.; Korber, D.R. Environmental biofilms as reservoirs for antimicrobial resistance. Front. Microbiol. 2021, 12, 3880. [Google Scholar] [CrossRef]
- Larsson, D.J.; Flach, C.F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Ruuskanen, M.; Muurinen, J.; Meierjohan, A.; Pärnänen, K.; Tamminen, M.; Lyra, C.; Kronberg, L.; Virta, M. Fertilizing with animal manure disseminates antibiotic resistance genes to the farm environment. J. Environ. Qual. 2016, 45, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Kousar, S.; Rehman, N.; Javed, A.; Hussain, A.; Naeem, M.; Masood, S.; Ali, H.A.; Manzoor, A.; Khan, A.A.; Iqbal, F.; et al. Intensive Poultry Farming Practices Influence Antibiotic Resistance Profiles in Pseudomonas Aeruginosa Inhabiting Nearby Soils. Infect. Drug Resist. 2021, 14, 4511–4516. [Google Scholar] [CrossRef]
- Muurinen, J.; Stedtfeld, R.; Karkman, A.; Parnanen, K.; Tiedje, J.; Virta, M. Influence of manure application on the environmental resistome under Finnish agricultural practice with restricted antibiotic use. Environ. Sci. Technol. 2017, 51, 5989–5999. [Google Scholar] [CrossRef]
- Baker, M.; Williams, A.D.; Hooton, S.P.T.; Helliwell, R.; King, E.; Dodsworth, T.; Baena-Nogueras, R.M.; Warry, A.; Ortori, C.A.; Todman, H.; et al. Antimicrobial resistance in dairy slurry tanks: A critical point for measurement and control. Environ. Int. 2022, 169, 107516. [Google Scholar] [CrossRef] [PubMed]
- Han, X.M.; Hu, H.W.; Chen, Q.L.; Yang, L.Y.; Li, H.L.; Zhu, Y.G.; Li, X.Z.; Ma, Y.B. Antibiotic resistance genes and associated bacterial communities in agricultural soils amended with different sources of animal manures. Soil Biol. Biochem. 2018, 126, 91–102. [Google Scholar] [CrossRef]
- Deng, W.; Zhang, A.; Chen, S.; He, X.; Jin, L.; Yu, X.; Yang, S.; Li, B.; Fan, L.; Ji, L.; et al. Heavy metals, antibiotics and nutrients affect the bacterial community and resistance genes in chicken manure composting and fertilized soil. J. Environ. Manag. 2020, 257, 109980. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, D.; Xue, J.; Weaver, L.; Wakelin, S.A.; Feng, Y.; Li, Z. Changes in microbial community structure during pig manure composting and its relationship to the fate of antibiotics and antibiotic resistance genes. J. Hazard. Mater. 2020, 389, 122082. [Google Scholar] [CrossRef] [PubMed]
- Sardar, M.F.; Zhu, C.; Geng, B.; Ahmad, H.R.; Song, T.; Li, H. The fate of antibiotic resistance genes in cow manure composting: Shaped by temperature-controlled composting stages. Bioresour. Technol. 2021, 320, 124403. [Google Scholar] [CrossRef]
- Wang, G.; Kong, Y.; Yang, Y.; Ma, R.; Li, L.; Li, G.; Yuan, J. Composting temperature directly affects the removal of antibiotic resistance genes and mobile genetic elements in livestock manure. Environ. Pollut. 2022, 303, 119174. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Feng, Y.; Liu, Y.; Xue, J.; Li, Z. Dynamics of oxytetracycline, sulfamerazine, and ciprofloxacin and related antibiotic resistance genes during swine manure composting. J. Environ. Manag. 2019, 230, 102–109. [Google Scholar] [CrossRef]
- Song, L.; Wang, C.; Jiang, G.Y.; Ma, J.B.; Li, Y.F.; Chen, H.; Guo, J.H. Bioaerosol is an important transmission route of antibiotic resistance genes in pig farms. Environ. Int. 2021, 154, 106559. [Google Scholar] [CrossRef] [PubMed]
- Karkman, A.; Do, T.T.; Walsh, F.; Virta, M.P. Antibiotic-resistance genes in waste water. Trends Microbiol. 2018, 26, 220–228. [Google Scholar] [CrossRef]
- Lerminiaux, N.A.; Cameron, A.D. Horizontal transfer of antibiotic resistance genes in clinical environments. Can. J. Microbiol. 2019, 65, 34–44. [Google Scholar] [CrossRef]
- Bakkeren, E.; Huisman, J.S.; Fattinger, S.A.; Hausmann, A.; Furter, M.; Egli, A.; Slack, E.; Sellin, M.E.; Bonhoeffer, S.; Regoes, R.R.; et al. Salmonella Persisters Promote the Spread of Antibiotic Resistance Plasmids in the Gut. Nature 2019, 573, 270–280. [Google Scholar] [CrossRef]
- Nhung, N.T.; Chansiripornchai, N.; Carrique-Mas, J.J. Antimicrobial resistance in bacterial poultry pathogens: A review. Front. Vet. Sci. 2017, 4, 126. [Google Scholar] [CrossRef] [PubMed]
- Van den Honert, M.S.; Gouws, P.A.; Hoffman, L.C. Importance and implications of antibiotic resistance development in livestock and wildlife farming in South Africa: A Review. S. Afr. J. Anim. Sci. 2018, 48, 401–412. [Google Scholar] [CrossRef]
- Manaia, C.M. Assessing the risk of antibiotic resistance transmission from the environment to humans: Non-direct proportionality between abundance and risk. Trends Microbiol. 2017, 25, 173–181. [Google Scholar] [CrossRef]
- Ghosh, S.; LaPara, T.M. The effects of subtherapeutic antibiotic use in farm animals on the proliferation and persistence of antibiotic resistance among soil bacteria. ISME J. 2007, 1, 191–203. [Google Scholar] [CrossRef]
- Balcázar, J.L.; Subirats, J.; Borrego, C.M. The role of biofilms as environmental reservoirs of antibiotic resistance. Front. Microbiol. 2015, 6, 1216. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.A.S.; Xu, J.; Moore, J.E.; Blair, I.S.; McDowell, D.A. Environmental stress and antibiotic resistance in food-related pathogens. Appl. Environ. Microbiol. 2007, 73, 211–217. [Google Scholar] [CrossRef]
- Seiler, C.; Berendonk, T.U. Heavy metal driven co-selection of antibiotic resistance in soil and water bodies impacted by agriculture and aquaculture. Front. Microbiol. 2012, 3, 399. [Google Scholar] [CrossRef]
- Komijani, M.; Shamabadi, N.S.; Shahin, K.; Eghbalpour, F.; Tahsili, M.R.; Bahram, M. Heavy metal pollution promotes antibiotic resistance potential in the aquatic environment. Environ. Pollut. 2021, 274, 116569. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, F.; Shirzad-Aski, H.; Ferreira, S. Antimicrobials and food-related stresses as selective factors for antibiotic resistance along the farm to fork continuum. Antibiotics 2021, 10, 671. [Google Scholar] [CrossRef]
- Kurenbach, B.; Hill, A.M.; Godsoe, W.; van Hamelsveld, S.; Heinemann, J.A. Agrichemicals and antibiotics in combination increase antibiotic resistance evolution. PeerJ 2018, 6, e5801. [Google Scholar] [CrossRef] [PubMed]
- Chopyk, J.; Nasko, D.J.; Allard, S.; Callahan, M.T.; Bui, A.; Ferelli, A.M.C.; Chattopadhyay, S.; Mongodin, E.F.; Pop, M.; Micallef, S.A.; et al. Metagenomic analysis of bacterial and viral assemblages from a freshwater creek and irrigated field reveals temporal and spatial dynamics. Sci. Total Environ. 2020, 706, 135395. [Google Scholar] [CrossRef] [PubMed]
- Bougnom, B.P.; Thiele-Bruhn, S.; Ricci, V.; Zongo, C.; Piddock, L.J.V. Raw wastewater irrigation for urban agriculture in three African cities increases the abundance of transferable antibiotic resistance genes in soil, including those encoding extended spectrum betalactamases (ESBLs). Sci. Total Environ. 2020, 698, 134201. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, G.; Chang, J.; Kong, Y.; Jiang, T.; Wang, J.; Yuan, J. Enrichment of antibiotic resistance genes after sheep manure aerobic heap composting. Bioresour. Technol. 2021, 323, 124620. [Google Scholar] [CrossRef]
- Christou, A.; Agüera, A.; Bayona, J.M.; Cytryn, E.; Fotopoulos, V.; Lambropoulou, D.; Manaia, C.M.; Michael, C.; Revitt, M.; Schröder, P.; et al. The potential implications of reclaimed wastewater reuse for irrigation on the agricultural environment: The knowns and unknowns of the fate of antibiotics and antibiotic resistant bacteria and resistance genes–a review. Water Res. 2017, 123, 448–467. [Google Scholar] [CrossRef] [PubMed]
- Fahrenfeld, N.L.; Ma, Y.; O’Brien, M.; Pruden, A. Reclaimed water as a reservoir of antibiotic resistance genes: Distribution system and irrigation implications. Front. Microbiol. 2013, 4, 130. [Google Scholar] [CrossRef] [PubMed]
- Gatica, J.; Cytryn, E. Impact of treated wastewater irrigation on antibiotic resistance in the soil microbiome. Environ. Sci. Pollut. Res. Int. 2013, 20, 3529–3538. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Xie, J.; He, T.; Wu, D.; Li, X. Airborne transmission as an integral environmental dimension of antimicrobial resistance through the “One Health” lens. Crit. Rev. Environ. Sci. Technol. 2021, 52, 4172–4419. [Google Scholar] [CrossRef]
- Salmenlinna, S.; Lyytikäinen, O.; Vainio, A.; Myllyniemi, A.L.; Raulo, S.; Kanerva, M.; Rantala, M.; Thomson, K.; Seppänen, J.; Vuopio, J. Human cases of methicillin-resistant Staphylococcus aureus CC398, Finland. Emerg. Infect. Dis. 2010, 16, 1626. [Google Scholar] [CrossRef]
- Deng, Y.; Zeng, Z.; Chen, S.; He, L.; Liu, Y.; Wu, C.; Chen, Z.; Yao, Q.; Hou, J.; Yang, T.; et al. Dissemination of incFII plasmids carrying rmtB and qepA in Escherichia coli from pigs, farm workers and the environment. Clin. Microbiol. Infect. 2011, 17, 1740–1745. [Google Scholar] [CrossRef]
- Liu, X.Q.; Wang, J.; Li, W.; Zhao, L.Q.; Lu, Y.; Liu, J.H.; Zeng, Z.L. Distribution of cfr in Staphylococcus spp. and Escherichia coli strains from pig farms in China and characterization of a novel cfr-carrying F43: A-: B-plasmid. Front. Microbiol. 2017, 8, 329. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, D.V.; Holst, G.J.; Basinas, I.; Elholm, G.; Schlünssen, V.; Linneberg, A.; Santl-Temkiv, T.; Finster, K.; Sigsgaard, T.; Marshall, I.P.G. Pig farmers’ homes harbor more diverse airborne bacterial communities than pig stables or suburban homes. Front. Microbiol. 2018, 9, 870. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Antibiotic-resistant bacteria: Prevalence in food and inactivation by food-compatible compounds and plant extracts. J. Agric. Food Chem. 2015, 63, 3805–3822. [Google Scholar] [CrossRef] [PubMed]
- Vines, J.; Cusco, A.; Napp, S.; Alvarez, J.; Saez-Llorente, J.L.; Rosas-Rodoreda, M.; Francino, O.; Migura-Garcia, L. Transmission of similar mcr-1 carrying plasmids among different Escherichia coli lineages isolated from livestock and the farmer. Antibiotics 2021, 10, 313. [Google Scholar] [CrossRef] [PubMed]
- Mbareche, H.; Veillette, M.; Pilote, J.; Letourneau, V.; Duchaine, C. Bioaerosols play a major role in the nasopharyngeal microbiota content in agricultural environment. Int. J. Environ. Res. Public Health 2019, 16, 375. [Google Scholar] [CrossRef] [PubMed]
- Luiken, R.E.; Van Gompel, L.; Bossers, A.; Munk, P.; Joosten, P.; Hansen, R.B.; Knudsen, B.E.; García-Cobos, S.; Dewulf, J.; Aarestrup, F.M.; et al. Farm dust resistomes and bacterial microbiomes in European poultry and pig farms. Environ. Int. 2020, 143, 105971. [Google Scholar] [CrossRef]
- Yang, F.; Gao, Y.; Zhao, H.; Li, J.; Cheng, X.; Meng, L.; Dong, P.; Yang, H.; Chen, S.; Zhu, J. Revealing the distribution characteristics of antibiotic resistance genes and bacterial communities in animal-aerosol-human in a chicken farm: From One-Health perspective. Ecotoxicol. Environ. Saf. 2021, 2241, 12687. [Google Scholar] [CrossRef]
- Navajas-Benito, E.V.; Alonso, C.A.; Sanz, S.; Olarte, C.; Martínez-Olarte, R.; Hidalgo-Sanz, S.; Somalo, S.; Torres, C. Molecular characterization of antibiotic resistance in Escherichia coli strains from a dairy cattle farm and its surroundings. J. Sci. Food Agric. 2017, 97, 362–365. [Google Scholar] [CrossRef]
- McEachran, A.D.; Blackwell, B.R.; Hanson, J.D.; Wooten, K.J.; Mayer, G.D.; Cox, S.B.; Smith, P.N. Antibiotics, bacteria, and antibiotic resistance genes: Aerial transport from cattle feed yards via particulate matter. Environ. Health Perspect. 2015, 123, 337–343. [Google Scholar] [CrossRef]
- He, Y.; Yuan, Q.; Mathieu, J.; Stadler, L.; Senehi, N.; Sun, R.; Alvarez, P.J. Antibiotic resistance genes from livestock waste: Occurrence, dissemination, and treatment. NPJ Clean Water 2020, 3, 4. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, W.; Yang, X.; Wang, J.; Lin, H.; Yang, Y. Microplastics are a hotspot for antibiotic resistance genes: Progress and perspective. Sci. Total Environ. 2021, 773, 145643. [Google Scholar] [CrossRef] [PubMed]
- Hamscher, G.; Pawelzick, H.T.; Sczesny, S.; Nau, H.; Hartung, J. Antibiotics in dust originating from a pig-fattening farm: A new source of health hazard for farmers? Environ. Health Perspect. 2003, 111, 1590–1594. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.Z.; Zou, H.Y.; Wu, D.L.; Chen, S.; He, L.Y.; Zhang, M.; Bai, H.; Ying, G.G. Swine farming elevated the proliferation of Acinetobacter with the prevalence of antibiotic resistance genes in the groundwater. Environ. Int. 2020, 136, 105484. [Google Scholar] [CrossRef] [PubMed]
- Al Salah, D.M.M.; Laffite, A.; Poté, J. Occurrence of bacterial markers and antibiotic resistance genes in sub-Saharan rivers receiving animal farm wastewaters. Sci. Rep. 2019, 9, 14847. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.J.; Zurek, L. Association of Escherichia coli O157:H7 with houseflies on a cattle farm. Appl. Environ. Microbiol. 2004, 70, 7578–7580. [Google Scholar] [CrossRef] [PubMed]
- Usui, M.; Iwasa, T.; Fukuda, A.; Sato, T.; Okubo, T.; Tamura, Y. The role of flies in spreading the extended-spectrum beta-lactamase gene from cattle. Microb. Drug Resist. 2013, 19, 415–420. [Google Scholar] [CrossRef]
- Srinivasan, V.; Nguyen, L.T.; Headrick, S.I.; Murinda, S.E.; Olive, S.P. Antimicrobial Resistance Patterns of Shiga Toxin-Producing Escherichia Coli O157:H7 and O157:H7- from Different Origins. Microb. Drug Resist. 2007, 13, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.M.; Jiménez, L.; Perea, J.M.; Arias, R.; Palop, M.L. Farming practices influence antibiotic resistance and biogenic amine capacity of staphylococci from bulk tank ewe’s milk. Animals 2020, 10, 1622. [Google Scholar] [CrossRef] [PubMed]
- Badul, S.; Abia, A.L.; Amoako, D.G.; Perrett, K.; Bester, L.A.; Essack, S.Y. From the farms to the dining table: The distribution and molecular characteristics of antibiotic-resistant Enterococcus spp. in intensive pig farming in South Africa. Microorganisms 2021, 9, 882. [Google Scholar] [CrossRef] [PubMed]
- Abgottspon, H.; Stephan, R.; Bagutti, C.; Brodmann, P.; Hachler, H.; Zurfluh, K. Characteristics of extended-spectrum cephalosporin-resistant Escherichia coli isolated from Swiss and imported poultry meat. J. Food Prot. 2014, 77, 112–115. [Google Scholar] [CrossRef]
- Adzitey, F.; Huda, N.; Rahmat Ali, G.R. Prevalence and antibiotic resistance of Campylobacter, Salmonella, and L. monocytogenes in ducks: A review. Foodborne Pathog. Dis. 2012, 9, 498–505. [Google Scholar] [CrossRef]
- Rahman, M.M.; Husna, A.; Elshabrawy, H.A.; Alam, J.; Runa, N.Y.; Badruzzaman, A.T.M.; Banu, N.A.; Al Mamun, M.; Paul, B.; Das, S.; et al. Isolation and Molecular Characterization of Multidrug-Resistant Escherichia Coli from Chicken Meat. Sci. Rep. 2020, 10, 21999. [Google Scholar] [CrossRef]
- Cornejo, J.; Pokrant, E.; Figueroa, F.; Riquelme, R.; Galdames, P.; Di Pillo, F.; Jimenez-Bluhm, P.; Hamilton-West, C. Assessing antibiotic residues in poultry eggs from backyard production systems in Chile, first approach to a non-addressed issue in farm animals. Animals 2020, 10, 1056. [Google Scholar] [CrossRef]
- Amoako, D.G.; Somboro, A.M.; Abia, A.L.; Molechan, C.; Perrett, K.; Bester, L.A.; Essack, S.Y. Antibiotic resistance in Staphylococcus aureus from poultry and poultry products in uMgungundlovu District, South Africa, using the “Farm to Fork” approach. Microb. Drug Resist. 2020, 26, 402–411. [Google Scholar] [CrossRef]
- Zhu, B.; Chen, Q.; Chen, S.; Zhu, Y.G. Does organically produced lettuce harbor higher abundance of antibiotic resistance genes than conventionally produced? Environ. Int. 2017, 98, 152–159. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic use in agriculture and its consequential resistance in environmental sources: Potential public health implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef]
- Gao, F.Z.; He, L.Y.; He, L.X.; Zou, H.Y.; Zhang, M.; Wu, D.L.; Liu, Y.S.; Shi, Y.J.; Bai, H.; Ying, G.G. Untreated swine wastes changed antibiotic resistance and microbial community in the soils and impacted abundances of antibiotic resistance genes in the vegetables. Sci. Total Environ. 2020, 741, 12. [Google Scholar] [CrossRef]
- Chen, Q.L.; Cui, H.L.; Su, J.Q.; Penuelas, J.; Zhu, Y.G. Antibiotic resistomes in plant microbiomes. Trends Plant Sci. 2019, 24, 530–541. [Google Scholar] [CrossRef]
- Stockwell, V.O.; Duffy, B. Use of antibiotics in plant agriculture. Rev. Sci. Tech.-Off. Int. Epizoot. 2012, 31, 199–210. [Google Scholar] [CrossRef]
- Sundin, G.W.; Wang, N. Antibiotic Resistance in Plant-Pathogenic Bacteria. Annu. Rev. Phytopathol. 2018, 56, 161–180. [Google Scholar] [CrossRef]
- Donato, J.J.; Moe, L.A.; Converse, B.J.; Smart, K.D.; Berklein, F.C.; McManus, P.S.; Handelsman, J. Metagenomic analysis of apple orchard soil reveals antibiotic resistance genes encoding predicted bifunctional proteins. Appl. Environ. Microbiol. 2010, 13, 4396–4401. [Google Scholar] [CrossRef] [PubMed]


| Type of Livestock | Antibiotics | References |
|---|---|---|
| Swine | Pleuromutilins, tetracyclines, macrolides | [15] |
| Swine | Penicillin, tetracyclines, cephalosporins, lincosamides, fluoroquinolones, amoxicillin, tulathromycin, tylosin, colistin | [19] |
| Poultry | Bacitracin, virginiamycin, salinomycin, tilmicosin | [20] |
| Cattle | β-lactams, aminoglycosides, quinolones, fluoroquinolones, macrolides, tetracyclines, sulfonamides, streptogramins, lincosamides | [20] |
| Cattle | Penicillins, tetracyclines and macrolides | [15] |
| Type of Livestock | Bacteria under Study | Resistance Level | Antibiotics | Detected Genes | References |
|---|---|---|---|---|---|
| Poultry | E. coli | 35% | 3rd generation cephalosporins | [16] | |
| Poultry and swine | E. coli | 29.5–54.7% | ampicillin, sulfonamide, tetracycline | [41] | |
| Poultry | E. coli | 57.6% | Ciprofloxacin | [41] | |
| Poultry | E. coli | 47.4% | Nalidixic acid | [41] | |
| Poultry | E. coli | >10% | Cefotaxim | [41] | |
| Poultry | E. coli | >50% | Sulfisoxazole, streptomycin | [41] | |
| Poultry | E. coli | >40 | Tetracycline, gentamicin | [41] | |
| Poultry | Enterobacteriaceae | 60–70% | Trimethoprim/sulfamethoxazol, tetracycline, chloramphenicol, amoxicillin/clavulanic acid | qnrS different tet genes | [42] |
| Poultry | Different isolates | >50% | Penicillin, ciprofloxacin, rifampicin, kanamycin, streptomycin, cefixime, erythromycin, ampicillin, Tetracyclin | [43] | |
| Sheep | Staphylococcus aureus | 23.5% | Methicillin, multi-resistant | [44] | |
| Cattle | E. coli | 24.5–30.6% | Ampicillin, streptomycin Sulfonamide, tetracycline | [41] | |
| Cattle and goats | Staphylococcus aureus | 17.6% | Methicillin, multi-resistant | [44] | |
| Cattle | E. coli Salmonella sp. | Significant resistance | Azithromycin, tetracycline, erythromycin, oxytetracycline, ertapenem | tetA, tetB ereA | [45] |
| Cattle | Faecal bacteria | 14.2–79.2% | Tetracycline | [46] | |
| Cattle | E. coli | cfrB optrA | [47] | ||
| Swine | Enterobacteriaceae | 80–90% | Tetracycline, trimethoprim/sulfamethoxazol | [42] | |
| Swine | Campylobacter spp. | 35.5–79.6 | Nalidixic acid, erythromycin, tetracycline, azitromycine, Ciprofloxacin | tetO ermB | [48] |
| Swine | E. coli | 70% | Tetracycline, streptomycin, florfenicol | [49] | |
| Swine | Enterococcus faecalis | 70–100% | Streptomycin, tetracycline | [50] | |
| Swine | Enterococcus faecium | Up to 97% | Ampicillin, oxytetracycline, gentamicin, streptomycin | [50] |
| Type of Wastes | Antibiotic Resistance | Resistance Genes | References |
|---|---|---|---|
| Poultry manure | ermB, sul2, tetA, sul1, and strB | [60] | |
| Swine and cattle manure | Tetracyclines, sulfonamides, aminoglycoside, macrolide antibiotics, beta-lactam antibiotics and chloramphenicol | Tet and sul genes | [61,62] |
| Poultry litter | Amoxiclav, doxycycline, cefotaxime, levofloxacin, ciprofloxacin, amikacin, meropenem, linezolid, chloramphenicol, cefuroxime, ceftriaxone | [63] |
| Type of Product | Resistant Microorganisms | Antibiotic Resistance Profile | References |
|---|---|---|---|
| Sheep milk | Staphylococci | Multi-resistant, tetM, ermB, ermC, and grlA genes | [120] |
| Pork meat | Enterococcus faecalis, E. faecium E. casseliflavus | 80% of the strains resistant to sulfamethoxazole/trimethoprim, over 5% resistant to levofloxacin. A total of 40% of E. faecium strains resistant to quinupristin–dalfopristin. A total of 78% of the isolates were multi-resistant strains. Around 90% containing resistance genes towards tetracycline, aminoglycoside and macrolide antibiotics. | [121] |
| Poultry meat | A total of 88% of isolates were antibiotic resistant. | [122] | |
| Chicken carcasses | E. coli, Salmonella sp. | Multi-resistant. | |
| Ready-to-cook poultry products | Salmonella sp. | More than 80% strains resistant to at least 5 antibiotics. | [63] |
| Duck meat | Campylobacter sp. | Antibiotic resistant strains detected. | [123] |
| Poultry meat | E. coli | Resistance genes detected: tetA (tetracycline), ereA (erythromycin), aac-3-IV (gentamicin), cmlA and catA1 (chloramphenicol) aadA1 (streptomycin). | [124] |
| Chicken eggs | β-lactams, macrolides, tetracyclines and aminoglycosides. | [125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karwowska, E. Antibiotic Resistance in the Farming Environment. Appl. Sci. 2024, 14, 5776. https://doi.org/10.3390/app14135776
Karwowska E. Antibiotic Resistance in the Farming Environment. Applied Sciences. 2024; 14(13):5776. https://doi.org/10.3390/app14135776
Chicago/Turabian StyleKarwowska, Ewa. 2024. "Antibiotic Resistance in the Farming Environment" Applied Sciences 14, no. 13: 5776. https://doi.org/10.3390/app14135776
APA StyleKarwowska, E. (2024). Antibiotic Resistance in the Farming Environment. Applied Sciences, 14(13), 5776. https://doi.org/10.3390/app14135776





