Effects of Different Tonic, Isometric and Isometric/Vibratory Strength Training Programs on Motor Symptomatology in People with Parkinson’s Disease: Study Protocol for a Randomized Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Protocol Registration
2.2. Eligibility Criteria
- Individuals of both sexes with a diagnosis of PD aged between 50 and 70 years, in stages I–III according to the Hoehn and Yahr classification.
- Authorized by their treating physician to participate in the intervention program.
- Medication-controlled.
- Capable of following verbal instructions and having motor autonomy.
- Mini-mental examination score greater than 24 points [4].
- Signed informed consent form to participate in the study.
2.3. Exclusion Criteria
- Participants with adherence below 80% to the different types of intervention sessions will not be included in the results analysis.
- People with orthopedic disabilities or other neurological conditions.
2.4. Sample Size
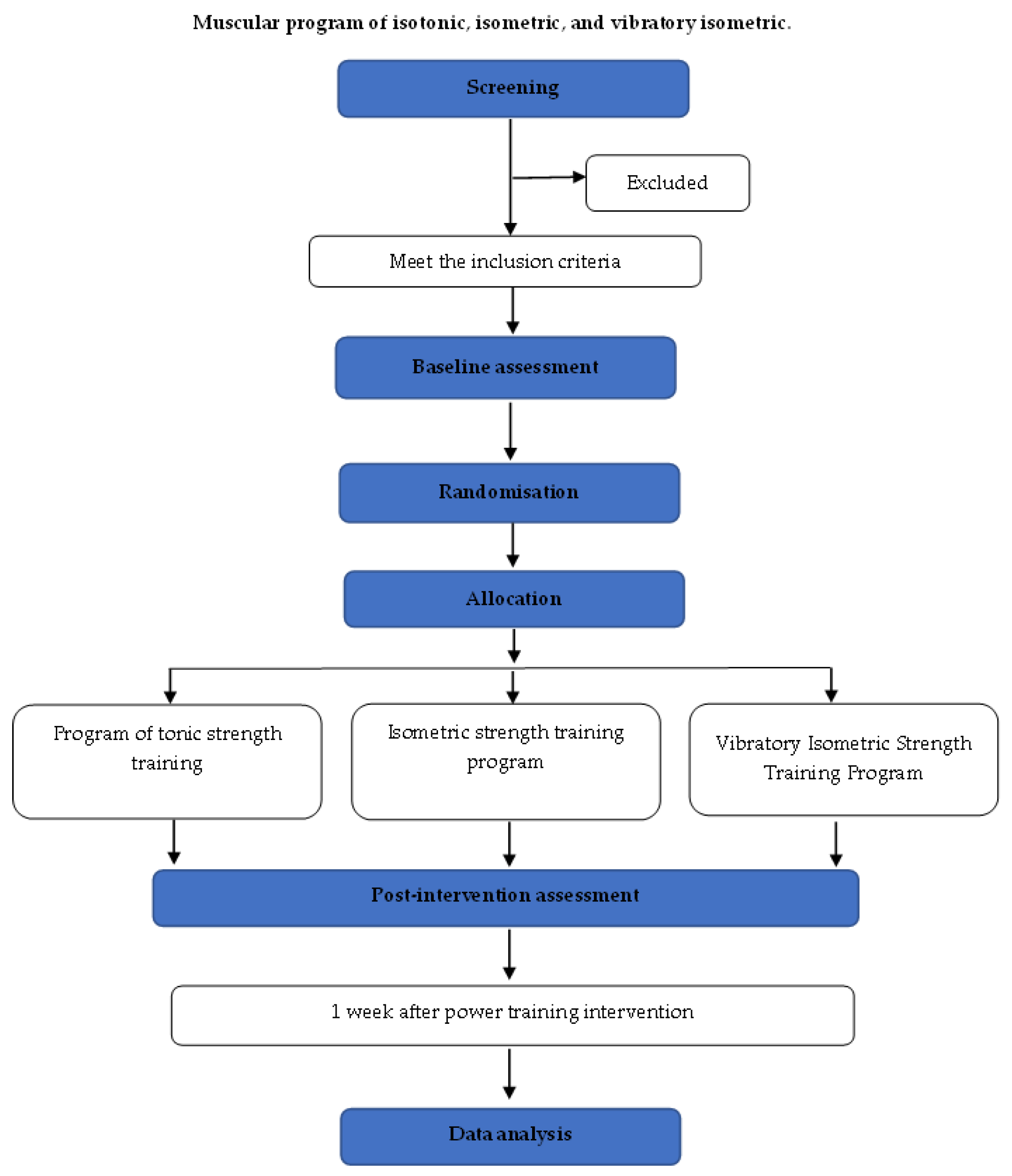
2.5. Randomization, Allocation, and Blinding
2.6. Intention to Treat
2.7. Procedures
2.8. Intervention
2.9. Experimental Groups
2.10. Data Collection
2.10.1. Motor Symptomatology Assessment Protocol
- (a)
- Resting tremor: Actigraphy accelerometer [25] in a 5-trial test of 20 s duration. The Fast Fourier Transform (FFT) will be calculated for the 15-s recording cutoff. The maximum power and initial maximum amplitude domain of the FFT will be reported within the limit of 1.5 to 9 Hz. From this signal, a quantity of the temblor will be extracted.
- (b)
- (c)
- Balance: The Mini-BESTest balance test [22] will be used, which assesses static-dynamic balance. The scale measures balance primarily with closed eyes, sitting, standing, performing a 360° turn, and a destabilizing stimulus, classifying the patient’s behavior based on points.
- (d)
- Gait speed: The fast speed of the Ten Meter Walk Test (TMW) [9] will be used, which measures the time in seconds that it takes a patient to run 10 m in a straight line. Sports clothing and shoes will be requested. Photocells (WITTY, Microgate® Bolzano, Italia) will be used to record speed, and a video camera will be used to record the step length and gait cadence.
2.10.2. Non-Motor Symptomatology Assessment Protocol
2.10.3. Anthropometry and Body Composition
2.10.4. Muscle Strength Evaluation
2.11. Statistical Analysis
3. Discussion
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erkkinen, M.; Kim, M.; Geschwind, M. Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2018, 1, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Leiva, A.; Martinez-Sanguinetti, M.; Troncoso-Pantoja, C.; Nazar, G.; Petermann-Rocha, F.; Celis-Morales, C. Carta al editor Chile lidera el ranking latinoamericano de prevalencia de enfermedad de Parkinson. Rev. Med. Chile. 2019, 147, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.W.; Choi, S.M.; Kim, B.C. Gender differences in motor and non-motor symptoms in early Parkinson disease. Medicine 2022, 101, e28643. [Google Scholar] [CrossRef] [PubMed]
- Moraes, R.; Alves, W.; da Costa, M.G.; Lima, T.A.; Alves, G.; Alves Filho, P.; Pimentel, C.P.; Sousa, E.C.; Cortinhas-Alves, E.A. The effect of resistance training on the anxiety symptoms and quality of life in elderly people with parkinson’s disease: A randomized controlled trial. Arq. Neuro-Psiquiatr. 2018, 76, 499–506. [Google Scholar]
- Linares-del Rey, M.; Vela-Desojo, L.; Cano-de la Cuerda, R. Mobile phone applications in Parkinson’s disease: A systematic review. Neurologia 2019, 34, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Stuckenschneider, T.; Askew, C.D.; Menêses, A.L.; Baake, R.; Weber, J.; Schneider, S. The effect of different exercise modes on domain-specific cognitive function in patients suffering from Parkinson’s disease: A systematic review of randomized controlled trials. J. Park. Dis. 2019, 9, 73–95. [Google Scholar] [CrossRef]
- Kadkhodaie, M.; Sharifnezhad, A.; Ebadi, S.; Marzban, S.; Habibi, S.A.; Ghaffari, A.; Forogh, B. Effect of eccentric-based rehabilitation on hand tremor intensity in Parkinson disease. Neurol. Sci. 2020, 41, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Gilbert, R.M. Epidemiology of Parkinson Disease. Neurol. Clin. 2016, 34, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Rafferty, M.R.; Prodoehl, J.; Robichaud, J.A.; David, F.J.; Poon, C.; Goelz, L.C.; Vaillancourt, D.E.; Kohrt, W.M.; Comella, C.L.; Corcos, D.M. Effects of 2 Years of Exercise on Gait Impairment in People with Parkinson Disease: The PRET-PD Randomized Trial. J. Neurol. Phys. Ther. 2017, 41, 21–30. [Google Scholar] [CrossRef]
- Mu, J.; Chaudhuri, K.R.; Bielza, C.; de Pedro-Cuesta, J.; Larrañaga, P.; Martinez-Martin, P. Parkinson’s disease subtypes identified from cluster analysis of motor and non-motor symptoms. Front. Aging Neurosci. 2017, 9, 1–10. [Google Scholar] [CrossRef]
- Criciotoiu, O.; Stanca, D.I.; Glavan, D.G.; Bondari, S.; Malin, R.D.; Ciolofan, M.S.; Bunescu, M.G.; Romanescu, F.M.; Schenker, M.; Georgescu, O.S.; et al. The relations between non-motor symptoms and motor symptoms in Parkinson disease. Rev. Chim. 2019, 70, 2652–2655. [Google Scholar] [CrossRef]
- Chung, C.; Thilarajah, S.; Tan, D. Effectiveness of resistance training on muscle strength and physical function in people with Parkinson’s disease: A systematic review and meta-analysis. Clin. Rehabil. 2016, 30, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Signorile, J.F.; Mooney, K.; Balachandran, A.; Potiaumpai, M.; Luca, C.; Moore, J.G.; Kuenze, C.M.; Eltoukhy, M.; Perry, A.C. Comparative Effect of Power Training and High-Speed Yoga on Motor Function in Older Patients with Parkinson Disease. Arch. Phys. Med. Rehabil. 2016, 97, 345–354. [Google Scholar] [CrossRef] [PubMed]
- de Lima, T.A.; Ferreira-Moraes, R.; Alves, W.M.G.d.C.; Alves, G.; Pimentel, C.P.; Sousa, E.C.; Abrahin, O.; Cortinhas-Alves, E. Resistance training reduces depressive symptoms in elderly people with Parkinson disease: A controlled randomized study. Scand. J. Med. Sci. Sports 2019, 29, 1957–1967. [Google Scholar] [CrossRef]
- Lacio, M.; Vieira, J.G.; Trybulski, R.; Campos, Y.; Santana, D.; Filho, J.E.; Novaes, J.; Vianna, J.; Wilk, M. Effects of resistance training performed with different loads in untrained and trained male adult individuals on maximal strength and muscle hypertrophy: A systematic review. Int. J. Environ. Res. Public Health 2021, 18, 11237. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Rubio, A.; Cabrera-Martos, I.; Torres-Sánchez, I.; Casilda-López, J.; López-López, L.; Valenza, M.C. Effects of a resistance training program on balance and fatigue perception in patients with Parkinson’s disease: A randomized controlled trial. Med. Clin. 2018, 15, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Ramazzina, I.; Bernazzoli, B.; Costantino, C. Systematic review on strength training in parkinson’s disease: An unsolved question. Clin. Interv. Aging 2017, 12, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.; Fernandez-Rio, J.; Winge, K.; Barragán-Pérez, B.; González-Gómez, L.; Rodríguez-Pérez, V.; González-Díez, V.; Lucía, A.; Iglesias-Soler, E.; Dopico-Calvo, X.; et al. Effects of progressive resistance exercise in akinetic-rigid Parkinson’s disease patients: A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2017, 53, 651–663. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, C.; Clifford, A.; Van De Ven, P.; Nelson, J. Chronic Neck Pain and Exercise Interventions: Frequency, Intensity, Time, and Type Principle. Arch. Phys. Med. Rehabil. 2014, 95, 770–783. [Google Scholar] [CrossRef]
- Cherup, N.P.; Buskard, A.N.L.; Strand, K.L.; Roberson, K.B.; Michiels, E.R.; Kuhn, J.E.; Lopez, F.A.; Signorile, J.F. Power vs. strength training to improve muscular strength, power, balance and functional movement in individuals diagnosed with Parkinson’s disease. Exp. Gerontol. 2019, 128, 110740. [Google Scholar] [CrossRef]
- Corcos, D.M.; Robichaud, J.A.; David, F.J.; Leurgans, S.E.; Vaillancourt, D.E.; Poon, C.; Rafferty, M.R.; Kohrt, W.M.; Comella, C.L. A Two-Year Randomized Controlled Trial of Progressive Resistance Exercise for Parkinson’s Disease. Mov. Disord. 2013, 28, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Prodoehl, J.; Rafferty, M.R.; David, F.J.; Poon, C.; Vaillancourt, D.E.; Comella, C.L.; Leurgans, S.E.; Kohrt, W.M.; Corcos, D.M.; Robichaud, J.A. Two-year exercise program improves physical function in Parkinson’s disease: The PRET-PD randomized clinical trial. Neurorehabilit. Neural Repair 2015, 29, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Strand, K.; Cherup, N.; Totillo, M.; Castillo, D.; Gabor, N.; Signorile, J. Periodized Resistance Training with and without Functional Training Improves Functional Capacity, Balance, and Strength in Parkinson’s Disease. J. Strength Cond. Res. 2021, 35, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Kwak, S.; Li, F.; Wu, C.; Chen, Y.; Yamamoto, Y.; Cai, D. Actigraphy monitoring of symptoms in patients with Parkinson’s disease. Physiol. Behav. 2013, 119, 156–160. [Google Scholar] [CrossRef]
- Soriano-Maldonado, A.; Carrera-Ruiz, Á.; Díez-Fernández, D.M.; Esteban-Simón, A.; Maldonado-Quesada, M.; Moreno-Poza, N.; García-Martínez, M.D.M.; Alcaraz-García, C.; Vázquez-Sousa, R.; Moreno-Martos, H.; et al. Effects of a 12-week resistance and aerobic exercise program on muscular strength and quality of life in breast cancer survivors: Study protocol for the EFICAN randomized controlled trial. Medicine 2020, 98, e17625. [Google Scholar] [CrossRef] [PubMed]
- Jerez-Mayorga, D.; Huerta-Ojeda, Á.; Chirosa-Ríos, L.J.; Guede-Rojas, F.; Guzmán-Guzmán, I.P.; Intelangelo, L.; Miranda-Fuentes, C.; Delgado-Floody, P. Test–retest reliability of functional electromechanical dynamometer on five sit-to-stand measures in healthy young adults. Int. J. Environ. Res. Public Health 2021, 18, 6829. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.A.; Goetz, C.G.; Stebbins, G.; Lees, A.J.; Schrag, A. Validation of the MDS-UPDRS Part I for nonmotor symptoms in Parkinson’s disease. Mov. Disord. 2012, 27, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chien, H.F.; Francato, D.C.V.; Barbosa, A.F.; Souza, C.d.O.; Voos, M.C.; Greve, J.M.D.; Barbosa, E.R. Effects of resistance training on postural control in Parkinson’s disease: A randomized controlled trial. Arq. Neuro-Psiquiatr. 2020, 79, 511–520. [Google Scholar] [CrossRef]
- Lima, D.P.; de Almeida, S.B.; Bonfadini, J.d.C.; Sobreira, E.S.T.; Damasceno, P.G.; Viana Júnior, A.B.; de Alencar, M.S.; de Luna, J.R.G.; Rodrigues, P.G.B.; Pereira, I.d.S.; et al. Effects of a power strength training using elastic resistance exercises on the motor and non-motor symptoms in patients with Parkinson’s disease H&Y 1-3: Study protocol for a randomised controlled trial (PARK-BAND Study). BMJ Open 2020, 10, 2–16. [Google Scholar]
- David, F.J.; Robichaud, J.A.; Vaillancourt, D.E.; Poon, C.; Kohrt, W.M.; Comella, C.L.; Corcos, D.M. Progressive resistance exercise restores some properties of the triphasic EMG pattern and improves bradykinesia: The PRET-PD randomized clinical trial. J. Neurophysiol. 2016, 116, 2298–2311. [Google Scholar] [CrossRef] [PubMed]
- Jukic, I.; Van Hooren, B.; Ramos, A.G.; Helms, E.R.; McGuigan, M.R.; Tufano, J.J. The Effects of Set Structure Manipulation on Chronic Adaptations to Resistance Training: A Systematic Review and Meta-Analysis. Sports Med. 2021, 51, 1061–1086. [Google Scholar] [CrossRef] [PubMed]

| Exercises | ||
|---|---|---|
| Warm-Up Phase | Aerobic Part | 5 min of low-intensity aerobic activity (50–65% HRR) on a stationary bike or elliptical machine |
| Joint mobility sets and repetitions: 2 × 8 | (performed in a seated position) Cervical rotation, acromio-humeral rotation, lumbar rotation, and flexion–extension coxofemoral rotation | |
| Development Phase | Core stability exercises (4–5 RPE) | Pelvic tilt in a sitting position, abdominal bracing in a sitting position with a fitball, abdominal bracing with a band in the transversal plane, bird–dog, humeral rotation without abduction in a sitting position |
| Resistance training (strength) | Bilateral seated bench, press unilateral isometric knee extension in closed, kinetic chain at 90°, unilateral open kinetic chain knee extension, bilateral seat row, biceps curl, triceps pushdown | |
| Cool-Down | Dynamic/static stretching of major muscle groups, sets and repetitions: 1 × 12 | Pectoralis major, dorsal width, quadriceps, hamstrings |
| Muscle Strength Training Program: Tonic | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Weeks | 1 | - | 4 | 5 | - | 8 | 9 | - | 12 |
| Intensity (1RM%) | 50% | 60% | 70% | ||||||
| Sets | 3 | 5 | 10 | ||||||
| Repetition | 10 | 6 | 3 | ||||||
| Rest period (s) | 60 | 36 | 18 | ||||||
| Muscle strength training program: Isometric | |||||||||
| Weeks | 1 | - | 4 | 5 | - | 8 | 9 | - | 12 |
| Intensity (1RM%) | 100% | 100% | 100% | ||||||
| Sets | 7 | 5 | 3 | ||||||
| Repetition (s) | 3 | 4.5 | 7 | ||||||
| Rest period (s) | 60 | 36 | 18 | ||||||
| Muscle strength training program: Vibratory Isometric | |||||||||
| Weeks | 1 | - | 4 | 5 | - | 8 | 9 | - | 12 |
| Intensity (1RM%) | 50% | 60% | 70% | ||||||
| Sets | 7 | 5 | 3 | ||||||
| Repetition (s) | 3 | 4.5 | 7 | ||||||
| Rest period (s) | 60 | 36 | 18 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrades-Ramírez, O.; Ulloa-Díaz, D.; Guede-Rojas, F.; Araya-Sierralta, S.; Muñoz-Bustos, G.; Arroyo-Jofré, P.; Chirosa-Ríos, L.-J. Effects of Different Tonic, Isometric and Isometric/Vibratory Strength Training Programs on Motor Symptomatology in People with Parkinson’s Disease: Study Protocol for a Randomized Trial. Appl. Sci. 2024, 14, 5923. https://doi.org/10.3390/app14135923
Andrades-Ramírez O, Ulloa-Díaz D, Guede-Rojas F, Araya-Sierralta S, Muñoz-Bustos G, Arroyo-Jofré P, Chirosa-Ríos L-J. Effects of Different Tonic, Isometric and Isometric/Vibratory Strength Training Programs on Motor Symptomatology in People with Parkinson’s Disease: Study Protocol for a Randomized Trial. Applied Sciences. 2024; 14(13):5923. https://doi.org/10.3390/app14135923
Chicago/Turabian StyleAndrades-Ramírez, Oscar, David Ulloa-Díaz, Francisco Guede-Rojas, Sergio Araya-Sierralta, Gustavo Muñoz-Bustos, Patricio Arroyo-Jofré, and Luis-Javier Chirosa-Ríos. 2024. "Effects of Different Tonic, Isometric and Isometric/Vibratory Strength Training Programs on Motor Symptomatology in People with Parkinson’s Disease: Study Protocol for a Randomized Trial" Applied Sciences 14, no. 13: 5923. https://doi.org/10.3390/app14135923
APA StyleAndrades-Ramírez, O., Ulloa-Díaz, D., Guede-Rojas, F., Araya-Sierralta, S., Muñoz-Bustos, G., Arroyo-Jofré, P., & Chirosa-Ríos, L.-J. (2024). Effects of Different Tonic, Isometric and Isometric/Vibratory Strength Training Programs on Motor Symptomatology in People with Parkinson’s Disease: Study Protocol for a Randomized Trial. Applied Sciences, 14(13), 5923. https://doi.org/10.3390/app14135923








