Serum Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand and the Cardiovascular Disease Continuum: Insights from Hypertensive Urgencies and Acute Heart Failure Events
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Setting
2.3. Protocol
2.4. Measurements
2.5. Data Analysis
3. Results
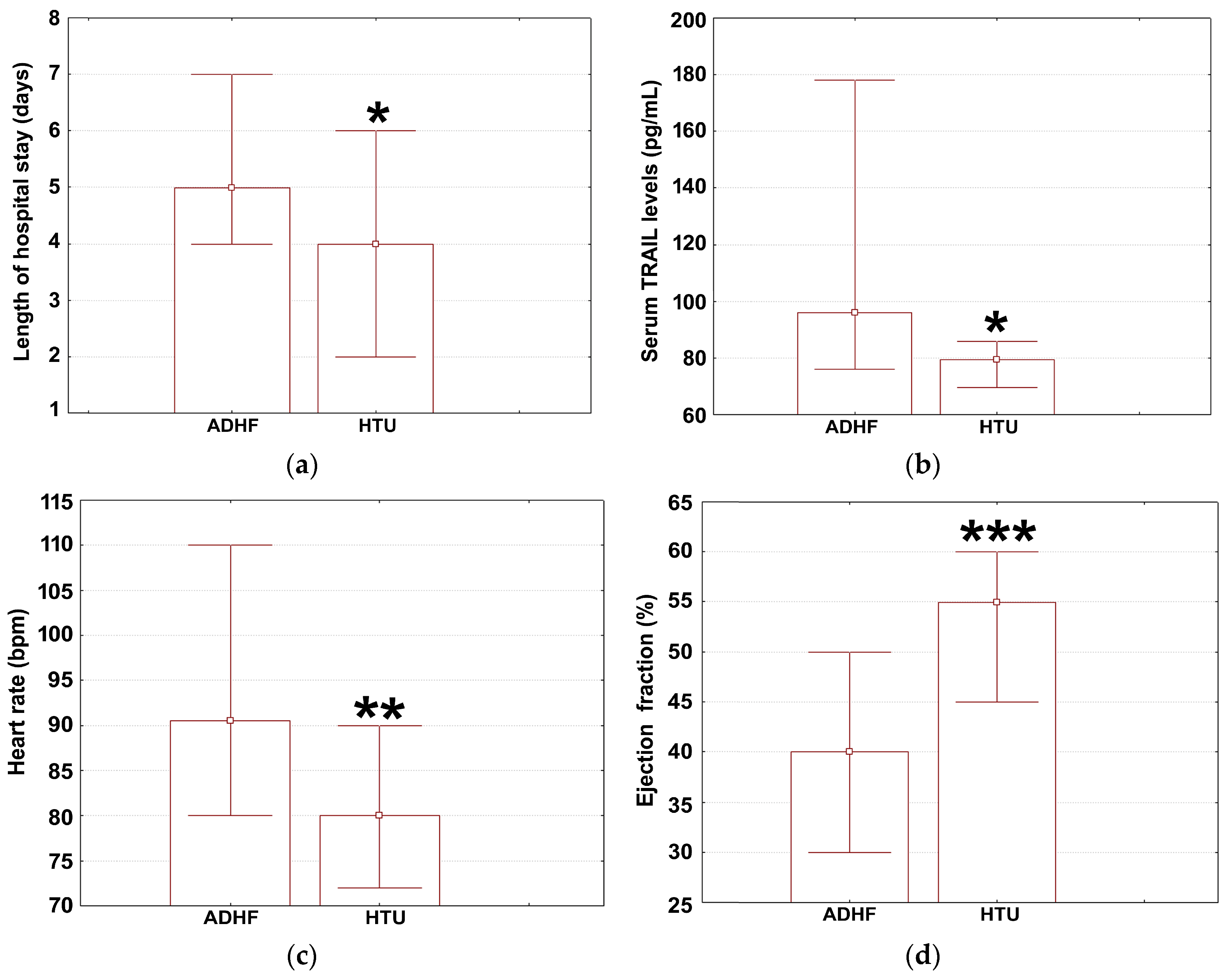
3.1. Health Profiles in HTU and ADHF
3.2. Variables Associated with TRAIL Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dzau, V.J.; Antman, E.M.; Black, H.R.; Hayes, D.L.; Manson, J.E.; Plutzky, J.; Popma, J.J.; Stevenson, W. The cardiovascular disease continuum validated: Clinical evidence of improved patient outcomes: Part I: Pathophysiology and clinical trial evidence (risk factors through stable coronary artery disease). Circulation 2006, 114, 2850–2870. [Google Scholar] [CrossRef]
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef] [PubMed]
- Liberale, L.; Montecucco, F.; Tardif, J.C.; Libby, P.; Camici, G.G. Inflamm-ageing: The role of inflammation in age-dependent cardiovascular disease. Eur. Heart J. 2020, 41, 2974–2982. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, R.; Vasan, R.S. Biomarkers in cardiovascular disease: Statistical assessment and section on key novel heart failure biomarkers. Trends Cardiovas. Med. 2017, 27, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Flack, J.M.; Adekola, B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovas. Med. 2020, 30, 160–164. [Google Scholar] [CrossRef]
- Jaffe, A.S.; Babuin, L.; Apple, F.S. Biomarkers in acute cardiac disease: The present and the future. J. Am. Coll. Cardiol. 2006, 48, 10–11. [Google Scholar]
- Neves, J.A.; Neves, J.A.; Oliveira, R.D.C.M. Biomarkers of endothelial function in cardiovascular diseases: Hypertension. J. Vasc. Bras. 2016, 15, 224–233. [Google Scholar] [CrossRef]
- Schiffrin, E.L. Vascular remodeling in hypertension: Mechanisms and treatment. Hypertension 2012, 59, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Fortuño, M.A.; Ravassa, S.; Fortuño, A.; Zalba, G.; Díez, J. Cardiomyocyte apoptotic cell death in arterial hypertension: Mechanisms and potential management. Hypertension 2001, 38, 1406–1412. [Google Scholar] [CrossRef] [PubMed]
- Narula, J.; Pandey, P.; Arbustini, E.; Haider, N.; Narula, N.; Kolodgie, F.D.; Dal, B.; Semigran, M.J.; Bielsa-Masdeu, A.; Dec, W.; et al. Apoptosis in heart failure: Release of cytochrome c from mitochondria and activation of caspase-3 in human cardiomyopathy. Proc. Natl. Acad. Sci. USA 1999, 96, 8144–8149. [Google Scholar] [CrossRef]
- Del Re, D.P.; Amgalan, D.; Linkermann, A.; Liu, Q.; Kitsis, R.N. Fundamental mechanisms of regulated cell death and implications for heart disease. Physiol. Rev. 2019, 99, 1765–1817. [Google Scholar] [CrossRef]
- Li, H.; Zhao, J.; Wang, A.; Li, Q.; Cui, W. Supramolecular assembly of protein-based nanoparticles based on tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) for cancer therapy. Colloids Surf. A: Physicochem. Eng. Asp. 2020, 590, 124486. [Google Scholar] [CrossRef]
- Grisanti, L.A. TRAIL and its receptors in cardiac diseases. Front. Physiol. 2023, 14, 1256852. [Google Scholar] [CrossRef]
- Hong, Y.; Li, Y.; Wang, X.M.; Liu, X.S.; Li, Y.N.; Ouyang, W.M.; Chen, C.S.; Jin, B.Q. Effects of perindopril on plasma soluble TRAIL and receptor soluble DR5 in patients with congestive heart failure. J. Cell. Mol. Immunol. 2003, 19, 366–368. [Google Scholar]
- Zauli, G.; Tisato, V.; Raffetto, J.D.; Vaccarezza, M. Inflammation and cardiovascular cross talk in ischemic vascular diseases. Mediators Inflamm. 2017, 1–2. [Google Scholar] [CrossRef]
- Hage, C.; Michaëlsson, E.; Linde, C.; Donal, E.; Daubert, J.-C.; Gan, L.-M.; Lund, L.H. Inflammatory biomarkers predict heart failure severity and prognosis in patients with heart failure with preserved ejection fraction. Circ. Cardiovasc. Genet. 2017, 10, e001633. [Google Scholar] [CrossRef]
- Niessner, A.; Sato, K.; Chaikof, E.L.; Colmegna, I.; Goronzy, J.J.; Weyand, C.M. Pathogen-sensing plasmacytoid dendritic cells stimulate cytotoxic T-cell function in the atherosclerotic plaque through interferon-α. Circulation 2006, 114, 2482–2489. [Google Scholar] [CrossRef] [PubMed]
- Secchiero, P.; Corallini, F.; Ceconi, C.; Parrinello, G.; Volpato, S.; Ferrari, R.; Zauli, G. Potential prognostic significance of decreased serum levels of TRAIL after acute myocardial infarction. PLoS ONE 2009, 4, e4442. [Google Scholar] [CrossRef]
- Kakareko, K.; Rydzewska-Rosołowska, A.; Zbroch, E.; Hryszko, T. TRAIL and cardiovascular disease—A risk factor or risk marker: A systematic review. J. Clin. Med. 2021, 10, 1252. [Google Scholar] [CrossRef]
- Mori, K.; Emoto, M.; Inaba, M. Multifunctional role of TRAIL in atherosclerosis and cardiovascular disease. In Advances in the Diagnosis of Coronary Atherosclerosis; Kiraç, S.F., Ed.; INTECH d.o.o.: Rijeka, Croatia, 2011; Volume 1, pp. 19–33. [Google Scholar]
- Brombo, G.; Volpato, S.; Secchiero, P.; Passaro, A.; Bosi, C.; Zuliani, G.; Zauli, G. Association of Soluble Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL) with central adiposity and low-density lipoprotein cholesterol. PLoS ONE 2013, 8, e58225. [Google Scholar] [CrossRef]
- Lorz, C.; Benito, A.; Ucero, A.C.; Santamaría, B.; Ortiz, A. Trail and kidney disease. Front. Biosci. 2009, 14, 3740–3749. [Google Scholar] [CrossRef] [PubMed]
- Korosteleva, O. Clinical Statistics: Introducing Clinical Trials, Survival Analysis, and Longitudinal Data Analysis; Jones & Bartlett Publishers: London, UK, 2008; pp. 120–166. [Google Scholar]
- Arad County Emergency Hospital. Available online: https://en.wikipedia.org/wiki/Arad_County_Clinical_Hospital (accessed on 22 May 2024).
- Popețiu, R.O.; Donath-Miklos, I.; Borta, S.M.; Rus, L.A.; Vîlcea, A.; Nica, D.V.; Pușchiță, M. Serum YKL-40 levels, leukocyte profiles, and acute exacerbations of advanced COPD. J. Clin. Med. 2023, 12, 6106. [Google Scholar] [CrossRef] [PubMed]
- Vîlcea, A.; Borta, S.M.; Popețiu, R.O.; Alexandra, R.L.; Pilat, L.; Nica, D.; Pușchiță, M. High ADMA Is associated with worse health profile in heart failure patients hospitalized for episodes of acute decompensation. Medicina 2024, 60, 813. [Google Scholar] [CrossRef] [PubMed]
- Grelus, A.; Nica, D.V.; Miklos, I.; Belengeanu, V.; Ioiart, I.; Popescu, C. Clinical significance of measuring global hydroxymethylation of white blood cell DNA in prostate cancer: Comparison to PSA in a pilot exploratory study. Int. J. Mol. Sci. 2017, 18, 2465. [Google Scholar] [CrossRef] [PubMed]
- Ginhină, C.; Vinereanu, D.; Popescu, B.A. Manual de Cardiologie al Societatii Romane de Cardiologie, 2nd ed.; Editura Medicală: Bucharest, Romania, 2020; pp. 456–510. [Google Scholar]
- Cleophas, T.J.; Zwinderman, A.H.; Cleophas, T.F.; Cleophas, E.P. Statistics Applied to Clinical Trials, 4th ed.; Springer: Dordrecht, The Netherlands, 2010; pp. 79–149. [Google Scholar]
- Suciu, M.; Gruia, A.T.; Nica, D.V.; Azghadi, S.M.; Mic, A.A.; Mic, F.A. Data on expression of lipoxygenases-5 and-12 in the normal and acetaminophen-damaged liver. Data Brief 2016, 7, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Korppi, M.; Nuolivirta, K. Exploratory and confirmatory studies have different targets and both are needed in clinical research. Acta Paediatr. 1992, 107, 734–735. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, M.; Drăghici, G.A.; Oancea, E.F.; Dehelean, C.A.; Şoica, C.; Vlăduţ, N.V.; Nica, D. Effects of cadmium sulfate on the Brown garden snail Cornu aspersum: Implications for DNA methylation. Toxics 2023, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Popețiu, R.O.; Donath-Miklos, I.; Borta, S.M.; Moldovan, S.D.; Pilat, L.; Nica, D.V.; Pușchiță, M. Serum YKL-40 levels in patients with asthma or COPD: A pilot study. Medicina 2023, 59, 383. [Google Scholar] [CrossRef] [PubMed]
- Filimon, M.N.; Nica, D.V.; Ostafe, V.; Bordean, D.M.; Borozan, A.B.; Vlad, D.C.; Popescu, R. Use of enzymatic tools for biomonitoring inorganic pollution in aquatic sediments: A case study (Bor, Serbia). Chem. Cent. J. 2013, 7, 1–14. [Google Scholar] [CrossRef]
- Drăghici, G.A.; Dehelean, C.A.; Moacă, A.E.; Moise, M.L.; Pînzaru, I.; Vladuţ, V.N.; Bănăţean-Dunea, I.; Nica, D. Cadmium nitrate and DNA methylation in gastropods: Comparison between ovotestis and hepatopancreas. PeerJ 2023, 11, e15032. [Google Scholar] [CrossRef]
- Richter, B.; Koller, L.; Hohensinner, P.J.; Zorn, G.; Brekalo, M.; Berger, R.; Mörtl, D.; Maurer, G.; Pacher, R.; Huber, K.; et al. A multi-biomarker risk score improves prediction of long-term mortality in patients with advanced heart failure. Int. J. Cardiol. 2013, 168, 1251–1257. [Google Scholar] [CrossRef]
- Zhou, C.; Long, Y.; Yang, H.; Zhu, C.; Ma, Q.; Zhang, Y. TRAIL is decreased before 20 weeks gestation in women with hypertensive disorders of pregnancy. Plos ONE 2015, 10, e0128425. [Google Scholar] [CrossRef] [PubMed]
- Varounis, C.; Katsi, V.; Nihoyannopoulos, P.; Lekakis, J.; Tousoulis, D. Cardiovascular hypertensive crisis: Recent evidence and review of the literature. Front. Cardiovasc. Med. 2017, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.C.; Ng, J.; Lei, L.; Raj, S.R. Autonomic dysfunction in cardiology—Pathophysiology, investigation, and management. Can. J. Cardiol. 2017, 33, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. AHA/ACC/HFSA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef] [PubMed]
- Zhirov, I.V.; Nasonova, S.N.; Tereshchenko, S.N. Acute decompensation of heart failure: State of the problem. Terapevt. Arkh. 2022, 94, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Hiki, M.; Iwata, H.; Takasu, K.; Nojiri, S.; Ishikawa, G.; Chikata, Y.; Mattson, P.C.; Kasai, T.; Miyazaki, T.; Inoue, K.; et al. Elevated heart rate in combination with elevated blood pressure predicts lower cardiovascular mortality in acute decompensated heart failure. Int. Heart J. 2020, 61, 308–315. [Google Scholar] [CrossRef]
- Jo, S.H. PS-BPC05-2: Admission and discharge blood pressure influence medical prescription and clinical outcomes of patients with decompensated heart failure. J. Hypertens. 2023, 41, e303. [Google Scholar] [CrossRef]
- Stamerra, C.A.; D’Elia, E.; Gori, M.; Roncali, F.; Cereda, A.; Gavazzi, A.; Ferri, C.; Senni, M. Red cell distribution width (RDW) is correlated to time of oxygen desaturation < 90% and length of sleep apneas in patients with sleep disorder breathing (SDB) and acute heart failure with preserved ejection fraction (HFpEF). Front. Cardiovasc. Med. 2023, 10, 1045702. [Google Scholar] [CrossRef]
- Kibel, A.; Lukinac, A.M.; Dambic, V.; Juric, I.; Selthofer-Relatic, K. Oxidative stress in ischemic heart disease. Oxid. Med. Cell. Longev. 2020, 6627144. [Google Scholar] [CrossRef]
- Griendling, K.K.; Camargo, L.L.; Rios, F.J.; Alves-Lopes, R.; Montezano, A.C.; Touyz, R.M. Oxidative stress and hypertension. Circ. Res. 2021, 128, 993–1020. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, R.; Govind, B.; Chowdary, N.V.S.; Sharma, S.; Satish, R.J.; Desai, S.V.; Probodh, V.S.; Aslami, A.N. HbA1c levels in patients of acute decompensated heart failure. Asian J. Med. Sci. 2016, 7, 55–58. [Google Scholar] [CrossRef]
- Khoo, K.; Lew, J.; Neef, P.; Kearney, L.; Churilov, L.; Robbins, R.; Tan, A.; Hachem, M.; Owen-Jones, L.; Lam, Q.; et al. Routine use of HbA1c amongst inpatients hospitalised with decompensated heart failure and the association of dysglycaemia with outcomes. Sci. Rep. 2018, 8, 13564. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K. Does HbA1c play a role in the development of cardiovascular diseases? Curr. Pharm. Des. 2018, 24, 2876–2882. [Google Scholar] [CrossRef]
- Mullens, W.; Nijst, P. Cardiac output and renal dysfunction: Definitely more than impaired flow. J. Am. Coll. Cardiol. 2016, 67, 2209–2212. [Google Scholar] [CrossRef] [PubMed]
- Mironova, O.I. Hyperuricemia and kidney damage in patients with cardiovascular disease: A review. Ter. Arkh. 2023, 94, 1426–1430. [Google Scholar] [CrossRef]
- Hong, C.; Zhu, H.; Zhou, X.; Zhai, X.; Li, S.; Ma, W.; Liu, K.; Shirai, K.; Sheerah, H.A.; Cao, J. Association of blood urea nitrogen with cardiovascular diseases and all-cause mortality in USA adults: Results from NHANES 1999–2006. Nutrients 2023, 15, 461. [Google Scholar] [CrossRef] [PubMed]
- Toth, P.P.; Philip, S.; Hull, M.; Granowitz, C. Elevated triglycerides (≥150 mg/dL) and high triglycerides (200–499 mg/dL) are significant predictors of new heart failure diagnosis: A real-world analysis of high-risk statin-treated patients. Cardiorenal Med. 2019, 9, 400–407. [Google Scholar] [CrossRef]
- Von Haehling, S.; Ebner, N.; Dos Santos, M.R.; Springer, J.; Anker, S.D. Muscle wasting and cachexia in heart failure: Mechanisms and therapies. Nat. Rev. Cardiol. 2017, 14, 323–341. [Google Scholar] [CrossRef]
- Valentova, M.; Anker, S.D.; von Haehling, S. Cardiac cachexia revisited: The role of wasting in heart failure. Heart Fail. Clin. 2020, 16, 61–69. [Google Scholar] [CrossRef]
- Sharma, Y.P.; Kaur, N.; Kasinadhuni, G.; Batta, A.; Chhabra, P.; Verma, S.; Panda, P. Anemia in heart failure: Still an unsolved enigma. Egypt. Heart J. 2021, 73, 75. [Google Scholar] [CrossRef] [PubMed]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory mechanisms contributing to endothelial dysfunction. Biomedicines 2023, 9, 781. [Google Scholar] [CrossRef] [PubMed]
- Masoudkabir, F.; Sarrafzadegan, N. The interplay of endothelial dysfunction, cardiovascular disease, and cancer: What we should know beyond inflammation and oxidative stress. Eur. J. Prev. Cardiol. 2020, 27, 2075–2076. [Google Scholar] [CrossRef] [PubMed]
- Vulesevic, B.; Lavoie, S.S.; Neagoe, P.E.; Dumas, E.; Räkel, A.; White, M.; Sirois, M.G. CRP induces NETosis in heart failure patients with or without diabetes. Immunohorizons 2019, 3, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Tromp, J.; Ter Maaten, J.M.; Damman, K.; O’Connor, C.M.; Metra, M.; Dittrich, H.C.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; Davison, B.; et al. Serum potassium levels and outcome in acute heart failure (data from the PROTECT and COACH trials). Am. J. Cardiol. 2017, 119, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Guo, Q.; Gao, Y.; Han, J.; Yan, B.; Peng, L.; Song, A.; Peng, L.; Wang, G. The relationship between red blood cell distribution width and blood pressure abnormal dipping in patients with essential hypertension: A cross-sectional study. BMJ Open 2016, 6, e010456. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yu, T.; Yan, Q.; Zhang, Q.; Wang, L. Association of Hemoglobin to Red Blood Cell Distribution Width-Standard Deviation (RDW-SD) Ratio and 3-month readmission in elderly chinese patients with heart failure: A retrospective cohort study. Int. J. Gen. Med. 2023, 16, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Azghadi, S.M.R.; Suciu, M.; Gruia, A.T.; Barbu-Tudoran, L.; Cristea, M.I.; Mic, A.A.; Muntean, D.; Nica, D.V.; Mic, F.A. Mesenchymal stromal cells support the viability and differentiation of thymocytes through direct contact in autologous co-cultures. Histochem. Cell. Biol. 2016, 146, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Sinha, I.P.; Sinha, S.K. Single-center trials in neonatology: Issues to consider. Semin. Fetal Neonatal Med. 2015, 20, 384–388. [Google Scholar] [CrossRef]
- Veas, F. Acute Phase Proteins as Early Non-Specific Biomarkers of Human and Veterinary Diseases, 1st ed.; INTECH d.o.o.: Rijeka, Croatia, 2011; pp. 127–175. [Google Scholar]
- Lakens, D. Sample size justification. Collabra Psychol. 2022, 8, 33267. [Google Scholar] [CrossRef]
- Bojin, L.A.; Georgescu, M.; Cojocaru, C.; Pascariu, M.C.; Purcarea, V.L.; Ivan, M.V.; Puiu, M.; Dehelean, C.; Serb, A.F.; Sisu, E.; et al. Structural investigation of raw and modified glycans by MALDI-TOF mass spectrometry. Farmacia 2020, 68, 891–897. [Google Scholar] [CrossRef]
- Popescu, I.M.; Mărgan, M.M.; Anghel, M.; Mocanu, A.; Laitin, S.M.D.; Mărgan, R.; Căpraru, I.D.; Tene, A.A.; Gal-Nadasan, E.G.; Cîrnațu, D.; et al. Developing prediction models for COVID-19 outcomes: A valuable tool for resource-limited hospitals. Int. J. Gen. Med. 2023, 16, 3053–3065. [Google Scholar] [CrossRef] [PubMed]
- Gruia, A.T.; Suciu, M.; Barbu-Tudoran, L.; Azghadi, S.M.R.; Cristea, M.I.; Nica, D.V.; Văduva, A.; Muntean, D.; Mic, A.A.; Mic, F.A. Mesenchymal stromal cells differentiating to adipocytes accumulate autophagic vesicles instead of functional lipid droplets. J. Cell. Physiol. 2016, 231, 863–875. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Strata | HTU Patients | ADHF Patients | p |
|---|---|---|---|---|
| Sex | Male | 24 (53.34%) | 28 (62.22%) | 0.521 |
| Female | 21 (46.66%) | 17 (37.78%) | ||
| Origin | Rural | 30 (66.67%) | 23 (51.12%) | 0.198 |
| Urban | 15 (33.33%) | 22 (48.88%) | ||
| Smoking status | Yes | 18 (40%) | 12 (26.67%) | 0.263 |
| No | 27 (60%) | 33 (72.32%) | ||
| Diabetes | Yes | 16 (35.56%) | 23 (51.12%) | 0.137 |
| No | 29 (64.44%) | 22 (48.88%) | ||
| Tachyarrythmia | Yes | 17 (37.78%) | 23 (48.78%) | 0.212 |
| No | 28 (63.22%) | 22 (50.22%) | ||
| Renal dysfunction | Yes | 12 (26.67%) | 26 (57.77%) | <0.001 *** |
| No | 33 (73.33%) | 19 (42.23%) |
| Characteristic | ADHF Patients | HTU Patients | Reference Range | p |
|---|---|---|---|---|
| Age (years) | 70 (63; 79) | 70 (62; 77) | 0.257 | |
| SBP (mm Hg) | 140 (130; 150) | 150 (140; 160) | 90–130 | 0.303 |
| DBP (mm Hg) | 80 (73; 90) | 80 (75; 90) | 60–80 | 0.659 |
| ADMA (ng/mL) | 111 (60; 240) | 105 (80; 219) | 0.941 | |
| NT-proBNP (pg/mL) | 5136 (2060; 8400) | 1570 (580; 5600) | 0.064 | |
| LAD (mm) | 43 (40; 80) | 41 (36; 44) | 25–53 | 0.093 |
| LVD (mm) | 51 (43; 58) | 47 (45; 59) | 39–59 | 0.216 |
| LVEDV (mL) | 125.5 (94; 173) | 120.5 (82.5; 120) | 46–150 | 0.619 |
| LVESV (mL) | 77 (55.5; 111.3) | 55 (42; 82.5) | 14–61 | 0.145 |
| IVSd (cm) | 1.16 (1; 1.3) | 1.2 (1; 1.3) | 0.6–1.2 | 0.792 |
| ALC (cells/μL) | 5365 (4110; 6139) | 6125 (5400; 7139) | 4000–11,000 | 0.013 ** |
| ANC (cells/μL) | 6175 (3820; 7400) | 4670 (3350; 6425) | 2500–7500 | 0.187 |
| RDW-CV (%) | 15.05 (14.1; 16.6) | 14.2 (13.2; 15.15) | 11.5–15.4 | 0.002 *** |
| RDW-SD (fL) | 46.4 (44.3; 50.55) | 43.90 (41.15; 47.95) | 39–46 | 0.006 ** |
| Hemoglobin (g/dL) | 12.8 (11.6; 13.8) | 14.8 (13; 15.2) | 12–18 | <0.001 *** |
| ESR (mm/h) | 30 (24; 43) | 26 (18;42) | 0–30 | 0.580 |
| CRP (mg/L) | 13 (5; 42) | 12 (1; 61) | <10 | 0.363 |
| Random glucose (mg/dL) | 133 (112; 170) | 119 (99; 161) | <200 | 0.229 |
| Hba1C (%) | 6.9 (6; 7.80) | 7.9 (6; 8.6) | <6.5 | 0.122 |
| GFR (mL/min) | 67.82 (47.94; 88.64) | 77.69 (96.85; 102.5) | >60 | 0.173 |
| Serum urea (mg/dL) | 53.67 (44; 68) | 37 (31; 65.8) | <49 | 0.007 ** |
| Serum uric acid (mg/dL) | 8.2 (7.2; 10.4) | 7.70 (5.5; 10.3) | 3–7 | 0.463 |
| Total cholesterol (mg/dL) | 147.8 (117;175) | 175 (128; 200) | <200 | 0.092 |
| LDL (mg/dL) | 88.3 (71; 120) | 118 (79; 135) | <130 | 0.109 |
| HDL (mg/dL) | 41 (34; 50) | 40.35 (35; 48.75) | >40 | 0.885 |
| Triglycerides (mg/dL) | 98 (80; 131) | 118 (98; 220) | <150 | 0.017 * |
| AST (UI/L) | 28.5 (20; 40) | 25.3 (19; 38) | 5–56 | 0.621 |
| ALT (UI/L) | 24 (17.45; 35) | 25.5 (16.5; 54.5) | 9–40 | 0.798 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vîlcea, A.; Borta, S.M.; Pop Moldovan, A.; Osser, G.; Dărăbanțiu, D.; Bănățean-Dunea, I.; Pușchiță, M. Serum Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand and the Cardiovascular Disease Continuum: Insights from Hypertensive Urgencies and Acute Heart Failure Events. Appl. Sci. 2024, 14, 5890. https://doi.org/10.3390/app14135890
Vîlcea A, Borta SM, Pop Moldovan A, Osser G, Dărăbanțiu D, Bănățean-Dunea I, Pușchiță M. Serum Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand and the Cardiovascular Disease Continuum: Insights from Hypertensive Urgencies and Acute Heart Failure Events. Applied Sciences. 2024; 14(13):5890. https://doi.org/10.3390/app14135890
Chicago/Turabian StyleVîlcea, Anamaria, Simona Maria Borta, Adina Pop Moldovan, Gyongyi Osser, Dan Dărăbanțiu, Ioan Bănățean-Dunea, and Maria Pușchiță. 2024. "Serum Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand and the Cardiovascular Disease Continuum: Insights from Hypertensive Urgencies and Acute Heart Failure Events" Applied Sciences 14, no. 13: 5890. https://doi.org/10.3390/app14135890
APA StyleVîlcea, A., Borta, S. M., Pop Moldovan, A., Osser, G., Dărăbanțiu, D., Bănățean-Dunea, I., & Pușchiță, M. (2024). Serum Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand and the Cardiovascular Disease Continuum: Insights from Hypertensive Urgencies and Acute Heart Failure Events. Applied Sciences, 14(13), 5890. https://doi.org/10.3390/app14135890







