A Precision Livestock Farming Technique from Breeding to Slaughter: Infrared Thermography in Pig Farming
Abstract
:1. Introduction
2. Application of Thermography in Pig Breeding
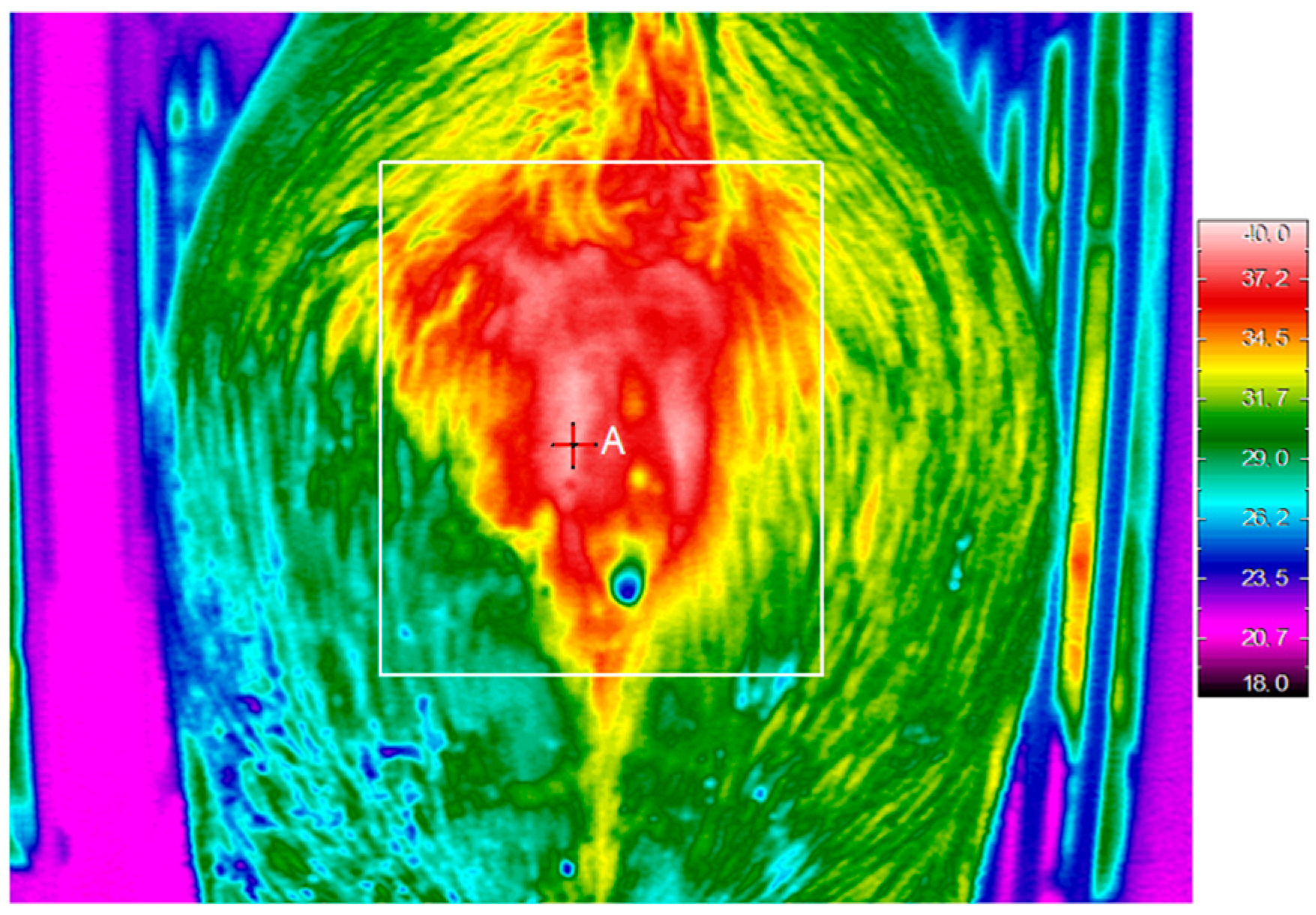
2.1. Oestrus Detection
2.2. Neonatal Mortality Reduction
2.3. Fever or Diseases Early Detection
2.4. Growing Pig Efficiency
2.5. Environmental Stress Assessment
2.6. Interaction or Restraint Stress Assessment
2.6.1. Assessment of Restraint-Related Stress
2.6.2. Assessment of Stress Related to Aggressive Interaction
2.7. Assessing the Stress Related to Pre-Slaughter Procedures
2.8. Meat Quality Prediction
3. Discussion
3.1. Individual Variables
3.2. Outer Variables
3.3. Technical Variables
4. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, S.; Zhou, C.; Jiang, X.; Huang, J.; Xu, D. Progress on Infrared Imaging Technology in Animal Production: A Review. Sensors 2022, 22, 705. [Google Scholar] [CrossRef]
- McManus, C.; Tanure, C.B.; Peripolli, V.; Seixas, L.; Fischer, V.; Gabbi, A.M.; Menegassi, S.R.O.; Stumpf, M.T.; Dias, G.J.; Costa, J.B.G. Infrared thermography in animal production: An overview. Comput. Electron. Agric. 2016, 123, 10–16. [Google Scholar] [CrossRef]
- Guevara, R.D.; Pastor, J.J.; Manteca, X.; Tedo, G.; Llonch, P. Systematic review of animal-based indicators to measure thermal, social, and immune-related stress in pigs. PLoS ONE 2022, 17, e0266524. [Google Scholar] [CrossRef]
- Yin, M.; Ma, R.; Hailing, L.; Jun, L.; Zhao, Q.; Zhang, M. Non-contact sensing technology enables precision livestock farming in smart farms. Comput. Electron. Agric. 2023, 212, 108171. [Google Scholar] [CrossRef]
- Maldagues, X.P.V.; Moore, P.O. Nondestructive Testing Handbook: Infrared and Thermal Testing, 3rd ed.; The American Society for Nondestructive Testing: Columbus, OH, USA, 2001. [Google Scholar]
- Scolari, S.; Evans, R.; Knox, R.; Tamassia, M.; Clark, S. Determination of the relationship between vulvar skin temperatures and time of ovulation in swine using digital infrared thermography. Reprod. Fertil. Dev. 2009, 22, 178. [Google Scholar] [CrossRef]
- Scolari, S.; Clark, S.; Knox, R.; Tamassia, M. Vulvar skin temperature changes significantly during estrus in swine as determined by digital infrared thermography. J. Swine Health Prod. 2011, 19, 151–155. [Google Scholar]
- Simões, V.; Lyazrhi, F.; Picard-Hagen, N.; Gayrard, V.; Martineau, G.; Waret-Szkuta, A. Variations in the vulvar temperature of sows during proestrus and estrus as determined by infrared thermography and its relation to ovulation. Theriogenology 2014, 82, 1080–1085. [Google Scholar] [CrossRef]
- Sykes, D.J.; Couvillion, J.S.; Cromiak, A.; Bowers, S.; Schenck, E.; Crenshaw, M.; Ryan, P.L. The use of digital infrared thermal imaging to detect estrus in gilts. Theriogenology 2012, 78, 147–152. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, D.H.; Yun, W.; Oh, H.J.; An, J.S.; Kim, Y.G.; Kim, G.M.; Cho, J.H. Quantifiable and feasible estrus detection using the ultrasonic sensor array and digital infrared thermography. J. Anim. Sci. Technol. 2019, 61, 163–169. [Google Scholar] [CrossRef]
- Schmitt, O.; Reigner, S.; Bailly, J.; Ravon, L.; Billon, Y.; Gress, L.; Bluy, L.; Canario, L.; Gilbert, H.; Bonnet, A.; et al. Thermoregulation at birth differs between piglets from two genetic lines divergent for residual feed intake. Animal 2021, 15, 100069. [Google Scholar] [CrossRef]
- Muns, R.; Nuntapaitoon, M.; Tummaruk, P. Non-infectious causes of pre-weaning mortality in piglets. Livest. Sci. 2016, 184, 46–57. [Google Scholar] [CrossRef]
- Llamas Moya, S.; Boyle, L.A.; Lynch, P.B.; Arkins, S. Influence of teeth resection on the skin temperature and acute phase response in newborn piglet. Anim. Welf. 2006, 15, 291–297. [Google Scholar] [CrossRef]
- Tabuarici, P.; Bunter, K.L.; Graser, H.-U. Thermal imaging as a potential tool for identifying piglets at risk. In Proceedings of the AGBU Pig Genetics Workshop, Armidale, Australia, 24–25 October 2012; pp. 23–30. [Google Scholar]
- Herpin, P.; Damon, M.; Le Dividich, J. Development of thermoregulation and neonatal survival in pigs. Livest. Prod. Sci. 2002, 78, 25–45. [Google Scholar] [CrossRef]
- Schmitt, O.; O’Driscoll, K. Use of infrared thermography to noninvasively assess neonatal piglet temperature. Transl. Anim. Sci. 2021, 5, txaa208. [Google Scholar] [CrossRef] [PubMed]
- Loughmiller, J.A.; Spire, M.F.; Tokach, M.D.; Dritz, S.S.; Nelssen, J.L.; Goodband, R.D.; Hogge, S.B. An Evaluation of Differences in Mean Body Surface Temperature with Infrared Thermography in Growing Pigs Fed Different Dietary Energy Intake and Concentration. J. Appl. Anim. Res. 2005, 28, 73–80. [Google Scholar] [CrossRef]
- Cook, N.; Chabot, B.; Liu, T.; Froehlich, D.; Basarab, J.; Juarez, M. Radiated temperature from thermal imaging is related to feed consumption, growth rate and feed efficiency in grower pigs. J. Therm. Biol. 2020, 94, 102747. [Google Scholar] [CrossRef]
- Lengling, A.; Alfert, A.; Reckels, B.; Steinhoff-Wagner, J.; Büscher, W. Feasibility Study on the Use of Infrared Thermography to Classify Fattening Pigs into Feeding Groups According Their Body Composition. Sensors 2020, 20, 5221. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gariepy, C.; Amiot, J.; Nadai, S. Ante-mortem detection of PSE and DFD by Infrared Thermography of pigs before stunning. Meat Sci. 1989, 25, 37–41. [Google Scholar] [CrossRef]
- Lawrence, T.E.; Spire, M.F.; Dikeman, M.E.; Hunt, M.C.; Hogge, S.B.; James, B.W. Utilizing Infrared Thermography to Predict Pork Quality. Swine Day 2001, 2001, 131–134. [Google Scholar]
- Schaefer, A.L.; Jones, S.D.M.; Murray, A.C.; Sather, A.P.; Tong, A.K.W. Infrared thermography of pigs with known genotypes for stress susceptibility in relation to pork quality. Can. J. Anim. Sci. 1989, 69, 491–495. [Google Scholar] [CrossRef]
- Rocha, L.M.; Devillers, N.; Maldague, X.; Kabemba, F.Z.; Fleuret, J.; Guay, F.; Faucitano, L. Validation of anatomical sites for the measurement of infrared body surface temperature variation in response to handling and transport. Animals 2019, 9, 425. [Google Scholar] [CrossRef]
- Dikeman, M.; Spire, M.; Hunt, M.; Lowak, S. Infrared Thermography of Market Hogs as a Predictor of Pork Quality; Research Report NP B #02-025; National Pork Board: Des Moines, IA, USA, 2003; pp. 1–9. [Google Scholar]
- Nanni Costa, L.; Stelletta, C.; Cannizzo, C.; Gianesella, M.; Lo Fiego, D.P.; Morgante, M. The use of thermography on the slaughter-line for the assessment of pork and raw ham quality. Ital. J. Anim. Sci. 2007, 6 (Suppl. S1), 704–706. [Google Scholar] [CrossRef]
- Johnson, S.R.; Dunbar, M.R. Infrared thermography: Its use and application for detecting infectious diseases in wildlife and domestic animals. In Proceedings of the InfraMation 2010, Las Vegas, NV, USA, 8–12 November 2010. [Google Scholar]
- Bashiruddin, J.B.; Mann, J.; Finch, R.; Zhang, Z.; Paton, D. Preliminary study of the use of thermal imaging to assess surface temperatures during foot-and-mouth disease virus infection in cattle, sheep and pigs. In Proceedings of the 2006 Session of the Research Group of the Standing Technical Committee of the European Commission for the Control of Foot-and-Mouth Disease (Appendix 46), Paphos, Cyprus, 17–20 October 2006; Food and Agriculture Organization: Rome, Italy, 2006; pp. 304–308. [Google Scholar]
- Wendt, M.; Eickhoff, K.; Koch, R. Die Messung der Hauttemperatur als Methode zur Erkennung fieberhaft erkrankter Schweine. Deut. Tierarztl. Woch. 1997, 104, 29–33. [Google Scholar]
- Loughmiller, J.A.; Spire, M.F.; Dritz, S.S.; Fenwick, B.W.; Hosni, M.H.; Hogge, S.B. Relationship between mean body surface temperature measured by use of infrared thermography and ambient temperature in clinically normal pigs and pigs inoculated with Actinobacillus pleuropneumoniae. Am. J. Vet. Res. 2001, 62, 676–681. [Google Scholar] [CrossRef]
- Friendship, R.; Poljak, Z.; McIntosh, K. Use of infrared thermography for early detection of disease causing sudden death in a swine finishing barn. In Proceedings of the 28th Annual Centralia Swine Research Update, Guelph, ON, Canada, 28 January 2009; pp. I27–I28. [Google Scholar]
- Nanni Costa, L.; Redaelli, V.; Magnani, D.; Cafazzo, S.; Amadori, M.; Razzuoli, E.; Verga, M.; Luzi, F. Preliminary study on the relationship between skin temperature of piglet measured by infrared thermography and environmental temperature in a vehicle in transit. In Veterinary Science. Current Aspects in Biology, Animal Pathology, Clinic and Food Hygiene; Springer-Verlag: Berlin/Heidelberg, Germany, 2012; pp. 193–198. [Google Scholar]
- Magnani, D.; Gatto, M.; Cafazzo, S.; Stelletta, C.; Morgante, M.; Costa, L. Difference of surface body temperature in piglets due to the backtest and environmental condition. In Animal Hygiene and Sustainable Livestock Production. In Proceedings of the XVth International Congress of the International Society for Animal Hygiene, Vienna, Austria, 3–7 July 2011; pp. 1029–1032. [Google Scholar]
- Warriss, P.; Pope, S.; Brown, S.; Wilkins, L.; Knowles, T. Estimating the body temperature of groups of pigs by thermal imaging. Vet. Rec. 2006, 158, 331–334. [Google Scholar] [CrossRef]
- Weschenfelder, A.V.; Saucier, L.; Maldague, X.; Rocha, L.M.; Schaefer, A.L.; Faucitano, L. Use of infrared ocular thermography to assess physiological conditions of pigs prior to slaughter and predict pork quality variation. Meat Sci. 2013, 95, 616–620. [Google Scholar] [CrossRef]
- Flores-Peinado, S.; Mota-Rojas, D.; Guerrero-Legarreta, I.; Mora-Medina, P.; Cruz-Monterrosa, R.; Gómez-Prado, J.; Hernández, M.G.; Cruz-Playas, J.; Martínez-Burnes, J. Physiological responses of pigs to preslaughter handling: Infrared and thermal imaging applications. Int. J. Vet. Sci. Med. 2020, 8, 71–84. [Google Scholar] [CrossRef]
- Boileau, A.; Farish, M.; Turner, S.P.; Camerlink, I. Infrared thermography of agonistic behaviour in pigs. Physiol. Behav. 2019, 210, 112637. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Liu, T. Study on body temperature detection of pig based on infrared technology: A review. Artif. Intell. Agric. 2019, 1, 14–26. [Google Scholar] [CrossRef]
- Reza, M.N.; Ali, M.R.; Kabir, M.S.N.; Karim, M.R.; Ahmed, S.; Kyoung, H.; Kim, G.; Chung, S.O. Thermal imaging and computer vision technologies for the enhancement of pig husbandry: A review. J. Anim. Sci. Technol. 2024, 66, 31–56. [Google Scholar] [CrossRef]
- Soerensen, D.D.; Clausen, S.; Mercer, J.B.; Pedersen, L.J. Determining the emissivity of pig skin for accurate infrared thermography. Comput. Electron. Agric. 2014, 109, 52–58. [Google Scholar] [CrossRef]
- De Santis, M.; Contalbrigo, L.; Borgi, M.; Cirulli, F.; Luzi, F.; Redaelli, V.; Stefani, A.; Toson, M.; Odore, R.; Vercelli, C.; et al. Equine Assisted Interventions (EAIs): Methodological Considerations for Stress Assessment in Horses. Vet. Sci. 2017, 4, 44. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perić, D.; Livada, B.; Perić, M.; Vujić, S. Thermal imager range: Predictions, expectations, and reality. Sensors 2019, 19, 3313. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, F.; Yang, R.; Wang, K. An Infrared Temperature Correction Method for the Skin Temperature of Pigs in Infrared Images. Agriculture 2023, 13, 520. [Google Scholar] [CrossRef]
- Cai, Z.; Cui, J.; Yuan, H.; Cheng, M. Application and research progress of infrared thermography in temperature measurement of livestock and poultry animals: A review. Comput. Electron. Agric. 2023, 205, 107586. [Google Scholar] [CrossRef]
- Küster, S.; Haverkamp, L.; Schlather, M.; Traulsen, I. An Approach towards a Practicable Assessment of Neonatal Piglet Body Core Temperature Using Automatic Object Detection Based on Thermal Images. Agriculture 2023, 13, 812. [Google Scholar] [CrossRef]
- Garrido, I.; Erazo-Aux, J.; Lagüela, S.; Sfarra, S.; Ibarra-Castanedo, C.; Pivarčiová, E.; Gargiulo, G.; Maldague, X.; Arias, P. Introduction of deep learning in thermographic monitoring of cultural heritage and improvement by automatic thermogram pre-processing algorithms. Sensors 2021, 21, 750. [Google Scholar] [CrossRef]
- Vardasca, R.; Bento, F.; Tereso, M.; Martinho, D. Infrared thermal imaging: A dataset definition towards decision making and intelligence. In Proceedings of the 16th Quantitative InfraRed Thermography Conference (QITC), Paris, France, 4–8 July 2022. [Google Scholar]



| Application | Area of Interest | References | |
|---|---|---|---|
| Enhance production | Oestrus Detection | Vulvar, Body, Eye | [6,7,8,9,10] |
| Neonatal mortality reduction | Dorsal, Body, Eye | [11,12,13,14,15,16] | |
| Growing pig efficiency | Dorsal, Eye, Body | [17,18,19] | |
| Meat quality prediction | Dorsal, Body, Eye | [20,21,22,23,24,25] | |
| Enhance animal welfare | Fever or diseases early detection | Eye, Ear, Body | [26,27,28,29,30] |
| Environmental stress assessment | Dorsal, Eye, Ear | [29,31] | |
| Interaction or restraint stress assessment | Dorsal, Eye, Ear | [32] | |
| Assessing the stress related to pre-slaughter procedures | Eye, Ear, Body | [33,34,35] |
| Individual variables | Skin emissivity |
| Body condition score | |
| Coat alterations | |
| Stress or disease | |
| Individual physiological variability | |
| Outer variables | Physical efforts |
| Kind and time of feeding | |
| Timetable of the surveys | |
| Dirt, sweat, or moisture on the skin | |
| Solar radiation or other heating sources nearby | |
| Wind | |
| Environmental temperature and humidity | |
| Technical variables | Distance |
| Viewing angle | |
| Type of device (microbolometric or not) | |
| Thermic and spatial resolution | |
| ROI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Redaelli, V.; Zaninelli, M.; Martino, P.; Luzi, F.; Costa, L.N. A Precision Livestock Farming Technique from Breeding to Slaughter: Infrared Thermography in Pig Farming. Appl. Sci. 2024, 14, 5780. https://doi.org/10.3390/app14135780
Redaelli V, Zaninelli M, Martino P, Luzi F, Costa LN. A Precision Livestock Farming Technique from Breeding to Slaughter: Infrared Thermography in Pig Farming. Applied Sciences. 2024; 14(13):5780. https://doi.org/10.3390/app14135780
Chicago/Turabian StyleRedaelli, Veronica, Mauro Zaninelli, Pieranna Martino, Fabio Luzi, and Leonardo Nanni Costa. 2024. "A Precision Livestock Farming Technique from Breeding to Slaughter: Infrared Thermography in Pig Farming" Applied Sciences 14, no. 13: 5780. https://doi.org/10.3390/app14135780





