Applications of Raman Microscopy/Spectroscopy-Based Techniques to Plant Disease Diagnosis
Abstract
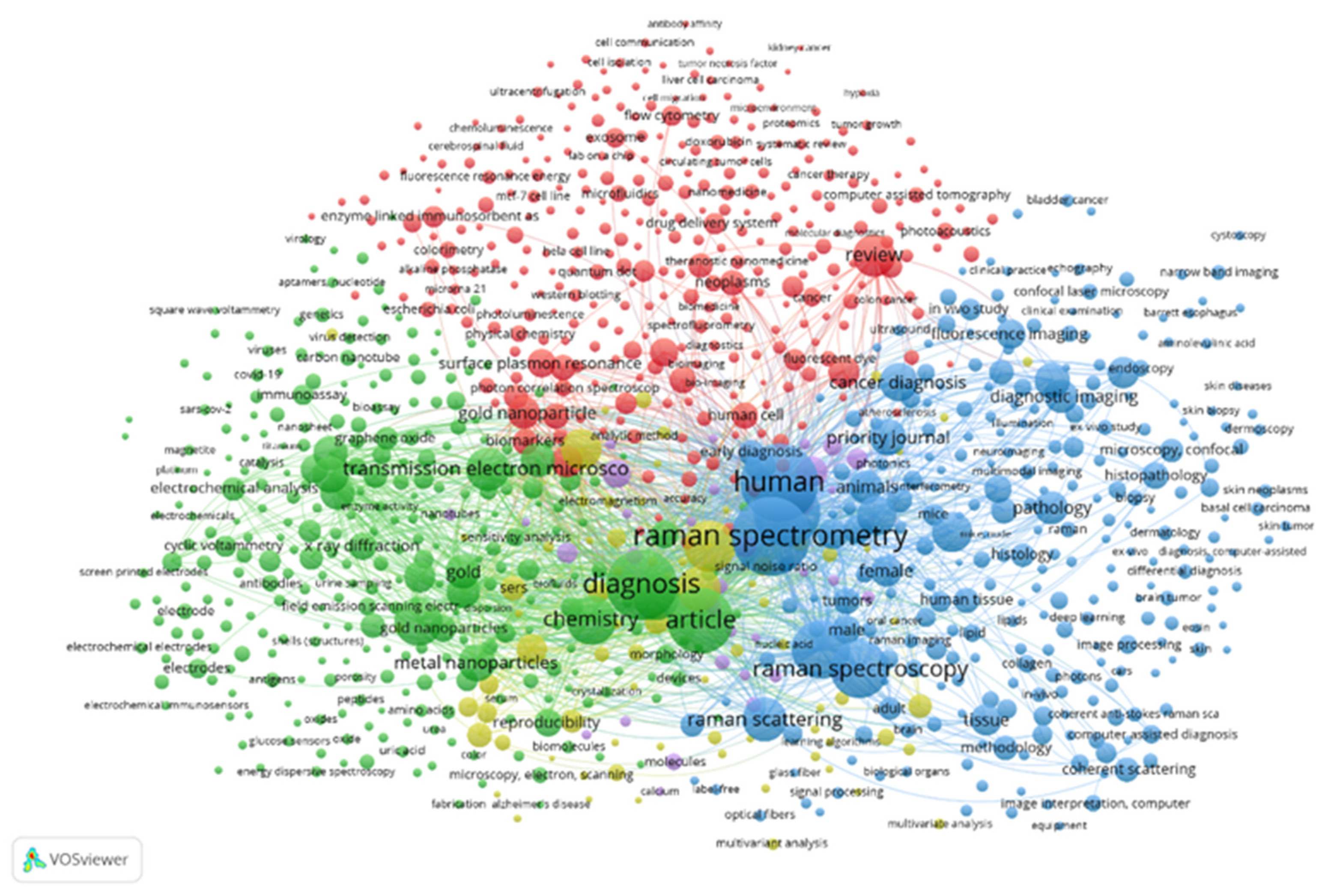
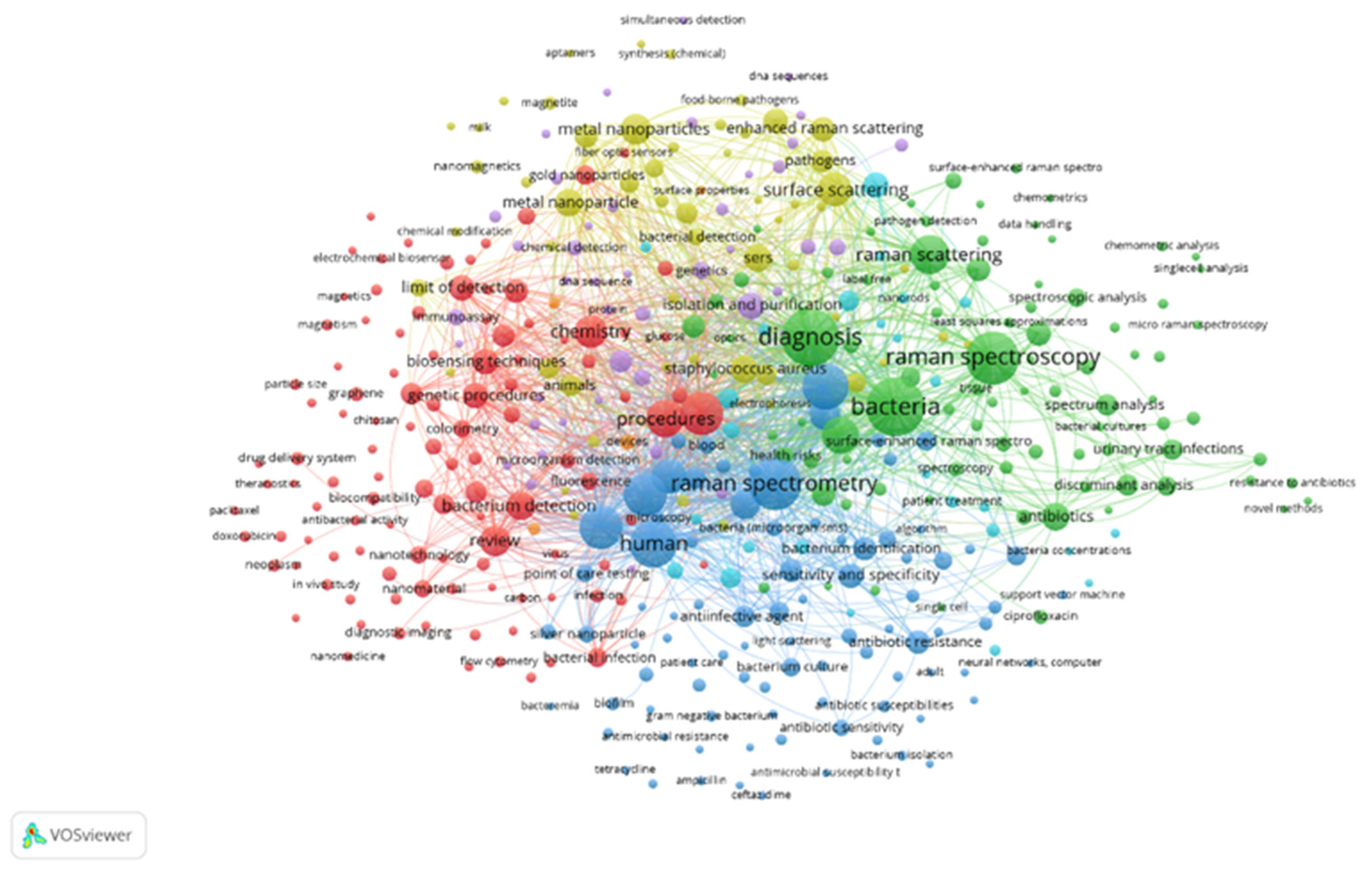
:1. Introduction
2. Plant Disease Diagnosis an Overview—Why Early Detection Matters—The Problem
- Advanced genomics and transcriptomics: Analyzing gene expression and genomic data to better understand pathogen–host interactions and disease development [30].
3. Raman Microscopy—A New Method Used to Study Cell Biology
4. Raman Spectroscopy—Principles of Raman Spectroscopy
5. Raman Microscopy in Single Cells—Single Microbial Cells
6. Raman Microscopy/Spectroscopy and Chemical Information
7. “Raman Shifts” in Cell Biology
8. Raman Spectroscopy in Cancer Research
9. Why Raman Techniques Are Important in Diagnosis
10. Raman Microspectroscopy in Plant Disease Diagnosis
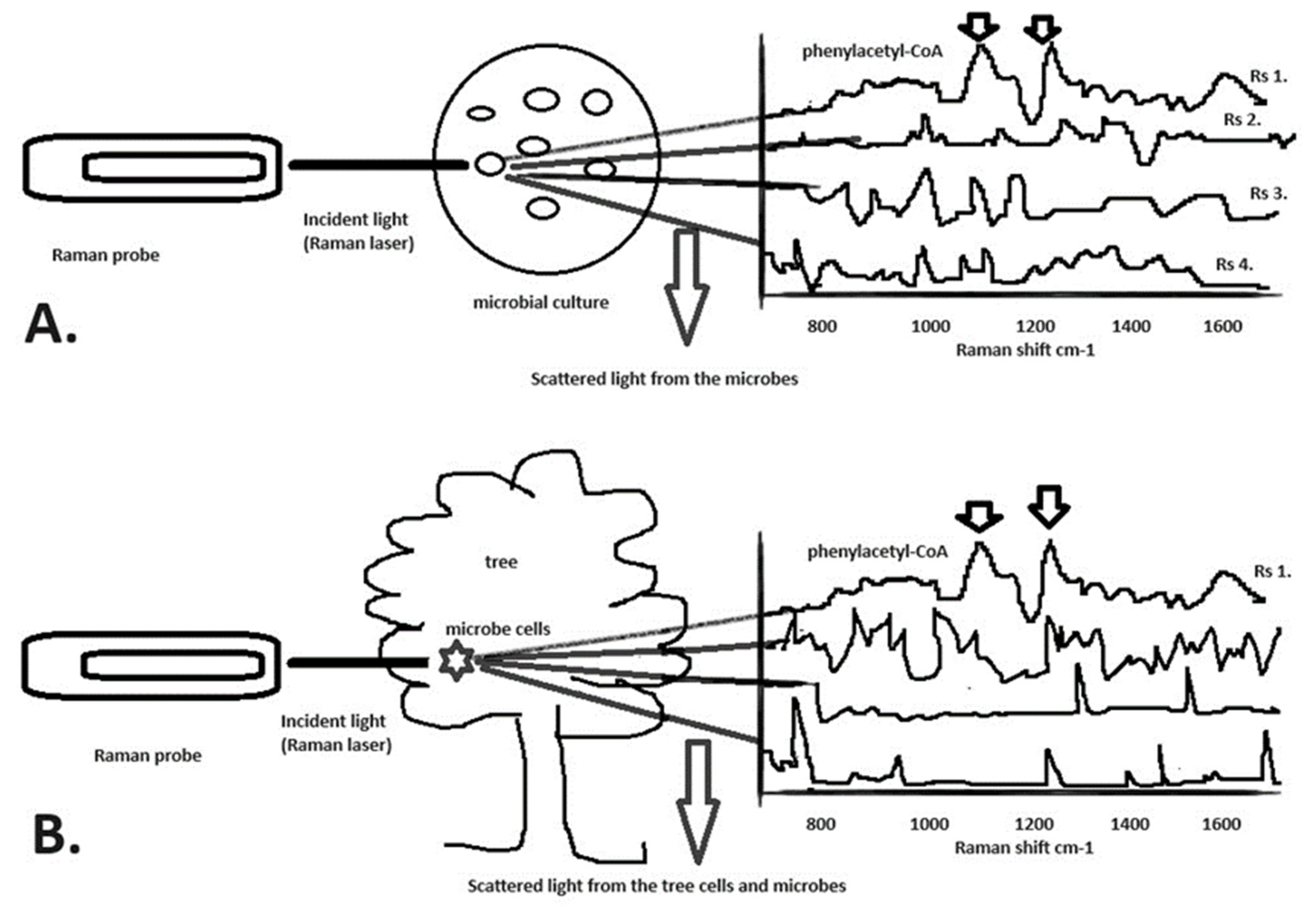
11. An Example Based on the Raman Microscopy Techniques Proposed in this Article
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Venbrux, M.; Crauwels, S.; Rediers, H. Current and emerging trends in techniques for plant pathogen detection. Front. Plant Sci. 2023, 14, 1120968. [Google Scholar] [CrossRef]
- Vagelas, I.; Papadimos, A.; Lykas, C. Pre-symptomatic disease detection in the vine, chrysanthemum, and rose leaves with a low-cost infrared sensor. Agronomy 2021, 11, 1682. [Google Scholar] [CrossRef]
- Sinclair, J.B. Latent infection of soybean plants and seeds by fungi. Plant Dis. 1991, 75, 220–224. [Google Scholar] [CrossRef]
- Tongsri, V.; Songkumarn, P.; Sangchote, S. Leaf spot characteristics of Phomopsis durionis on durian (Durio zibethinus Murray) and latent infection of the pathogen. Acta Univ. Agric. Silvic. Mendel. Brun. 2016, 64, 185–193. [Google Scholar] [CrossRef]
- Aylward, J.; Steenkamp, E.T.; Dreyer, L.L.; Roets, F.; Wingfield, B.D.; Wingfield, M.J. A plant pathology perspective of fungal genome sequencing. IMA Fungus 2017, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Baltrus, D.A.; McCann, H.C.; Guttman, D.S. Evolution, genomics and epidemiology of Pseudomonas syringae: Challenges in bacterial molecular plant pathology. Mol. Plant Pathol. 2017, 18, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Pérez, A.; Pintado, A.; Murillo, J.; Caballo-Ponce, E.; Tegli, S.; Moretti, C.; Rodríguez-Palenzuela, P.; Ramos, C. Host range determinants of Pseudomonas savastanoi pathovars of woody hosts revealed by comparative genomics and cross-pathogenicity tests. Front. Plant Sci. 2020, 11, 973. [Google Scholar] [CrossRef] [PubMed]
- Aragona, M.; Haegi, A.; Valente, M.T.; Riccioni, L.; Orzali, L.; Vitale, S.; Luongo, L.; Infantino, A. New-generation sequencing technology in diagnosis of fungal plant pathogens: A dream comes true? J. Fungi 2022, 8, 737. [Google Scholar] [CrossRef] [PubMed]
- Panchal, P.; Raman, V.C.; Mantri, S. Plant diseases detection and classification using machine learning models. In Proceedings of the 4th International Conference on Computational Systems and Information Technology for Sustainable Solution (CSITSS), Bengaluru, India, 20–21 December 2019; Volume 4, pp. 1–6. [Google Scholar] [CrossRef]
- Nagaraju, M.; Chawla, P. Systematic review of deep learning techniques in plant disease detection. Int. J. Syst. Assur. Eng. Manag. 2020, 11, 547–560. [Google Scholar] [CrossRef]
- Sarkar, C.; Gupta, D.; Gupta, U.; Hazarika, B.B. Leaf disease detection using machine learning and deep learning: Review and challenges. Appl. Soft Comput. 2023, 145, 110534. [Google Scholar] [CrossRef]
- Shah, S.K.; Kumbhar, V.; Singh, T.P. A Systematic Review on Crop Leaf Disease Identification Using Machine Learning and Deep Learning Techniques. In Proceedings of the 7th International Conference On Computing, Communication, Control and Automation (ICCUBEA), Pune, India, 18–19 August 2023; pp. 1–7. [Google Scholar] [CrossRef]
- Omran, E.E. Early sensing of peanut leaf spot using spectroscopy and thermal imaging. Arch. Agron. Soil Sci. 2017, 63, 883–896. [Google Scholar] [CrossRef]
- Conrad, A.O.; Li, W.; Lee, D.; Wang, G.; Rodriguez-Saona, L.E.; Bonello, P. Machine learning-based presymptomatic detection of rice sheath blight using spectral profiles. Plant Phenom. 2020, 2020, 8954085. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.A. Plant Pathology and Plant Pathogens, 4th ed.; Willey-Blackwell: Chichester, UK, 2020; p. 432. [Google Scholar]
- Agrios, G.N. Plant Pathology, 5th ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2005; p. 952. [Google Scholar]
- Pathak, R.K.; Kumar Singh, S.; Tak, A.; Gehlot, P.S. Impact of climate change on host, pathogen and plant disease adaptation regime: A review. Biosci. Biotechnol. Res. Asia 2018, 15, 529–540. [Google Scholar] [CrossRef]
- Liaqat, W.; Barutçular, C.; Farooq, M.U.; Ahmad, H.; Jan, M.F.; Ahmad, Z.; Nawaz, H.; Li, M. Climate change in relation to agriculture: A review. Span. J. Agric. Res. 2022, 20, e03R01. [Google Scholar] [CrossRef]
- Henson, J.M.; French, R.C. The polymerase chain reaction and plant disease diagnosis. Annu. Rev. Phytopathol. 1993, 31, 81–109. [Google Scholar] [CrossRef] [PubMed]
- Balodi, R.; Bisht, S.; Ghatak, A.; Rao, K.H. Plant disease diagnosis: Technological advancements and challenges. Indian Phytopathol. 2017, 70, 275–281. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, Q.; Zahr, K.; Harding, M.W.; Feindel, D.; Feng, J. Impact of DNA extraction efficiency on the sensitivity of PCR-based plant disease diagnosis and pathogen quantification. Europ. J. Plant Pathol. 2021, 159, 583–591. [Google Scholar] [CrossRef]
- Buja, I.; Sabella, E.; Monteduro, A.G.; Chiriacò, M.S.; Bellis, L.D.; Luvisi, A.; Maruccio, G. Advances in plant disease detection and monitoring: From traditional assays to in-field diagnostics. Sensors 2021, 21, 2129. [Google Scholar] [CrossRef]
- Martins, L.; Fernandes, C.; Albuquerque, P.M.; Tavares, F. Assessment of Xanthomonas arboricola pv. juglandis bacterial load in infected walnut fruits by quantitative PCR. Plant Dis. 2019, 103, 2577–2586. [Google Scholar] [CrossRef]
- Scortichini, M.; Marchesi, U.; Prospero, P.D. Genetic diversity of Xanthomonas arboricola pv. juglandis (synonyms: X. campestris pv. juglandis; X. juglandis pv. juglandis) strains from different geographical areas shown by repetitive polymerase chain reaction genomic fingerprinting. J. Phytopathol. 2001, 149, 325–332. [Google Scholar] [CrossRef]
- Moragrega, C.; Llorente, I. Effects of leaf wetness duration, temperature, and host phenological stage on infection of walnut by Xanthomonas arboricola pv. juglandis. Plants 2023, 12, 2800. [Google Scholar] [CrossRef] [PubMed]
- Manthos, I.; Sotiropoulos, T.; Vagelas, I. Is the artificial pollination of walnut trees with drones able to minimize the presence of Xanthomonas arboricola pv. juglandis? A Review. Appl. Sci. 2024, 14, 2732. [Google Scholar] [CrossRef]
- John, M.A.; Bankole, I.; Ajayi-Moses, O.; Ijila, T.; Jeje, O.; Lalit, P. Relevance of advanced plant disease detection techniques in disease and pest management for ensuring food security and their implication: A Review. Am. J. Plant Sci. 2023, 14, 1260–1295. [Google Scholar] [CrossRef]
- Jafar, A.; Bibi, N.; Naqvi, R.A.; Sadeghi-Niaraki, A.; Jeong, D. Revolutionizing agriculture with artificial intelligence: Plant disease detection methods, applications, and their limitations. Front. Plant Sci. 2024, 15, 1356260. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, L.; Mohapatra, D.; Raghuvanshi, H.R.; Kumar, S.; Kaur, R.; Chawla, R.; Afreen, N. Transforming agriculture through artificial intelligence: Advancements in plant disease detection, applications and challenges. J. Adv. Biol. Biotechnol. 2024, 27, 381–388. [Google Scholar] [CrossRef]
- Mena, E.; Garaycochea, S.; Stewart, S.; Montesano, M.; Ponce de León, I. Comparative genomics of plant pathogenic Diaporthe species and transcriptomics of Diaporthe caulivora during host infection reveal insights into pathogenic strategies of the genus. BMC Genom. 2022, 23, 175. [Google Scholar] [CrossRef]
- Das, S.; Pattanayak, S.; Behera, P.R. Application of machine learning: A recent advancement in plant diseases detection. J. Plant Prot. Res. 2023, 62, 122–135. [Google Scholar] [CrossRef]
- Précigout, P.; Claessen, D.; Makowski, D.; Robert, C. Does the latent period of leaf fungal pathogens reflect their trophic type? A meta-analysis of biotrophs, hemibiotrophs and necrotrophs. Phytopathology 2020, 110, 345–361. [Google Scholar] [CrossRef]
- Routis, G.; Michailidis, M.; Roussaki, I. Plant disease identification using machine learning algorithms on single-board computers in IoT environments. Electronics 2024, 13, 1010. [Google Scholar] [CrossRef]
- Takahashi, H.; Fukuhara, T.; Kitazawa, H.; Kormelink, R. Virus latency and the impact on plants. Front. Microbiol. 2019, 10, 2764. [Google Scholar] [CrossRef]
- Jeger, M.J.; Pautasso, M.; Holdenrieder, O.; Shaw, M.W. Modelling disease spread and control in networks: Implications for plant sciences. New Phytol. 2007, 174, 279–297. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, E.; Caffi, T.; Rossi, V.; Salotti, I.; Fedele, G. Plant disease models and forecasting: Changes in principles and applications over the last 50 years. Phytopathology 2023, 113, 678–693. [Google Scholar] [CrossRef] [PubMed]
- Pezzotti, G. Raman spectroscopy in cell biology and microbiology. J. Raman Spectrosc. 2021, 52, 2348–2443. [Google Scholar] [CrossRef]
- Matthäus, C.; Bird, B.; Miljković, M.; Chernenko, T.; Romeo, M.J.; Diem, M. Chapter 10: Infrared and Raman microscopy in cell biology. Methods Cell Biol. 2008, 89, 275–308. [Google Scholar] [CrossRef] [PubMed]
- Radić, D. Characterization of Microorganisms Using Raman Microscopy. In Application of Molecular Methods and Raman Microscopy/Spectroscopy in Agricultural Sciences and Food Technology; Vucelić Radović, B., Lazić, D., Nikšić, M., Eds.; Ubiquity Press: London, UK, 2019; pp. 161–165. [Google Scholar] [CrossRef]
- Shigeto, S.; Takeshita, N. Raman microspectroscopy and imaging of filamentous fungi. Microbes Environ. 2022, 37, ME22006. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Zhang, S.; Yue, S. Raman spectroscopy and imaging for cancer diagnosis. J. Healthc. Eng. 2018, 2018, 8619342. [Google Scholar] [CrossRef]
- Hanna, K.; Krzoska, E.; Shaaban, A.M.; Muirhead, D.K.; Abu-Eid, R.; Speirs, V. Raman spectroscopy: Current applications in breast cancer diagnosis, challenges and future prospects. Br. J. Cancer 2022, 126, 1125–1139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Z.; Gao, B.; Zhang, F.; Tian, L.; Zeng, H.; Wang, S. Raman microspectroscopy based TNM staging and grading of breast cancer. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 285, 121937. [Google Scholar] [CrossRef]
- Vriens, L. Raman scattering cross sections for In and T1 atoms and multiphoton processes in Sr. Opt. Commun. 1974, 11, 396–401. [Google Scholar] [CrossRef]
- Langer, J.; Aberasturi, D.J.; Aizpurua, J.; Álvarez-Puebla, R.A.; Auguié, B.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.; Boisen, A.; et al. Present and future of surface-enhanced Raman scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef]
- Dodo, K.; Fujita, K.; Sodeoka, M. Raman spectroscopy for chemical biology research. J. Am. Chem. Soc. 2022, 144, 19651–19667. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Visualizing Cells. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. Available online: https://www.ncbi.nlm.nih.gov/books/NBK21048/ (accessed on 1 June 2024).
- Cuny, A.P.; Schlottmann, F.P.; Ewald, J.C.; Pelet, S.; Schmoller, K.M. Live cell microscopy: From image to insight. Biophys. Rev. 2022, 3, 021302. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, H.; Hobson, C.M.; Chew, T.; Aaron, J.S. Imagining the future of optical microscopy: Everything, everywhere, all at once. Commun. Biol. 2023, 6, 1096. [Google Scholar] [CrossRef] [PubMed]
- Gierlinger, N.; Keplinger, T.; Harrington, M. Imaging of plant cell walls by confocal Raman microscopy. Nat. Protoc. 2012, 7, 1694–1708. [Google Scholar] [CrossRef] [PubMed]
- Lević, S. Materials Characterization by Raman Microscopy. In Application of Molecular Methods and Raman Microscopy/Spectroscopy in Agricultural Sciences and Food Technology; Vucelić Radović, B., Lazić, D., Nikšić, M., Eds.; Ubiquity Press: London, UK, 2019. [Google Scholar] [CrossRef]
- Mateu, B.P.; Bock, P.; Gierlinger, N. Raman imaging of plant cell walls. Methods Mol. Biol. 2020, 2149, 251–295. [Google Scholar] [CrossRef] [PubMed]
- Nitta, N.; Iino, T.; Isozaki, A.; Yamagishi, M.; Kitahama, Y.; Sakuma, S.; Suzuki, Y.; Tezuka, H.; Oikawa, M.; Arai, F.; et al. Raman image-activated cell sorting. Nat. Commun. 2020, 11, 3452. [Google Scholar] [CrossRef] [PubMed]
- Derely, L.; Collart Dutilleul, P.; Michotte de Welle, S.; Szabo, V.; Gergely, C.; Cuisinier, F. Raman confocal microscopy and AFM combined studies of cancerous cells treated with Paclitaxel. In Proceedings of the Nanoscale Imaging, Sensing, and Actuation for Biomedical Applications, Event: SPIE BiOS, San Francisco, CA, USA, 22–27 January 2011; Volume 7908. [Google Scholar] [CrossRef]
- Yan, S.; Cui, S.; Ke, K.; Zhao, B.; Liu, X.; Yue, S.; Wang, P. Hyperspectral stimulated Raman scattering microscopy unravels aberrant accumulation of saturated fat in human liver cancer. Anal. Chem. 2018, 90, 6362–6366. [Google Scholar] [CrossRef]
- Ramesh, G.; Paul, W.; Valparambil Puthanveetil, V.; Raja, K.; Embekkat Kaviyil, J. Raman spectroscopy as a novel technique for the identification of pathogens in a clinical microbiology laboratory. Spectrosc. Lett. 2022, 55, 546–551. [Google Scholar] [CrossRef]
- Dinçtürk, E. Determination of Raman spectrum under different culture conditions: Preliminary research on bacterial fish pathogens. Anim. Biotechnol. 2024, 35, 2299733. [Google Scholar] [CrossRef]
- Wang, L.; Liu, W.W.; Tang, J.; Wang, J.; Liu, Q.; Wen, P.; Wang, M.; Pan, Y.; Gu, B.; Zhang, X. Applications of Raman spectroscopy in bacterial infections: Principles, advantages, and shortcomings. Front. Microbiol. 2021, 12, 683580. [Google Scholar] [CrossRef]
- Rebrošová, K.; Samek, O.; Kizovský, M.; Bernatová, S.; Holá, V.; Růžička, F. Raman spectroscopy—A novel method for identification and characterization of microbes on a single-cell level in clinical settings. Front. Cell. Infect. Microbiol. 2022, 12, 866463. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Zhu, D.; Ji, Y.; Wang, T.; McIlvenna, D.; Yin, H.; Xu, J.; Huang, W.E. Probing and sorting single cells—The application of a Raman-activated cell sorter. Spectrosc. Eur. 2013, 25, 16–20. [Google Scholar]
- Yuan, Y.; Wang, D.; Zhang, J.; Liu, J.; Chen, J.; Zhang, X. Raman spectra of the GFP-like fluorescent proteins. Biophys. Rep. 2018, 4, 265–272. [Google Scholar] [CrossRef]
- Auner, G.W.; Koya, S.K.; Huang, C.; Broadbent, B.; Trexler, M.; Auner, Z.; Elias, A.; Mehne, K.C.; Brusatori, M.A. Applications of Raman spectroscopy in cancer diagnosis. Cancer Metastasis Rev. 2018, 37, 691–717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, M.; Yang, G.; Liu, X.; Yu, Z.; Peng, S. Protocatechuic acid, ferulic acid and relevant defense enzymes correlate closely with walnut resistance to Xanthomonas arboricola pv. juglandis. BMC Plant Biol. 2022, 22, 598. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Chen, Y.; Li, J.; Wang, H.; Ning, T.; Wang, S. A graphical user interface (NWUSA) for Raman spectral processing, analysis and feature recognition. J. Biophotonisc. 2021, 14, e202000456. [Google Scholar] [CrossRef]
- Vagelas, I.; Leontopoulos, S. A bibliometric analysis and a citation mapping process for the role of soil recycled organic matter and microbe interaction due to climate change using Scopus database. AgriEngineering 2023, 5, 581–610. [Google Scholar] [CrossRef]
- Lykas, C.; Vagelas, I. Innovations in agriculture for sustainable agro-systems. Agronomy 2023, 13, 2309. [Google Scholar] [CrossRef]
- Sánchez, L.R.; Pant, S.R.; Mandadi, K.K.; Kurouski, D. Raman spectroscopy vs quantitative polymerase chain reaction in early stage Huanglongbing diagnostics. Sci. Rep. 2020, 10, 10101. [Google Scholar] [CrossRef]
- Sánchez, L.R.; Pant, S.R.; Xing, Z.; Mandadi, K.K.; Kurouski, D. Rapid and noninvasive diagnostics of Huanglongbing and nutrient deficits on citrus trees with a handheld Raman spectrometer. Anal. Bioanal. Chem. 2019, 411, 3125–3133. [Google Scholar] [CrossRef] [PubMed]
- Meena, M.K.; Zehra, A.; Swapnil, P.; Dubey, M.K.; Patel, C.B.; Upadhyay, R.S. Effect on lycopene, β-carotene, ascorbic acid and phenolic content in tomato fruits infected by Alternaria alternata and its toxins (TeA, AOH and AME). Arch. Phytopathol. Plant Protect. 2017, 50, 317–329. [Google Scholar] [CrossRef]
- Jiang, S.; Han, S.; He, D.; Cao, G.; Fang, K.; Xiao, X.; Yi, J.; Wan, X. The accumulation of phenolic compounds and increased activities of related enzymes contribute to early defense against walnut blight. Physiol. Mol. Plant Pathol. 2019, 108, 101433. [Google Scholar] [CrossRef]
- Koyama, Y.; Umemoto, Y.; Akamatsu, A.; Uehara, K.; Tanaka, M. Raman spectra of chlorophyll forms. J. Mol. Struct. 1986, 146, 273–287. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, H.; Lin, Y. Construction of Raman spectroscopic fingerprints for the detection of Fusarium wilt of banana in Taiwan. PLoS ONE 2020, 15, e0230330. [Google Scholar] [CrossRef] [PubMed]
- Payne, W.Z.; Kurouski, D. Raman-Based Diagnostics of Biotic and Abiotic Stresses in Plants. A Review. Front. Plant Sci. 2021, 11, 616672. [Google Scholar] [CrossRef] [PubMed]
- Parlamas, S.; Goetze, P.K.; Humpal, D.M.; Kurouski, D.; Jo, Y. Raman Spectroscopy Enables Confirmatory Diagnostics of Fusarium Wilt in Asymptomatic Banana. Front. Plant Sci. 2022, 13, 922254. [Google Scholar] [CrossRef]
- Jehlička, J.; Edwards, H.G.; Osterrothová, K.; Novotná, J.; Nedbalová, L.; Kopecký, J.; Němec, I.; Oren, A. Potential and limits of Raman spectroscopy for carotenoid detection in microorganisms: Implications for astrobiology. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2014, 372, 20140199. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vagelas, I.; Manthos, I.; Sotiropoulos, T. Applications of Raman Microscopy/Spectroscopy-Based Techniques to Plant Disease Diagnosis. Appl. Sci. 2024, 14, 5926. https://doi.org/10.3390/app14135926
Vagelas I, Manthos I, Sotiropoulos T. Applications of Raman Microscopy/Spectroscopy-Based Techniques to Plant Disease Diagnosis. Applied Sciences. 2024; 14(13):5926. https://doi.org/10.3390/app14135926
Chicago/Turabian StyleVagelas, Ioannis, Ioannis Manthos, and Thomas Sotiropoulos. 2024. "Applications of Raman Microscopy/Spectroscopy-Based Techniques to Plant Disease Diagnosis" Applied Sciences 14, no. 13: 5926. https://doi.org/10.3390/app14135926





